Abstract
Background
There are notable changes in the numbers of white blood cells (WBCs) after stroke, but the primary mediators of these changes are unclear. In this study we assessed the role of the neuroendocrine and sympathetic nervous systems in stroke induced changes of WBCs within distinct leukocyte subsets as well as the effect of these changes on stroke outcomes.
Methods
Patients were recruited within 72 hrs after ischemic stroke; complete blood count with differential was obtained at set time points. Relationships between leukocyte numbers, cortisol, adrenocorticotrophic hormone (ACTH), interleukin (IL)-6 and metanephrines were assessed at 72 hrs after stroke. Associations between abnormal leukocyte counts at 72 hrs, post-stroke infection and 3 month outcomes were determined.
Results
A total of 114 subjects were enrolled. Severe stroke was associated with leukocytosis, neutrophilia, monocytosis, lymphopenia and eosinopenia. At 72 hrs after stroke, increased serum cortisol was independently associated with neutrophilia and lymphopenia. Abnormal leukocyte counts were not independently predictive of post-stroke infection, but lymphopenia was associated with poor outcome (modified Rankin Score >3) at 3 months after stroke (OR = 22.86 [1.95, 267.65]; P=0.01).
Conclusions
Increased serum cortisol is independently associated with neutrophilia and lymphopenia after stroke. Lymphopenia is not an independent predictor of infections but is independently associated with worse outcome.
Keywords: leukocytes, lymphocytes, eosinophils, cortisol, metanephrines
A variety of immunologic perturbations occur after stroke, with one of the most notable being that of changes in the numbers of white blood cells (WBCs) within different leukocyte subsets. Data consistently show that stroke induces leukocytosis, which is predominantly driven by an increase polymorphonuclear cells (PMNs), and to a lesser extent, an increase in mononuclear cells.[1, 2] At the same time, there is a decrease in the number of lymphocytes, and the degree of this decrease correlates with stroke severity.[1, 3–6] The decrease in circulating lymphocytes is thought to be mediated by activation of the sympathetic nervous system as well as by an increase in systemic glucocorticoids.[7] Adrenocorticotrophic hormone (ACTH) is produced and secreted by the anterior pituitary gland and is responsible for increasing the production and release of cortisol from the adrenal cortex. Following stroke, however, the elevation in cortisol appears to be partly mediated by IL-6.[8]
A standard leukocyte differential also includes information about the number of eosinophils. There are limited data regarding the contribution of eosinophils to stroke-related outcomes. Recent studies suggest that lower eosinophil numbers after stroke are associated with an increased risk of infection as well as worse outcomes.[9, 10] These studies, however, did not control for stroke severity and have other methodological issues that limit interpretation of the findings.
In this study we assessed the correlation between stroke severity, cell counts within distinct leukocyte subsets, the risk of infection and functional outcome in patients with ischemic stroke. Further, we tested the strength of the association between these cell counts and systemic levels of cortisol, ACTH, IL-6 and metanephrines.
Methods
The patient cohort has been described elsewhere.[5, 11] Briefly, patients with ischemic stroke admitted within 72 hours of symptom onset were enrolled in a prospective study evaluating post-ischemic immune responses. Patients with immunodeficiency (HIV) or on immunomodulatory treatments were excluded. The study was approved by the Institutional Review Board; all patients or their surrogates provided informed consent.
Clinical Data
Stroke severity was determined by the National Institutes of Health Stroke Scale (NIHSS) score and outcome by the modified Rankin Scale (mRS) at 3 months. Intervention was defined as the use of intravenous alteplase or endovascular therapy. Poor outcome was considered to be an mRS>3. Infarct volume was calculated on diffusion weighted MRI by a neuroradiologist using the ABC/2 method.[12]
Laboratory Studies
WBC count and differential was determined by the clinical laboratory. Classification of abnormal cell counts were based on the laboratory’s normative data as follows: WBCs>10,000/μL, PMNs >7,000/μL, lymphocytes <1,000/μL, monocytes >800/μL and eosinophils <50/μL (there is no universally accepted lower limit for eosinophils, this number is thus somewhat arbitrary). The concentrations of ACTH and cortisol were also determined by the clinical laboratory using standard methodologies. Interleukin (IL)-6, was measured with a cytometric bead-based system (Fluorokine MAP; R&D Systems); the lower limit of detection was 1.1 pg/mL. Standard enzyme linked immunoassay (ELISA) was used to determine plasma concentrations of plasma metanephrines (IBL-America; sensitivity = 14.9 pg/mL).
