Abstract
Purpose
To report a highly-interrupted radial variant of balanced steady-state free precession (bSSFP) imaging, termed Fast Interrupted Steady-State (FISS), for decreasing flow artifact as well as fat signal conspicuity with respect to bSSFP, and saturation effects vis-à-vis fast low-angle shot (FLASH) imaging.
Methods
Numerical simulations, phantom studies, and human studies were conducted to examine the imaging contrast, off-resonance behavior, and flow properties of FISS. Human studies applied FISS for cine cardiac imaging and ungated nonenhanced magnetic resonance angiography (MRA) of the legs, neck and brain. Comparisons were made with bSSFP and FLASH imaging.
Results
Simulations revealed that FISS retains the high signal levels of bSSFP for stationary on-resonant spins, while reducing undesirable signal heterogeneity from flowing spins. Phantom studies agreed with the simulations, and showed that FISS reduces fat signal and flow artifact with respect to bSSFP imaging. FISS imaging in human subjects agreed with the simulations and phantom studies, and showed reduced saturation artifact as compared to FLASH imaging.
Conclusion
FISS imaging reduces flow artifact and fat signal conspicuity with respect to bSSFP imaging, and ameliorates arterial signal saturation observed with FLASH imaging. Potential clinical applications include fat-suppressed cine imaging and ungated nonenhanced MRA.
Keywords: FISS, bSSFP, interrupted, steady-state, radial
INTRODUCTION
Fast low-angle shot (FLASH) (1) and balanced steady-state free precession (bSSFP) (2) are two rapid techniques often used in clinical cardiovascular magnetic resonance imaging. In addition to speed, strengths of FLASH include robustness against artifacts from main magnetic field (B0) inhomogeneity as well as blood flow, while a prominent strength of bSSFP is its high signal-to-noise efficiency. Despite these strengths, these two methods carry some limitations. For instance, due to spoiling of transverse magnetization before each radiofrequency (RF) excitation, FLASH can suffer from low signal-to-noise ratio in tissues with prolonged T1 times. On the other hand, bSSFP imaging largely avoids the signal saturation artifact that is associated with FLASH imaging, but is highly sensitive to artifacts from B0 inhomogeneity and from signals that arise from flowing spins that have left the imaging slice (3,4).
Over a decade ago, Derbyshire and colleagues reported a variant of bSSFP referred to as spectrally selective suppression with SSFP (S5FP) to improve fat suppression (5). The method applied a series of short Cartesian bSSFP echo trains sandwiched between tailored opening and closing subsequences of RF pulses and gradients, with each series repeating at a rate of ≈10-20 Hz. This innovative work, however, did not gain clinical traction, perhaps in part due to the use of Cartesian k-space sampling and need for data scaling during the image reconstruction process to avoid image artifacts in the phase-encoding direction. Moreover, as S5FP was optimized for imaging efficiency, the bSSFP echo train lengths advocated by the approach were too long to adequately offset the sensitivity of bSSFP imaging to flow artifact (6).
We therefore hypothesized that a more frequently interrupted variant of bSSFP imaging leveraging radial k-space sampling and regular spoiling of spins with undesirable phase accumulation might prove useful for reducing flow artifact, suppressing fat, and ultimately improving the image quality of bSSFP-based cardiovascular imaging and nonenhanced MR angiography (MRA). We evaluated this highly-interrupted radial imaging approach, to which we refer as ‘Fast Interrupted Steady-State’ (FISS), using numerical simulations as well as imaging studies of phantoms and human subjects.
METHODS
Fast Interrupted Steady-State Pulse Sequence
The basic pulse sequence diagram for FISS imaging is shown in Figure 1. The FISS sequence can be viewed as a hybrid of bSSFP and spoiled gradient-echo imaging in that it consists of a series of modules in the generic form of: [α/2,α,(readout,α)n, α/2,spoil], where α denotes the flip angle of the radiofrequency (RF) pulse, the RF pulses are phase alternated, and n (≥1) denotes the number of bSSFP repetitions (i.e., readouts) per FISS module. Analogous to bSSFP, the FISS technique applies a vanishing zeroth gradient moment between the RF pulses within each module. However, akin to spoiled gradient-echo imaging, the cumulative zeroth gradient moment across the entire module (equaling the shaded gray area shown in Figure 1) is non-zero in the slice direction. At the end of each FISS module, this non-vanishing gradient moment spoils residual transverse magnetization within the slice (which is non-negligible for some off-resonant spins), and for spins that have flowed in and through the slice. Spoiling of transverse magnetization may be strengthened by extending the shaded gradients in Figure 1 and/or by applying RF spoiling across (but not within) FISS modules. The module shown in Figure 1 is repeated (without insertion of quiescent periods between successive modules) until the prescribed radial k-space is sampled.
Figure 1.
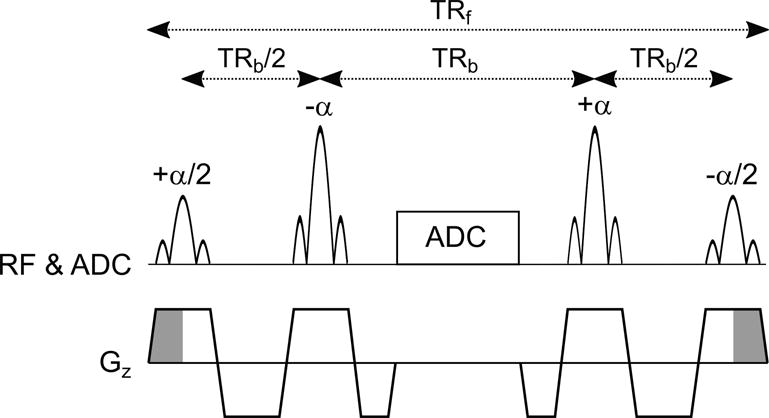
Pulse sequence timing diagram showing one FISS module in the form [+α/2,-α,ADC,+ α,−α/2], where α denotes the RF flip angle. The signs of the RF pulses indicate the applied phase. The unbalanced slice-select gradient area (shaded in gray) at the beginning and end of each FISS module spoils remnant transverse magnetization. The sequence shown repeats using a radial readout gradient (not shown) until the prescribed radial k-space is sampled. The number of bSSFP repetitions (i.e., readouts) per FISS module, n, equals one in this example. TRf=FISS repetition time. TRb=bSSFP repetition time. RF=radiofrequency. ADC=k-space sampling. Gz=slice-select gradient.
The use of store/restore α/2 pulses and gradient spoiling takes cues from prior work (6,7), but differs in that the FISS technique deliberately interrupts (i.e., stops and starts) the bSSFP echo train to a greater degree (≈50-100 Hz), thereby reducing sensitivity to flow-related artifact and phase accrual across the echo train. In further distinction between prior work, radial k-space sampling is used with FISS in lieu of Cartesian k-space sampling. With respect to Cartesian sampling, radial k-space sampling reduces flow and motion sensitivity (8), and avoids ghost artifacts (observable in the phase-encoding direction with Cartesian k-space sampling and n>1) caused by mismatched echo intensities arising from repeated interruption of the bSSFP echo train.
