Abstract
Astrocytes are an abundant and evolutionarily conserved central nervous system cell type. Despite decades of evidence that astrocytes are integral to neural circuit function, it seems as though astrocytic and neuronal biology continue to advance in parallel to each other, to the detriment of both. Recent advances in molecular biology and optical imaging are being applied to astrocytes in new and exciting ways but without fully considering their unique biology. From this perspective, we explore the reasons that astrocytes remain enigmatic, arguing that their responses to neuronal and environmental cues shape form and function in dynamic ways. Here, we provide a roadmap for future experiments to explore the nature of astrocytes in situ.
Keywords: astrocyte, glia, neural circuits, physiology, identity, morphology, evolution
INTRODUCTION
Astrocytes—the most abundant type of non-neuronal cells in the brain—have received increased attention as researchers have developed and applied more tools to unravel their roles in the central nervous system (CNS). Mammalian astrocytes begin to be produced during mid-embryogenesis and are therefore poised to contribute to neural circuit development and function through much of the life span. During development, astrocytes are a key source of trophic molecules that promote neuronal excitatory synapse formation (1–3). In adulthood, astrocytes provide neuronal circuits with trophic and metabolic support, regulate cerebrovascular tone, and play key roles in circuit-level brain state transitions, such as those that occur between sleep and wake (4–6). Despite these findings, it seems as though progress in astrocyte biology has continued in parallel with the rest of neuroscience but excluded from working models of a unified theory of brain function.
A major hurdle in current astrocyte research is the dynamic nature of the astrocyte itself at both the molecular and physiological levels. We posit that this both distinguishes their function in neural circuits and inhibits our current understanding of their varied roles in the nervous system. To illustrate this idea, we refer to Dynamism of a Dog on a Leash, a painting by the futurist Giacomo Balla from 1912 (Figure 1). In this work, Balla presents the moving parts of a dog and the woman walking it down the street, but we do not get a clear picture of either: They only exist in motion. In the same way, we argue that astrocytes are a type of brain cell in constant flux and are defined by their responses to the neural circuits they support.
Figure 1.
Dynamism of a Dog on a Leash by Giacomo Balla from 1912. This painting provides a graphic analogy for astrocytes in a state of constant flux. These cells are often defined by their responses to the particular neural circuit in which they exist. Reproduced with permission from Albright-Knox Art Gallery.
In this review, we discuss the literature on astrocyte molecular identity and function, with particular attention to blind spots—areas that we have ignored or in which we applied a biased lens to the defining parameter. We then provide thoughts on how we might modify these parameters to guide future studies. Rather than reiterating the many excellent recent reviews on astrocytes, we focus on new conceptual and methodological approaches to examine the intricacies of astrocyte identity and function. We focus on protoplasmic (or synapse-associated) astrocytes and their emerging roles in neural circuit development and function, with particular attention to in vivo data. Finally, although much of this review depends on findings from rodent astrocytes, we conclude with a discussion of how the conservation of astrocytes across model organisms reflects their essential roles within the CNS.
THE SHIFTING MOLECULAR IDENTITY OF ASTROCYTES
Astrocyte Lineage Determination
Are astrocytes a distinct cell lineage? Although all cells are molecularly defined by a balance of cell-autonomous and cell-extrinsic cues, we propose that cell-extrinsic cues play a larger role in astrocyte identity and function relative to other brain cell types (Figure 2). As previously reviewed (7, 8), neural stem cells generate neurons first, followed by glia, and progliogenic transcription regulators including the Notch pathway act at least in part by repressing neuronal fate. From this generic gliogenic pool emerge the two major macroglial cell types in the brain: astrocytes and oligodendrocytes. Difficulties in defining astrocyte lineage have often been attributed to the relative lack of markers to positively identify astrocytes and their intermediate progenitors and to the fact that most astrocyte markers are also expressed in neural stem cells. In addition, although there is strong evidence that astrocytes are developmentally patterned (9, 10), it is not clear that these patterning transcription factors generate stable molecular and functional diversity in astrocytes, as they clearly do in neurons (11, 12). Efforts to understand the specification of mammalian astrocytes from neural stem cells have identified cues that make a cell competent to become an astrocyte, but they have so far found no evidence of a cell-intrinsic program that instructively directs progenitors toward an astrocyte fate.
Figure 2.
Cellular identity as a balance between cell-autonomous cues and cell-extrinsic cues. In contrast to neurons, few transcription factors that define the astrocytes as a unique lineage have been identified. This raises the question of whether astrocyte molecular identity is primarily state dependent and thus responsive to changes in their local environment.
In the many studies on glial fate determination, no single transcription factor has emerged as a unique positive regulator of astrocyte fate, in striking contrast to oligodendrocytes, their nearest relatives (13, 14). For example, several recent studies have used progliogenic transcription factors to identify regulatory cascades that determine whether oligodendrocytes or astrocytes are produced. Sox9 is a gliogenic transcription factor (15) that regulates induction of nuclear factor IA (NFIA) (16), a transcription factor necessary and sufficient for gliogenesis in the embryonic spinal cord (17). NFIA promotes astrogenesis via interactions with Sox9 and by antagonizing the oligodendrocyte-specific transcription factor Sox10 (18). However, although Sox9 is expressed in many astrocytes (19), it is required for both astrocyte and oligodendrocyte generation (15), perhaps via maintenance of multipotent neural progenitors (20). Epigenetic modifications can also alter astrocyte-oligodendrocyte fate choice, such as the histone deacetylase Hdac3, which promotes oligodendrocyte fate commitment and concurrently prevents astrogenesis by repressing the transcription factor Stat3 (21).