Statistics
Descriptive data are presented as mean ± standard deviation (sd) or median and interquartile range (IQR); group comparisons were performed using ANOVA or the Kruskal-Wallis H test as appropriate. Correlations are presented using Spearman’s rho. Categorical data are compared by χ2. Logistic regression was used to assess the predictors of clinically abnormal leukocyte counts, infection and poor outcome at 3 months after stroke onset. Biologically plausible variables were included in the models. Significance was set at P<0.05.
Results
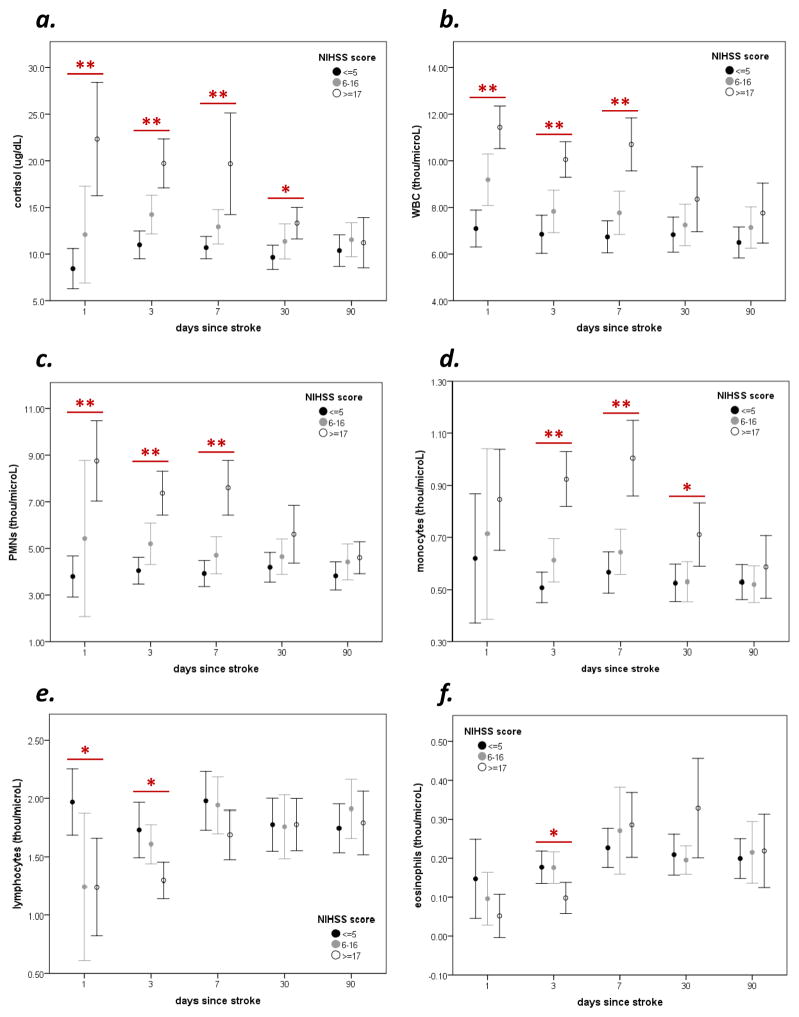
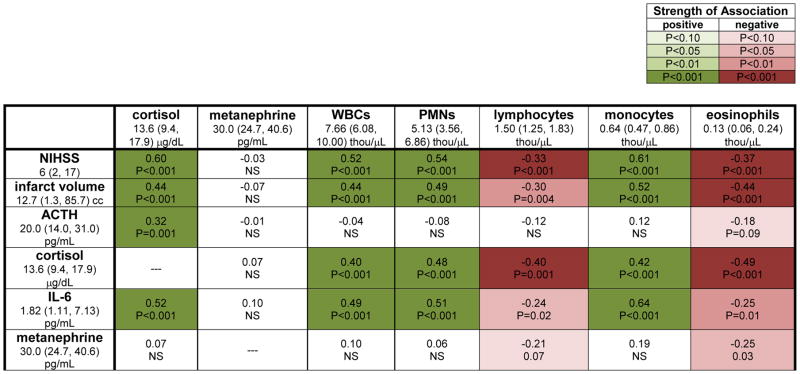
A total of 114 patients were enrolled. There was a prolonged elevation in plasma cortisol in patients with severe stroke (Figure 1a). More severe strokes were also associated with an increase in WBCs (Figure 1b), PMNs Figure 1c) and monocytes Figure 1d) that last for at least a week after stroke onset, as well as a more short-lived decrease in the numbers of lymphocytes and eosinophils (Figure 1e and f). The correlations between ACTH, IL-6, cortisol, metanephrines, WBCs and leukocyte subsets with stroke severity (NIHSS score, infarct volume) at 72 hrs after stroke are shown in Figure 2. (The median value and interquartile range are provided for each biomarker of stress/inflammation.) ACTH and IL-6 are both highly correlated with cortisol levels, which in turn are highly correlated to stroke severity. In general, changes in PMNs and monocytes track together while changes in lymphocytes and eosinophils track together, and all are highly correlated with both cortisol and IL-6, but not ACTH. Metanephrines were inversely correlated with lymphocyte and eosinophil numbers. “Clinically abnormal” cell counts are prevalent on the first day after stroke, while at least 50% of patients with severe stroke continue to manifest abnormal neutrophil and monocyte levels at day 3 after stroke (Table 1); laboratory defined lymphopenia is less common than laboratory defined neutrophilia and monocytosis. Table 2 shows the predictors of “clinically abnormal” cell counts at 3 days after stroke. After controlling for stroke severity (NIHSS and/or infarct volume), cortisol is the only independent predictor of neutrophilia and lymphopenia.
