Abstract
Background
In 2001, the United States revised the arsenic maximum contaminant level for public drinking water systems from 50 μg/L to 10 μg/L. This study aimed to examine temporal trends in urinary arsenic concentrations in the U.S. population from 2003 to 2014 by drinking water source among individuals aged 12 years and older who had no detectable arsenobetaine - a biomarker of arsenic exposure from seafood intake.
Methods
We examined data from 6 consecutive cycles of the National Health and Nutrition Examination Survey (2003–2014; N=5,848). Total urinary arsenic (TUA) was calculated by subtracting arsenobetaine’s limit of detection and detectable arsenocholine from total arsenic. Additional sensitivity analyses were conducted using a second total urinary arsenic index (TUA2, calculated by adding arsenite, arsenate, monomethylarsonic acid, dimethylarsinic acid). We classified drinking water source using 24-hour dietary questionnaire data as community supply (n=3,427), well or rain cistern (n=506), and did not drink tap water (n=1,060).
Results
Geometric means (GM) of survey cycles were calculated from multivariate regression models adjusting for age, gender, race/ethnicity, BMI, income, creatinine, water source, type of water consumed, recent smoking, and consumption of seafood, rice, poultry, and juice. Compared to 2003–2004, adjusted TUA was 35.5% lower in 2013–2014 among the general U.S. population. Stratified analysis by smoking status indicated that the trend in lower TUA was only consistent among non-smokers. Compared to 2003–2004, lower adjusted TUA was observed in 2013–2014 among non-smoking participants who used community water supplies (1.98 vs 1.16 μg/L, p<0.001), well or rain cistern users (1.54 vs 1.28 μg/L, p<0.001) and who did not drink tap water (2.24 vs 1.53 μg/L, p<0.001). Sensitivity analyses showed consistent results for participants who used a community water supplier and to a lesser extent those who did not drink tap water. However, the sensitivity analysis showed overall exposure stayed the same or was higher among well or rain cistern users. Finally, the greatest decrease in TUA was among participants within the highest exposure percentiles (e.g. 95th percentile had 34% lower TUA in 2013/2014 vs 2003/2004, p<0.001).
Conclusions
Overall, urinary arsenic levels in the U.S. population declined over a 12-year period that encompassed the adoption of the revised Arsenic Rule. The most consistent trends in declining exposure were observed among non-smoking individuals using public community water systems. These results suggest regulation and prevention strategies to reduce arsenic exposures in the U.S. may be succeeding.
Keywords: Arsenic, urinary biomarker, population surveillance, United States, Safe Drinking Water Act
1. Introduction
Arsenic is a public health concern worldwide including in the United States. Chronic exposure to inorganic arsenic (iAs) is associated with adverse health effects such as various cancers, skin disorders, cardiovascular disease, and immunotoxicity (ATSDR 2007; Cardenas et al. 2015; Cardenas et al. 2016; IARC 2012; Naujokas et al. 2013). A naturally occurring element, there are numerous anthropogenic and natural sources of iAs in the United States. Exposures to iAs can come from contaminated soils or dust, emissions from industrial smelting processes or specialized glass manufacturers, mining effluents, or household pesticides and chemicals (ATSDR 2007). Inorganic As is also a common drinking water contaminant. Elevated concentrations of iAs in groundwater occurs throughout the United States although it is more prevalent in the Northeast, Midwest, and Western regions of the country (ATSDR 2007; Frost et al. 2003; Nielsen 2010). While drinking water contaminated with iAs is a major route of exposure in the United States (ATSDR 2007; Naujokas et al. 2013), people can also be exposed to iAs from eating rice or rice-based products (Davis et al. 2012; Navas-Acien et al. 2011). Other dietary sources of arsenic include seafood, grains, fruits, and various juice products (Davis et al. 2012; deCastro et al. 2014; Navas-Acien et al. 2011). People can also be exposed to iAs from cigarette smoke (Caruso et al. 2013; Pappas 2011).
Arsenic has been regulated in drinking water in the United States since 1942 when the United States Public Health Service set a standard of 50 μg/L (USPHS 1943). Amendments to the 1996 Safe Drinking Water Act (PL 104–182) required the United States Environmental Protection Agency (EPA) to issue a primary drinking water regulation for arsenic based on additional evidence of its health effects, occurrence, and treatment costs at low concentrations in drinking water. In 2001, the EPA adopted the revised Arsenic Rule, which reduced the maximum contaminant level (MCL) to 10μg/L for public water systems. This rule became enforceable in January 2006, but many small or highly affected water systems were provided renewable 3-year waivers to reach compliance (EPA 2001a). The EPA estimated that the revised arsenic MCL would affect more than 4,000 water systems serving at least 12.7 million people (EPA 2001a). Water systems required to comply with the revised MCL include community water systems serving at least 25 people year-round (e.g. most cities and towns) or with at least 15 connections and non-transient, non-community water systems that serve at least 25 of the same people for at least 6 months per year (e.g. schools, churches, and businesses). The EPA does not regulate or monitor water sources considered private, which typically includes domestic wells serving a single or a limited number of homes (Nielsen 2010). Thus, approximately 12% of the U.S. population who are served by domestic wells were not required to comply with the revised MCL even though it is estimated that 11–19% of private wells contain arsenic in excess of 10μg/L (Focazio et al. 2006; Kumar et al. 2010; Montgomery et al. 2003). It is likely, therefore, that the revised Arsenic Rule would reduce arsenic exposure only among people who receive their drinking water from a community water source.
Given the adoption of the Arsenic Rule, we hypothesized that there would be a population level decrease in iAs exposure following its implementation. We examined urinary arsenic levels among the general U.S. population to evaluate these trends in exposure among different water users by using 6 consecutive cycles of the National Health and Nutrition Examination Survey (NHANES) spanning from 2003–2004 to 2013–2014. NHANES collects biological monitoring data that is used to evaluate trends in population-level exposure to chemicals and NHANES urinary arsenic measurements have been used by multiple studies to improve our understanding of the health effects of iAs exposure (Cardenas et al. 2015; Jones et al. 2011; Navas-Acien et al. 2011) and sources of iAs exposure (Davis et al. 2012; deCastro et al. 2014; Mantha et al. 2017; Xue et al. 2010). Here we examined temporal urinary arsenic trends among people who receive their drinking water from a community supply, wells or rain cisterns, or who did not drink tap water. We hypothesized that decreased arsenic exposure would be greatest for individuals using public water systems (impacted by the revised Arsenic Rule) compared to individuals using wells (not impacted by the rule change).
2. Materials and Methods
2.1. Study design
NHANES data is collected annually and publicly released in two-year cycles by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The survey uses a complex multistage probability sample design to select a representative sample of the civilian, non-institutionalized U.S. population (CDC 2015). Each cycle includes multiple survey stages that include questionnaires, physical exam, biospecimen collection and a variety of laboratory tests (Zipf et al. 2013). All participants provided informed consent and study protocols were approved by the NCHS research ethics review board (CDC 2012).
We used publicly available data from six NHANES cycles: 2003–2004 (n=2,554), 2005–2006 (n=2,568), 2007–2008 (n=2,545), 2009–2010 (n=2,855), 2011–2012 (n=2,501), and 2013–2014 (n=2,640) cycles. This analysis was restricted to participants who had data on urinary arsenic speciation, which was first collected by NHANES in the 2003–2004 cycle (Caldwell et al. 2009). Urinary arsenic concentrations were measured in a random one-third subsample of participant’s ≥6 years of age (N=15,663 from the 6 pooled NHANES survey cycles) (Caldwell et al. 2009). We further restricted our analysis to individuals who had urinary arsenobetaine concentrations below the limit of detection. Arsenobetaine (AsB) is the dominant form of organic arsenic found in seafood with 90% of the ingested AsB excreted in urine within 66 hours (Molin et al. 2015; Schmeisser et al. 2006). Thus, by restricting to individuals without detectable AsB levels in the urine we are greatly reducing the potential for seafood intake to confound our analyses. Finally, to account for the potential confounding by smoking status we further restricted the sample to individuals who completed a smoking history questionnaire. This questionnaire was only asked to participants ≥12 years old. Thus, our final sample size was (N= 5,848 from the 6 pooled NHANES survey cycles). We compared the selected socio-demographic and exposure characteristics between the unrestricted and restricted analysis and observed no difference in underlying population characteristics (data not shown).