Numerical Simulations
Numerical simulations were performed to compute the magnitude of the transverse magnetization (i.e., MRI signal magnitude) provided by FISS as a function of off-resonance frequency, flip angle and through-plane flow velocity. The matrix formalism of Hinshaw (9) was used in carrying out the simulations, incorporating the effects of the RF pulse shape and slice-selection gradient. The simulated transverse magnetization was computed as the complex sum of transverse magnetization in the slice direction. Simulation parameters for FISS were: FISS TR (TRf)/bSSFP TR (TRb)/TE=9 ms/4 ms/2 ms, α∈[30°, 60°, 90°], n=1, 1.0-ms-long Hanning-windowed-sinc RF pulses with one zero crossing, T1=1600 ms (10), T2 for in-slice magnetization=180 ms (11), T2* for out-of-slice magnetization=45 ms, slice thickness=3 mm, isochromat density in the slice direction=30 mm−1, off-resonance frequencies spanning −500 Hz to +500 Hz in 5 Hz increments, time step=5 μs.
A first simulation examined the impacts of off-resonance frequency and α on the steady-state transverse magnetization of stationary spins; the number of simulated FISS modules was set to 1000. A second simulation examined the impact of flow through the imaging slice and its dependence on off-resonance frequency. Parameters for this simulation were: flow velocities of 0-30 cm/s in 10 cm/s increments, α=60°, number of simulated FISS modules (i.e., echoes)=50.
The magnitude of signal fluctuations in the transient state was examined by computing the median absolute inter-view complex signal difference (MISD) across the first 50 echoes, defined as: MISD = median(|S1-S2|, |S2-S3|, …, |S49-S50|), where Si denotes the complex signal of the ith FISS echo. Simulations of FISS were supplemented with simulations of bSSFP and FLASH imaging. Simulations of bSSFP used identical TRb, TE and α values as FISS. Unlike the other simulations, simulations of FLASH (TR/TE=9 ms/2 ms) assumed instantaneous RF nutation and perfect spoiling between successive RF pulses. The flip angle used for FLASH equaled the Ernst angle (12) and 30° in simulations of stationary and flowing spins, respectively.
Additional simulations of FISS (α=60°) were performed to examine the impacts of n, the number of bSSFP readouts per FISS module, and the use of RF spoiling across FISS modules (phase increment of 117°). All simulations were performed in MATLAB software (version R2015a, Mathworks Inc., Natick, MA).
Overall Study Design
The prototype radial FISS sequence was tested in phantom and human subject studies. Human subjects (n=5, 4 male; mean age=39 years) were imaged after providing written informed consent under an Institutional Review Board-approved protocol.
Imaging was done on a whole-body 3.0T MRI system (MAGNETOM SkyraFit, Siemens, Erlangen, Germany). FISS imaging was acquired using radial k-space sampling providing azimuthally equidistant views of ≈15° (13), 2-fold k-space oversampling in the readout direction, and, unless noted otherwise, one bSSFP radial view per FISS module (i.e., n=1) and without RF spoiling across modules. Comparisons of FISS were made with corresponding radial bSSFP and/or radial flow-compensated FLASH readouts matched for field-of-view (FOV), reconstruction matrix, slice thickness (SL), and, unless noted differently, number of acquired radial views (rn) (phantom studies) or scan time per imaging slice (human subject studies).
Reconstructed FOV and matrix values provided in subsequent sections are as reconstructed on the MRI system after removal of oversampling in the readout direction and without zero-filling interpolation. The radial undersampling factor (R) was computed by dividing the number of radial views required to satisfy the Nyquist sampling requirements, by rn.
Image Contrast and Signal-to-Noise
The image contrast provided by FISS was probed by imaging the thigh of one volunteer using flip angles of 30°, 60° and 90°, with the following additional parameters: TRf/TRb/TE=10.0 ms/3.8 ms/1.9 ms, rn=1000, azimuthal view angle increment (rθ)=14.94°, FOV=400 mm×400 mm, matrix=384×384, 1.04 mm in-plane spatial resolution, R=0.6, SL=3 mm, receiver bandwidth (BW)=930 Hz/pixel, RF spoiling. One thousand views were collected to capture a near steady-state image appearance. Contrast between arterial blood in the femoral artery and the background tissues of muscle and fat were computed as SA/SB-1, where SA and SB denote the mean signals of arterial blood and the background tissue, respectively. FISS scans using a flip angle of 60° were acquired twice in the thigh and brain to measure signal-to-noise ratio (SNR) for skeletal muscle, arterial blood, fat, gray matter and white matter (14). Comparisons were made with bSSFP imaging. Signal measurements were made in ImageJ (version 1.51n, National Institutes of Health, Bethesda, MA).
Fat Suppression Phantom
An oil phantom (T1/T2=296/132 ms) was imaged to examine the appearance of fat during FISS imaging and its dependence on TRb, the use of RF spoiling across FISS modules, and n. Values of TRb tested ranged from 2.2 ms to 5.0 ms in 0.1 ms increments, FISS imaging was applied with and without RF spoiling (with RF spoiling applied across FISS modules), and n ranged from 1 to 3. Other imaging parameters were: rn=500, rθ=14.76°, α=45°, FOV=400 mm×400 mm, matrix=128×128, 3.13 mm in-plane spatial resolution, R=0.4, SL=10 mm, BW=280-1955 Hz/pixel (depending on the TRb).
Flow Phantom Study
A phantom study was conducted to examine the flow properties of FISS when incorporated into a quiescent-interval slice-selective (QISS) MRA sequence (15). In brief, QISS MRA is a 2D technique that applies a thin saturation or inversion pulse to suppress background signal within the imaging slice, an inflow time (TI) to allow fully magnetized arterial spins to enter the imaging slice, and a readout (normally bSSFP or FLASH) to image arterial inflow into the slice. The scan is repeated over multiple contiguous slices to produce the angiogram. The time spanning from the saturation pulse to the end of data acquisition comprises one QISS “shot.”
The flow phantom consisted of flexible tubing (6.35 mm inner diameter) containing a 50% diameter stenosis that was filled with a blood analog (30% glycerin and 70% distilled water, T1/T2=1226/546 ms). The phantom was connected to a computer-controlled pump (PD-1100, BDC Labs, Wheat Ridge, CO) providing pulsatile flow (60 Hz frequency) at rate of 300 mL/min. The mean flow velocity was 91 cm/s at the location of greatest stenosis. QISS imaging was synchronized with pulsatile flow waveform of the pump.
The number of acquired bSSFP views per FISS module, n, was varied from 1, 3, 6, 12 and 24; TRf values for these five configurations were 7.6 ms, 16.2 ms, 27.6 ms, 50.4 ms and 96.0 ms, respectively. Other imaging parameters were: rn=96, rθ=13.125°, FOV=384 mm×384 mm, matrix=384×384, 1.00 mm in-plane spatial resolution, R=6.3, SL=3 mm, TRb/TE/α=3.8 ms/1.9 ms/45°, TI=400 ms, BW=1002 Hz/pixel, QISS shots per slice=2, slices=40, scan time=80 s. A 2.6 ms gradient (strength=7.8 mT/m) was applied in slice direction between FISS modules.
