Figure 1.
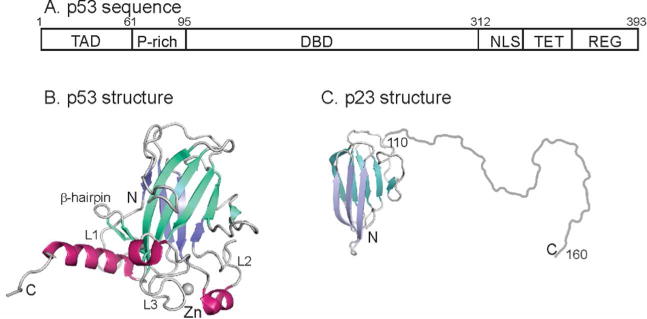
A. Schematic diagram showing the domain structure of human p53. Residues 95-312 comprise the folded DNA-binding domain. TAD – trans-activation domain; P-rich – proline rich domain; DBD – DNA-binding domain; NLS – nuclear localization sequence; TET – tetramerization domain; REG – C-terminal regulatory domain. B. Cartoon representation of the solution structure of the DBD of human p53 (2FEJ4)helices are shown in red, and the strands of the two β-sheets are colored in blue and turquoise. C. Representation of the structure of human p23. The folded domain (residues 1-109) is derived from the crystal structure (1EJF6) and the disordered C-terminus (residues 110-160) is shown schematically as a loop. The strands of the two β-sheets are colored in blue and turquoise.