Figure 1.
Patients with severe stroke (NIHSS≥17) experience a prolonged increase in cortisol (a) as well as an increase in the overall numbers of white blood cells (b), neutrophils (d) and monocytes (d). Severe strokes are also associated with a less prolonged decrease in the numbers of lymphocytes (e) and eosinophils (f). Statistics are by ANOVA; *P<0.05, *P<0.01.
Figure 2.
Correlations between leukocyte numbers, cortisol and metanephrines with stroke severity and biomarkers of inflammation/stress at 3 days after stroke. The median value (interquartile range) for each variable at this time point is displayed. Correlations are by Spearman rank order.
Table 1.
Proportion of patients with “clinically abnormal” leukocyte counts* after stroke.
| Days after Stroke: | 1 day | 3 days | 7 days | 30 days | 90 days | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIHSS: | ≤5 | 6–16 | ≥17 | P | ≤5 | 6–16 | ≥17 | P | ≤5 | 6–16 | ≥17 | P | ≤5 | 6–16 | ≥17 | P | ≤5 | 6–16 | ≥17 | P |
| WBCs>10,000 | 2/37 | 13/34 | 28/39 | <0.001 | 3/39 | 7/36 | 17/39 | 0.001 | 2/33 | 7/33 | 17/35 | <0.001 | 2/33 | 3/26 | 9/32 | 0.04 | 1/26 | 1/24 | 3/25 | NS |
| (5%) | (38%) | (72%) | (7%) | (19%) | (44%) | (6%) | (21%) | (49%) | (6%) | (12%) | (28%) | (4%) | (4%) | (12%) | ||||||
| PMNs>7,000 | 0/10 | 1/5 | 8/11 | 0.002 | 1/36 | 8/33 | 16/32 | <0.001 | 1/30 | 4/29 | 13/30 | <0.001 | 2/32 | 1/23 | 7/31 | 0.06 | 1/24 | 2/24 | 2/24 | NS |
| (0) | (20%) | (73%) | (3%) | (24%) | (50%) | (3%) | (14%) | (43%) | (6%) | (4%) | (23%) | (4%) | (8%) | (8%) | ||||||
| lymphocytes<1,000 | 0/10 | 1/5 | 3/11 | NS | 3/36 | 3/33 | 9/32 | 0.04 | 1/30 | 3/29 | 4/30 | NS | 3/31 | 2/23 | 3/31 | NS | 2/24 | 0/24 | 0/24 | NS |
| (0) | (20%) | (27%) | (8%) | (9%) | (28%) | (3%) | (10%) | (13%) | (10%) | (9%) | (10%) | (8%) | (0) | (0) | ||||||
| monocytes>800 | 3/10 | 2/5 | 6/11 | NS | 4/35 | 7/33 | 19/32 | <0.001 | 5/30 | 8/29 | 17/30 | 0.003 | 3/32 | 3/23 | 8/30 | NS | 1/24 | 1/24 | 4/24 | NS |
| (30%) | (40%) | (54%) | (11%) | (21%) | (59%) | (17%) | (28%) | (57%) | (9%) | (13%) | (27%) | (4%) | (4%) | (17%) | ||||||
| eosinophils<50 | 1/10 | 1/5 | 8/11 | 0.008 | 2/36 | 4/33 | 14/31 | <0.001 | 1/30 | 1/29 | 1/29 | NS | 1/32 | 0/23 | 0/30 | NS | 0/24 | 1/24 | 1/24 | NS |
| (10%) | (20%) | (73%) | (6%) | (12%) | (45%) | (3%) | (3%) | (3%) | (3%) | (0) | (0) | (0) | (4%) | (4%) | ||||||
counts are presented as total number per μL, WBCs=white blood cells, PMNs=polymorphonuclear cells, NIHSS=National Institutes of Health Stroke Scale, NS=not significant (P>0.10)
Table 2.