Human Imaging
Human studies consisted of applying FISS for cine imaging of the heart and for ungated QISS-based MRA (15). The heart was imaged using a retrospective cardiac-gated radial cine FISS protocol with the following parameters: rn=108, rθ=11.667°, FOV=260 mm×260 mm, matrix=144×144, 1.81 mm in-plane spatial resolution, R=2.1, SL=5 mm, RF-spoiled FISS with n=3, TRf/TRb/TE/α=15.5 ms/3.4 ms/1.7 ms/54°, BW=1447 Hz/pixel, scan time=12 heartbeats, 14 calculated phases, temporal resolution=46.5 ms. Comparison was made with an analogous retrospective cardiac-gated radial bSSFP cine protocol.
QISS FISS was also applied for ungated pelvic, carotid, and intracranial MRA using the parameters provided in Table 1. Comparisons of ungated QISS FISS MRA were made with analogous ungated radial QISS bSSFP or FLASH protocols matched for basic imaging parameters (FOV, matrix, SL, TI), scan time per imaging slice and, unless noted otherwise, rn. Ungated radial QISS bSSFP used the same flip angle and TRb values as FISS, whereas QISS FLASH used a flip angle of 30° (16). During pelvic MRA, a commercially-available cardiac-gated Cartesian QISS bSSFP protocol (15,17) was also acquired for reference purposes. Blood contrast with respect to fat, muscle or brain tissue was computed.
Table 1.
Imaging Parameters used for Ungated Nonenhanced QISS FISS MRA
| Parameter | Pelvic MRA | Carotid MRA | Intracranial MRA |
|---|---|---|---|
| FISS TRf (ms) | 17.6 | 17.3 | 10.7 |
| TRb (ms) | 3.8 | 3.9 | 4.0 |
| TE (ms) | 1.9 | 1.9 | 2.0 |
| Flip angle (°) | 70 | 70 | 67 |
| FISS n | 3 | 3 | 1 |
| FISS RF spoiling | yes | no | no |
| QISS TI (ms) | 1600 | 505 | 400 |
| Field of view (mm × mm) | 400 × 400 | 256 × 256 | 256 × 256 |
| Reconstructed Matrix | 384 × 384 | 256 × 256 | 256 × 256 |
| rn, radial views | 147/441a | 132 | 192 |
| rθ, view angle increment (°) | 15.918/15.102a | 17.727 | 15.938 |
| Radial undersampling factorb | 4.1/1.4a | 3.0 | 2.1 |
| Slice thickness (mm) | 3 | 3 | 1 |
| Slice overlap (mm) | – | 1.5 | 0.5 |
| Imaging shots per slice | 1/3a | 1 | 4 |
| Bandwidth (Hz/pixel) | 930 | 930 | 975 |
| Number of slices | 120 | 120 | 120 |
| Scan time (per slice) | 2.07/6.21 sa | 1.01 s | 2.58 s |
| Scan time (total) | 9 min 40 s | 2 min 1 s | 5 min 10 s |
| FISS Gspoil duration (ms) | 1.4 | 5.1 | – |
| FISS Gspoil strength (mT/m) | 7.8 | 7.8 | – |
Values provided in the form: lower 40 slices / upper 80 slices.
With respect to satisfying the Nyquist sampling requirements. FISS Gspoil refers to an additional gradient applied between FISS modules (slice axis). n=number of bSSFP views acquired per FISS module; QISS=quiescent-interval slice-selective; TI=inflow time. Field of view and matrix values are after removal of oversampling in readout direction and without zero-filling interpolation.
RESULTS
Numerical Simulations
The magnitude of the steady-state signal for an archetypal FISS protocol (n=1, no RF spoiling) is shown in Figure 2. A cyclical signal versus frequency spectrum was observed with FISS, with two cycles shown in Figure 2a. Compared with bSSFP (Figure 2b), simulations predict that FISS provides similar signal magnitude for spins within ±0.25/TRb of on-resonance (±63 Hz for TRb=4 ms), while providing “V-shaped” notch-like suppression of off-resonant spins centered approximately at ±(0.60-0.65)/TRb and ±(1.35-1.40)/TRb (±150-163 Hz and ±338-350 Hz for TRb=4 ms). Simulations also show that FISS can provide substantial signal from isochromats displaced ±1/TRb (±250 Hz for TRb=4 ms) from on-resonance; signal at this locus was most prominent at the lower flip angle of 30° (as compared to 60° and 90°).
Figure 2.

Plots of steady-state signal magnitude for (a) FISS and (b) bSSFP as a function of off-resonance frequency and flip angle for stationary spins. Compared with bSSFP, FISS demonstrates similar signal for on-resonant spins, and wider notches of suppression of off-resonant spins centered at approximately ±150-163 Hz and ±338-350 Hz (±0.6-0.65/TRb and ±1.35-1.40/TRb, dependent on the flip angle), instead of ±125 Hz and ±375 Hz (±0.5/TRb and ±1.5/TRb) which is customary for bSSFP. Plots of median inter-view complex signal difference (MISD) for (c) FISS and (d) bSSFP in the transient state. FISS demonstrates smaller signal variability (i.e., lower MISD values) than bSSFP across the off-resonance frequency spectrum. Simulation parameters: number of FISS modules/bSSFP repetitions=1000, TRf/TRb/TE = 9 ms/4 ms/2 ms, T1/T2=1600 ms/180 ms, FISS n=1 without RF spoiling. Dashed gray lines show values for FLASH (TR/TE=9 ms/2 ms, Ernst α=6°).
In the transient state, simulations revealed that FISS (Figure 2c), as compared against bSSFP (Figure 2d), dramatically reduces inter-view signal fluctuations from off-resonant spins as assessed by MISD values. For off-resonant isochromats resonating between 0.4/TRb and 1/TRb (100 Hz-250 Hz for TRb=4 ms), median reductions in MISD values obtained with FISS with respect to bSSFP were 91%, 77% and 74% for flip angles of 30°, 60° and 90°, respectively.
Additional simulations (Supporting Figure S1) show that the use of RF spoiling across FISS modules widens and deepens the notches of signal suppression for off-resonant spins, while the acquisition of n bSSFP views per FISS module yields n local signal maxima in the off-resonance band spanning from ≈±0.5/TRb to ±1.5/TRb (±125 to ±375 Hz for TRb=4 ms).
Figure 3 summarizes numerical simulations of FISS and bSSFP for flowing spins. Whereas as bSSFP demonstrates a ≈5-fold increase in signal from out-of-slice magnetization near the off-resonant frequencies of ±0.5/TRb and ±1.5/TRb (±125 Hz and ±375 Hz for TRb=4 ms) (4,18) (Figure 3b), FISS avoids this phenomenon and provides a more uniform signal spectrum (Figure 3a). Importantly, relevant to the imaging of flow in areas of off-resonance, the notches of suppressed off-resonance frequencies that are predicted for FISS imaging of stationary spins (refer to Figure 2a) are eliminated for flowing spins. FISS also considerably reduces MISD values as compared with bSSFP (Figures 3c and 3d).
Figure 3.