2.2. Urinary arsenic analysis
The methods describing urinary arsenic collection and measurement have been described previously (Caldwell et al. 2009; CDC 2014). Briefly, spot urine samples were collected during the survey physical examination and shipped to the CDC’s National Center for Environmental Health where the samples were analyzed within three weeks of collection by high-performance liquid chromatography and inductively coupled-plasma dynamic reaction cell-mass spectrometry. NHANES provides the following urinary arsenic measurements: total arsenic, arsenite (AsIII), arsenate (AsV), arsenobetaine (AsB), arsenocholine (AsC), monomethylarsonic acid (MMA), dimethylarsenic acid (DMA), and trimethylarsine oxide (CDC 2014). The arsenic metabolites AsB and AsC are commonly found in seafood and understood to be relatively nontoxic (Caldwell et al. 2009; Choi et al. 2010; deCastro et al. 2014). Thus, we defined total urinary arsenic (TUA) as total arsenic minus AsB and AsC the sum of AsIII, AsV, MMA, and DMA. We also constructed a second total urinary arsenic composite (TUA2) by the sum of AsIII, AsV, MMA, and DMA and re-ran all models as a sensitivity analysis (Supplemental Tables S4–S6, Figure S1). These composite measures of urinary arsenic have been used by the CDC and in previous studies to represent iAs (Cardenas et al. 2015; CDC 2017).
The limits of detection (LOD) for the three arsenic species used to compute TUA varied by survey cycle (Table S1). To enable unbiased comparisons of TUA across cycles, we assumed the maximal LOD for each arsenic species observed in one cycle (i.e. Total As = 0.88 μg/L, AsB = 0.84 μg/L, AsC = 0.42 μg/L, AsIII=0.85 μg/L, AsV = 0.71 μg/L, DMA = 1.35 μg/L, MMA = μg/L) and applied it to each cycle. Then, any samples below the maximal LOD were assigned the maximal LOD divided by the square root of 2 prior to analysis. This method has been used by the CDC and produces reasonably non-biased estimates (CDC 2013; Hornung and Reed 1990; Zota et al. 2014). We opted to restrict the adjusted analyses to individuals with AsB concentrations below the intercycle LOD (i.e. ≤0.84 μg/L). Finally, urine dilution was accounted for using urinary creatinine as a separate independent variable in regression analyses (Barr et al. 2005).
2.3. Drinking water source
All participants are asked to complete a 24-hour dietary recall which includes quantity and types of water consumed in the 24-hour period preceding the physical examination and urine collection (CDC 2015). NCHS provides summed quantities (grams) of specific water types (e.g. plain, tap, bottled) from all portions of the 24-hour dietary recall interview. Participants were also asked additional questions regarding the source of their drinking water. NHANES made a slight change to the wording of this question between the 2003–2004 cycle and all subsequent cycles (2005–2006 to 2013–2014). In the 2003–2004 cycle, interviewers asked participants “Was the main source of the tap water you drank yesterday the community water supply, a well or rain cistern, a spring, or something else.” In the subsequent cycles, interviewers asked participants “When you drink tap water, what is the main source of the tap water? Is the city water supply (community water supply), a well or rain cistern, a spring, something else, or I don’t drink tap water?” Thus, in the 2003–2004 cycle only participants who reported consuming tap water in the previous 24-hours were asked about the primary source of their drinking water; while in the 2005–2014 cycles participants were asked about the main source of their drinking water regardless of their water consumption during the previous day.
To address this issue, we used an additional question within the 2003–2004 cycle housing characteristics questionnaire that asks participants “What is the source of tap water in this home? Is it a private or public water company, a private or public well, or something else?” (Table S2). The housing characteristics questionnaire was administered before the physical examination and collected data on features of the physical environment of the participant’s home. The similar wording between the two questions allowed us to compare the consistency with which participants responded to their source of tap water in the 24-hour dietary recall in 2003–2004. We cross-tabulated these 2 questions to compare the consistency for community supply and well or rain cistern supply responses. For example, a response was considered consistent if the participant said “community supply” in the 24-hour dietary questionnaire and “company supply” in the housing questionnaire. To reduce misclassification, participants were excluded from our analysis if they reported “other source” (n=572), “don’t know” (n=115), or “spring” (n=180). Thus, we categorized participants into: i) community supply (n=3,427), ii) well or rain cistern (n=506), iii) and did not drink water (n=1,060). If participants did not drink tap water in the 24-hour dietary recall questionnaire (2003–2004) or responded that they did not drink tap water ever (2005–2006 to 2013–2014), they were categorized as “did not drink water”.
2.4. Covariates
We examined the following covariates due to their potential to influence urinary arsenic: survey cycle, age, gender, race and ethnicity, body mass index (BMI), family poverty-income ratio (PIR), type of water consumed, recent smoking status, and recent seafood, rice, poultry, and juice consumption. Age at examination was categorized as 12–19 years, 20–39 years, and over 40 years. Participants self-reported as non-Hispanic white, non-Hispanic black, Mexican American, other Hispanics, other race, or multiracial. We combined “other Hispanics” and “other race” into a single category to increase sample size and allow for comparisons between cycle years. BMI was calculated by dividing measured weight in kilograms by measured height in meters squared. BMI was classified as underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30). Participants under 20 years of age were assigned BMI classifications as defined by the CDC growth charts for age and sex specific cutoffs. PIR was calculated as a ratio of self-reported family income compared to the US Census poverty threshold. We classified participants’ PIR status as low (<1.8), medium (>1.8–<3.9), or high (3.9–5). Smoking status (i.e. smoker vs non-smoker) was determined from a single question that asked the participants if he/she had used tobacco or nicotine products (e.g. cigarettes, pipes, cigars, chewing tobacco, snuff) within the last 5 days. This question corresponds to NHANES question SMQ680 in the 2003–2012 cycles and question SMDANY in the 2013–2014 cycle. While serum cotinine is a preferred method of adjusting for current smoking status, this data was unavailable for the 2013–2014 cycle at the time of analysis. However, cross-tabulations of smokers defined by serum cotinine (i.e. >10ng/mL) with the survey question in the 2003–2012 cycles showed that the self-reported classification had high accuracy (i.e. sensitivity: >91%; specificity: >96%).
To account for dietary arsenic exposure, we used data from the 24-hour dietary recall to create covariates for intake of seafood, rice, poultry, or juice (yes/no) using USDA food codes (Table S3). Specifically, we adopted the seafood classification scheme developed by Navas-Acien et al. (2011). Finally, we used the USDA food codes to create 4 mutually exclusive categories of type of water consumed: i) “Tap water only” if they drank plain or filtered tap water or from a drinking fountain; ii) “Bottle/Spring water only” if they drank bottled or spring water; iii) “Mixed sources” if they drank plain or filtered water, from a drinking fountain, bottled and/or spring water; and iv) “None” if they did not drink water from any of these sources.
2.5 Statistical analysis
All statistical analyzes were conducted in Stata with the survey command to account for NHANES complex sampling design that includes the sampling weights, primary sampling units, and strata (Stata version 14; StatCorp LP, College Station, TX). All statistical tests were two-tailed and type 1 error was set at α=0.05. Standard errors (SE) and confidence intervals were estimated using the Taylor linearization method. Unweighted sample sizes and weighted proportions for covariates were computed for all covariates. TUA concentration and urinary creatinine were right-skewed and were ln-transformed to account for the non-normal distributions before regression analysis. Multivariate regression models were used to assess the association between ln-transformed TUA and cycle year where 2003–2004 cycle was the reference group. Models were adjusted by including all a priori covariates (i.e. age, gender, race, BMI, PIR, tap water source, type of water consumed, recent smoking, and recent consumptions of seafood, rice, poultry, and juice), NHANES sampling cycle, and urinary creatinine. The Stata margins command was used after performing regression analysis to provide cycle-specific adjusted geometric means (GMs) of TUA concentrations from fully adjusted models.
We used a multiplicative interaction between NHANES cycle and source of drinking water within fully adjusted regression model to evaluate differences in TUA concentrations by water source. This approach allowed group means to vary between cycles. We further stratified this analysis by smoking status (i.e. non-smoker, smoker) to evaluate trends in these groups separately. Wald tests were used to estimate the statistical significance of the overall comparison of TUA between survey cycles within the different categories of drinking water source. Finally, we examined TUA concentration within exposure percentiles using linear predictions from fully adjusted models to estimate TUA concentrations to calculate the 95th, 75th, and 50th percentiles for each source of drinking water strata for each survey cycle.
3. Results
The average TUA concentration in each survey cycle was relatively low (Table 1). After restricting the study population to those with AsB concentrations below the LOD, the adjusted GM in: 2003–2004 was 2.17 μg/L (95%CI: 1.58, 2.96 μg/L), 2005–2006 was 2.43 μg/L (95% CI: 2.12, 2.79 μg/L), 2007–2008 was 2.77 μg/L (95% CI: 2.23, 3.42 μg/L), 2009–2010 was 2.98 μg/L (95% CI: 2.52, 3.53 μg/L), 2011–2012 was 1.69 μg/L (95% CI: 1.33, 2.16 μg/L), and 2013–2014 was 1.4 μg/L (95% CI: 1.03, 1.91 μg/L). Factors that were associated with average TUA in at least one cycle were age, gender, race/ethnicity, PIR, seafood consumption, rice intake, poultry intake, and juice intake. Within each cycle, the majority of participants reported using a community supply (range: 53%–69%); whereas the proportion of participants who reported using a well or rain cistern (range: 10%–18%) or who did not drink tap water in the past 24-hours (range: 12%–32%) varied more between survey cycle.