With no evidence of any cell-autonomous factors that uniquely regulate astrocyte identity, we are left with only the proastrogenic Stat3, a latent (i.e., signal-dependent) transcription factor (22) better known as a regulator of astrocyte reactivity (23). Although this review focuses on physiological roles of astrocytes, there is a vast literature on the transition from resting to reactive astrocyte under conditions of stress, injury, or neurodegeneration (24). Reactive astrocytes, induced under pathological conditions—typically hypertrophy—proliferate and upregulate markers, including glial fibrillary acidic protein (GFAP) and vimentin. Recent research has begun to molecularly dissect this response, identifying strikingly different gene expression profiles of reactive astrocytes induced by diverse injuries, such as hypoxia-ischemia or endotoxemia (25), and identifying functional differences between physiologic and reactive astrocytes (26). The next step is the recognition of astrocyte reactivity along a spectrum of normal to pathological, wherein changes in circuit function, circuit rearrangements, and circuit stress all may engage a type of astrocyte reaction that can be molecularly and functionally characterized.
Although it is possible that the astrocyte-defining transcription factors in mammals are yet to be discovered, this absence of evidence itself may be the message. Astrocyte identity may indeed be state dependent, which is a function of its context in the brain: unperturbed versus reactive, cortical versus subcortical, etc. Unlike neurons, which are highly methylated, the glial methylome resembles the fetal methylome throughout life, suggesting the possibility of dynamic transcriptional flexibility (27). State-dependent transcription factors such as Stat3 and NF-κB are typically broadly expressed across cell types and often respond to more than one upstream signaling pathway. How multiple inputs can converge on one regulatory pathway while still generating diverse cellular responses is unknown. However, detailed studies of NF-kB signaling in response to two distinct input signals reveal distinct temporal patterns of protein phosphorylation in response to each and reveal dynamic decision making when faced with simultaneous inputs, suggesting that these pathways are more than simple on/off switches (28). It seems likely that astrocytes respond to multiple upstream cues that dynamically affect gene expression and cellular function. Understanding this in the context of astrocytes in vivo will require a better understanding of the types of environmental cues that astrocytes can sense and how sensing these cues may translate to functional diversity, as discussed below.
Astrocyte Molecular Dynamism in Response to Cell Extrinsic Cues
What molecular cues might drive astrocyte functional diversity? Astrocytes in different brain regions have been shown to coordinate with region-specific neuronal functions, such aspHsensing in respiratory areas (29), indicating that their location within the nervous system or proximity to certain types of neurons may determine their functional roles. In the ventral spinal cord, developing astrocytes secrete the guidance cue Sema3a to promote motor neuron survival and synaptogenesis (30). Cortical astrocytes can be segregated based on cell surface markers into distinct populations that vary in their synaptogenic potential (31). Using different tools, these case reports from different CNS regions suggest that astrocytes can be highly specialized to support subtypes of neurons. Might this heterogeneity be in response to the neurons themselves? One elegant example is suggested in a study of the role of the well-known embryonic patterning factor Sonic hedgehog (Shh) in diversifying two molecularly and morphologically distinct subtypes of cerebellar astrocytes: vellate astrocytes and Bergmann glia. The authors showed that Shh continues to be expressed by cerebellar Purkinje neurons into adulthood and helps to promote the molecular and functional identity of the adjacent Bergman glia as distinct from vellate astrocytes (32). These data suggest that one well-described developmental patterning pathway operates postnatally as a neuronal signal that regulates astrocyte identity.
Although gene expression data indicate that astrocytes express receptors for a wide variety of neurotransmitters, hormones, patterning factors, and immune cues (33), there are few to no data on protein levels or on the downstream molecular or functional implications of the loss of such signaling. Several research groups have proposed that neuronal activity could regulate myelination by oligodendrocytes in the CNS, though this has been contentious (34). Could neuronal activity also regulate astrocyte molecular identity or function? The answer to this question is unknown, but if so, this communication would presumably be mediated by molecular cues released from neurons in an activity-dependent manner. Astrocytes express most neurotransmitter receptors, including metabotropic glutamate receptors [particularly mGluR5 developmentally and mGluR2/3 in adulthood (35)], GABA receptors (36), and adrenergic receptors (37). Diminished glutamatergic signaling with global VGluT1 deficiency leads to a modest decrease in astrocyte size, implicating glutamatergic signaling in their functional maturation (38). How activation of these receptors may in turn activate downstream signaling pathways to regulate astrocyte identity or function is unknown. What other molecular pathways might modulate astrocyte physiology? In the setting of injury, astrocytes clearly respond to cytokine signaling from microglia and other sources (26), raising the question of whether similar immune cues could play a physiologic role. Given that protoplasmic astrocytes contact blood vessels as part of the glia limitans, circulating factors or blood-derived cues also have the potential to regulate astrocyte function. Exploring these questions may lead to a different understanding of astrocyte identity—one that is defined by their functional adaptations to a dynamic environment.