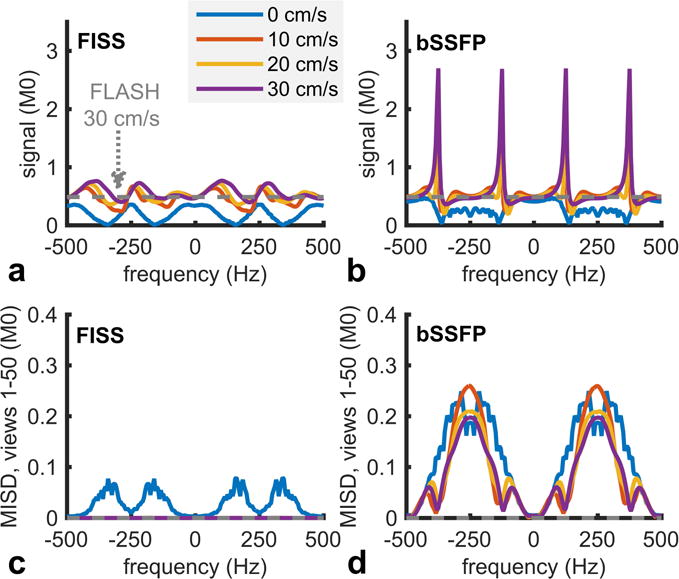
Magnitude of (a) FISS and (b) bSSFP signals as a function of off-resonance frequency and through-plane flow velocity at the 50th view. Whereas bSSFP demonstrates large increases in signal from out-of-slice spins for frequencies near ±125 Hz and ±375 Hz (i.e., ±0.5/TRb and ±1.5/TRb), FISS avoid this phenomenon and provides more uniform signal across off-resonance frequencies and flow rates. (c) FISS reduces the median inter-view complex signal difference (MISD) as compared to (d) bSSFP imaging. Simulations parameters: number of FISS modules/bSSFP repetitions=50, SL=3 mm, TRf/TRb/TE/α = 9 ms/4 ms/2 ms/60°, T1=1600 ms, T2/T2* for in-slice/out-of-slice isochromats=180 ms/45 ms, FISS n=1 without RF spoiling. Dashed gray lines show values for FLASH (TR/TE=9 ms/2 ms, α=30°) imaging of flow at velocity of 30 cm/s.
Image Contrast and Signal-to-Noise
The imaging contrast provided by FISS and bSSFP is shown in Figure 4. In agreement with simulations, FISS improved the degree of fat suppression with respect to bSSFP. At flip angles of 30°(60°)[90°], arterial-to-muscle and arterial-to-fat contrast values obtained with FISS were 3.2(8.4)[16.4] and 1.0(2.6)[5.8], respectively. Corresponding arterial-to-muscle and arterial-to-fat contrast values for bSSFP were 2.9(7.6)[13.6] and 1.1(1.9)[3.5], respectively. At the flip angle of 60° SNR values obtained with FISS/bSSFP were 16.8/20.9 in skeletal muscle, 42.3/76.5 in fat, 83.8/60.3 in arterial blood, 28.4/31.5 in gray matter, and 23.2/25.0 in white matter.
Figure 4.
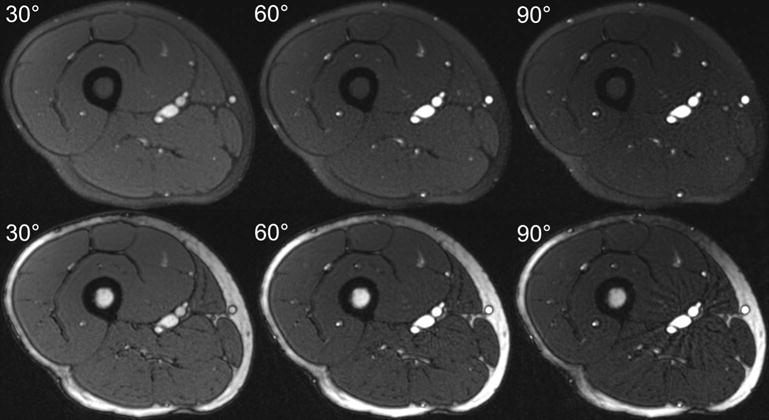
Axial images of the thigh illustrating the imaging contrasts provided by FISS (upper panel) and bSSFP (lower panel) at flip angles of 30°, 60° and 90°. FISS images were acquired with n=1 and RF spoiling.
Phantom Studies
Signal from the oil phantom study is plotted in Figure 5. In agreement with the simulations shown in Supporting Figure S1, fat suppression with FISS depended on the TRb, the use of RF spoiling, and the number of bSSFP views acquired per FISS module (n). Compared with bSSFP, FISS substantially broadened the range of TRb values that suppressed the oil phantom.
Figure 5.
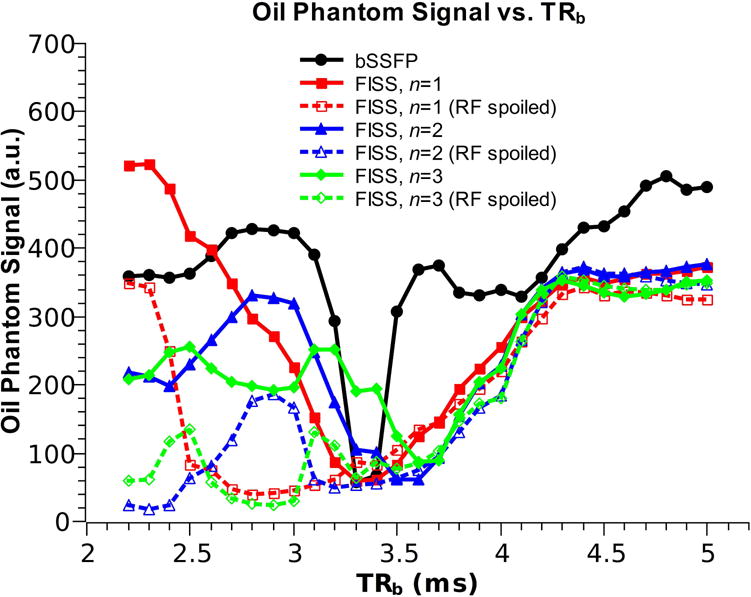
Plot of signal from an oil phantom illustrating the dependence on TRb, RF spoiling across FISS modules, and the number of bSSFP readouts per FISS module (n). With respect to bSSFP imaging, FISS suppresses fat signal over a wider range of TRb values. The degree of fat signal suppression improves with the application of RF spoiling across FISS modules (dashed lines), and depends on n, the number of bSSFP readouts acquired in each FISS module.
Results of the flow phantom experiment are shown in Figure 6. Compared with a bSSFP acquisition, FISS substantially reduced flow artifact near the stenosis (Figure 6a). FISS portrayed the stenosis more similar to a flow-compensated FLASH acquisition. Figure 6b shows the impact of n on the display of the stenosis. The acquisition of only one bSSFP view per FISS module (i.e., n=1) increased signal intensity and reduced flow artifact as compared to larger values of n.
Figure 6.