Table 1.
Population characteristics in six NHANES cycles from 2003–2014 from the total population (n=15,663) and from the restricted population (n=5,848) of those with urinary AsB concentrations below the maximum intercycle LOD (i.e. 0.84). Urinary arsenic is defined as TUA† and estimated geometric means (GM) and 95% confidence intervals adjusted for urinary creatinine are presented.
| % | 2003 –04 GM (95% CI) |
p‡ | % | 2005 –06 GM (95% CI) |
p‡ | % | 2007 –08 GM (95% CI) |
p‡ | % | 2009 –10 GM (95% CI) |
p‡ | % | 2011 –12 GM (95% CI) |
p‡ | % | 2013 –14 GM (95% CI) |
p‡ | p‡‡ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total sample size (n) | 2,554 | 2,568 | 2,545 | 2,855 | 2,501 | 2,640 | |||||||||||||
| Sample size <AsB LOD (n) | 957 | 869 | 1,033 | 1,072 | 996 | 921 | |||||||||||||
| Age group (years) | 0.900 | 0.107 | 0.364 | 0.004 | 0.020 | 0.671 | <0.001 | ||||||||||||
| 12–19yrs | 18.1% | 3.56 (2.87, 4.43) | 20.0% | 3 (2.1, 4.28) | 18.5% | 3.24 (2.38, 4.42) | 19.5% | 2.11 (1.52, 2.94) | 17.2% | 1.13 (0.74, 1.72) | 5.8% | 1.79 (1.04, 3.11) | |||||||
| 20–39yrs | 30.9% | 2.12 (1.34, 3.35) | 32.4% | 2.59 (1.95, 3.43) | 30.9% | 2.62 (2.01, 3.41) | 34.0% | 2.48 (1.78, 3.45) | 32.7% | 1.7 (1.2, 2.4) | 37.5% | 1.44 (0.97, 2.14) | |||||||
| >40 yrs | 51.1% | 1.32 (0.73, 2.39) | 47.7% | 1.84 (1.48, 2.29) | 50.6% | 1.91 (1.45, 2.52) | 46.6% | 2.46 (2.1, 2.88) | 50.1% | 1.28 (0.88, 1.86) | 56.8% | 1.05 (0.77, 1.43) | |||||||
| Gender | 0.358 | 0.691 | 0.702 | 0.75 | 0.881 | 0.011 | 0.137 | ||||||||||||
| Male | 44.8% | 2.21 (1.59, 3.08) | 50.3% | 3.16 (2.58, 3.88) | 43.7% | 2.77 (2.15, 3.55) | 48.3% | 2.85 (2.32, 3.49) | 49.8% | 1.82 (1.27, 2.59) | 46.0% | 1.29 (0.94, 1.77) | |||||||
| Female | 55.2% | 1.34 (0.82, 2.22) | 49.7% | 1.32 (1.02, 1.7) | 56.3% | 1.71 (1.32, 2.21) | 51.7% | 1.8 (1.51, 2.14) | 50.3% | 0.93 (0.66, 1.31) | 54.0% | 1.07 (0.77, 1.49) | |||||||
| Race/ethnicity | 0.064 | 0.009 | 0.005 | 0.027 | 0.008 | <0.001 | 0.220 | ||||||||||||
| Non-Hispanic white | 77.5% | 1.41 (0.99, 2.01) | 76.3% | 1.41 (0.99, 2.01) | 73.9% | 1.78 (1.4, 2.28) | 69.3% | 2.01 (1.64, 2.46) | 69.0% | 0.98 (0.64, 1.48) | 66.5% | 0.87 (0.57, 1.32) | |||||||
| Non-Hispanic black | 8.2% | 3.18 (2.69, 3.75) | 8.5% | 3.18 (2.69, 3.75) | 10.8% | 3.91 (3.46, 4.43) | 10.0% | 2.47 (1.88, 3.25) | 10.1% | 2.17 (1.47, 3.21) | 9.3% | 2.36 (1.82, 3.05) | |||||||
| Mexican American | 8.4% | 2.81 (1.93, 4.11) | 9.9% | 2.81 (1.93, 4.11) | 9.1% | 2.79 (2.16, 3.61) | 11.0% | 2.92 (2.46, 3.47) | 9.2% | 2.33 (1.73, 3.12) | 12.7% | 2.01 (1.48, 2.73) | |||||||
| Other, multiple races | 5.9% | 2.85 (1.19, 6.83) | 5.3% | 2.85 (1.19, 6.83) | 6.2% | 3.72 (2.47, 5.61) | 9.8% | 3.13 (1.97, 4.98) | 11.6% | 2.28 (1.61, 3.21) | 11.6% | 1.84 (1.08, 3.11) | |||||||
| BMI status (kg/m2) | 0.335 | 0.106 | 0.336 | 0.327 | 0.153 | 0.193 | 0.010 | ||||||||||||
| Underweight (<18.5) | 3.1% | 1.5 (0.68, 3.34) | 4.2% | 1.68 (0.73, 3.9) | 3.0% | 3.1 (1.6, 6.01) | 1.5% | 1.22 (0.65, 2.29) | 1.6% | 1.76 (1.03, 3) | 1.3% | 0.56 (0.09, 3.41) | |||||||
| Normal weight (18.5–24.9) | 34.1% | 1.77 (1.06, 2.96) | 38.6% | 1.6 (1.05, 2.44) | 34.7% | 1.66 (1.21, 2.29) | 36.7% | 1.82 (1.35, 2.46) | 33.6% | 1.17 (0.84, 1.63) | 28.8% | 1.02 (0.63, 1.66) | |||||||
| Overweigh t (25–29.9) | 32.0% | 1.39 (0.82, 2.38) | 27.6% | 2.4 (1.74, 3.3) | 32.0% | 2.23 (1.76, 2.83) | 27.7% | 2.58 (2.15, 3.1) | 30.4% | 1.48 (0.94, 2.33) | 33.8% | 1.3 (0.9, 1.89) | |||||||
| Obese (≥30) | 30.7% | 2.1 (1.37, 3.21) | 29.6% | 2.31 (1.58, 3.38) | 30.3% | 2.76 (2.35, 3.23) | 34.2% | 2.65 (2.29, 3.07) | 34.4% | 1.3 (0.84, 2.01) | 36.2% | 1.35 (1.03, 1.76) | |||||||
| Family poverty-income ratio | 0.030 | 0.487 | 0.160 | 0.160 | 0.894 | 0.972 | 0.304 | ||||||||||||
| Low (0–1.8) | 31.7% | 2.53 (1.89, 3.4) | 30.9% | 2.27 (1.74, 2.97) | 29.6% | 1.97 (1.38, 2.83) | 35.3% | 2.13 (1.65, 2.76) | 38.7% | 1.49 (1.16, 1.92) | 36.3% | 1.38 (1.05, 1.81) | |||||||
| Medium (1.8–3.9) | 31.3% | 1.95 (1.22, 3.13) | 34.8% | 2.1 (1.6, 2.76) | 31.5% | 1.79 (1.4, 2.28) | 30.9% | 2.52 (2.19, 2.89) | 28.3% | 1.43 (0.89, 2.29) | 28.1% | 1.23 (0.84, 1.82) | |||||||
| High (3.9–5) | 32.3% | 1.01 (0.57, 1.79) | 31.6% | 1.69 (1.26, 2.28) | 32.5% | 2.69 (1.82, 3.98) | 27.9% | 2.05 (1.45, 2.89) | 27.6% | 1.11 (0.52, 2.35) | 30.3% | 0.96 (0.59, 1.58) | |||||||
| Missing | 4.8% | 2.23 (1.73, 2.86) | 2.7% | 2.26 (1.06, 4.81) | 6.4% | 2.46 (1.76, 3.44) | 6.0% | 3.62 (2.43, 5.4) | 5.4% | 0.72 (0.27, 1.91) | 5.2% | 2.06 (1.25, 3.38) | |||||||
| Seafood consumptionA | 0.003 | 0.076 | 0.002 | 0.289 | 0.002 | <0.001 | 0.605 | ||||||||||||
| No | 94.5% | 1.69 (1.15, 2.48) | 96.4% | 2.07 (1.81, 2.38) | 94.5% | 2.08 (1.72, 2.53) | 94.7% | 2.22 (1.87, 2.65) | 94.2% | 1.22 (0.88, 1.69) | 92.8% | 1.13 (0.83, 1.53) | |||||||
| Yes | 3.6% | 7.82 (3.25, 18.83) | 2.2% | 3.26 (1.39, 7.66) | 3.8% | 4.55 (3.15, 6.58) | 4.4% | 3.35 (1.31, 8.59) | 2.8% | 4.37 (2.53, 7.55) | 3.3% | 6.2 (3.16, 12.15) | |||||||
