Abstract
Background
Patients with heart failure with reduced ejection fraction (HFrEF) exhibit lower efficiency, dyspnea, and diminished peak O2 uptake (VO2peak) during exercise. Dietary nitrate (NO3-), a source of nitric oxide (NO), has improved these measures in some studies of other populations. We determined the effects of acute NO3- ingestion on exercise responses in eight patients with HFrEF using a randomized, double-blind, placebo-controlled, crossover design.
Methods and Results
Plasma NO3-, nitrite (NO2-), and breath NO were measured at multiple time points and respiratory gas exchange was determined during exercise after ingestion of beetroot juice containing or devoid of 11.2 mmol of NO3-. NO3- intake increased (P<0.05-0.001) plasma NO3- and NO2- and breath NO by 1469±245, 105±34, and 60±18%, respectively. Efficiency and ventilation during exercise were unchanged. However, NO3- ingestion increased (P<0.05) VO2peak by 8±2%, i.e., from 21.4±2.1 to 23.0±2.3 mL.min-1.kg-1. Time to fatigue improved (P<0.05) by 7±3 %, i.e., from 582±84 to 612±81 s.
Conclusions
Acute dietary NO3- intake increases VO2peak and performance in patients with HFrEF. These data, in conjunction with our recent data demonstrating that dietary NO3- also improves muscle contractile function, suggest that dietary NO3- supplementation may be a valuable means of enhancing exercise capacity in this population.
Keywords: Nitric oxide, heart failure, VO2peak, exercise
Introduction
Tens of millions of men and women around the world suffer from heart failure (HF), a disabling and often deadly affliction (2). In approximately half of all such individuals, the ejection fraction (EF) of the heart is reduced (2). However, regardless of the precise nature or etiology of the disease, i.e., HF with reduced EF (HFrEF) or HF with preserved EF (HFpEF), patients with HF exhibit dyspnea and diminished peak oxygen (O2) uptake (VO2peak) during exercise (18,19). Along with declines in maximal muscle speed and power (12), these abnormalities in aerobic exercise responses play a major role in the disability, loss of independence, and reduced quality of life that accompany HF. Perhaps more importantly, elevations in ventilatory demand and decreases in VO2peak (and in skeletal muscle contractile function (22)) are highly predictive of mortality in patients with HF (3,13,26,40).
One factor contributing to the exercise intolerance of HF – especially HFrEF - may be a reduction in nitric oxide (NO) signaling. Along with its well-recognized role as a vasodilator, NO modulates numerous other physiological functions relevant to exercise performance, e.g., muscle contractility (10,11,12,41). There is considerable evidence, however, that NO bioavailability is diminished in HFrEF, as a result of both reduced NO production via the NO synthase (NOS) pathway (45) and more rapid destruction of NO due to increased oxidative stress (33). For example, endothelial dysfunction is common in HFrEF (48), indicative of blunted NO activity. Breath NO levels, a biomarker of whole-body NO production, are also lower in patients with HFrEF (1,8,9), as are the circulating concentrations of nitrite (NO2-) (31,46), the immediate degradation product of NO. The conversion of 15N-labeled arginine to 15N-labeled nitrate (NO3-) has also been shown to be diminished in patients with HFrEF (24), demonstrating directly that NOS-mediated NO production is impaired. Finally, increasing NO bioavaiability via L-arginine supplementation has been shown to improve 6 min walk distance in patients with HFrEF (39). Collectively, these data suggest that reduced NO signaling in HFrEF may contribute to the altered exercise responses described above.
Although most of the NO in the body is produced via the NOS pathway, it is now recognized that production of NO from dietary NO3- is an important source as well (28). In this enterosalivary pathway, ingested NO3- is reduced to NO2- with aid of the mouth microbiota and then to NO in the tissues via a number of endogenous catalysts (43). This last step is enhanced at low pO2 and low pH, conditions that regularly exist in exercising muscle. A number of studies have therefore examined the effects of dietary NO3- supplementation, often in the form of beetroot juice (BRJ), on physiological responses and performance during exercise (23). Many, but not all, of these studies have reported that NO3- ingestion can enhance efficiency, reduce ventilatory demands, and/or increase performance during exercise in at least some populations, including patients with HFpEF (15,50,51). On the other hand, results of previous studies of patients with HFrEF (20,25) have been equivocal.
The purpose of the present proof-of-concept study was to test the hypothesis that acute dietary NO3- intake would reduce ventilatory demands, increase VO2peak, and improve exercise performance in patients with HFrEF. We chose to study patients with HFrEF instead of HFpEF because evidence of reduced NO bioavailability is strongest in this population (vida supra). We focused specifically on ventilatory responses and VO2peak because of their importance as determinants of exercise capacity and predictors of survival in patients with HFrEF (3,13,26,40). Furthermore, since improvements in economy or efficiency are believed to be an important mechanism by which dietary NO3- enhances performance in other subject groups (5,36), we designed our study to carefully quantify not only gross but also delta efficiency during exercise, as the latter is a more direct indicator of muscle contractile efficiency (41).