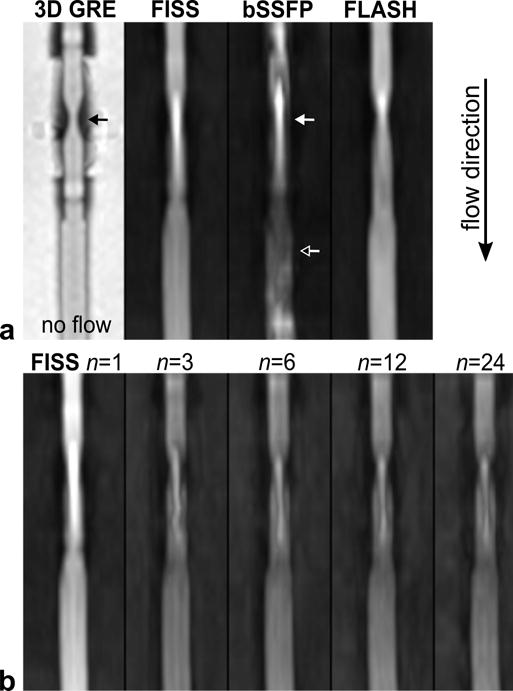
Signal behavior of FISS in a stenotic flow phantom. (a) Comparison of FISS, bSSFP and FLASH acquisitions during QISS-based imaging of a tubular flow phantom with a 50% diameter stenosis. Images are thin maximum intensity projections. The geometry of the stenosis (solid black arrow) is shown on a 3D gradient recalled-echo (GRE) scan. When imaging during flow (flow direction: top to bottom), the use of a FISS readout (α=60°) reduces flow artifact compared with bSSFP (α=60°) at and distal to the stenosis (solid and hollow arrows), and more closely resembles the appearance of FLASH (α=30°). Images in top panel were shown after equalizing the maximum signal intensity in each image. (b) Impact of n, the number of bSSFP repetitions per FISS module, on image quality. FISS applied with n=1 increases signal-to-noise and reduces flow artifact compared with n>1. The windowing is identical for all images.
Human Imaging
Cine frames of the heart obtained with FISS are shown in Figure 7. Whereas bSSFP imaging demonstrated bright subcutaneous and epicardial fat, fat appeared dark in FISS cine images. Left ventricular blood-to-epicardial fat contrast values for FISS and bSSFP were 2.2 and −0.4, respectively. Blood-to-myocardial contrast values for FISS and bSSFP equaled 1.3.
Figure 7.
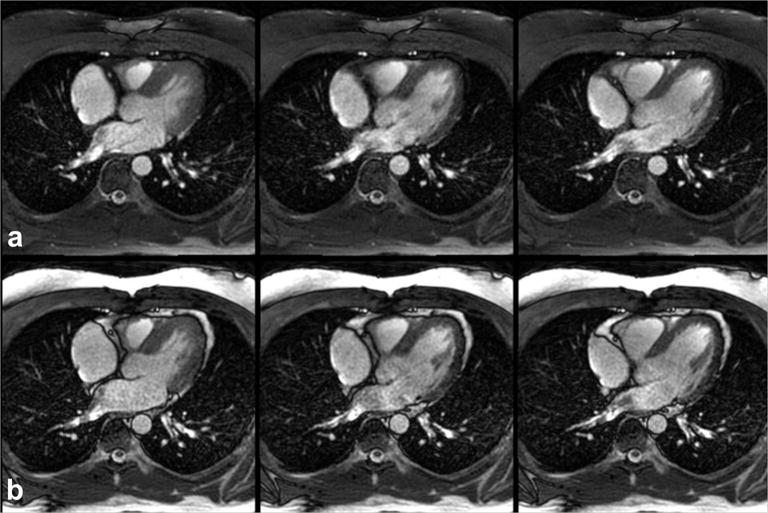
Fat-suppressed radial cine FISS imaging. Three of fourteen frames obtained with (a) radial cine FISS (n=3, α=54°, RF spoiling) and (b) radial cine bSSFP (α=54°). Note the strong suppression of subcutaneous and epicardial fat signal obtained with FISS in comparison with bSSFP.
Figure 8a shows a QISS FISS MRA of the pelvis obtained without cardiac gating. Ungated QISS FISS eliminated the severe flow artifacts observed with ungated QISS bSSFP MRA (Figure 8b), and showed excellent correlation with ECG-gated Cartesian QISS MRA (Figure 8c). Blood-to-muscle and blood-to-fat contrast values obtained with ungated QISS FISS were 5.1 and 9.1, respectively. Corresponding values for ungated QISS bSSFP were 9.4 and 1.0.
Figure 8.
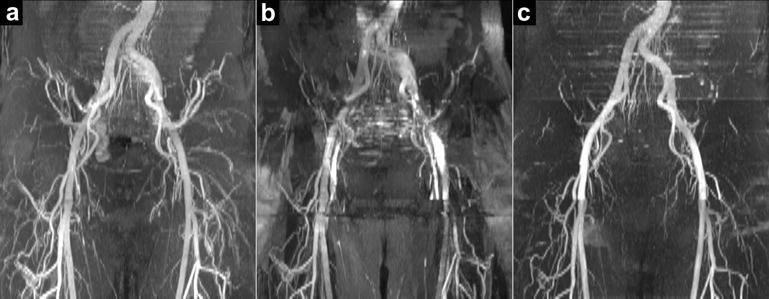
Ungated free-breathing QISS FISS MRA of the pelvis. Comparison of (a) ungated radial QISS FISS MRA (α=70°, n=3, RF spoiling) and (b) ungated radial QISS bSSFP MRA (α=70°; 1.6-fold more views acquired in each shot to match the readout duration of QISS FISS) with respect to a reference exam of (c) cardiac-gated breath-hold Cartesian QISS bSSFP MRA (α=80°). Acquisitions in (a) and (b) were acquired without breath-holding, while the upper-most slices in (c) were acquired with breath-holding. Whereas ungated radial QISS bSSFP MRA shows severe artifact, ungated radial QISS FISS MRA displays the pelvic arteries, and agrees with the cardiac-gated breath-hold Cartesian QISS bSSFP MRA.
Images of the carotid bifurcation obtained with ungated QISS FISS MRA are shown in Supporting Figure S2. The use of a FISS readout markedly improved the image quality of the carotid bifurcation as compared with a bSSFP readout (which showed severe artifact), and reduced saturation of signal within the carotid bulb that was observed with a FLASH readout. Contrast values between the internal carotid arterial bulb and stenocleidomastoid muscle for FISS, bSSFP and FLASH readouts were 6.0, 5.7 and 4.3, respectively.
In the brain, QISS FISS MRA improved the display of the proximal middle cerebral arteries as compared with QISS bSSFP (Figure 9a), and avoided the severe artifact that was observed with QISS bSSFP in the anterior cerebral artery near the paranasal sinuses (Figure 9b) caused by B0 inhomogeneity and out-of-slice magnetization. QISS FISS MRA improved the projective and source conspicuity of some small cortical arteries as compared with QISS FLASH MRA (Figure 9c). Arterial-to-brain contrast in the M2 segment of the middle cerebral artery was 3.3, 1.6 and 1.8 for FISS, bSSFP and FLASH readouts, respectively. We speculate that the improved contrast provided by QISS FISS with respect to QISS bSSFP was due to increased magnetization transfer effects imparted by the FISS echo train, and partial suppression of stationary brain tissue with FISS due to B0 inhomogeneity.