PHYSIOLOGICAL DYNAMISM
Astrocytes play increasingly appreciated roles in population-level neural circuit functions, including arousal-state and sleep-wake-state transitions (4, 6, 39–41), whereas synapse-specific functions previously described for astrocytes, such as the vesicular release of neurotransmitter via a SNARE-dependent mechanism (gliotransmission), remain controversial (42). The very fact that a potentially fundamental function of this cell is currently debated points to the lack of a basic understanding of how this cell functions in information flow in neural circuits. Like any other cell type integrated in a larger tissue structure, astrocytes are not limited to one particular role. Their importance in extracellular neurotransmitter clearance and potassium (K+) homeostasis, for example, is not contentious. However, the challenge for the future, when considering their raison d’être, will be to address how all of their physiological roles fit together, when and where each function is deployed, and how can they be experimentally dissected. The overarching question will be how potentially disparate functions are integrated and integral to neural circuit computation over time and space. Below, we discuss recent developments that have begun to address these physiological concerns.
Modern fluorescent imaging tools, although largely developed to visualize and manipulate neuronal activity, have revealed rich calcium (Ca2+) signals in astrocytes and extracellular glutamate signals (among others), thus greatly increasing our ability to interrogate astrocyte function in intact neural circuits. However, it is important to bear in mind that much of the astrocytic information being obtained with these tools (43, 44) is circumscribed by the context in which the tools were developed: to study neurons, a different cell type, whose fundamental function in neural circuits is comparatively clear. Neurons integrate input from other neurons and output (via action potential-triggered neurotransmitter release) to downstream neurons. Optical techniques applied to neurons interrogate various steps in this sequence, either by monitoring or manipulating their activities. For astrocytes, the correspondence of optical imaging data to biological function is still being discovered. Technically and conceptually, these tools are thus carrying out very different scientific discovery functions in neurons and astrocytes. We argue that this situation is one exemplified by the illustration used at the beginning of this review (Figure 1), wherein the time and place at which the astrocyte is observed optically may impart incomplete or potentially misleading information about astrocytic function.
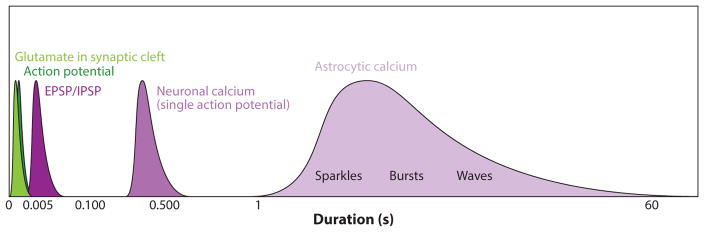
Perhaps the most salient difference between neuronal and astrocytic activity in neural circuits stems from the fact that neurons are electrically excitable, whereas astrocytes are largely electrophysiologically silent, although they exhibit spatiotemporally rich intracellular Ca2+ activity. A corollary to this observation is that neuronal and astrocytic activities occur at vastly different timescales, with the many observed types of astrocyte Ca2+ activity lasting up to orders of magnitude longer, thus spanning a much wider temporal window than neuronal action potentials or postsynaptic events (Figure 3). It has been suggested that the differing timescales of observed astrocytic Ca2+ events likely reflect widely varying cellular functions that have yet to be uncovered. In this framework, astrocytic Ca2+ would relay qualitatively different signals than neuronal somatic Ca2+ does in mature neural circuits, where changes in the amplitude and duration of fluorescence [measured either by fluorescent dyes or genetically encoded indicators (43, 45)] largely reflect differences in neuronal firing frequency. In the case of astrocytic Ca2+, rather than indicating a change in degree of activity, differences in duration of events might signify differing responses to extracellular or intracellular molecular events. However, these hypotheses remain largely untested, as astrocyte biologists are continuing to disambiguate the circuit signals driving each particular astrocytic Ca2+ event observed and the downstream events that astrocytic Ca2+ signals precipitate at any given place and time. Because many excellent recent reviews discuss the state of the field of astrocytic Ca2+ signaling (46, 47), we focus here on gaps in our understanding of astrocyte activity dynamics and potential avenues for future progress.
Figure 3.
Schematic of the wide range of time scales for various basic neuronal (action potential, EPSP, IPSP, and calcium change in response to action potential using genetically encoded sensors) and astrocytic calcium events. Astrocyte calcium excitability tends to be slower than many neuronal events. Abbreviations: EPSP, excitatory post-synaptic potential; IPSP, inhibitory post-synaptic potential.
Spatially, investigations of astrocyte activity have tended to fall into two distinct groups: those that examine the dynamics of a single cell and/or its subcellular compartments (48, 49) and those that take a larger view of the astrocytic population in a particular brain structure (4, 6, 39, 40). Both perspectives are critical to understand how astrocytes work, and although it remains technically difficult to examine both cell- and population-level resolutions simultaneously, we believe that tools to allow this kind of integrated analysis will be necessary to answer outstanding physiology questions. However, astrocyte researchers can be hindered in these endeavors—particularly on the analysis side—by the great spatiotemporal heterogeneity of astrocyte signals, both within and among astrocytes. To make progress on overcoming these analysis hurdles, astrocyte-specific analytical techniques will need to move away from region-of-interest (ROI)-based analyses, which assume a fixed size of the compartment to analyze (6, 49–53). Although ROI-based approaches are sufficient for most neuronal (whether cell soma or dendritic) imaging analyses, they cannot account for the heterogeneity of astrocyte signals. For example, a small-amplitude, short-duration event in an astrocyte may overlap spatially, at a later time point, with a large-amplitude, longer event. In addition, many observed astrocytic events are propagative, so static ROI-based analyses are insufficient. We propose that a satisfying computational solution to these problems would take into account these dynamics, potentially applying mathematical models that have not been developed with neurons in mind (54). Furthermore, we posit that analytical tools built around these astrocyte-specific constraints may address current research discrepancies in this field and allow researchers to link cellular- and circuit-level physiology in meaningful ways.