Predictors of abnormal cell counts at day 3 after stroke.
| WBCs >10,000/μL | PMNs >7,000/μL | Lymphocytes <1,000/μL | Monocytes >800/μL | Eosinophils <50/μL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| NIHSS (per point) | 1.12 (1.06–1.18) | <0.001 | 1.13 (1.07–1.20) | <0.001 | 1.08 (1.03–1.15) | 0.004 | 1.15 (1.09–1.22) | <0.001 | 1.15 (1.08–1.22) | <0.001 |
| Total infarct volume (per 10 cc) | 1.05 (1.01, 1.08) | 0.01 | 1.01 (1.00, 1.01) | <0.001 | 1.08 (1.03, 1.13) | 0.001 | 1.10 (1.05, 1.16) | <0.001 | 1.12 (1.06, 1.18) | <0.001 |
| Cortisol (per μg/dL) | 1.15 (1.07–1.24) | <0.001 | 1.16 (1.08–1.26) | <0.001 | 1.14 (1.05–1.23) | 0.001 | 1.14 (1.06–1.23) | <0.001 | 1.16 (1.07–1.26) | <0.001 |
| ACTH (per pg/mL) | 1.01 (0.99–1.04) | NS | 1.00 (0.98–1.03) | NS | 1.02 (0.99–1.04) | NS | 1.01 (0.99–1.03) | NS | 1.01 (0.98–1.03) | NS |
| IL-6 (per pg/mL) | 1.03 (1.00–1.06) | 0.02 | 1.03 (1.00–1.05) | 0.02 | 1.02 (1.00–1.04) | 0.03 | 1.04 (1.01–1.08) | 0.02 | 1.04 (1.01–1.07) | 0.01 |
| Metanephrines (per pg/mL) | 0.99 (0.95–1.04) | NS | 0.92 (0.86–0.0.99) | 0.02 | 1.00 (0.94–1.06) | NS | 1.00 (0.96–1.04) | NS | 0.99 (0.94, 1.05) | NS |
| Multivariate – Model 1 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| NIHSS (per point) | 1.09 (1.00–1.18) | 0.04 | 1.14 (1.03, 1.26) | 0.009 | 1.04 (0.94–1.15) | NS | 1.17 (1.07–1.29) | <0.001 | 1.14 (1.04, 1.25) | 0.004 |
| Cortisol (per μg/dL) | 1.10 (1.00–1.22) | 0.05 | 1.17 (1.03, 1.32) | 0.02 | 1.18 (1.05–1.34) | 0.007 | 1.05 (0.94–1.17) | NS | 1.05 (0.94, 1.18) | NS |
| IL-6 | 1.00 (0.97–1.03) | NS | 0.98 (0.95–1.02) | NS | 0.99 (0.96–1.02) | NS | 0.99 (0.96–1.03) | NS | 1.01 (0.97–1.05) | NS |
| Metanephrines (per pg/mL) | 1.01 (0.95–1.06) | NS | 0.92 (0.84–1.02) | NS | 1.01 (0.94–1.08) | NS | 1.02 (0.96–1.08) | NS | 1.00 (0.94, 1.07) | NS |
| Multivariate – Model 2 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Total infarct volume | 1.00 (0.94, 1.06) | NS | 1.04 (0.98, 1.11) | NS | 1.06 (1.00, 1.13) | <0.05 | 1.09 (1.02, 1.16) | 0.010 | 1.12 (1.05, 1.21) | 0.001 |
| Cortisol (per μg/dL) | 1.14 (1.04–1.27) | 0.08 | 1.20 (1.06, 1.35) | 0.004 | 1.17 (1.03–1.32) | 0.01 | 1.07 (0.97, 1.19) | NS | 1.05 (0.94, 1.18) | NS |
| IL-6 | 1.01 (0.98–1.03) | NS | 1.00 (0.97–1.03) | NS | 0.99 (0.96–1.02) | NS | 1.01 (0.98–1.05) | NS | 1.02 (0.99–1.06) | NS |
| Metanephrines (per pg/mL) | 0.99 (0.94–1.05) | NS | 0.90 (0.81–1.00) | 0.04 | 1.01 (0.94–1.08) | NS | 1.01 (0.96–1.06) | NS | 1.00 (0.94, 1.07) | NS |
| Multivariate – Model 3 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| NIHSS (per point) | 1.14 (1.03, 1.26) | 0.01 | 1.16 (1.03, 1.32) | 0.02 | 0.91 (0.74, 1.11) | NS | 1.17 (1.05, 1.31) | 0.006 | 1.03 (0.89, 1.19) | NS |
| Total infarct volume (per 10 cc) | 0.94 (0.87, 1.02) | NS | 0.98 (0.90, 1.06) | NS | 1.11 (0.98, 1.26) | 0.09 | 1.00 (0.93, 1.08) | NS | 1.10 (1.00, 1.23) | 0.06 |
| Cortisol (per μg/dL) | 1.11 (1.01–1.24) | 0.03 | 1.17 (1.03, 1.32) | 0.01 | 1.19 (1.04–1.35) | 0.009 | 1.05 (0.95, 1.18) | NS | 1.05 (0.93, 1.18) | NS |
| IL-6 | 1.00 (0.97–1.03) | NS | 0.98 (0.95–1.02) | NS | 1.00 (0.96–1.05) | NS | 0.99 (0.96–1.03) | NS | 1.02 (0.98–1.06) | NS |
| Metanephrines (per pg/mL) | 1.00 (0.95–1.06) | NS | 0.93 (0.84–1.02) | NS | 1.00 (0.93–1.08) | NS | 1.02 (0.93, 1.07) | NS | 1.00 (0.94, 1.07) | NS |
| Multivariate – Model 4 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Cortisol (per μg/dL) | 1.15 (1.04–1.26) | 0.005 | 1.22 (1.08–1.37) | 0.001 | 1.20 (1.07–1.35) | 0.002 | 1.10 (1.01–1.22) | 0.03 | 1.10 (1.00–1.22) | 0.06 |
| IL-6 | 1.01 (0.98–1.04) | NS | 1.00 (0.97–1.03) | NS | 0.99 (0.96–1.02) | NS | 1.02 (0.98–1.05) | NS | 1.03 (0.99–1.06) | NS |
| Metanephrines (per pg/mL) | 1.00 (0.94–1.05) | NS | 0.89 (0.81–0.99) | 0.03 | 1.00 (0.93–1.07) | NS | 1.00 (0.95–1.05) | NS | 0.98 (0.92–1.05) | NS |