Figure 9.
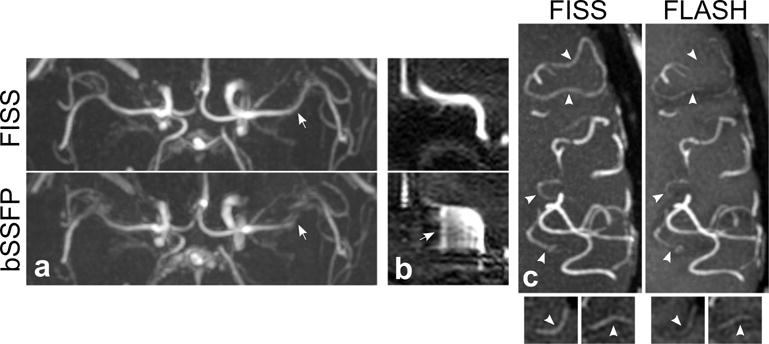
Ungated QISS FISS MRA of the brain. (a) Axial 50-mm-thick maximum intensity projections (MIPs) demonstrate improved display of the proximal middle cerebral arteries with FISS (α=67°, n=1) as compared with bSSFP (α=67°) (arrows). (b) Sagittal source reformations through the anterior cerebral artery show that FISS suppresses out-of-slice magnetization that causes severe artifacts with bSSFP imaging (arrow). (c) Axial 10-mm-thick MIPs show improved conspicuity of some small cortical arteries (arrowheads) with FISS as compared with FLASH (α=30°). Small panels at bottom show source (i.e., non-projective) image data at the locations of the uppermost two arrowheads.
DISCUSSION
In this work, we evaluated FISS, an interrupted radial variant of bSSFP imaging, using numerical simulations, phantom studies, and human studies. The FISS method maintained the high signal levels associated with bSSFP imaging while greatly reducing its sensitivity to flow artifacts from off-resonant and out-of-slice spins.
Balanced steady-state free precession imaging is a mainstay of clinical magnetic resonance of the heart and blood vessels for reasons of imaging speed and high signal-to-noise ratio (19–21). Despite these strengths, a well-recognized drawback of bSSFP imaging is its sensitivity to flow artifacts, especially near arterial stenoses (22,23) and in regions of B0 inhomogeneity (4,18). Out-of-slice magnetization further contributes to flow artifacts with bSSFP imaging, especially in areas of off-resonance (4,24,25). These artifacts were evident in our flow phantom study and in vivo (e.g., when imaging the circle of Willis near the air-filled paranasal sinuses). We have also observed such flow artifacts in cine bSSFP images of the heart and great vessels during periods of rapid systolic flow; these artifacts become more severe as the slice thickness is reduced.
The FISS technique, which spoils residual transverse magnetization between one FISS module and the next, markedly reduced the flow artifacts characteristic of conventional bSSFP imaging. We attribute the reduced flow artifact with FISS to spoiling-mediated suppression of unwanted flow-induced magnitude and phase variations over the echo train, as well as to the suppression of out-of-slice magnetization. With bSSFP, on the other hand, these unwanted sources of MR signal variation carry over from one repetition to the next. FISS also substantially reduced signal oscillations from off-resonant spins that occur with bSSFP imaging in the transition to the steady-state (26–28).
The use of α/2 RF pulses to, in effect, pause and resume the bSSFP echo train was reported by Scheffler and colleagues (at a rate of ≈5-6 Hz) to achieve fat-suppressed bSSFP imaging with the intermittent application of chemically-selective fat saturation RF pulses (7). FISS represents an extreme implementation of this strategy, applying α/2 flip-down and flip-up RF pulses at a rate between 50 and 100 Hz, depending on the number of bSSFP views acquired in each FISS module.
In related work, Derbyshire and colleagues described a Cartesian spectrally selective suppression with steady-state free precession technique (S5FP) that provided fat saturated bSSFP-based imaging at 1.5T (5). FISS and S5FP differ in several respects. First, instead of applying simple α/2 flip-down and flip-up RF pulses, the S5FP method applied more complex subsequences of RF pulses before and after the bSSFP readouts as well as data rescaling to equalize echo amplitudes. Second, as compared with FISS, which collects only one to a few bSSFP repetitions between the flip-down and flip-up RF pulses, S5FP employed a longer bSSFP echo train (typically, n=24) to minimize the scan time penalty with respect to conventional uninterrupted bSSFP imaging. As observed in the flow phantom study, however, the use of larger n augments the sensitivity of the FISS method to flow artifact.
A key difference between FISS and S5FP is that FISS uses a radial k-space trajectory, whereas S5FP used a Cartesian k-space trajectory. The use of radial k-space sampling trajectory is beneficial in two respects. First, due to the oversampling of central k-space, radial sampling is less sensitive than Cartesian sampling to motion and arterial pulsation artifact (8). Whereas Cartesian-based QISS FISS MRA requires the use of cardiac synchronization to eliminate arterial pulsation artifact (not shown), radial QISS FISS MRA does not. Second, when multiple bSSFP readouts are collected in each FISS module (i.e., n>1), radial sampling is much less sensitive than Cartesian sampling to artifacts caused by signal fluctuations arising from the interrupted nature of the FISS acquisition. With Cartesian k-space sampling, these signal fluctuations produce ghost artifact in the phase-encoding direction. Conversely, these signal variations produce minimal to no apparent artifact with radial sampling.
The image contrasts of bSSFP and FISS differed in vivo, with FISS providing greater suppression of fat tissue in cine and non-cine acquisitions. With radial FISS imaging applying one bSSFP readout per module, TRb values that provide ≈50% or greater intrinsic suppression of fat signal at 3.0T (i.e., without requiring Dixon (29) or phase-based post-processing (30)) range from ≈3.0 ms-3.7 ms without RF spoiling, and ≈2.4 ms-3.8 ms with RF spoiling. With bSSFP, on the other hand, inherent suppression of fat is more difficult due to the much narrower spectral bands of low signal and the extent of B0 inhomogeneity present across a typical FOV (≈100Hz).
Results of in vivo studies also showed that FISS reduces spin saturation compared with FLASH imaging. This effect was evident during QISS-based imaging of the internal carotid bulb, where slow, recirculatory flow is common (31), and in the display of small intracranial arteries with slow blood flow.
The FISS method is not without limitations. First, due to the continuously interrupted nature of acquisition, FISS is approximately two-fold less efficient than conventional bSSFP imaging when one bSSFP view is collected in each FISS module. However, the imaging efficiency of FISS can be substantially improved with the acquisition of just a few bSSFP views per FISS module. Second, because more RF pulses are applied to acquire one view of k-space, FISS entails greater total RF energy deposition than bSSFP. All other things being equal, bSSFP would therefore be able to use larger flip angles than FISS in magnetization-prepared single-shot or multi-shot imaging scenarios (e.g., such as QISS MRA) assuming equal numbers of echoes. On the other hand, because the rate of RF energy deposition (i.e., energy per time) of continuously-running FISS and bSSFP acquisitions are approximately the same, cine FISS should closely match the flip angles achievable with cine bSSFP.