Finally, although neurons and astrocytes are two distinct cell types, they are intimately connected in neural circuits and communicate within and between themselves as part of ongoing neuronal physiology. Thus, disentangling their functions is a challenging technical and conceptual problem that will require simultaneous imaging of both in vivo. In the past, astrocyte physiology at their fine branches could be assessed only in the context of simultaneous neuronal electrophysiological recordings. However, with the introduction of high signal-to-noise sensors that are excitable outside of the green spectrum (55), there are opportunities to assess fine-scale astrocyte and neuronal activity simultaneously and at high spatial resolution (56–58). In addition, advances in in vivo two-photon imaging techniques in three dimensions, as well as in deep brain structures (59), should expand the reach of fluorescent and analytical tools beyond the most commonly studied brain structures, nuclei, and layers. Applying these tools to neural circuits throughout the nervous system will allow researchers to assess interactions of astrocytes and neurons on a moment-to-moment basis and to dissect the interplay of these two cell types across time and cytoarchitectural space.
MORPHOLOGICAL DYNAMISM
The physiological and molecular dynamism of astrocytes discussed in the previous sections are potentially the cause or consequence of another type of cellular dynamics: morphological. The first suggestion that astrocytic branches might be motile likely came from Cajal, who hypothesized that astrocytes invade the synaptic cleft during sleep and separate the pre- and postsynaptic neurons to halt synaptic transmission, while retracting during wake (60). Although there is no evidence that this occurs and that sleep is not characterized by a lack of synaptic transmission, some recent clues suggest that morphological dynamics of astrocytes may underlie differences between sleep and wake. First, the Nedergaard group (5) has demonstrated that there is a dramatic shift in the perivascular movement of cerebrospinal fluid through the brain parenchyma between sleep and wake and that this difference is correlated with a change in extracellular space—one that may be mediated by astrocytes. Although not shown directly, it is likely that astrocyte branches may move during the course of these large-scale, tissue-wide expansions and retractions over sleep and wake (5). Second, ribosome profiling of astrocytes demonstrated that many more genes were uniquely expressed in wake compared to sleep, and these wakefulness astrocyte-specific genes included some with the potential to mediate branch elongation: Trio, Synj2, and Gem (61). Furthermore, ultrastructural evidence indicates that sleep deprivation is accompanied by increased astrocytic coverage of dendritic spines, demonstrating that astrocytes have the potential to be motile over the time course of days. It is worth noting that, when evaluating electron microscopy data, that a recent paper found an almost twofold difference in astrocytic coverage of synapses in tissues prepared via cryofixation (~34%) when compared to traditional chemical fixation methods (~62%) (62). These data suggest that the tissue shrinkage that occurs with chemical fixation may be driving astrocytic branches into synapses and may not reflect the in vivo morphology of these synapses. In addition, they should encourage a comparison between these fixation methods for future ultrastructural studies of astrocytes.
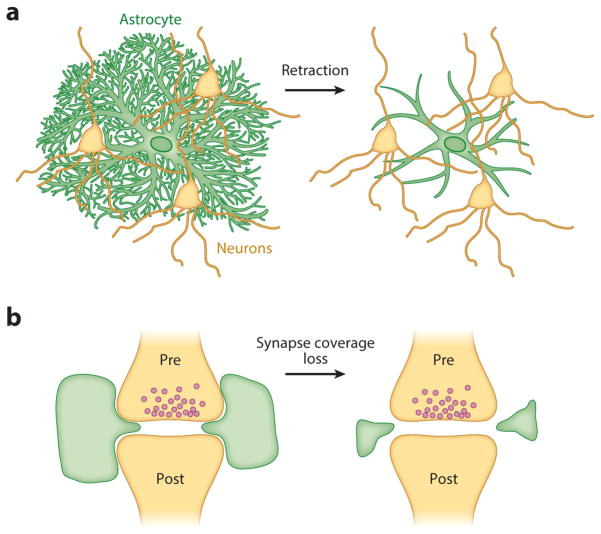
A dramatic example of morphological dynamism documented in astrocytes has been observed in the hypothalamus and in the supraoptic and paraventricular nuclei (summarized in 63). Compared to the subtler changes in synaptic coverage discussed above, the astrocytes in these regions— identified by GFAP labeling—fully retract their branches during lactation, parturition, and dehydration on a rapid timescale, within hours of the onset of these processes that stimulate oxytocin and/or vasopressin secretion (Figure 4a). These morphological changes are reversible, with the speed of reversal negatively correlated with duration of the stimulation. For example, astrocytes in the supraoptic nucleus revert back to their basal morphology within one month if rats have lactated, but this reversion takes two months if the animals have undergone two consecutive gestation and lactation periods. The morphological astrocytic dynamics exhibited in this circuit have enabled elegant work in which neuronal activity in the presence and absence of astrocyte branches can be quantified, yielding a greater understanding of astrocytes’ roles in volume transmission, neurotransmitter uptake, and synaptic plasticity (64, 65).