OR=odds ratio, CI=confidence interval, NIHSS=National Institutes of Health Stroke Scale, NS=not significant (P>0.10)
Of the 114 patients enrolled in the study, 7 developed an infection in the first 3 days after stroke and 1 died in the week after presentation. Of the remaining patients 106 patients, 22 (21%) developed an infection by day 15.[5] “Clinically abnormal” cells counts at day 3 were not independently predictive of infection (Table 3). For the 94 patients for whom 3 month follow-up was available, 20 (21%) had poor outcome (mRS>3), and lymphopenia at day 3 was independently predictive of poor outcome at day 90 after stroke (Table 3).
Table 3.
Abnormal cell counts at day 3 after stroke and their ability to predict infection and good outcome. Data are displayed as the odds ratio (OR) and 95% confidence interval (CI).
| Infection by Day 15* | uncontrolled | controlled for NIHSS | controlled for NIHSS and age | controlled for NIHSS, age and cortisol | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| WBCs | 5.46 | 0.001 | 1.89 | NS | 1.98 | NS | 1.92 | NS | ||
| (>10,000/μL) | (1.96, 15.20) | (0.52, 6.89) | (0.52, 7.52) | (0.45, 8.24) | ||||||
| PMNs | 3.92 | 0.01 | 0.94 | NS | 0.94 | NS | 0.65 | NS | ||
| (>7,000/μL) | (1.34, 11.44) | (0.23, 3.88) | (0.21, 4.27) | (0.12, 3.54) | ||||||
| lymphocytes | 2.99 | 0.09 | 1.42 | NS | 1.02 | NS | 0.58 | NS | ||
| (<1,000/μL) | (0.85, 10.51) | (0.24, 6.42) | (0.16, 6.43) | (0.07, 5.01) | ||||||
| monocytes | 8.52 | <0.001 | 2.57 | NS | 2.78 | NS | 2.08 | NS | ||
| (>800/μL) | (2.78, 26.15) | (0.65, 10.11) | (0.65, 11.94) | (0.43, 10.15) | ||||||
| eosinophils | 4.66 | 0.008 | 0.76 | NS | 0.97 | NS | 0.53 | NS | ||
| (<50/μL) | (1.51, 14.39) | (0.16, 1.29) | (0.17, 5.67) | (0.07, 3.88) | ||||||
| Bad Outcome (mRS>3) at 90 days | uncontrolled | controlled for NIHSS | controlled for NIHSS and age | controlled for NIHSS, age and cortisol | controlled for NIHSS, age cortisol and infection | |||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| WBCs | 5.73 | 0.002 | 1.39 | NS | 1.74 | NS | 1.43 | NS | 0.73 | NS |
| (>10,000/μL) | (1.93, 16.96) | (0.31, 6.12) | (0.34, 8.73) | (0.25, 8.26) | (0.10, 5.66) | |||||
| PMNs | 5.83 | 0.003 | 1.27 | NS | 1.41 | NS | 1.22 | NS | 0.99 | NS |
| (>7,000/μL) | (1.84, 18.42) | (0.27, 5.92) | (0.29, 7.00) | (0.22, 6.72) | (0.14, 6.89) | |||||
| lymphocytes | 11.20 | <0.001 | 15.57 | 0.01 | 17.21 | 0.009 | 17.74 | 0.01 | 22.86 | 0.01 |
| (<1,000/μL) | (3.00, 41.83) | (1.94, 124.68) | (2.06, 144.00) | (1.79, 138.81) | (1.95, 267.65) | |||||
| monocytes | 9.97 | <0.001 | 1.22 | NS | 1.15 | NS | 0.78 | NS | 0.44 | NS |
| (>800/μL) | (2.98, 33.31) | (0.23, 6.55) | (0.20, 6.44) | (0.12, 5.19) | (0.05, 4.13) | |||||
| eosinophils | 5.10 | 0.008 | 0.64 | NS | 0.71 | NS | 0.49 | NS | 0.51 | NS |
| (<50/μL) | (1.52, 17.12) | (0.11, 3.68) | (0.12, 4.40) | (0.07, 3.32) | (0.06, 4.30) | |||||
NIHSS=National Institutes of Health Stroke Scale score, WBCs=white blood cells, PMNs=polymorphonuclear cells, NS=not significant (P>0.10).
patients with infection up to day 3 are excluded
Discussion
Changes in leukocyte numbers after stroke are well described. Most studies, however, tend to look at changes in cell counts as a continuous variable, yet the clinical implications of changes within the normal clinical range are unclear. For instance, it would seem unlikely that a decrease in lymphocyte count from 1,500/μL to 1,400/μL (or 1,300/μL) would be independently associated with a change in the risk of infection (or any other outcome). We thus chose to evaluate changes in leukocyte counts categorically – within laboratory norms or not. We also aimed to determine the most important factors associated with those changes – including clinical stroke severity (NIHSS and infarct volume), serum cortisol and metanephrines.