Materials and Methods
Subjects
The subjects in this study were patients ≥18 y of age with HFrEF (i.e., EF <45%) who were on stable medical therapy (i.e., no addition, removal, or change in medication dose of >100% in the last 3 mo). Each underwent a physical exam, medical history, and blood tests for fasting chemistries. In addition, to document the presence of HFrEF a resting echocardiogram was obtained from those who had not undergone cardiac imaging for clinical purposes in the last 12 mo. Subjects were excluded if they had major organ system disease or dysfunction other than HF, were pregnant, smoked, or had significant orthopedic limitations or other contraindications to exercise. In addition, subjects using antacids or proton pump, xanthine oxidase, or phosphodiesterase inhibitors (e.g., sildenafil) were excluded, as these can affect reduction of NO3- and NO2- to NO (30). Finally, subjects treated with organic nitrates (e.g., trinitroglycerin) were also excluded. After screening of 33 subjects, 10 subjects were enrolled in the study, with eight completing the entire protocol as planned (Fig. 1). One subject was unable to achieve a steady-state at even 20 W, such that their gross and delta efficiency and VT could not be determined. Data from another subject were excluded when subsequent analysis of their plasma samples demonstrated that they inadvertently received NO3- during both trials. Approval for the study was obtained from the Human Subjects Office at Indiana University and the Human Research Protection Office at Washington University School of Medicine, and all subjects provided written, informed consent.
Figure 1.
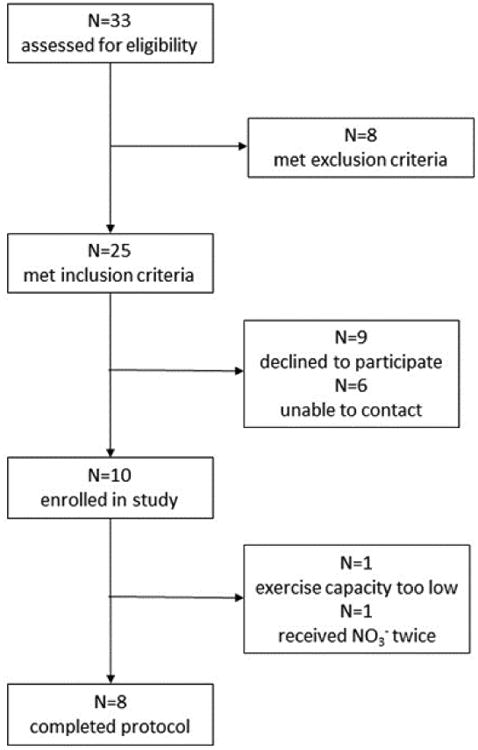
CONSORT diagram illustrating flow of subjects through the study.
Experimental design and protocol
Upon enrollment, each patient was studied using a randomized, double-blind, placebo-controlled, crossover design (Fig. 2, top panel). During one trial, they were tested 2 h after ingesting 140 mL of a concentrated BRJ supplement (Beet It Sport®, James White Drinks, Ipswich, UK) containing 11.2 mmol of NO3-. During another trial, they were tested after ingesting the same volume of NO3--depleted BRJ. This placebo is prepared by the manufacturer by extracting NO3- from BRJ using an ion exchange resin and is indistinguishable from the standard product in packaging, color, texture, taste, and smell, and does not alter plasma NO3- or NO2- concentrations or breath NO levels. There was a minimum 1 wk washout period between trials. To limit variation in baseline NO3-, NO2-, and NO levels, subjects were instructed to avoid high NO3- foods for 10 d prior to intervention and throughout the study. Subjects were also instructed to avoid food, caffeine, alcohol, and exercise for 12 h prior to each trial, and to not chew gum or use mouthwash on study days, as these products can block conversion of NO3- to NO2- and hence to NO via the enterosalivary pathway (17).
Figure 2.
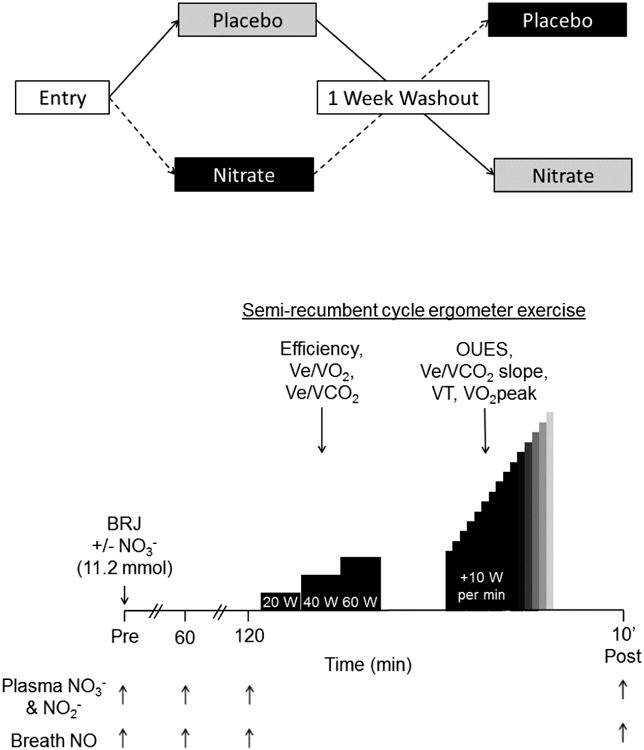
Experimental design (top panel) and protocol (bottom panel).