Figure 4.
Schematic of the different possible types of morphological changes, including (a) full-scale, whole-cell branch retraction and (b) subtler changes in the astrocytic coverage of synapses. Pre- and post- refer to the pre- and post-synaptic cells. Both large-scale retraction (a) and smaller-scale synaptic coverage changes (b) can affect neuronal signaling, but these have not yet been explored in many brain regions.
Whether the effects on neurons due to the types of changes observed in these hypothalamic nuclei can be generalized to astrocytes in other areas remains unclear. In addition, it is unknown whether the morphological changes observed in these hypothalamic astrocytes represent an active (astrocyte-driven) or a passive (neuron-driven) process from the perspective of the astrocyte. Simultaneous two-color imaging of astrocyte processes and dendritic spines in hippocampus has shown that astrocytes can extend and retract their fine processes at a rapid timescale (on the order of minutes) to engage and disengage with dendritic spines (66) (Figure 4b). This astrocytic motility is more dynamic than that of the apposed spines (66), suggesting the possibility of an astrocyte-intrinsic motility. More recent work, in which astrocyte and neuronal components were again tracked using two-color fluorescence and accompanied by astrocytic manipulations, observed extensive structural plasticity in the astrocytic processes. These dynamics were shown to be causally related to increased spine stability and astrocytic spine coverage (67).
Related work addresses the larger concept that fine-scale changes in astrocyte morphology can drive synaptic physiological differences. One of the connexin proteins [connexin 30 (Cx30)] that forms gap junctions between astrocytes can influence synaptic strength via the degree of astrocytic invasion into the synaptic space (68). Because these conclusions were drawn from fixed brain sections of wild-type and Cx30 knockout mice rather than live imaging experiments, the timescale of these effects remains unclear, but a significant change in astrocyte branch length and complexity is evident. Together with the aforementioned work, these data strongly implicate astrocytes as active players in morphological dynamics at the synapse. However, not all evidence points toward synaptic stability as an outcome of astrocyte-based motility. Indeed, astrocyte motility may play an important role in synapse elimination, as based on evidence showing that astrocytes engulf synapses wholesale—pre- and postsynaptic compartments together—in a neuronal activity-dependent manner (69). Besides raising fascinating questions about the function of astrocytes in neural circuits, this research also reinforces the notion that the motility of astrocytes at synapses is a critical component of their physiological functions.
ASTROCYTES IN AN EVOLUTIONARY CONTEXT
Astrocyte biology is no different from the rest of neuroscience in its reliance on rodent models (70), and this bias is reflected in almost all the aforementioned studies. How might perspectives from different model organisms contribute to our understanding of astrocyte biology? Many excellent recent reviews have addressed how astrocytes differ across model organisms (71–73). Here, we focus instead on conserved features that can inform our understanding of astrocyte function. Given the vagaries of glial lineage determination (discussed in Physiological Dynamism), perhaps it is not surprising that glial specification is not conserved across species. The gene glial cells missing (gcm), which is necessary and sufficient for gliogenesis in Drosophila melanogaster (74, 75), is not expressed in the mammalian brain and does not affect gliogenesis (76), nor is it obviously homologous with mechanisms of glial specification in other organisms such as Caenorhabditis elegans (77).
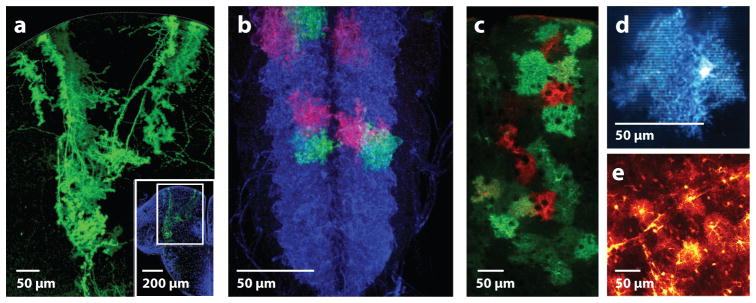
A focus on conserved function—rather than lineage—of astrocytes across species has been more informative. Glial diversity is more limited in lower organisms, in which the central and peripheral nervous systems are not as fully diverged. In some species, including C. elegans, a defined number of glia (~45 versus 302 neurons) play roles in positioning and guidance of neurons (78, 79); these roles are echoed in mammals (30). C. elegans glia also share roles in ensheathing and providing trophic support for neuronal dendrites (77, 78). However, these glia share few of the morphologic features of mammalian astrocytes, and it is not clear to what extent the molecular pathways mediating these effects may be conserved across species. Several species, including birds (80) and zebrafish (81), are not thought to have astrocytes at all but instead have postnatal persistence of a radial glial-like cell that spans the pial to ventricular surface. In the mature zebrafish, these cells develop fine processes strikingly similar to mammalian protoplasmic astrocytes (see Figure 5). Similar to neurons, mammalian protoplasmic astrocytes carry out local translation of mRNA in their fine processes (82), raising the question of whether astrocytic fine processes may act as semiautonomous subdomains when they interact with synapses or neural circuits. In such a scenario, zebrafish radial glia could have conserved functional roles with mammalian protoplasmic astrocytes with respect to synaptic and vascular interactions.
Figure 5.