Another drawback is that the frequency response of FISS can suppress stationary spins, and by consequence, very slow flowing vascular spins located in areas of B0 inhomogeneity. Instead of manifesting as sharp, narrow “bands” of low signal, as is the case with bSSFP imaging, B0 inhomogeneity during FISS imaging produces a gradual loss of static tissue signal over a broader region. The degree to which this phenomenon may be undesirable for imaging of static tissues and vessels containing very slow or in-plane flow (e.g., through a loss of sensitivity), and advantageous for MRA of vessels containing brisker through-plane flow (e.g., via improved background signal suppression and the potential for reduced partial volume artifact) requires further study and has uncertain diagnostic implications. Also, because the use of RF spoiling across FISS modules narrows the on-resonant band of high signal, FISS implemented without inter-modular RF spoiling is expected to be advantageous for imaging regions of increased B0 inhomogeneity, although at the cost of less fat suppression.
In the context of ungated nonenhanced MRA of the lower extremities, it should be noted that approaches other than 2D radial QISS FISS have been described in the literature (13,32–38). These methods, some of which are 3D and flow-independent acquisitions, however have not yet gained clinical traction for a variety of reasons including technical complexity, long scan times, motion sensitivity and lack of clinical validation.
Further study may be helpful to possibly define an analytic equation that describes the steady-state FISS signal at arbitrary flip angles, repetition times, off-resonance frequencies, and T1 and T2 values. Initial efforts to this end however did not reveal a closed-form equation accounting for all of these variables, as well as the number of acquired bSSFP views per module (n). Moreover, unlike bSSFP, where the assumption of infinitesimally short RF pulses can often be used in numerical simulations (39), we have observed that simulations of FISS ought to incorporate the impacts of finite-length RF pulses and the slice selection gradient. Finally, it should be noted that although the present study focused on the application of FISS at 3.0T, the approach is also feasible at 1.5T.
CONCLUSION
In conclusion, radial FISS-based imaging was found to retain the high on-resonant signal of bSSFP-based imaging, while reducing flow artifacts and signal fluctuations associated with off-resonant spins. Furthermore, FISS reduced saturation artifact that was observed with FLASH imaging, and showed promise for fat-suppressed cine imaging and nonenhanced MRA. Additional studies are needed to evaluate the clinical utility of radial FISS imaging in various cardiovascular imaging applications.
Supplementary Material
Supporting Figure S1. Plot of steady-state signal versus off-resonant frequency for FISS examining the impacts of the number of bSSFP readouts acquired per FISS module (n) and the application of RF spoiling across successive FISS modules. Simulation parameters: TRf/TRb/TE/α= 9-17 ms (for n=1-3)/4 ms/2 ms/60°, T1/T2=1600 ms/180 ms.
Supporting Figure S2. Ungated radial QISS MRA of the carotid artery bifurcation obtained with (a) FISS (α=70°, n=3) (b) bSSFP (α=70°) and (c) FLASH (α=30°) readouts. Images are maximum intensity projections. FISS provides better image quality than bSSFP, which shows severe signal heterogeneity, and reduces saturation of signal in internal carotid arterial bulb that is observed with FLASH.
Acknowledgments
Study was funded by NIH grants R01 HL130093 and R21 HL126015.
References
- 1.Haase A, Frahm J, Matthaei D, Hanicke W, Merboldt KD. FLASH imaging: rapid NMR imaging using low flip-angle pulses. 1986. J Magn Reson. 2011;213(2):533–541. doi: 10.1016/j.jmr.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Carr HY. Steady-state free precession in nuclear magnetic resonance. Physical Review. 1958;112(5):1693–1701. [Google Scholar]
- 3.Sekihara K. Steady-State Magnetizations in Rapid NMR Imaging Using Small Flip Angles and Short Repetition Intervals. IEEE Trans Med Imaging. 1987;6(2):157–164. doi: 10.1109/TMI.1987.4307816. [DOI] [PubMed] [Google Scholar]
- 4.Markl M, Alley MT, Elkins CJ, Pelc NJ. Flow effects in balanced steady state free precession imaging. Magn Reson Med. 2003;50(5):892–903. doi: 10.1002/mrm.10631. [DOI] [PubMed] [Google Scholar]
- 5.Derbyshire JA, Herzka DA, McVeigh ER. S5FP: spectrally selective suppression with steady state free precession. Magn Reson Med. 2005;54(4):918–928. doi: 10.1002/mrm.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derbyshire DA, Guttman MA, Lederman RJ, McVeigh ER. Reduction of Flow Artifacts in Balanced SSFP Imaging Using S5FP. Proceedings of the 16th Annual Meeting of ISMRM; Toronto. 2008; p. 211. [Google Scholar]
- 7.Scheffler K, Heid O, Hennig J. Magnetization preparation during the steady state: fat-saturated 3D TrueFISP. Magn Reson Med. 2001;45(6):1075–1080. doi: 10.1002/mrm.1142. [DOI] [PubMed] [Google Scholar]
- 8.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med. 1992;28(2):275–289. doi: 10.1002/mrm.1910280209. [DOI] [PubMed] [Google Scholar]
- 9.Hinshaw WS. Image formation by nuclear magnetic resonance: The sensitive‐point method. Journal of Applied Physics. 1976;47(8):3709–3721. [Google Scholar]
- 10.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52(3):679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 11.Chen JJ, Pike GB. Human whole blood T2 relaxometry at 3 Tesla. Magn Reson Med. 2009;61(2):249–254. doi: 10.1002/mrm.21858. [DOI] [PubMed] [Google Scholar]
- 12.Ernst RR, Anderson WA. Application of Fourier Transform Spectroscopy to Magnetic Resonance. Review of Scientific Instruments. 1966;37(1):93–102. [Google Scholar]
- 13.Edelman RR, Giri S, Murphy IG, Flanagan O, Speier P, Koktzoglou I. Ungated radial quiescent-inflow single-shot (UnQISS) magnetic resonance angiography using optimized azimuthal equidistant projections. Magn Reson Med. 2014;72(6):1522–1529. doi: 10.1002/mrm.25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firbank MJ, Coulthard A, Harrison RM, Williams ED. A comparison of two methods for measuring the signal to noise ratio on MR images. Phys Med Biol. 1999;44(12):N261–264. doi: 10.1088/0031-9155/44/12/403. [DOI] [PubMed] [Google Scholar]