| Missing | 1.9% | 2.33 (1.64, 3.33) | 1.3% | 1.16 (0.25, 5.38) | 1.8% | 3.09 (1.44, 6.64) | 0.9% | 2.26 (1.57, 3.25) | 3.0% | 2.51 (1.62, 3.89) | 3.9% | 1.16 (0.52, 2.6) | |||||||
| Water SourceB | 0.805 | 0.087 | 0.487 | 0.139 | 0.099 | 0.964 | <0.001 | ||||||||||||
| Community supply | 52.6% | 1.6 (1.05, 2.44) | 63.7% | 1.89 (1.63, 2.21) | 66.8% | 2.33 (1.84, 2.95) | 69.3% | 2.21 (1.76, 2.78) | 66.1% | 1.33 (1.05, 1.69) | 69.4% | 1.19 (0.85, 1.67) | |||||||
| Well or rain cistern | 10.9% | 1.86 (0.64, 5.39) | 18.1% | 2.18 (1.57, 3.04) | 14.7% | 1.18 (0.67, 2.09) | 11.2% | 1.83 (1.32, 2.55) | 13.1% | 0.52 (0.18, 1.49) | 9.8% | 1.13 (0.45, 2.84) | |||||||
| Don’t drink tap water | 32.3% | 1.96 (1.35, 2.84) | 12.6% | 2.18 (1.07, 4.46) | 13.2% | 2.71 (1.97, 3.72) | 14.3% | 2.67 (2.21, 3.22) | 13.3% | 2.21 (1.63, 3.02) | 13.6% | 1.33 (0.92, 1.92) | |||||||
| Missing | 4.2% | 1.17 (0.51, 2.66) | 5.6% | 2.34 (1.44, 3.79) | 5.4% | 1.69 (1.02, 2.82) | 5.3% | 2.46 (1.68, 3.6) | 7.5% | 1.35 (0.8, 2.28) | 7.2% | 0.86 (0.4, 1.86) | |||||||
| Type of water consumption C | 0.776 | 0.382 | 0.738 | 0.129 | 0.975 | 0.502 | <0.001 | ||||||||||||
| No water | 12.5% | 1.98 (1.12, 3.49) | 24.7% | 2.41 (1.74, 3.35) | 24.0% | 2.27 (1.61, 3.19) | 26.4% | 2.05 (1.51, 2.77) | 20.4% | 1.86 (1.24, 2.79) | 18.1% | 1.14 (0.73, 1.79) | |||||||
| Tap only | 48.9% | 1.56 (0.94, 2.57) | 44.8% | 1.77 (1.41, 2.23) | 42.1% | 2 (1.43, 2.79) | 40.2% | 2.17 (1.86, 2.53) | 43.9% | 1.05 (0.69, 1.58) | 41.3% | 1.07 (0.74, 1.55) | |||||||
| Bottled/sp ring only | 19.8% | 1.97 (1.25, 3.1) | 21.2% | 2.17 (1.23, 3.81) | 25.4% | 2.37 (1.78, 3.16) | 27.4% | 2.57 (2.06, 3.19) | 26.3% | 1.48 (0.89, 2.44) | 27.1% | 1.65 (1.19, 2.29) | |||||||
| Mixed source | 17.7% | 1.8 (1.04, 3.1) | 8.1% | 2.09 (1.26, 3.47) | 6.8% | 1.92 (1.21, 3.05) | 5.0% | 2.59 (1.3, 5.19) | 6.4% | 0.62 (0.22, 1.77) | 9.6% | 0.71 (0.25, 1.99) | |||||||
| Missing | 1.2% | 2.37 (1.37, 4.11) | 1.3% | 1.16 (0.25, 5.38) | 1.8% | 3.09 (1.44, 6.64) | 0.9% | 2.26 (1.57, 3.25) | 3.0% | 2.51 (1.62, 3.89) | 3.9% | 1.16 (0.52, 2.6) | |||||||
| RiceA | <0.001 | 0.030 | <0.001 | <0.001 | <0.001 | 0.001 | 0.501 | ||||||||||||
| No | 92.2% | 1.61 (1.11, 2.33) | 90.0% | 1.98 (1.68, 2.34) | 91.1% | 2.08 (1.72, 2.52) | 91.5% | 2.12 (1.76, 2.55) | 90.1% | 1.18 (0.86, 1.62) | 86.7% | 1.08 (0.79, 1.49) | |||||||
| Yes | 5.9% | 5.22 (3.73, 7.3) | 8.1% | 2.89 (1.74, 4.79) | 7.1% | 3.71 (2.6, 5.31) | 7.3% | 5.06 (3.51, 7.3) | 6.9% | 2.78 (1.98, 3.92) | 9.2% | 2.57 (1.64, 4.02) | |||||||
| Missing | 1.9% | 2.33 (1.64, 3.33) | 1.9% | 0.95 (0.38, 2.35) | 1.8% | 3.09 (1.44, 6.64) | 1.2% | 1.53 (0.47, 4.96) | 3.0% | 2.51 (1.62, 3.89) | 4.1% | 1.17 (0.55, 2.5) | |||||||
| PoultryA | 0.118 | 0.011 | 0.152 | 0.042 | 0.914 | 0.003 | 0.726 | ||||||||||||
| No | 64.6% | 1.62 (1.08, 2.44) | 65.7% | 1.79 (1.48, 2.16) | 63.2% | 2.01 (1.64, 2.45) | 64.1% | 2 (1.55, 2.58) | 59.6% | 1.23 (0.89, 1.69) | 59.2% | 0.99 (0.65, 1.51) | |||||||
| Yes | 33.5% | 1.99 (1.43, 2.79) | 32.4% | 2.63 (2.17, 3.19) | 35.0% | 2.43 (1.89, 3.12) | 34.7% | 2.82 (2.4, 3.31) | 37.4% | 1.28 (0.84, 1.94) | 36.7% | 1.56 (1.17, 2.09) | |||||||
| Missing | 1.9% | 2.33 (1.64, 3.33) | 1.9% | 0.95 (0.38, 2.35) | 1.8% | 3.09 (1.44, 6.64) | 1.2% | 1.53 (0.47, 4.96) | 3.0% | 2.51 (1.62, 3.89) | 4.1% | 1.17 (0.55, 2.5) | |||||||
| JuiceA | 0.442 | 0.793 | 0.012 | 0.683 | 0.350 | 0.693 | 0.001 | ||||||||||||
| No | 62.6% | 1.65 (1.19, 2.27) | 58.9% | 1.96 (1.58, 2.42) | 60.8% | 1.76 (1.35, 2.28) | 57.9% | 2.16 (1.81, 2.57) | 63.6% | 1.15 (0.81, 1.62) | 68.4% | 1.15 (0.82, 1.61) | |||||||
| Yes | 35.5% | 1.9 (1.13, 3.22) | 39.2% | 2.15 (1.69, 2.74) | 37.4% | 2.94 (2.47, 3.5) | 40.9% | 2.39 (1.86, 3.08) | 33.5% | 1.44 (1.01, 2.06) | 27.5% | 1.25 (0.77, 2.02) | |||||||
| Missing | 1.9% | 2.33 (1.64, 3.33) | 1.9% | 0.95 (0.38, 2.35) | 1.8% | 3.09 (1.44, 6.64) | 1.2% | 1.53 (0.47, 4.96) | 3.0% | 2.51 (1.62, 3.89) | 4.1% | 1.17 (0.55, 2.5) | |||||||
| Smoking last 5 days D | 0.101 | 0.060 | 0.056 | 0.212 | 0.538 | 0.775 | 0.760 | ||||||||||||
| No | 69.4% | 1.77 (1.21, 2.59) | 72.4% | 2.21 (1.87, 2.62) | 74.2% | 2.39 (2.1, 2.73) | 72.6% | 2.38 (2.03, 2.78) | 73.4% | 1.31 (0.97, 1.78) | 72.8% | 1.08 (0.73, 1.59) | |||||||
| Yes | 30.6% | 1.88 (1.29, 2.75) | 27.6% | 1.92 (1.41, 2.6) | 25.8% | 1.83 (1.16, 2.88) | 27.4% | 2.14 (1.44, 3.19) | 26.6% | 1.47 (1.03, 2.09) | 27.2% | 1.5 (0.98, 2.29) | |||||||
| Missing*** | 19.8% | 3.13 (2.26, 4.35) | 19.5% | 3.44 (2.72, 4.34) | 17.8% | 3.34 (2.69, 4.14) | 21.0% | 3.64 (3.14, 4.23) | 16.7% | 2.58 (1.96, 3.4) | 26.1% | 1.34 (1.12, 1.6) |
TUA = Total arsenic - AsB – AsC
The p-values obtained from Wald Test that represent the overall comparison of GM within categories for non-missing covariates.
The p-values obtained from chi-square tests that test the difference between population size between NHANES cycles for each study covariate.