Astrocytes across model organisms display extensive elaboration of fine branches. (a) Zebrafish radial glia in an adult dorsal telencephalon sparse labeled with a membrane-bound fluorescent reporter. Image courtesy of Marion Coolen and Laure Bally-Cuif, Institut Pasteur, Paris. (b) Third instar larval astrocytes in Drosophila ventral nerve cord: Astrocytes are blue and sparse labeled in red and green using twin-spot MARCM (mosaic analysis with a repressible cell marker). Image courtesy of Tobias Stork and Marc Freeman, Vollum Institute, Portland, Oregon. (c) Adult mouse cortical astrocytes tiling across cortical layers and sparse labeled in red and green using a confetti:hGFAPcre construct. Image by Kimberly Hoi and Anna Molofsky. (d) Single juvenile murine cortical astrocyte labeled with a membrane-bound fluorescent protein. Image by Kira Poskanzer. (e) Partially penetrant EAAT2-tdTomato BAC transgenic cortical astrocytes indicating fine process labeling and astrocyte-vascular contacts (98). Image by Michelle Cahill and Kira Poskanzer.
Drosophila larval astrocytes are elegant models for the synapse-ensheathing morphology of mammalian protoplasmic astrocytes (Figure 5b). Their branches project into a dense neuropil rich in synapses but devoid of neuronal cell bodies, providing a unique opportunity to study their synapse-supporting functions. Their association with synapses is a morphologic specialization that requires the fibroblast growth factor (FGF) receptor heartless (83), reminiscent of the role of Fgfr3 in mammalian gliogenesis (84). Drosophila astrocytes modulate dopaminergic neuronal signaling (56) and mammalian astrocytes also involved in dopaminergic signaling (85). Drosophila astrocytes regulate excitatory/inhibitory balance via the GABA transporter, whose developmental expression is in turn regulated by neuronal activity (86). Mammalian astrocytes express Gat1 and Gat3 and regulate diffusion of GABA away from synaptic sites (87, 88); the roles of mammalian astrocytes in inhibitory synapse development and the maturation of astrocyte functional properties are major issues being addressed in the field. A developmentally distinct population of ensheathing astrocytes dependent on Notch signaling (89) reside outside the neuropil and provide neuronal support as well as engulfing degenerating axons via the engulfment receptor draper (90), whose mammalian analog Megf10 regulates synapse engulfment by astrocytes (69). These examples illustrate multiple conserved molecular pathways from flies to mammals.
Other morphologic features of rodent astrocytes are variably conserved across species, raising the question of whether these differences are functionally relevant. Rodent and fly astrocytes tile in discrete domains (Figure 5b,c), in contrast to some domain overlap in human astrocytes (91). When examined with membrane-tagged labels, astrocytes in most species have extensive synapse-contacting fine processes (Figure 5) and interact closely with vasculature (Figure 5e). However, several groups have described specialized processes in primate astrocytes, some of which span and possibly connect different cortical layers, as in the so-called interlaminar astrocytes (92). This could confer entirely new primate-specific properties in astrocytes. Likewise, human astrocytes are bigger than rodent and other primate astrocytes (91) and may support higher cognitive function (93). These morphological differences suggest that the extent of astrocyte-synaptic connectivity could vary among species, buffering neurotransmitters and modulating neuronal synchrony in unique and possibly primate-specific ways. Exploring these questions is largely impossible in intact primate brains. However, studies of the numerous gene expression differences between human and mouse astrocytes (33) begin to hint at molecular pathways that could be explored in emerging organoid cultures (94, 95) and could yield insights into primate-specific astrocyte properties.
CONCLUSION
In summary, the explosion of molecular and optical tools in neuroscience is poised to change our understanding of how astrocytes participate in neural function, if we allow it to. As with any data set, biological complexity can be obliterated by the analysis parameters applied to the question. For example, the discovery of transcription factors required for circadian rhythms (96) and the description of transcript accumulation and decay over the course of a 24-h cycle (97) were only possible when performing experiments with the defining parameter in mind: time of day. As we argue in this review, the neuron-centric assumption of a fixed lineage may obscure the amazing capacity of astrocytes to react to an enormous range of extracellular cues. Likewise, being married to a strict input–output functional relationship akin to neuronal integration can obscure potential subcellular functional units in astrocytes. Conversely, investigating astrocytic involvement in state switching over a long timescale has opened up areas of inquiry previously believed to be solely in the domain of neurons. Much remains to be learned about how astrocytes respond to their environment, how they communicate with each other over space and time, and the link between their molecular identity and physiology. We hope that a directed focus on the uniqueness of the astrocyte will shed light on these questions and many others.