Subjects arrived at the Clinical Research Unit of Washington University School of Medicine in the morning after fasting overnight. Baseline heart rate and blood pressure were first measured, after which an antecubital venous catheter was inserted and a blood sample was obtained. Plasma was rapidly separated via centrifugation and frozen at -80° C for subsequent determination of NO3- and NO2- concentrations using a dedicated HPLC system (ENO-30, Eicom USA, San Diego, CA). Briefly, plasma was thawed on ice, mixed 1:1 with methanol, and centrifuged at 4° C for 10 min at 10,000 g. A 10 μL aliquot of the protein-poor supernatant was then injected into the HPLC, wherein NO3- and NO2- were isolated via a separation column, NO3- reduced to NO2- on a cadmium column, and both reacted with Griess reagent then detected spectrophotometrically at 540 nm. Plasma NO3- and NO2- concentrations were calculated based on integrated peak areas compared to those of authentic standards. This method was highly reproducible, with test-retest correlation coefficients of 0.99 and 0.98 for NO3- and NO2-, respectively. To further reduce variability, all samples from a single subject were analyzed together. Breath NO level, a biomarker of whole-body NO production (1,8,9,35), was also measured once at this time using a portable electrochemical analyzer (NIOX VERO, Circassia Pharmaceuticals Inc., Chicago, IL) following American Thoracic Society guidelines. These measurements were repeated 1 and 2 h after the subject had ingested the BRJ, and also 10 min after completion of all exercise testing (i.e., at ∼3 h). The latter consisted of submaximal steady-state and maximal incremental exercise on a semi-recumbent cycle ergometer (Lode, Gronigen, The Netherlands) (Fig. 2, bottom panel). Semi-recumbent cycle ergometry was chosen to minimize use of upper body musculature, thus aiding interpretation of any observed changes in exercise efficiency. After adjustment of the seat position, subjects first pedaled the ergometer at 60 rpm for 6 min each at 20, 40, and 60 W while respiratory gas exchange was measured continuously using a ParvoMedics 2900 metabolic cart (ParvoMedics, Sandy, UT). Heart rate, blood pressure, and perceived exertion (7) were determined during the last 30 s of each stage. Following 10 min of rest, subjects resumed pedaling at 60 W for 1 min, after which the power output was incremented by 10 W/min (47) until volitional fatigue. Respiratory gas exchange and heart rate were monitored continuously and blood pressure was measured periodically throughout the test and also immediately following cessation of exercise.
Data analyses
Respiratory gas exchange data collected during the final 2 min of each stage of the submaximal exercise test were averaged and used in all subsequent analyses. Gross efficiency was calculated as the ratio of external power to metabolic power (37), multiplied by 100%. Delta efficiency, i.e., the slope of the relationship between external and metabolic power, and the metabolic cost of unloaded cycling, i.e., the y intercept of this relationship, were determined by regression analysis (40). Similarly, during the maximal exercise test the oxygen uptake efficiency slope (OUES; Ref. 4) was calculated by regressing VO2 (in L/min) on the log of ventilation (Ve; also in L/min), both being measured at 15 s intervals. The Ve/VCO2 slope (3) was calculated in a similar fashion. Ventilatory threshold (VT) was determined using the V-slope method (6). Peak power was defined as the average power during the last 1 min of exercise. VO2peak was defined as the highest VO2 measured over any 1 min period.
Statistical analyses were performed using GraphPad Prism version 7.02 (GraphPad Software, La Jolla, CA). Normality of data distribution was first tested using the D'Agostino-Pearson omnibus test. Data were subsequently analyzed using two-way (treatment × order) ANOVA, with subject as a repeated measures factor within treatment. A P value of <0.05 was considered significant. Primary outcome variables were changes in ventilatory responses and VO2peak in response to dietary NO3-. Secondary outcome variables were changes in exercise performance and efficiency; all other variables measured were considered tertiary.
Results
Patient characteristics
Characteristics of the patients are shown in Table 1. All had mild-to-moderate nonischemic HFrEF (based on NYHA class, MLWHFQ score, and EF). All were under stable, standard-of-care therapy, including use a β-blocker and, in six out of eight, treatment with an angiotensin converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB).
Table 1.
Patient characteristics.
| N (M/F) | 8 (6/2) |
|---|---|
| Age (y) | 52 ± 5 |
| Height (m) | 1.79 ± 0.03 |
| Body mass (kg) | 107.6 ± 14.1 |
| BMI (m/kg2) | 33.1 ± 3.5 |
| Duration of HF (y) | 6 ± 3 |
| NYHA class (I/II/III/IV) | 3/2/3/0 |
| MLWHFQ (score) | 35 ± 8 |
| Ejection fraction (%) | 34 ± 2 |
| B-blocker | 8/8 |
| ACEi/ARB | 6/8 |
| Spironolactone | 6/8 |
| Statin | 2/8 |
Values are mean±S.E. for n=8. NYHA, New York Heart Association. MLWHFQ, Minnesota Living with Heart Failure Questionaire. ACEi, angiotensin converting enzyme inhibitor. AR, angiotensin receptor blocker.
Plasma NO3- and NO2- and breath NO
No changes in plasma NO3- or NO2- concentration (Fig. 3, top and middle panels) or in breath NO levels (Fig. 3, bottom panel) occurred during the placebo trial. In contrast, ingestion of NO3--containing BRJ elevated (P < 0.01) plasma NO3- concentrations approximately 10-fold after 1 h, with this increase being maintained for the remainder of the experiment (Fig. 3, top panel). Concentrations of the downstream metabolites of NO3-, i.e., plasma NO2- and breath NO, were also significantly elevated by NO3- intake, albeit to a much lesser degree (Fig. 3, middle and bottom panels). The increase in plasma NO2- also seemed to lag behind that of NO3-, achieving statistical significance only after 2 h and peaking at 10 min post-exercise. These findings are consistent with the important rate-limiting role played by oral bacteria in the enterosalivary pathway of NO production (28).
Figure 3.