Participants who reported consuming food category based on USDA food codes from day 1 dietary interview. If participant reported consuming any food that corresponded to USDA food codes within category (Supplemental Table S3) they were categorized as “yes” for consumption in previous 24 hours.
The three categories of source of tap water consumption were determined from a dietary questionnaire in which participants were asked to indicate the main source of the tap water they consumed in the previous 24 hours. Participants were asked to identify if a community water supply, a well or rain cistern, or other supply (not included) was their primary tap water source. Participants who indicated they either did not drink tap water from home in previous 24 hours (2003–04 cycle) or do not drink home tap water in general (2005–2014) were labeled as “Don’t drink tap water.”
The four categories of water consumption are mutually exclusive and were determined by summed values of USDA food codes corresponding to different water consumption categories. Participants were labeled as Tap only if they consumed plain tap water, filtered tap water, or water from a drinking fountain in the previous 24 hours. The original NHANES plain water definition included plain tap water, water from a drinking fountain, water from a water cooler, bottled water, or spring water. However, for the purposes of this analysis, those participants who did not report drinking from any tap water sources, but did report consuming bottled water or spring water were labeled as Plain only. Participants were labeled as Plain & tap water if they consumed some tap water source and plain water source. Participants were labeled as No water if they reported no consumption of any of these water sources in the previous 24 hours.
Smoking status determined from self-reported nicotine use within the previous 5 days among all study participants ≥12 years of age. The missing category represents all study participants age 6–12 years with TUA measurements available.
Overall, TUA concentrations decreased over time (Table 2). Compared to 2003–2004, the overall adjusted geometric mean TUA was 22.1% and 35.5% lower in the 2011–2012 and 2013–2014 cycles, respectively, after adjusting for creatinine, age, sex, race/ethnicity, BMI, PIR, smoking status, water source, type of water consumed, and consumption of seafood, rice, poultry, or juice. The percent reduction in TUA differed by water source (Table 3). Furthermore, effect modification was observed for smoking status but not seafood, rice, poultry, or juice intake. When the trend in TUA was examined by source of drinking water stratified by smoking status, we only observed a consistent downward trend in TUA among non-smokers (Table 3). Compared to 2003–2004, TUA in 2013–2014 was 41.4%, 16.9%, and 31.7% lower among non-smokers who reported using a community water supply, well/rain cisterns, or did not drink tap water, respectively, after adjusting for covariates. The lowest observed TUA concentrations were in the most recent cycles (2011–2012 and 2013–2014) for participants using a community water supply, but the trend was not as consistent among those who used a well/rain cistern or reported not drinking any tap water. In our sensitivity analysis that used a different TUA metric (i.e. TUA2), this downward trend was observed only for participants that used a community water supply or did not drink tap water, but not for well/rain cistern users (Supplemental Table S5). Among non-smokers, this analysis showed a consistent decrease between cycle years among those who used a community water supply (−10.6%) and an overall decrease among those who did not drink tap water (−9.6%), but no change (0.0%) was observed among well/rain users.
Table 2.
Trends of unweighted (uGM) and weighted geometric means (GM) of urinary arsenic (μg/L) in the US general population from NHANES, 2003–2014. Urinary arsenic levels are defined as total urinary arsenic (TUA)† and have been corrected for the maximal LOD of each composite urinary arsenic metabolite.
| Year | Total population
|
p-Value‡ | |||
|---|---|---|---|---|---|
| uGMA (95% CI) | uGMB (95% CI) | aGMC (95% CI) | % ChangeD | ||
| 2003–04 | 3.32 (2.59,4.25) | 1.69 (1.17,2.44) | 2.17 (1.58,2.96) | (ref) | <0.001 |
| 2005–06 | 3.8 (3.25,4.46) | 1.94 (1.66,2.26) | 2.43 (2.12,2.79) | 12.0% | |
| 2007–08 | 3.8 (3.27,4.42) | 2.19 (1.8,2.68) | 2.77 (2.23,3.42) | 27.6% | |
| 2009–10 | 4.45 (3.92,5.04) | 2.31 (1.93,2.76) | 2.98 (2.52,3.53) | 37.3% | |
| 2011–12 | 2.28 (1.7,3.05) | 1.3 (0.98,1.72) | 1.69 (1.33,2.16) | −22.1% | |
| 2013–14 | 1.69 (1.35,2.11) | 1.18 (0.89,1.55) | 1.4 (1.03,1.91) | −35.5% | |
TUA = Total arsenic - AsB – AsC
P-value for the overall comparison between interaction groups was assessed by the Wald Test.
Adjusted for natural log-transformed creatinine and intercycle changes in limits of detection. Final sample size for the unadjusted model was 15,663.
The sample was limited to those individuals with urinary AsB concentrations below the intercycle LOD (i.e. <0.84 μg/L) and smoking status data available (i.e. age ≥12 years). The regression was adjusted for natural log-transformed creatinine and the final sample size was 5,848.
Linear regression for log transformed TUA were adjusted for age, sex, race/ethnicity, BMI (categorical), family poverty income ratio, natural log-transformed urinary creatinine, tap water source, type of water consumed, self-reported smoking in the previous 5 days, and consumption of seafood, rice, poultry, or juice. Final sample size for the adjusted model was 4,999.
Percent change in the adjusted GM of TUA after LOD correction by NHANES cycle represent the percentage difference between the 2003–04 cycle from other cycles for each category.
Table 3.
Association between adjusted weighted geometric means (GM) of urinary arsenic (μg/L) and NHANES cycles (2003–2014) by main source of tap waterC, defined as those who receive tap water from a community water supply, well or rain cistern, or those who do not drink tap water from six continuous NHANES cycles between 2003–2014 (n=4,993). The sample was restricted to those individuals with urinary AsB concentrations below the limit of detection (i.e. ≤0.84) and stratified by non-smokers (n=3,746) and smokers (n=1,247). Urinary arsenic is defined as total urinary arsenic (TUA)†.
| Year | Non-smokers | Smokers | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| aGMA (95% CI) | % ChangeB | p-Valueǂ | aGMA (95% CI) | % ChangeB | p-Valueǂ | ||
| Community Water Supply | 2003–04 | 1.98 (1.45,2.73) | (ref) | <0.001 | 1.31 (0.72,2.37) | (ref) | <0.001 |
| 2005–06 | 2.14 (1.74,2.64) | 8.1% | 2.03 (1.53,2.69) | 55.0% | |||
| 2007–08 | 2.68 (2.19,3.27) | 35.4% | 3.22 (2.14,4.83) | 145.8% | |||
| 2009–10 | 2.83 (2.35,3.4) | 42.9% | 2.46 (1.58,3.85) | 87.8% | |||
| 2011–12 | 1.53 (1.17,1.99) | −22.7% | 1.68 (1.27,2.22) | 28.2% | |||
| 2013–14 | 1.16 (0.81,1.65) | −41.4% | 1.61 (1.02,2.52) | 22.9% | |||
| Well/Rain | 2003–04 | 1.54 (0.54,4.41) | (ref) | <0.001 | 4.84 (1.32,17.69) | (ref) | <0.001 |
| 2005–06 | 2.85 (2.06,3.94) | 85.1% | 1.82 (0.76,4.37) | −62.4% | |||
| 2007–08 | 1.82 (1.43,2.32) | 18.2% | 0.39 (0.09,1.65) | −91.9% | |||
| 2009–10 | 1.92 (1.51,2.44) | 24.7% | 3.11 (1.56,6.23) | −35.7% | |||
| 2011–12 | 0.68 (0.26,1.77) | −55.8% | 0.89 (0.26,3.05) | −81.6% | |||
| 2013–14 | 1.28 (0.61,2.67) | −16.9% | 0.75 (0.1,5.47) | −84.5% | |||
| Don’t drink 1tap water | 2003–04 | 2.24 (1.64,3.07) | (ref) | <0.001 | 2.3 (1.35,3.91) | (ref) | <0.001 |
| 2005–06 | 2.78 (1.72,4.51) | 24.1% | 1.22 (0.28,5.26) | −47.0% | |||
| 2007–08 | 3.57 (2.44,5.24) | 59.4% | 2.02 (0.74,5.46) | −12.2% | |||
| 2009–10 | 3.73 (2.95,4.72) | 66.5% | 3.39 (2.27,5.07) | 47.4% | |||
| 2011–12 | 2.94 (2.04,4.24) | 31.3% | 1.73 (0.97,3.07) | −24.8% | |||
| 2013–14 | 1.53 (0.97,2.41) | −31.7% | 1.66 (1.06,2.61) | −27.8% | |||
TUA = Total arsenic - AsB – AsC
P-value for the overall comparison between cycle years interaction groups was assessed by the Wald Test.
P-value for the overall comparison within category for interaction groups was assessed by the Wald Test.
Multivariate linear regression for interaction between NHANES sampling cycles and tap water source adjusted for log-transformed urinary creatinine (continuous), age, sex, race/ethnicity, BMI (categorical), PIR, type of water consumed (categorical), and consumption of seafood, rice, poultry, or juice.