Clinical stroke severity and cortisol appear to be the primary driver of neutrophilia. The relationship between glucocorticoids and neutrophilia has been appreciated for decades and is thought to be secondary to a demargination of polymorphonuclear cells from the bone marrow.[13, 14] Data also suggest that an increase in glucocorticoids leads to an increase in monocyte counts, although the mechanism is not clear.[14, 15] We did not find an independent link between cortisol and elevated monocyte counts in our study.
Cortisol was the most important mediator of lymphopenia in our patient cohort. We did not find a relationship between plasma metanephrines and lymphocyte counts, while other studies that found lymphocyte numbers to be less in patients with higher circulating normetanephrines.[2, 16] That we did not find an independent effect of metanephrines on lymphocyte numbers could be related to a different effect of metanephrines versus normetanephrines on lymphocyte counts, different time points for metanephrines/normetanephrine measurement in the studies, different patient populations being studied, and/or different types of treatments received by patients in the immediate post-stroke period. For instance, because lymphocytes express β2 receptors[7, 17], it is possible that early use of β-blockers might affect lymphocyte numbers (and function). Similar to our findings, Mracsko and colleagues found that an increase in glucocorticoids was more important in causing post-stroke lymphopenia than activation of the sympathetic nervous system in an animal model of severe stroke.[7] And unlike the findings from other studies[2, 16]. we found no link between plasma metanephrines and infection risk
A link between post-stroke lymphopenia and an increased risk of infection has been well described.[1, 3–5] It is difficult, however, to separate the contribution of the severe stroke that predisposes to infection (by virtue of aspiration or instrumentation), from the activation of the sympathetic nervous system and neuroendocrine system that induce lymphopenia (which are both driven by stroke severity), and the lymphopenia itself. We tried to determine the most important contributors to infection in our patient cohort by building different multivariate models, but could not demonstrate an independent effect of lymphopenia on infection risk. Post-stroke infections are generally bacterial pneumonias (PNAs) and urinary tract infections (UTIs).[5] Most data suggest that it is neutropenia, as opposed to lymphopenia, that is associated with an increased risk of bacterial infections.[18] In patients with lymphopenia due to therapeutic myelosuppression (for treatment of malignancy or autoimmune disease) or acquired immunodeficiency (like HIV), it is unusual viral and fungal infections (ie. opportunistic infections), not bacterial PNAs and UTIs, that are of concern. Further, individuals treated chronically with natalizumab, an antibody that binds α4 and inhibits lymphocyte adhesion and migration to sites of inflammation, are not at increased risk of bacterial infection.[19] And finally, a recent randomized controlled trial of natalizumab for treatment of acute ischemic stroke found no increase in infection in anti-α4 treated patients.[20] The relative numbers of CD4+ cells to other types of lymphocytes may provide a more accurate assessment of infection risk, but in general, hospital acquired bacterial infections would be seemingly unusual in patients who experience only a transient decrease in lymphocyte counts. Lymphocyte function, as opposed to lymphocyte number, might also be an important predictor of post-stroke infection[21], but because stroke severity is intrinsically linked to lymphocyte dysfunction, it is again difficult to attribute any increase in the risk of infection to lymphocyte dysfunction as opposed to those risks associated with the severe stroke (ie. aspiration and instrumentation).