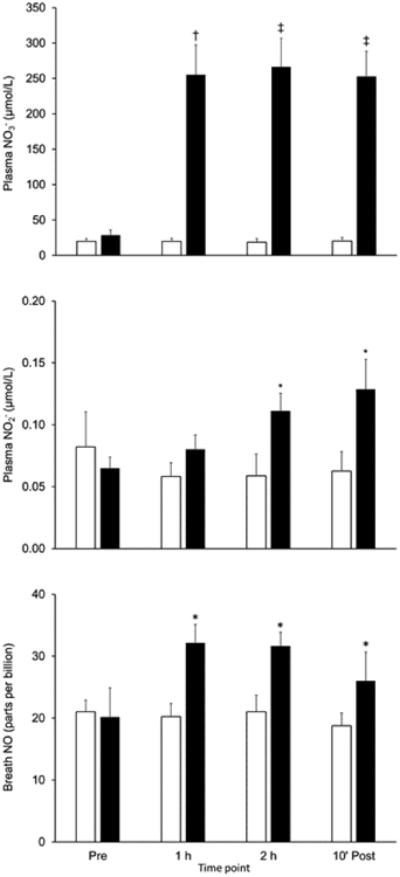
Effect of acute ingestion of beetroot juice either devoid of (Placebo; open bars) or containing (Nitrate; solid bars) 11.2 mmol of NO3- on plasma NO3- (top panel) and NO2- (middle panel) concentrations and breath NO levels (bottom panel) in patients with heart failure with reduced ejection fraction. Values are mean ± SE for n=8. 10′ Post = 10 min post-exercise. Nitrate significantly higher than than Placebo at same time point: *P<0.05, †P<0.01, ‡P<0.001.
Responses to submaximal exercise
Despite the increase in NO bioavailability resulting from NO3- ingestion, no differences were observed in VO2, ventilation, ventilatory equivalents (i.e., Ve/VO2 and Ve/VCO2), respiratory exchange ratio, or gross efficiency during submaximal steady-state exercise (Table 2). Delta efficiency was also unaffected by dietary NO3- intake, averaging 26.2 ± 2.5 and 24.9 ± 1.8% in the placebo and nitrate trials, respectively (P = NS). The metabolic cost of unloaded cycling was also unchanged, averaging 200 ± 27 W, or 1.87 ± 0.07 W/kg, in the placebo trial and 215 ± 27 W, or 2.06 ± 0.14 W/kg, in the nitrate trial (P = NS). Finally, no significant differences were observed in heart rate, systolic or diastolic blood pressures, or in perceived exertion (Table 2).
Table 2.
Cardiorespiratory and perceptual responses to steady-state exercise.
| Power output (W) | ||||
|---|---|---|---|---|
| Trial | 20 | 40 | 60 | |
| VO2 (L/min) | Placebo | 0.81 ± 0.09 | 1.00 ± 0.09 | 1.26 ± 0.12 |
| Nitrate | 0.87 ± 0.10 | 1.08 ± 0.11 | 1.34 ± 0.13 | |
| VO2 (mL.min-1.kg-1) | Placebo | 7.8 ± 0.4 | 9.8 ± 0.7 | 12.3 ± 1.0 |
| Nitrate | 8.4 ± 0.5 | 10.5 ± 0.7 | 13.1 ± 1.0 | |
| % of VO2peak | Placebo | 38.5 ± 3.9 | 48.1 ± 5.0 | 60.6 ± 6.6 |
| Nitrate | 38.0 ± 3.2 | 47.5 ± 4.0 | 59.1 ± 4.9 | |
| Ve (L/min) | Placebo | 22.5 ± 2.7 | 26.8 ± 2.9 | 33.9 ± 4.1 |
| Nitrate | 24.1 ± 3.6 | 29.5 ± 4.6 | 36.1 ± 5.3 | |
| Ve/VO2 (L/L) | Placebo | 27.5 ± 1.0 | 26.5 ± 1.0 | 26.6 ± 1.3 |
| Nitrate | 27.2 ± 1.5 | 26.6 ± 1.7 | 26.4 ± 1.7 | |
| Ve/VCO2 (L/L) | Placebo | 33.9 ± 1.2 | 31.8 ± 1.3 | 30.6 ± 1.2 |
| Nitrate | 33.5 ± 1.7 | 32.2 ± 1.6 | 30.9 ± 1.6 | |
| Respiratory exchange ratio | Placebo | 0.81 ± 0.01 | 0.83 ± 0.01 | 0.87 ± 0.02 |
| Nitrate | 0.81 ± 0.02 | 0.82 ± 0.02 | 0.85 ± 0.02 | |
| Gross efficiency (%) | Placebo | 7.6 ± 0.8 | 12.1 ± 1.2 | 14.4 ± 1.4 |
| Nitrate | 7.2 ± 0.6 | 11.3 ± 0.9 | 13.6 ± 1.1 | |
| Heart rate (beats/min) | Placebo | 85 ± 3 | 98 ± 7 | 103 ± 5 |
| Nitrate | 96 ± 5 | 101 ± 4 | 107 ± 9 | |
| Systolic blood pressure (mmHg) | Placebo | 136 ± 7 | 141 ± 7 | 143 ± 8 |
| Nitrate | 132 ± 9 | 137 ± 9 | 136 ± 8 | |
| Diastolic blood pressure (mmHg) | Placebo | 76 ± 5 | 75 ± 5 | 76 ± 5 |
| Nitrate | 84 ± 5 | 81 ± 4 | 78 ± 5 | |
| Perceived exertion (units) | Placebo | 8 ± 1 | 9 ± 1 | 10 ± 1 |
| Nitrate | 8 ± 1 | 10 ± 1 | 12 ± 1 | |
Values are mean±S.E. for n=8. VO2, oxygen uptake. Ve, ventilation. VCO2, carbon dioxide production.