Percent change in the adjusted GM of total urinary arsenic (TUA) by NHANES cycle represent the percentage difference between the 2003–04 cycle from other cycles for each category.
The three categories of source of tap water consumption were determined from a dietary questionnaire in which participants were asked to indicate the main source of the tap water they consumed in the previous 24 hours. Participants were asked to identify if a community water supply, a well or rain cistern, or other supply (not included) was their primary tap water source. Participants who indicated they either did not drink tap water from home in previous 24 hours (2003–04 cycle) or do not drink home tap water in general (2005–2014) were labeled as “Don’t drink tap water.”
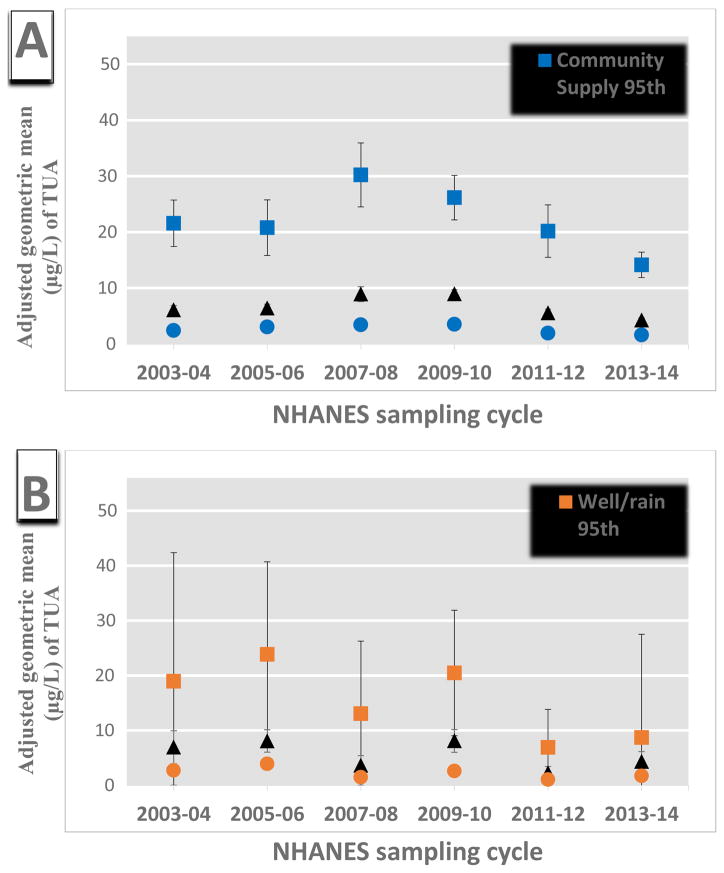
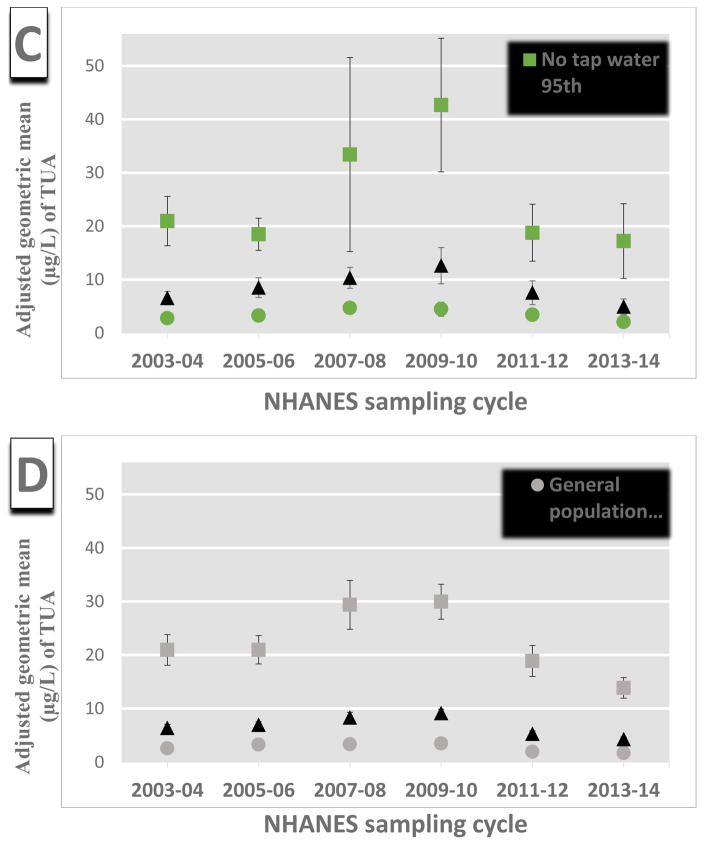
We further examined the change in TUA by survey cycle among different percentiles of arsenic exposure within the water sources groups (Figure 1, Supplementary Figure S1, and Supplementary Table S6). Overall, the largest decrease in TUA over time was amongst those participants with the highest arsenic exposure (e.g. in the 75th or 95th percentile). This trend was observed among participants who used a community water supply and who did not drink tap water, both of which had at least a 34% and 18% decrease in TUA among the 95th percentile for 2013–2014 vs 2003–2004 cycles, respectively (Supplementary Table S6). Additionally, the 50th and 75th percentiles also decreased over time within each of these water source groups. Among participants who reported that their source of drinking water was a well or a rain cistern using the TUA composite, there was a relatively large decrease in each percentile distribution with the largest in the 95th percentile. However, this trend was not consistent in our sensitivity analyses using TUA2, where TUA2 levels seemed to increase or stay the same within each exposure percentile (Supplementary Figure S1, Table S6).
Figure 1.
Association between adjusted weighted geometric means of urinary arsenic (μg/L) and NHANES cycles in the general U.S. population by percentiles of exposure (i.e. 95, 75, 50th) for those who consume tap water from a (A) community supply (n=3,427), (B) well or rain cistern (n=506), or (C) do not consume tap water (n=1,060), and (D) among the general U.S. population (n=4,993). The population is restricted to those individuals with urinary AsB concentrations below the limit of detection (i.e. ≥0.84) Estimates are from linear multivariate regression model with an interaction term between NHANES sampling cycle and tap water source adjusted for log-transformed urinary creatinine (continuous), age, sex, race/ethnicity, BMI (categorical), PIR, type of water consumed (categorical), recent smoking, and consumption of seafood, rice, poultry, or juice.. Urinary arsenic is defined as total urinary arsenic (TUA) which is total arsenic minus AsB and AsC.
4. Discussion
In this sample of the U.S. population ≥12 years of age, we observed that arsenic exposure decreased by approximately 35% over a 12-year period from 2003–2004 to 2013–2014 after adjusting for other exposure risk factors. The most consistent reduction in arsenic exposure was observed among non-smokers who received their drinking water from a community water supply or who do not drink tap water. Furthermore, this trend was most apparent among those participants who had the highest exposure levels in each cycle (i.e. 75th and 95th percentiles). This finding is noteworthy as the majority of the U.S. population does not have high levels of arsenic exposure and reducing exposure to arsenic would have substantial health benefits. A concurrent analysis by a separate research group observed a similar reduction in arsenic exposure using the same NHANES data while employing different modeling assumptions (Nigra et al. 2017).
Interestingly, TUA concentrations were found to decrease most sharply in the US population after the 2009–2010 NHANES cycle with strong reductions in the 2011–2012 and 2013–2014 cycles. Due to the nature of the cross-sectional study design and reliance on urine samples to assess exposure, we cannot determine the exposure sources. Additionally, NHANES did not start measuring urinary arsenic until the 2003–2004 survey cycle which is after the Arsenic Rule was adopted in 2001, though before it went into full effect in 2006. However, smaller water systems serving ≤3,300 people, which were much more likely to have been impacted by the standard change resulting from the Arsenic Rule, could apply for multiple 3-year exemptions up to an additional 9 years in order to achieve full compliance (EPA 2001a). This variance was permitted because smaller water systems are more likely to be in rural regions, serve lower income populations, and require greater costs relative to larger water systems to implement arsenic removal systems (EPA 2001b, 2002; Flanagan et al. 2015; Frost et al. 2003). The latter scenario signifies that certain small water systems in violation of the arsenic standard could potentially have until January 2015 to attain financial assistance and implement necessary compliance strategies, amounting to 14 years after the initial Arsenic Rule was published. This could explain why we observed the lowest TUA concentrations in the 2011–2012 and 2013–2014 cycles.