Surprisingly, we found that lymphopenia at day 3 after stroke was independently associated with poor outcome at 3 months. To our knowledge, the association between post-stroke lymphopenia and stroke outcome has not previously been reported. While lymphopenia may be just a marker of stroke severity, the multivariate model should control for this association. Lymphocytes are a heterogeneous group of cells that include T cells (CD4+ and CD8+), NK cells and B cells; these cells may also function as TH1, TH2, TH17 or TREG cells. Data suggest that regulatory T cells limit inflammation and might be important for stroke recovery, so a decrease in these cell numbers could have long-term consequences.[22, 23] The subclass of lymphocytes most affected by stroke is therefore likely to be of importance.
Eosinophils comprise less than 6% of white blood cells and have a very short life span (~36 hrs).[24] Eosinophils have been traditionally regarded as playing a role in combating parasites and in mediating allergic reactions, but an expanded role of eosinophils in the immune response is now appreciated.[25] These cells have the ability to respond to danger signals and serve as antigen presenting cells. They may also be involved in tissue repair and remodeling, as well as in modulation of the adaptive immune response. A rapid decrease in eosinophil numbers can be induced by infusion of either glucocorticoids [26, 27] or adrenaline.[28] The consequences of eosinopenia, which is variably defined, are unclear. Eosinopenia is common in sepsis and is linked to worse outcome/increased mortality.[29–31] Eosinopenia is also associated with worse outcome following traumatic ICH in children.[32]
There are scant data regarding changes in eosinophils in stroke. In a relatively small study of 50 patients with ischemic stroke, Hug and colleagues found no changes in circulating eosinophil counts.[1] Another study found that insular strokes were more likely to lead to a decrease in eosinophils than non-insular strokes.[33] More recent publications show that patients with lower eosinophil counts after stroke have more functional impairment [10] as well as increased rates of infection and increased mortality.[9] While these studies were large, methodological issues (ie. failure to control for stroke severity) limit the interpretation of the data. Given the effect of glucocorticoids and adrenaline on eosinophil counts[26–28], it is not surprising that we saw decreases in eosinophil counts in subjects with severe strokes (and higher cortisol levels).[26–28] And as for the situation with lymphopenia, parsing out the relative contributions of eosinopenia to stroke outcome from that of the stroke severity (and the hypercortisolemia and activation of the sympathetic nervous system which may the eosinopenia), is difficult.
In summary, we found that severe stroke is associated with neutrophilia and monocytosis as well as lymphopenia and eosinopenia. Cortisol was more important than metanephrines in driving these changes in WBC counts after stroke. We also identified transient eosinopenia to be a common sequela of severe stroke. Our data call into question an independent role for lymphopenia in the risk of post-stroke infection as well as the importance of the sympathetic nervous system in driving lymphopenia or predisposing to post-stroke infection. Finally, lymphopenia appears to be an independent predictor of poor outcome, a finding that deserves further evaluation.
Acknowledgments
Funding
This study was funded by National Institutes of Neurological Disorders and Stroke R01 NS049197.