Responses to maximal exercise
Ingestion of NO3- did not alter ventilatory responses during the incremental exercise test, regardless of whether the data were analyzed to determine the OUES, Ve/VCO2 slope, or VT (Table 3). Respiratory exchange ratio, heart rate, and systolic and diastolic blood pressures at peak exercise were also unchanged (Table 3). The patients were, however, able to achieve a higher (P<0.05) peak power (Table 3) and exercise longer (P<0.05; Fig. 4, top panel) following acute dietary NO3- intake. This improvement in exercise performance was accompanied by a moderate, but potentially clinically-significant (see Discussion), increase in VO2peak, expressed in either L/min (P<0.05; Table 3) or in mL.min-1.kg-1 (P < 0.05; Fig. 4, bottom panel). Notably, NO3- ingestion increased VO2peak in seven out of the eight patients, with individual increases ranging from 0.8 to 3.9 mL.min-1.kg-1, or 5 to 19%. VO2peak in the remaining patient, who weighed the most and hence received the smallest dose of NO3- per kilogram of body mass, was essentially unchanged. For the group as a whole, however, no statistically significant correlations were observed between the magnitude of the increase in VO2peak and the dose of NO3- provided or the increase in plasma NO3-/plasma NO2-/breath NO. The highest correlation was between the relative increase in plasma NO2- and the relative increase in VO2peak (r = 0.64; P = 0.09).
Table 3.
Responses to incremental exercise.
| Placebo | Nitrate | |
|---|---|---|
| OUES (L/log L) | 2.73 ± 0.39 | 2.77 ± 0.39 |
| Ve/VCO2 slope (L/L) | 25.6 ± 1.9 | 24.6 ± 2.5 |
| VT (L/min) | 1.42 ± 0.15 | 1.49 ± 0.19 |
| VT (mL.min-1.kg-1) | 14.4 ± 2.1 | 14.9 ± 1.8 |
| VT (% of VO2peak) | 66.1 ± 3.7 | 64.1 ± 2.5 |
| Peak respiratory exchange ratio | 1.05 ± 0.03 | 1.05 ± 0.02 |
| Peak heart rate (bts/min) | 134 ± 6 | 139 ± 7 |
| Peak systolic blood pressure (mmHg) | 158 ± 8 | 155 ± 10 |
| Peak diastolic blood pressure (mmHg) | 90 ± 11 | 82 ± 6 |
| Peak power (W) | 154 ± 14 | 160 ± 14* |
| Peak power (Wkg) | 1.53 ± 0.24 | 1.57 ± 0.23* |
| VO2peak (L/min) | 2.42 ± 0.34 | 2.60 ± 0.35* |
Figure 4.
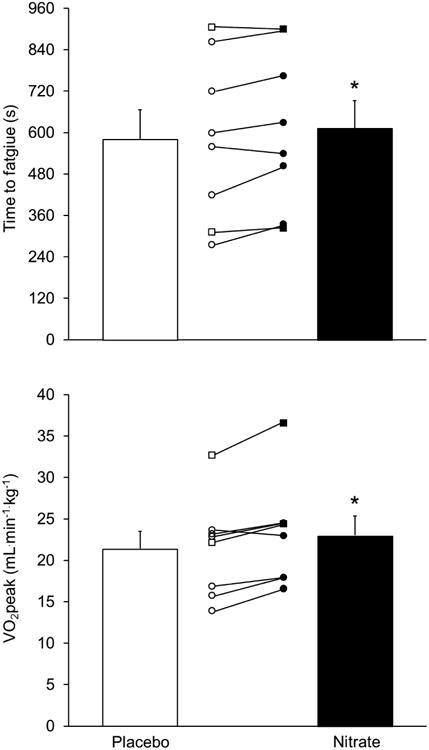
Effect of acute ingestion of beetroot juice either devoid of (Placebo; open bar or symbols) or containing (Nitrate; solid bar or symbols) 11.2 mmol of NO3- on time to fatigue (top panel) and peak O2 consumption (VO2peak; bottom panel) during an incremental exercise test in patients with heart failure with reduced ejection fraction. Values are mean ± SE for n=8; individual results are also shown (circles, men; squares, women). *Nitrate significantly higher than Placebo: P<0.05.
Discussion
The purpose of the present study was to determine the effects of dietary NO3- supplementation on the responses to aerobic exercise in patients with HFrEF. Using a double-blind, placebo-controlled, crossover design, we found that acute ingestion of 11.2 mmol of NO3- resulted in significant increases in exercise duration, peak power, and VO2peak during an incremental cycle ergometer exercise test. Contrary to our initial hypothesis, however, this was not accompanied by any changes in the ventilatory response (i.e., ventilatory equivalents, OUES, Ve/VCO2 slope, or VT) during submaximal or maximal exercise. There were also no changes in either gross or delta efficiency during steady-state exercise.
As stated above, we found that acute ingestion of NO3- enabled patients with non-ischemic HFrEF to exercise longer and to achieve a higher peak power output during incremental exercise. This improvement in performance was accompanied by an increase in VO2peak. The former is in keeping with the results of Kerley et al. (25), who reported that acute NO3- intake enhanced performance during an incremental shuttle walk test in patients with non-ischemic cardiomyopathy. In contrast, Hirai et al. (20) found that repeated ingestion of NO3- did not improve performance or VO2peak in patients with HFrEF primarily of ischemic origin. The reason for this discrepancy is not clear, but it may be due to this difference in disease etiology. On the other hand, it appears unrelated to disease severity, as even the three paients we studied with baseline VO2peak values of ∼15 mL.min-1.kg-1, i.e., comparable to those studied by Hirai et al. (20), demonstrated increases in VO2peak and in performance following NO3-ingestion.