Acknowledgments
The authors thank Gregory Chin for expert assistance with figure preparation, and we acknowledge essential input from colleagues and collaborators who contributed to the ideas in this review. A.V.M is supported by the National Institute of Mental Health (grant K08MH104417) and the Burroughs Wellcome Fund. K.E.P. is supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant R01NS099254) and National Science Foundation (grant 1604544).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486(7403):410–14. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–33. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, et al. Astrocytes assemble thalamocortical synapses by bridging NRX1α and NL1 via hevin. Cell. 2016;164(1–2):183–96. doi: 10.1016/j.cell.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82(6):1263–70. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–77. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. PNAS. 2016;113(19):E2675–84. doi: 10.1073/pnas.1520759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molofsky AV, Deneen B. Astrocyte development: a guide for the perplexed. Glia. 2015;63(8):1320–29. doi: 10.1002/glia.22836. [DOI] [PubMed] [Google Scholar]
- 8.Molofsky AV, Krenick R, Ullian E, Tsai H, Deneen B, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133(3):510–22. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai H-H, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337(6092):358–62. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- 12.Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74(6):975–89. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Lu QR, Sun T, Zhu Z, Ma N, Garcia M, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109(1):61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 15.Stolt CC, Lommes P, Sock E, Chaboissier M-C, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17(13):1677–89. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang P, Lee HK, Glasgow SM, Finley M, Donti T, et al. Sox9 andNFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. 2012;74(1):79–94. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52(6):953–68. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Glasgow SM, Zhu W, Stolt CC, Huang T-W, Chen F, et al. Mutual antagonism between Sox10 and NFIA regulates diversification of glial lineages and glioma subtypes. Nat Neurosci. 2014;17(10):1322–29. doi: 10.1038/nn.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun W, Cornwell A, Li J, Peng S, Osorio MJ, et al. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci. 2017;37(17):4493–507. doi: 10.1523/JNEUROSCI.3199-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13(10):1181–89. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, He X, Liu L, Jiang M, Zhao C, et al. Hdac3 interaction with p300 histone acetyltransferase regulates the oligodendrocyte and astrocyte lineage fate switch. Dev Cell. 2016;36(3):316–30. doi: 10.1016/j.devcel.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy DE, Darnell JE. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3(9):651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 23.Ben Haim L, Ceyzériat K, Carrillo-de Sauvage MA, Aubry F, Auregan G, et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J Neurosci. 2015;35(6):2817–29. doi: 10.1523/JNEUROSCI.3516-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–48. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–87. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellogg RA, Tian C, Etzrodt M, Tay S. Cellular decision making by non-integrative processing of TLR inputs. Cell Rep. 2017;19(1):125–35. doi: 10.1016/j.celrep.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329(5991):571–75. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molofsky AV, Kelley KW, Tsai H-H, Redmond SA, Chang SM, et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509(7499):189–94. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John Lin C-C, Yu K, Hatcher A, Huang T-W, Lee HK, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20(3):396–405. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, et al. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science. 2016;351(6275):849–54. doi: 10.1126/science.aab3103. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89(1):37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mount CW, Monje M. Wrapped to adapt: experience-dependent myelination. Neuron. 2017;95(4):743–56. doi: 10.1016/j.neuron.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun W, McConnell E, Pare J-F, Xu Q, Chen M, et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339(6116):197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia. 2008;56(10):1127–37. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- 37.Bekar LK, He W, Nedergaard M. Locus coeruleus α-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18(12):2789–95. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morel L, Higashimori H, Tolman M, Yang Y. VGluT1+ neuronal glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. J Neurosci. 2014;34(33):10950–62. doi: 10.1523/JNEUROSCI.1167-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, et al. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18(5):708–17. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62(3):400–12. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, et al. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54(6):387–94. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11(4):227–38. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 43.Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10(2):162–70. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeves AMB, Shigetomi E, Khakh BS. Bulk loading of calcium indicator dyes to study astrocyte physiology: key limitations and improvements using morphological maps. J Neurosci. 2011;31(25):9353–58. doi: 10.1523/JNEUROSCI.0127-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19(2):182–89. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 47.Shigetomi E, Patel S, Khakh BS. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol. 2016;26(4):300–12. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, et al. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci. 2015;18(2):219–26. doi: 10.1038/nn.3901. [DOI] [PubMed] [Google Scholar]
- 49.Bindocci E, Savtchouk I, Liaudet N, Becker D, Carriero G, Volterra A. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science. 2017;356(6339):eaai8185. doi: 10.1126/science.aai8185. [DOI] [PubMed] [Google Scholar]
- 50.Haustein MD, Kracun S, Lu X-H, Shih T, Jackson-Weaver O, et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron. 2014;82(2):413–29. doi: 10.1016/j.neuron.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal A, Wu P-H, Hughes EG, Fukaya M, Tischfield MA, et al. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron. 2017;93(3):587–605. e7. doi: 10.1016/j.neuron.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armbruster M, Hanson E, Dulla CG. Glutamate clearance is locally modulated by presynaptic neuronal activity in the cerebral cortex. J Neurosci. 2016;36(40):10404–15. doi: 10.1523/JNEUROSCI.2066-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hefendehl JK, LeDue J, Ko RWY, Mahler J, Murphy TH, MacVicar BA. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat Comm. 2016;7:13441. doi: 10.1038/ncomms13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D, Miller D, Poskanzer K, Tian L, Yu G. Graphical time warping for joint alignment of multiple curves. Adv Neural Inf Process Syst. 2016;29:3648–56. [Google Scholar]