- 15.Edelman RR, Sheehan JJ, Dunkle E, Schindler N, Carr J, Koktzoglou I. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: Technical considerations and clinical feasibility. Magn Reson Med. 2010;63(4):951–958. doi: 10.1002/mrm.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koktzoglou I, Murphy IG, Giri S, Edelman RR. Quiescent interval low angle shot magnetic resonance angiography of the extracranial carotid arteries. Magn Reson Med. 2016;75(5):2072–2077. doi: 10.1002/mrm.25791. [DOI] [PubMed] [Google Scholar]
- 17.Hodnett PA, Koktzoglou I, Davarpanah AH, Scanlon TG, Collins JD, Sheehan JJ, Dunkle EE, Gupta N, Carr JC, Edelman RR. Evaluation of peripheral arterial disease with nonenhanced quiescent-interval single-shot MR angiography. Radiology. 2011;260(1):282–293. doi: 10.1148/radiol.11101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storey P, Li W, Chen Q, Edelman RR. Flow artifacts in steady-state free precession cine imaging. Magn Reson Med. 2004;51(1):115–122. doi: 10.1002/mrm.10665. [DOI] [PubMed] [Google Scholar]
- 19.Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur Radiol. 2003;13(11):2409–2418. doi: 10.1007/s00330-003-1957-x. [DOI] [PubMed] [Google Scholar]
- 20.Finn JP, Nael K, Deshpande V, Ratib O, Laub G. Cardiac MR imaging: state of the technology. Radiology. 2006;241(2):338–354. doi: 10.1148/radiol.2412041866. [DOI] [PubMed] [Google Scholar]
- 21.Wheaton AJ, Miyazaki M. Non-contrast enhanced MR angiography: physical principles. J Magn Reson Imaging. 2012;36(2):286–304. doi: 10.1002/jmri.23641. [DOI] [PubMed] [Google Scholar]
- 22.Zavodni AE, Emery DJ, Wilman AH. Performance of steady-state free precession for imaging carotid artery disease. J Magn Reson Imaging. 2005;21(1):86–90. doi: 10.1002/jmri.20225. [DOI] [PubMed] [Google Scholar]
- 23.Koktzoglou I, Giri S, Piccini D, Grodzki DM, Flanagan O, Murphy IG, Gupta N, Collins JD, Edelman RR. Arterial spin labeled carotid MR angiography: A phantom study examining the impact of technical and hemodynamic factors. Magn Reson Med. 2015 doi: 10.1002/mrm.25611. [DOI] [PubMed] [Google Scholar]
- 24.Markl M, Pelc NJ. On flow effects in balanced steady-state free precession imaging: pictorial description, parameter dependence, and clinical implications. J Magn Reson Imaging. 2004;20(4):697–705. doi: 10.1002/jmri.20163. [DOI] [PubMed] [Google Scholar]
- 25.Staehle F, Leupold J, Hennig J, Markl M. Off-resonance-dependent slice profile effects in balanced steady-state free precession imaging. Magn Reson Med. 2008;59(5):1197–1202. doi: 10.1002/mrm.21557. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura DG, Vasanawala S. Analysis and reduction of the transient response in SSFP imaging. Proceedings of the 8th Annual Meeting of ISMRM; Denver. 2000; p. 301. [Google Scholar]
- 27.Hennig J, Speck O, Scheffler K. Optimization of signal behavior in the transition to driven equilibrium in steady-state free precession sequences. Magn Reson Med. 2002;48(5):801–809. doi: 10.1002/mrm.10274. [DOI] [PubMed] [Google Scholar]
- 28.Deshpande VS, Chung YC, Zhang Q, Shea SM, Li D. Reduction of transient signal oscillations in true-FISP using a linear flip angle series magnetization preparation. Magn Reson Med. 2003;49(1):151–157. doi: 10.1002/mrm.10337. [DOI] [PubMed] [Google Scholar]
- 29.Huang TY, Chung HW, Wang FN, Ko CW, Chen CY. Fat and water separation in balanced steady-state free precession using the Dixon method. Magn Reson Med. 2004;51(2):243–247. doi: 10.1002/mrm.10686. [DOI] [PubMed] [Google Scholar]
- 30.Hargreaves BA, Vasanawala SS, Nayak KS, Hu BS, Nishimura DG. Fat-suppressed steady-state free precession imaging using phase detection. Magn Reson Med. 2003;50(1):210–213. doi: 10.1002/mrm.10488. [DOI] [PubMed] [Google Scholar]
- 31.Reneman RS, van Merode T, Hick P, Hoeks AP. Flow velocity patterns in and distensibility of the carotid artery bulb in subjects of various ages. Circulation. 1985;71(3):500–509. doi: 10.1161/01.cir.71.3.500. [DOI] [PubMed] [Google Scholar]
- 32.Brittain JH, Olcott EW, Szuba A, Gold GE, Wright GA, Irarrazaval P, Nishimura DG. Three-dimensional flow-independent peripheral angiography. Magn Reson Med. 1997;38(3):343–354. doi: 10.1002/mrm.1910380302. [DOI] [PubMed] [Google Scholar]
- 33.Edelman RR, Koktzoglou I. Unenhanced flow-independent MR venography by using signal targeting alternative radiofrequency and flow-independent relaxation enhancement. Radiology. 2009;250(1):236–245. doi: 10.1148/radiol.2493080496. [DOI] [PubMed] [Google Scholar]
- 34.Koktzoglou I, Edelman RR. Ghost magnetic resonance angiography. Magn Reson Med. 2009;61(6):1515–1519. doi: 10.1002/mrm.21943. [DOI] [PubMed] [Google Scholar]
- 35.Cukur T, Shimakawa A, Yu H, Hargreaves BA, Hu BS, Nishimura DG, Brittain JH. Magnetization-prepared IDEAL bSSFP: a flow-independent technique for noncontrast-enhanced peripheral angiography. J Magn Reson Imaging. 2011;33(4):931–939. doi: 10.1002/jmri.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bangerter NK, Cukur T, Hargreaves BA, Hu BS, Brittain JH, Park D, Gold GE, Nishimura DG. Three-dimensional fluid-suppressed T2-prep flow-independent peripheral angiography using balanced SSFP. Magn Reson Imaging. 2011;29(8):1119–1124. doi: 10.1016/j.mri.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon KT, Wu HH, Shin T, Cukur T, Lustig M, Nishimura DG. Three-dimensional magnetization-prepared imaging using a concentric cylinders trajectory. Magn Reson Med. 2014;71(5):1700–1710. doi: 10.1002/mrm.24823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D, Seo H, Cho J, Kwon K, Han Y, Park H. Non-contrast-enhanced peripheral MR angiography using velocity-selective excitation. Magn Reson Med. 2017 doi: 10.1002/mrm.26732. [DOI] [PubMed] [Google Scholar]
- 39.Bieri O, Scheffler K. SSFP signal with finite RF pulses. Magn Reson Med. 2009;62(5):1232–1241. doi: 10.1002/mrm.22116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. Plot of steady-state signal versus off-resonant frequency for FISS examining the impacts of the number of bSSFP readouts acquired per FISS module (n) and the application of RF spoiling across successive FISS modules. Simulation parameters: TRf/TRb/TE/α= 9-17 ms (for n=1-3)/4 ms/2 ms/60°, T1/T2=1600 ms/180 ms.
Supporting Figure S2. Ungated radial QISS MRA of the carotid artery bifurcation obtained with (a) FISS (α=70°, n=3) (b) bSSFP (α=70°) and (c) FLASH (α=30°) readouts. Images are maximum intensity projections. FISS provides better image quality than bSSFP, which shows severe signal heterogeneity, and reduces saturation of signal in internal carotid arterial bulb that is observed with FLASH.