Regardless of the above, an increase in VO2peak of the magnitude that we observed, i.e., +1.6 ± 0.5 mL.min-1.kg-1, or +8 ± 2%, may prove to be clinically significant. In particular, in a previous cross-sectional study of patients with HFrEF one of us (LRP) found that for every 1 mL.min-1.kg-1 increase in VO2peak there was a 5% decrease in the annual risk of death or transplantation (38). A quantitatively-similar relationship was observed between changes in VO2peak and disease outcome in the longitudinal HF-ACTION trial (44). At least theoretically, then, the acute dietary NO3--induced increase in VO2peak observed in the present study would translate into almost a 10% reduction in annual risk. Additional research will be needed to test this hypothesis, especially in those at greatest risk (such as the three patients mentioned above).
It is also worth noting that the magnitude of the improvement in VO2peak that we observed is comparable to that typically resulting from standard-of-care drug therapies or from endurance exercise training in patients with HF, both of which provide salutatory effects. Specifically, a number of previous studies have demonstrated that chronic treatment of HFrEF patients with a beta blocker or ACEi/ARB increases VO2peak by approximately 10% (e.g., 14,16,32). Improvements in VO2peak with exercise training are also similar (22). Intriguingly, the 8% enhancement of VO2peak that we found in response to acute dietary NO3- intake occurred in patients with HFrEF already on optimal medical therapy, including use of a beta blocker and, in most cases, an ACEi/ARB, indicative of an additive effect. Future studies will be required to determine whether the impact of dietary NO3- on VO2peak is also additive (or perhaps even synergistic) to that of exercise training in patients with HF.
Although the present results indicate that acute dietary NO3- intake increases VO2peak in patients with HFrEF, the specific mechanisms responsible for this beneficial response cannot be determined from the present data. From the perspective of the cardiovascular Fick equation, though, an increase in VO2peak could only result from an increase in heart rate, stroke volume (SV), and/or arteriovenous O2 difference (a-vO2diff) at peak exercise. Indeed, at peak exercise heart rate tended to be higher and diastolic blood pressure tended to be lower, suggesting that the dietary NO3--induced increase in VO2peak we observed may have been the result of a greater cardiac output in a setting of reduced total peripheral resistance. Given the direct effects of NO on arteriolar smooth muscle, the latter response might be expected. In addition, recent data indicate that dietary NO3- intake also enhances vasodilation in contracting muscle by reducing sympathetic nerve activity (36). Again, however, in the absence of direct measurements the mechanism(s) responsible for the increase in VO2peak observed in the present study remain unknown.
Although acute dietary NO3- intake resulted in a significant increase in performance and VO2peak, there were no changes in the ventilatory response to exercise, quantified as either Ve/VO2 or Ve/VCO2 during steady-state exercise or as OUES, Ve/VCO2 slope, or VT during incremental exercise. The effects of dietary NO3- on these parameters in patients with HFrEF have not been previously reported. The present results, however, are generally comparable to previous similar studies of patients with HFpEF (15,51), although Zamani et al. (50) found that dietary NO3- supplementation resulted in a significant increase in VT. It should be noted, however, that the increase in VT in their study was only 0.5 ± 0.2 mL.min-1.kg-1, which is nearly identical to the 0.4 ± 0.5 mL.min-1.kg-1 difference (P=NS) that we observed. Thus, the effects of dietary NO3- on ventilatory responses in patients with HFrEF or HFpEF would at best seem equivocal.
As indicated previously, studies of dietary NO3- supplementation in healthy individuals have often, although not always, reported improvements in exercise economy or efficiency (36). The mechanism responsible for this O2-sparing effect is not clear, however, with some data suggesting that it results from direct inhibition of mitochondrial respiration (29) and other data implicating a decrease in ATP utilization by contracting muscle (5). In any case, given the compromised circulatory function of HF patients, any reduction in the demand for delivery of O2-carrying blood during exercise would seem beneficial. Hirai et al. (20), however, did not observe any dietary NO3--induced changes in submaximal VO2 during exercise. Despite using a protocol carefully designed to account for the slower VO2 kinetics found in HF, minimize involvement of non-active tissues, and allow assessment of not only gross but also delta efficiency, we also found acute dietary NO3- intake did not alter the energy requirements of submaximal exercise. As suggested by Zamani et al. (50), this may reflect differences between patients with HF and young, healthy control subjects in age or in the factors controlling mitochondrial respiration during exercise. Regardless, the present data demonstrate that, at least in patients with HFrEF, acute dietary NO3- intake can increase performance and VO2peak even in the absence of any changes in energy demand at a given power output.
There are a number of limitations to the present study. First, we studied a relatively small number of individuals, and therefore may have failed to detect some true effects of NO3-supplementation, e.g., a decrease in blood pressure. However, our sample size was comparable to those of similar previous studies of dietary NO3- intake on exercise responses in patients with HFrEF (20,25), and was adequate to detect changes in one of our primary outcomes, i.e., VO2peak. Second, as previously discussed we did not directly measure central or peripheral determinants of VO2peak, and therefore cannot determine the mechanisms responsible for the improvement that was observed. This does not, however, negate our primary finding that dietary NO3- supplementation increases exercise capacity and VO2peak in patients with HFrEF. Finally, we studied only the effects of acute ingestion of NO3- at a single, fixed dose, and therefore cannot draw any conclusions on the effects of longer-term treatment and/or other doses. Answering such questions will therefore require additional research.