Footnotes
Disclosures and Conflicts of Interest
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–32. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- 2.Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–8. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- 3.Vogelgesang A, Grunwald U, Langner S, Jack R, Broker BM, Kessler C, et al. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39:237–41. doi: 10.1161/STROKEAHA.107.493635. [DOI] [PubMed] [Google Scholar]
- 4.Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. 2009;158:1174–83. doi: 10.1016/j.neuroscience.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, et al. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14:244–52. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Raedt S, De Vos A, Van Binst AM, De Waele M, Coomans D, Buyl R, et al. High natural killer cell number might identify stroke patients at risk of developing infections. Neurol Neuroimmunol Neuroinflamm. 2015;2:e71. doi: 10.1212/NXI.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mracsko E, Liesz A, Karcher S, Zorn M, Bari F, Veltkamp R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun. 2014;41:200–9. doi: 10.1016/j.bbi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Johansson A, Olsson T, Carlberg B, Karlsson K, Fagerlund M. Hypercortisolism after stroke--partly cytokine-mediated? J Neurol Sci. 1997;147:43–7. doi: 10.1016/s0022-510x(96)05308-7. [DOI] [PubMed] [Google Scholar]
- 9.Hori YS, Kodera S, Sato Y, Shiojiri T. Eosinopenia as a Predictive Factor of the Short-Term Risk of Mortality and Infection after Acute Cerebral Infarction. J Stroke Cerebrovasc Dis. 2016 doi: 10.1016/j.jstrokecerebrovasdis.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Guo LB, Liu S, Zhang F, Mao GS, Sun LZ, Liu Y. The role of eosinophils in stroke: a pilot study. Eur Rev Med Pharmacol Sci. 2015;19:3643–8. [PubMed] [Google Scholar]
- 11.Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42:2763–9. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cream JJ. Prednisolone-induced granulocytosis. Br J Haematol. 1968;15:259–67. doi: 10.1111/j.1365-2141.1968.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 14.Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71:773–8. doi: 10.1016/0002-9343(81)90363-6. [DOI] [PubMed] [Google Scholar]
- 15.Barker S, Scott M, Chan GT. Corticosteroids and monocytosis. N Z Med J. 2012;125:76–8. [PubMed] [Google Scholar]
- 16.Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Gomez-Choco M, et al. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, et al. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–9. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estcourt LJ, Stanworth SJ, Hopewell S, Doree C, Trivella M, Massey E. Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. 2016;4:CD005339. doi: 10.1002/14651858.CD005339.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopylov U, Afif W. Risk of infections with biological agents. Gastroenterol Clin North Am. 2014;43:509–24. doi: 10.1016/j.gtc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16:217–26. doi: 10.1016/S1474-4422(16)30357-X. [DOI] [PubMed] [Google Scholar]
- 21.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–36. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–9. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 23.Liesz A, Zhou W, Na SY, Hammerling GJ, Garbi N, Karcher S, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci. 2013;33:17350–62. doi: 10.1523/JNEUROSCI.4901-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohnmacht C, Pullner A, van Rooijen N, Voehringer D. Analysis of eosinophil turnover in vivo reveals their active recruitment to and prolonged survival in the peritoneal cavity. J Immunol. 2007;179:4766–74. doi: 10.4049/jimmunol.179.7.4766. [DOI] [PubMed] [Google Scholar]
- 25.Ravin KA, Loy M. The Eosinophil in Infection. Clin Rev Allergy Immunol. 2016;50:214–27. doi: 10.1007/s12016-015-8525-4. [DOI] [PubMed] [Google Scholar]
- 26.Sabag N, Castrillon MA, Tchernitchin A. Cortisol-induced migration of eosinophil leukocytes to lymphoid organs. Experientia. 1978;34:666–7. doi: 10.1007/BF01937022. [DOI] [PubMed] [Google Scholar]
- 27.Sevitt S. The spleen and blood eosinopenia. J Clin Pathol. 1955;8:42–6. doi: 10.1136/jcp.8.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godlowski ZZ. Eosinopenia caused by adrenaline infusion and by insulin hypoglycaemia. Br Med J. 1948;1:46–8. doi: 10.1136/bmj.1.4540.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaaban H, Daniel S, Sison R, Slim J, Perez G. Eosinopenia: Is it a good marker of sepsis in comparison to procalcitonin and C-reactive protein levels for patients admitted to a critical care unit in an urban hospital? J Crit Care. 2010;25:570–5. doi: 10.1016/j.jcrc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12:R59. doi: 10.1186/cc6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS ONE. 2012;7:e42860. doi: 10.1371/journal.pone.0042860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hori YS, Fukuhara T, Aoi M, Namba Y. Eosinopenia in Children following Traumatic Intracranial Hemorrhage Is Associated with Poor Prognosis and Prolonged Hospital Admission. Pediatr Neurosurg. 2016;51:57–60. doi: 10.1159/000441390. [DOI] [PubMed] [Google Scholar]
- 33.Walter U, Kolbaske S, Patejdl R, Steinhagen V, Abu-Mugheisib M, Grossmann A, et al. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol. 2013;20:153–9. doi: 10.1111/j.1468-1331.2012.03818.x. [DOI] [PubMed] [Google Scholar]