This delay in compliance with the Arsenic Rule has been documented using data collected in the EPA’s Safe Drinking Water Information System. These studies identified community water systems in violation of the Arsenic Rule and showed that the number of systems reporting arsenic concentrations above the 10 μg/L MCL increased each year between 2003 to 2010, with approximately 2,500 violations occurring in both 2009 and 2010 (McGavisk et al. 2013). Data from subsequent years showed that there was a decline in the number of violations however, there were still 500 violations reported in 2012 (McGavisk et al. 2013). A separate analysis of EPA data demonstrated that the estimated population served by community water systems in violation of the Arsenic Rule followed the same general trend between 2003 and 2012, with the number of people increasing through 2010 and decreasing in subsequent years (Alfredo et al. 2014). If our observed changes in TUA in the U.S. population are related to reduction in exposure to arsenic-contaminated drinking water, the compliance schedule of community water supplies could help explain why we found decreases in the more recent NHANES survey cycles and among participants who report using drinking water from a community water system.
Our results partially confirmed our hypothesis that arsenic exposure did not decrease among individuals receiving drinking water from a well or rain cistern, as trends in TUA and TUA2 were inconsistent. Private domestic wells are not regulated by the EPA and users are required to monitor their own water quality. In some states, arsenic testing is required when completing real estate transactions (Flanagan et al. 2015). The change in the Arsenic Rule and subsequent real estate translation laws likely increased awareness about arsenic in private wells during the study period and could have led to an increase in well testing by homeowners, especially in regions known to have higher groundwater arsenic. However, studies show that private domestic well owners do not regularly conduct water tests and may not implement water treatment if presented evidence of arsenic contamination because of real or perceived financial and technical difficulties (Flanagan et al. 2015; Straub and Leahy 2014; Walker et al. 2006). When we calculated the exposure using TUA, which removed seafood-related organoarsenicals, we observed that TUA decreased among well or rain cistern users. However, when we used the most commonly accepted proxy for iAs, TUA2, there was no decrease in TUA in this water source group. Although it should be noted that TUA2 is more impacted by the percentage of samples below the LOD, which can systematically bias arsenic assessment amongst those individuals with the lowest exposure. Conversely, the total arsenic used in TUA has very few samples below the LOD (Supplemental Table S1), especially after restricting the study sample to those with AsB levels below the LOD. Thus, subtracting AsC and AsB from total arsenic (i.e. TUA2) produces a deflation in the upper end of the exposure distribution. This could explain the inconsistency in the overall urinary arsenic patterns between our composite measures.
Surprisingly, participants who reported not drinking any water in the previous 24-hours, or never drinking tap water, had the highest overall urinary arsenic concentrations among the three groups. Yet, this group also experienced declines in TUA. There are several potential contributing reasons for this observation. Arsenic is known to be a contaminant within apple juice, wine, rice milk, and other beverage products and recent studies using NHANES data has shown that these beverages are associated with elevated urinary arsenic (Davis et al. 2012; deCastro et al. 2014; Navas-Acien et al. 2011). Our statistical adjustments made for rice and juice consumption likely helped to reduce the potential differential contribution of those dietary arsenic exposures. However, the potential for these beverages to contribute to arsenic exposure received considerable media attention which could have influenced manufacturing and/or public awareness and in turn lowered arsenic exposure from these sources. It is also possible that this group was exposed to community water supplies and did not know it since this is the major source of publicly-available potable water in the United States (Kumar et al. 2010). However, spatiotemporal studies are needed to investigate this hypothesis.
Our study has several strengths including the use of a large representative sample of the U.S. population with relevant environmental exposures to arsenic. The rigorous quality control procedures implemented in NHANES provides a significant strength to the quality of data presented. Urinary arsenic measurement provides individual level exposure and the total urinary arsenic composite measure that we used excludes non-toxic compounds found in seafood (i.e. AsB and AsC). Additionally, we restricted our study sample to those with AsB concentrations below the LOD and adjusted for recent seafood consumption. While recent seafood consumption was related to higher TUA concentrations compared to not consuming seafood (Table 1), the overall trends in TUA were similar between groups (data not shown). Consumption of seafood can affect urinary arsenic levels for up to three days (Navas-Acien et al. 2011), making it possible that the 24-hour dietary recall used to identify seafood consumption includes some misclassification. However, we expect this misclassification to be non-differential and minimal because of the sample restriction we performed regarding AsB. We also made adjustments for some of the most common sources of dietary arsenic including rice, poultry, and juice consumption. Rice has been shown to contribute to appreciable urinary concentrations of DMA and MMA within NHANES (Davis et al. 2012; deCastro et al. 2014; Mantha et al. 2017; Xue et al. 2010). The dichotomous categorization we made for rice did not account for the relative amount or type of rice consumed, both of which have been shown to affect urinary arsenic levels (Mantha et al. 2017). However, the misclassification that could arise from not accounting for these factors are likely to be non-differential between types of drinking water supplies. While rice can potentially account for up to 40% of iAs exposure in the general U.S. population and be higher among certain ethnic subgroups, our models also adjusted for race/ethnicity (Xue et al. 2010). Additionally, a comprehensive probabilistic study of the estimated relative contributions of rice and drinking water confirmed that drinking water is the largest exposure source for the U.S. population (Mantha et al. 2017).
Finally, our sensitivity analysis that used the second composite measure TUA2 yielded consistent results among participants who used community well water supplies. By demonstrating similar overall effects with both measures it provides additional strength to the evidence that both the upper and lower ends of the arsenic exposure distribution are decreasing within the general U.S. population. However, the differences between TUA and TUA2 for non-smokers who receive their drinking water from a well or rain cistern requires further evaluation. There was a high proportion of samples below the limit of detection for AsIII, AsV, and MMA (Supplementary Table S1). Therefore, it is possible that this measure is providing more biased estimates than the TUA measure.
There were other limitations to our study. The cross-sectional study design of NHANES does not include repeated measures within individuals, limiting our ability to determine potential causal aspects of temporal trends in arsenic exposures. Urinary arsenic metabolites have short half-lives that typically represent arsenic ingestion in the previous three days, so chronic exposure patterns cannot be determined from this data (Buchet et al. 1981). Additionally, by restricting our study sample to those participants with only AsB measurements below the LOD we may have differentially excluded populations residing in coastal regions. A previous study using NHANES data found that blood mercury concentrations were higher among female populations in coastal regions and this was likely due to higher levels fish consumption in those regions (Mahaffey et al. 2009). Our conservative approach of restricting to participants undetectable AsB concentrations likely reduced the proportion of seafood consumers included in this analysis. This restriction may have affected the trends we have observed in the U.S. population if these populations had different trends in drinking water arsenic exposures. NHANES is designed to be representative of the general U.S. population and has sampled broadly from regions known to have elevated groundwater arsenic concentrations, such as the Northeast, Midwest, West, and South (CDC 2015). However, because geographical variables are restricted by NCHS due to disclosure concerns, geographical comparisons were not included in this study. Therefore, we cannot rule out the possibility that observed trends in urinary arsenic may have occurred because of the geographical heterogeneity of arsenic concentrations in groundwater between NHANES primary sampling units.
Additionally, the proportion of participants who reported using a well or rain cistern as their source of drinking water was relatively small compared to the other two groups. The participant selection process by NHANES oversamples certain subpopulations to better capture their variability, but domestic well users are not among the subpopulations currently oversampled. Furthermore, changes to the dietary recall questionnaire in regards to source of tap water likely resulted in some misclassification of source of tap water categorization. The largest potential for misclassification would be in the 2003–2004 cycle, as those who reported no water consumption in the 24-hour dietary recall questionnaire were not asked about their tap water source and were given the “do not drink tap water” classification. However, we evaluated the consistency of individual responses to the source of tap water between the two similar questions in different study questionnaires and observed high consistency in response rates across cycles (Table S2). This means that the misclassification between community water users versus well or rain cistern users would likely be non-differential, thus biasing estimates towards the null for tap water comparisons. Furthermore, we found a similar overall proportion (average of 13% over 6 cycles) of well or rain cistern users as previous survey data suggests (Focazio et al. 2006; Kumar et al. 2010; Montgomery et al. 2003). Separately, those that are labeled as “do not drink tap water” may have had additional water ingestion from water used for cooking or preparing beverages, potentially underestimating their exposures from a given tap water source.
5. Conclusions
We observed that urinary arsenic concentrations have become lower in the general U.S. population over the 12-year time period stretching from 2003–2004 to 2013–2014. The magnitude of the decrease was most consistent among people who reported using a community public water supply or who did not report drinking tap water. Results from individuals consuming water from a well or rain cistern had decreasing trends in exposure using one composite measure for urinary arsenic, but limited to increasing trends with the second composite measure. Future efforts to reduce arsenic exposure should focus on private domestic well users because this population is more vulnerable to arsenic contamination in drinking water and they did not experience the same decreasing trends in urinary arsenic exposure as people who used a community water supply. Our study provides evidence that overall arsenic exposures in the U.S. are decreasing, especially among those groups who had the highest exposure. These results show that regulation and prevention strategies to reduce large-scale population exposures to arsenic in the U.S. may be succeeding.
Supplementary Material
Highlights.
We examined trends in arsenic exposure US general population between 2003–2014.
Exposure among US general population decreased by over 35% over the 12-year period.