To summarize, the results of this proof-of-concept study demonstrate that acute ingestion of 11.2 mmol of NO3- (in the form of a concentrated BRJ supplement) increases aerobic exercise performance and VO2peak, but does not alter ventilatory responses or gross or delta efficiency during exercise, in patients with mild-to-moderate HFrEF. Along with our previous data demonstrating that acute dietary NO3- intake results in comparable improvements in muscle contractile function in this population (10), these suggest that dietary NO3- supplementation may be a valuable adjunctive treatment for exercise intolerance in this population. Larger, i.e., multi-center trials are needed to confirm the present findings and to determine whether longer-term dietary NO3- treatment improves physical activity levels, quality of life, and perhaps even survival in patients with HFrEF.
Highlights.
Acute dietary NO3- intake increased VO2peak in patients with HF by 8±2% (P<0.05).
Time to fatigue during exercise improved by 7±3 % (P<0.05).
Dietary NO3- may be a means of enhancing exercise capacity in patients with HF.
Acknowledgments
This study was supported by the Barnes-Jewish Hospital Foundation, the Washington University Mentors in Medicine and C-STAR programs, and Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adachi H, Nguyen PH, Belardinelli R, Hunter D, Jung T, Wasserman K. Nitric oxide production during exercise in chronic heart failure. Am Heart J. 1997;134:196–202. doi: 10.1016/s0002-8703(97)70124-8. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J. 2004;147:354–360. doi: 10.1016/j.ahj.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–72. doi: 10.1016/s0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 6.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 7.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 8.Bussotti M, Andreini D, Agostoni P. Exercise-induced changes in exhaled nitric oxide in heart failure. Eur J Heart Fail. 2004;6:551–554. doi: 10.1016/j.ejheart.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Clini E, Volterrani M, Pagani M, Bianchi L, Porta R, Gile' LS, Giordano A, Ambrosino N. Endogenous nitric oxide in patients with chronic heart failure (CHF): relation to functional impairment and nitrate-containing therapies. Int J Cardiol. 2000;73:123–130. doi: 10.1016/s0167-5273(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 10.Coggan AR, Leibowitz JL, Anderson Spearie C, Kadkhodayan A, Thomas DP, Ramamurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: a double-blind, placebo-controlled, randomized trial. Circ Heart Fail. 2015;8:914–920. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggan AR, Leibowitz JL, Kadkhodayan A, Thomas DT, Ramamurthy S, Anderson Spearie C, Waller S, Farmer M, Peterson LR. Effect of acute dietary nitrate intake on knee extensor speed and power in healthy men and women. Nitric Oxide. 2015;48:16–21. doi: 10.1016/j.niox.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coggan AR, Peterson LR. Dietary nitrate and skeletal muscle contractile function in heart failure. Curr Heart Fail Rep. 2016;13:158–165. doi: 10.1007/s11897-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrá U, Giordana A, Mezzani A, Gnemmi M, Pistono M, Caruso R, Giannuzzi P. Cardiopulmonary exercise testing and prognosis in heart failure due to systolic dysfunction: a validation study of the European Society of Cardiology Guidelines and Recommendations (2008) and further developments. Eur J Prev Cardiol. 2012;18:32–40. doi: 10.1177/1741826710393994. [DOI] [PubMed] [Google Scholar]
- 14.Dayi SU, Akbulut T, Akgoz H, Terzi S, Sayar N, Aydin A, Bilsel T, Ciloglu F. Long-term combined therapy with losartan and an angiotensin-converting enzyme inhibitor improves functional capacity in patients with left ventricular dysfunction. Acta Cardiol. 2005;60:373–37758. doi: 10.2143/AC.60.4.2004985. [DOI] [PubMed] [Google Scholar]
- 15.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4:428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis GR, Nightingale AK, Blackman DJ, Anderson RA, Mumford C, Timmins G, Lang D, Jackson SK, Penney MD, Lewis MJ, Frenneaux MP, Morris-Thurgood J. Addition of candesartan to angiotensin converting enzyme inhibitor therapy in patients with chronic heart failure does not reduce levels of oxidative tress. Eur J Heart Fail. 2002;4:193–199. doi: 10.1016/s1388-9842(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 17.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Haykowsky MJ, Kitzman DW. Exercise physiology in heart failure and preserved ejection fraction. Heart Fail Clin. 2014;10:445–452. doi: 10.1016/j.hfc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 2015;119:739–744. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai DM, Zelt JT, Jones JH, Castanhas LG, Bentley RF, Earle W, Staples P, Tschakovsky ME, McCans J, O'Donnell DE, Neder JA. Dietary nitrate supplementation and exercise tolerance in patients with heart failure with preserved ejection fraction. Am J Physiol Regul Integr Comp Physiol. 2017;312:R13–R22. doi: 10.1152/ajpregu.00263.2016. [DOI] [PubMed] [Google Scholar]