- 55.Dana H, Mohar B, Sun Y, Narayan S, Gordus A, et al. Sensitive red protein calcium indicators for imaging neural activity. eLife. 2016;5:413. doi: 10.7554/eLife.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature. 2016;539(7629):428–32. doi: 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enger R, Dukefoss DB, Tang W, Pettersen KH, Bjørnstad DM, et al. Deletion of aquaporin-4 curtails extracellular glutamate elevation in cortical spreading depression in awake mice. Cereb Cortex. 2017;27(1):24–33. doi: 10.1093/cercor/bhw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron. 2017;93(6):1420–25. doi: 10.1016/j.neuron.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang W, Yuste R. In vivo imaging of neural activity. Nat Methods. 2017;14(4):349–59. doi: 10.1038/nmeth.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cajal SR. Algunas conjeturas sobre el mecanismo anatómico de la ideación, asociación y atención. Rev Med Cir Pract. 1895;36:497–508. [Google Scholar]
- 61.Bellesi M, de Vivo L, Tononi G, Cirelli C. Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol. 2015;13(1):66. doi: 10.1186/s12915-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korogod N, Petersen CCH, Knott GW. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. eLife. 2015;4:241. doi: 10.7554/eLife.05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Theodosis DT, Poulain DA. Activity-dependent neuronal-glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience. 1993;57(3):501–35. doi: 10.1016/0306-4522(93)90002-w. [DOI] [PubMed] [Google Scholar]
- 64.Gordon GRJ, Iremonger KJ, Kantevari S, Ellis-Davies GCR, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64(3):391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panatier A, Theodosis DT, Mothet J-P, Touquet B, Pollegioni L, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–84. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 66.Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 26:8881–91. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernardinelli Y, Randall J, Janett E, Nikonenko I, König S, et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol. 2014;24(15):1679–8845. doi: 10.1016/j.cub.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 68.Pannasch U, Freche D, Dallérac G, Ghézali G, Escartin C, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17(4):549–58. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 69.Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504(7480):394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keifer J, Summers CH. Putting the “biology” back into “neurobiology”: the strength of diversity in animal model systems for neuroscience research. Front Syst Neurosci. 2016;10:69. doi: 10.3389/fnsys.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron. 2013;80(3):613–23. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasile F, Dossi E, Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct. 2017;222:2017–29. doi: 10.1007/s00429-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verkhratsky A, Nedergaard M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Phil Trans R Soc B. 2016;371(1700):20150428. doi: 10.1098/rstb.2015.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosoya T, Takizawa K, Nitta K, Hotta Y. Glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82(6):1025–36. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- 75.Jones BW, Fetter RD, Tear G, Goodman CS. Glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82(6):1013–23. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 76.Kim J, Jones BW, Zock C, Chen Z, Wang H, et al. Isolation and characterization of mammalian homologs of the Drosophila gene glial cells missing. PNAS. 1998;95(21):12364–69. doi: 10.1073/pnas.95.21.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaham S. Glial development and function in the nervous system of Caenorhabditis elegans. Cold Spring Harb Perspect Biol. 2015;7(4):a020578. doi: 10.1101/cshperspect.a020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell. 2005;8(6):893–906. doi: 10.1016/j.devcel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Shao Z, Watanabe S, Christensen R, Jorgensen EM, Colón-Ramos DA. Synapse location during growth depends on glia location. Cell. 2013;154(2):337–50. doi: 10.1016/j.cell.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264(2):159–70. doi: 10.1002/cne.902640203. [DOI] [PubMed] [Google Scholar]
- 81.Lyons DA, Talbot WS. Glial cell development and function in zebrafish. Cold Spring Harb Perspect Biol. 2014;7(2):a020586. doi: 10.1101/cshperspect.a020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakers K, Lake AM, Khazanchi R, Ouwenga R, Vasek MJ, et al. Astrocytes locally translate transcripts in their peripheral processes. PNAS. 2017;114(19):E3830–38. doi: 10.1073/pnas.1617782114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR. Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron. 2014;83(2):388–403. doi: 10.1016/j.neuron.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pringle NP, Yu W-P, Howell M, Colvin JS, Ornitz DM, Richardson WD. Fgfr3 expression by astrocytes and their precursors: evidence that astrocytes and oligodendrocytes originate in distinct neuroepithelial domains. Development. 2003;130(1):93–102. doi: 10.1242/dev.00184. [DOI] [PubMed] [Google Scholar]
- 85.Jennings A, Tyurikova O, Bard L, Zheng K, Semyanov A, et al. Dopamine elevates and lowers astroglial Ca2+ through distinct pathways depending on local synaptic circuitry. Glia. 2017;65(3):447–59. doi: 10.1002/glia.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muthukumar AK, Stork T, Freeman MR. Activity-dependent regulation of astrocyte GAT levels during synaptogenesis. Nat Neurosci. 2014;17(10):1340–50. doi: 10.1038/nn.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beenhakker MP, Huguenard JR. Astrocytes as gatekeepers of GABAB receptor function. J Neurosci. 2010;30(45):15262–76. doi: 10.1523/JNEUROSCI.3243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15(1):70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peco E, Davla S, Camp D, Stacey SM, Landgraf M, van Meyel DJ. Drosophila astrocytes cover specific territories of the CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development. 2016;143(7):1170–81. doi: 10.1242/dev.133165. [DOI] [PubMed] [Google Scholar]
- 90.Doherty J, Logan MA, Taşdemir ÖE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009;29(15):4768–81. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oberheim NA, Takano T, Han X, He W, Lin JHC, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29(10):3276–87. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Colombo JA, Reisin HD. Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res. 2004;1006(1):126–31. doi: 10.1016/j.brainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 93.Han X, Chen M, Wang F, Windrem M, Wang S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12(3):342–53. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Birey F, Andersen J, Makinson CD, Islam S, Wei W, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545(7652):54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 2017;95(4):779–90. e6. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–35. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–79. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, et al. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia. 2011;59(2):200–7. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]