Decreased exposure shown among community water supply and non-tap water users.
Inconsistent change in arsenic exposure among well or rain cistern populations.
Acknowledgments
The authors would like to thank the Centers for Disease Control and Prevention’s National Center for Health Statistics and the participants and study staff of the NHANES, without whom this work would not be possible. The authors also thank Dr. Isabel Canette for excellent technical assistance.
Funding Sources
This research was partially funded by the United States National Institutes of Environmental Health Science (P30 ES000210 and R01 ES023441) and by the National Center for Advancing Translational Sciences of the National Institutes of Health (TL1TR002371). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations
- iAs
inorganic arsenic
- AsIII
arsenite
- AsV
arsenate
- MMA
monomethylarsonic acid
- DMA
dimethylarsenic acid
- AsB
arsenobetaine
- AsC
arsenocholine
- TUA
Total urinary arsenic minus AsB and AsC
- TUA2
Sum of AsIII, AsV, MMA, and DMA
- SDWA
Safe Drinking Water Act
- EPA
United States Environmental Protection Agency
- BMI
body mass index (kg/m2)
- PIR
family poverty-income ratio
Footnotes
Human subjects statement
All participants provided informed consent prior to conducting the study and study protocols were approved by the National Center for Health Statistic’s research ethics review board (CDC 2012).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfredo K, Seidel C, Roberson JA. Reviewing the occurrence data used in the revised arsenic rule. J Am Water Works Ass. 2014;106:67–68. [Google Scholar]
- ATSDR (U.S. Department of Health and Human Services) Toxicological profile for arsenic. U.S. Department of Health and Human Services; Atlanta, GA: 2007. [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ Health Persp. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1981;48:71–79. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD. Levels of urinary total and speciated arsenic in the US population: National health and nutrition examination survey 2003–2004. J Expo Sci Env Epid. 2009;19:59–68. doi: 10.1038/jes.2008.32. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Smit E, Houseman EA, Kerkvliet NI, Bethel JW, Kile ML. Arsenic exposure and prevalence of the varicella zoster virus in the United States: NHANES (2003–2004 and 2009–2010) Environ Health Persp. 2015;123:590–596. doi: 10.1289/ehp.1408731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Smit E, Bethel JW, Houseman EA, Kile ML. Arsenic exposure and the seroprevalence of total hepatitis a antibodies in the US population: NHANES, 2003–2012. Epidemiol Infect. 2016;144:1641–1651. doi: 10.1017/S0950268815003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso RV, O’Connor RJ, Stephens WE, Cummings KM, Fong GT. Toxic metal concentrations in cigarettes obtained from u.S. Smokers in 2009: Results from the international tobacco control (itc) United States survey cohort. Int J Environ Res Public Health. 2013;11:202–217. doi: 10.3390/ijerph110100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) National center for health statistics. [accessed February 20 2014];NCHS research ethics review board (ERB) approval. 2012 Available: http://www.cdc.gov/nchs/nhanes/irba98.htm.
- CDC (Centers for Disease Control and Prevention) National health and nutrition examination survey: Analytic guidelines, 2011–2012. CDC National Center for Health Statistics; 2013. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) [accessed February 25 2014];2011–2012 lab methods. 2014 Available: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/lab_methods_11_12.htm.
- CDC (Centers for Disease Control and Prevention) [accessed August 3 2017];Questionnaires, datasets, and related documentation. 2015 Available: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- CDC (Centers for Disease Control and Prevention) Fourth report on human exposure to environmental chemicals, updated tables, January 2017. 2017;1:4,196–197. [Google Scholar]
- Choi B-S, Choi S-J, Kim D-W, Huang M, Kim N-Y, Park K-S, et al. Effects of repeated seafood consumption on urinary excretion of arsenic species by volunteers. Archives of environmental contamination and toxicology. 2010;58:222–229. doi: 10.1007/s00244-009-9333-8. [DOI] [PubMed] [Google Scholar]
- Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in u.S. Children. Environ Health Perspect. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCastro BR, Caldwell KL, Jones RL, Blount BC, Pan Y, Ward C, et al. Dietary sources of methylated arsenic species in urine of the United States population, NHANES 2003–2010. Plos One. 2014:9. doi: 10.1371/journal.pone.0108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (U.S. Environmental Protection Agency) Technical fact sheet: Final rule for arsenic in drinking water. EPA Office of Water; 2001a. EPA 815-F-00-016. [Google Scholar]
- EPA (Agency USEP) National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Agency USEP; 2001b. [Google Scholar]
- EPA (U.S. Environmental Protection Agency) Implementation guidance for the arsenic rule: Appendix g-exemptions and the arsenic rule. EPA Office of Water; 2002. EPA 816-R-02-021. [Google Scholar]
- Flanagan SV, Marvinney RG, Zheng Y. Influences on domestic well water testing behavior in a central maine area with frequent groundwater arsenic occurrence. Sci Total Environ. 2015;505:1274–1281. doi: 10.1016/j.scitotenv.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focazio MJ, Tipton D, Shapiro SD, Geiger LH. The chemical quality of self-supplied domestic well water in the United States. Ground Water Monit R. 2006;26:92–104. [Google Scholar]
- Frost FJ, Muller T, Petersen HV, Thomson B, Tollestrup K. Identifying US populations for the study of health effects related to drinking water arsenic. J Expo Anal Environ Epidemiol. 2003;13:231–239. doi: 10.1038/sj.jea.7500275. [DOI] [PubMed] [Google Scholar]
- Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- IARC (International Agency for Risk on Cancer) Part c: Arsenic, metals, fibres, and dusts. Lyon, France: IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; 2012. A review of human carinogens; pp. 100C–6. [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Tellez-Plaza M, Sharrett AR, Guallar E, Navas-Acien A. Urine arsenic and hypertension in US adults: The 2003–2008 national health and nutrition examination survey. Epidemiology. 2011;22:153–161. doi: 10.1097/EDE.0b013e318207fdf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Adak P, Gurian PL, Lockwood JR. Arsenic exposure in US public and domestic drinking water supplies: A comparative risk assessment. J Expo Sci Env Epid. 2010;20:245–254. doi: 10.1038/jes.2009.24. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Jeffries RA. Adult women’s blood mercury concentrations vary regionally in the United States: Association with patterns of fish consumption (NHANES 1999–2004) Environ Health Perspect. 2009;117:47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantha M, Yeary E, Trent J, Creed PA, Kubachka K, Hanley T, et al. Estimating inorganic arsenic exposure from u.S. Rice and total water intakes. Environ Health Perspect. 2017;125:057005. doi: 10.1289/EHP418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavisk E, Roberson JA, Seidel C. Using community economics to compare arsenic compliance and noncompliance. Journal AWWA. 2013:E115–E126. [Google Scholar]
- Molin M, Ulven SM, Meltzer HM, Alexander J. Arsenic in the human food chain, biotransformation and toxicology--review focusing on seafood arsenic. J Trace Elem Med Biol. 2015;31:249–259. doi: 10.1016/j.jtemb.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Montgomery DL, Ayotee JD, Carrol PR, Hamlin P. Arsenic concentrations in private bedrock wells in southeastern new hampshire. Reston, VA: U.S. Geological Survey Fact Sheet 051-03; 2003. [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environmental Research. 2011;111:110–118. doi: 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MG, Lombard PJ, Schalk LF. U.S. Geological survey scientific investigations report 2010–5199. 2010. Assessment of arsenic concentrations in domestic well water, by town, in maine, 2005–09. [Google Scholar]
- Nigra AE, Sanchez TR, Nachman KE, Harvey DE, Chillrud SN, Graziano JH, et al. The effect of the environmental protection agency maximum contaminant level on arsenic exposure in the USA from 2003 to 2014: An analysis of the national health and nutrition examination survey (NHANES) The Lancet Public Health. 2017;2:e513–e521. doi: 10.1016/S2468-2667(17)30195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas RS. Toxic elements in tobacco and in cigarette smoke: Inflammation and sensitization. Metallomics. 2011;3:1181–1198. doi: 10.1039/c1mt00066g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser E, Goessler W, Francesconi KA. Human metabolism of arsenolipids present in cod liver. Anal Bioanal Chem. 2006;385:367–376. doi: 10.1007/s00216-006-0401-x. [DOI] [PubMed] [Google Scholar]
- Straub CL, Leahy JE. Application of a modified health belief model to the pro-environmental behavior of private well water testing. J Am Water Resour As. 2014;50:1515–1526. [Google Scholar]
- USPHS (U.S. Public Health Service) Public health service drinking water standards. Public Health Reports (1896–1970) 1943;58:58–69. [Google Scholar]
- Walker M, Shaw WD, Benson M. Arsenic consumption and health risk perceptions in a rural western US area. J Am Water Resour As. 2006;42:1363–1370. [Google Scholar]
- Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Perspect. 2010;118:345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 2013;1:1–37. [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environ Health Perspect. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.