- 21.Hülsmann M, Quittan M, Berger R, Crevenna R, Springer C, Nuhr M, Mörtl D, Moser P, Pacher R. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6:101–107. doi: 10.1016/j.ejheart.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Ismail H, McFarlane JR, Nojoumian AH, Dieberg G, Smart NA. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure. JACC Heart Fail. 2013;1:514–522. doi: 10.1016/j.jchf.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med. 2014;44:S35–S45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz SD, Khan T, Zeballos GA, Mathew L, Potharlanka P, Knecht M, Whelan J. Decreased activity of the L-arginine-nitric oxide metabolic pathway in patients with congestive heart failure. Circulation. 1999;99:2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 25.Kerley CP, O'Neill JO, Reddy Bijjam V, Blaine C, James PE, Cormican L. Dietary nitrate increases exercise tolerance in patients with non-ischemic, dilated cardiomyopathy-a double-blind, randomized, placebo-controlled, crossover trial. J Heart Lung Transplant. 2016;35:922–926. doi: 10.1016/j.healun.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell TR, Piña IL, Leifer ES, Fleg JL, Blackburn G, Fonarow GC, Chase PJ, Piner L, Vest M, O'Connor CM, Ehrman JK, Walsh MN, Eqald G, Bensimhom D, Russell SD HF-ACTION Investigators. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol. 2016;67:780–789. doi: 10.1016/j.jacc.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Gödecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 28.Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017;105:48–67. doi: 10.1016/j.freeradbiomed.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastic nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maher AR, Arif S, Madhani M, Abozguia K, Ahmed I, Fernandez BO, Feelisch M, O'Sullivan AG, Christopoulos A, Sverdlov AL, Ngo D, Dautov R, James PE, Horowitz JD, Frenneaux MP. Impact of chronic congestive heart failure on pharmacokinetics and vasomotor effects of infused nitrite. Br J Pharmacol. 2013;169:659–670. doi: 10.1111/bph.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: A prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102:546–551. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- 33.Münzel T, Gori T, Keaney JF, Jr, Maack C, Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J. 2015 7;36:2555–2564. doi: 10.1093/eurheartj/ehv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Notay K, Incognito AV, Millar PJ. Acute beetroot juice supplementation on sympathetic nerve activity: a randomized, double-blind, placebo-controlled proof-of-concept study. Am J Physiol Heart Circ Physiol. 2017;313:H59–H65. doi: 10.1152/ajpheart.00163.2017. [DOI] [PubMed] [Google Scholar]
- 35.Olin AC, Aldenbratt A, Ekman A, Ljungkvist G, Jungersten L, Alving K, Torén K. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Resp Med. 2001;95:153–158. doi: 10.1053/rmed.2000.1010. [DOI] [PubMed] [Google Scholar]
- 36.Pawlak-Chaouch M, Boissière J, Gamelin FX, Cuvelier G, Berthoin S, Aucouturier J. Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: A systematic review and a meta-analysis. Nitric Oxide. 2016;53:65–76. doi: 10.1016/j.niox.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- 38.Peterson LR, Schechtman KB, Ewald GA, Geltman EM, Meyer T, Krekeler P, Rogers JG. The effect of beta-adrenergic blockers on the prognostic value of peak exercise oxygen uptake in patients with heart failure. J Heart Lung Transplant. 2003;22:70–77. doi: 10.1016/s1053-2498(02)00473-4. [DOI] [PubMed] [Google Scholar]
- 39.Rector TS, Bank AJ, Mullen KA, Tschumperlin LK, Sih R, Pillai K, Kubo SH. Randomized, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135–2141. doi: 10.1161/01.cir.93.12.2135. [DOI] [PubMed] [Google Scholar]
- 40.Reger M, Peterman JE, Kram R, Byrnes WC. Exercise efficiency of low power output cycling. Scand J Med Sci Sports. 2013;23:713–721. doi: 10.1111/j.1600-0838.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- 41.Rimer EG, Peterson LR, Coggan AR, Martin JC. Acute dietary nitrate supplementation increases maximal cycling power in athletes. Int J Sports Physiol Perf. 2016;11:715–720. doi: 10.1123/ijspp.2015-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafiq A, Brawner CA, Aldred HA, Lewis B, Williams CT, Tita C, Schairer JR, Ehrman JK, Velez M, Selektor Y, Lanfear DE, Keteyian SJ. Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry Ford HospITal CardioPulmonary EXercise Testing (FIT-CPX) project. Am Heart J. 2016;174:167–172. doi: 10.1016/j.ahj.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiva S. Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 2013;1:40–44. doi: 10.1016/j.redox.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swank AN, Horton J, Fleg JL, Fonarow GC, Ketayian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou MC, Vest M, Ewald G, Blackburn G, Leifer E, Cooper L Kraus WE; HE-ACTION Investigators. Modest increase in VO2peak is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. 2012;5:579–585. doi: 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang L, Wang H, Ziolo MT. Targeting NOS as a therapeutic approach for heart failure. Pharmacol Ther. 2014;142:306–315. doi: 10.1016/j.pharmthera.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Wennmalm A, Benthin G, Edlund A, Jungersten L, Kieler-Jensen N, Lundin S, Westfelt UN, Petersson AS, Waagstein F. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study Circ Res. 1993;73:1121–1127. doi: 10.1161/01.res.73.6.1121. [DOI] [PubMed] [Google Scholar]
- 47.Working Group on Cardiac Rehabilitation & Exercise Physiology and Working Group on Heart Failure of the European Society of Cardiology. Recommendations for exercise testing in chronic heart failure patients. European Heart J. 2001;22:37–45. doi: 10.1053/euhj.2000.2388. [DOI] [PubMed] [Google Scholar]
- 48.Wray DW, Amann M, Richardson RS. Peripheral vascular function, oxygen delivery and utilization: the impact of oxidative stress in aging and heart failure with reduced ejection fraction. Heart Fail Rev. 2017;22:149–166. doi: 10.1007/s10741-016-9573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamani P, Tan V, Soto-Calderon H, Beraun M, Brandimarto JA, Trieu L, Varakantam S, Doulias PT, Townsend RR, Chittams J, Margulies KB, Cappola TP, Poole DC, Ischiropoulos H, Chirinos JA. Pharmacokinetics and pharmacodynamics of inorganic nitrate in heart failure with preserved ejection fraction. Circ Res. 2017;120:1151–1161. doi: 10.1161/CIRCRESAHA.116.309832. [DOI] [PMC free article] [PubMed] [Google Scholar]


