Abstract
Left ventricular assist devices (LVADs) significantly improve outcomes of advanced heart failure patients. However, patients continue to have high readmission rates due to complications ranging from bleeding, thrombosis, heart failure, and infection. Considering that the hallmark benefit of LVAD therapy is improvement in hemodynamics (cardiac unloading and increased cardiac output), hemodynamic assessment on LVAD support is key to better understand these difficult complications and may serve as a tool to resolving them. In this review, we will discuss the hemodynamic changes following LVAD implantation, and the implications and prognostic impact of hemodynamic optimization on outcomes and complications.
Keywords: Left ventricular assist device, HeartMate, Ramp, Heart failure
Introduction
Left ventricular assist devices (LVADs) have become the mainstay therapy for advanced heart failure (HF) patients, both as a bridge to transplantation and as destination therapy [1]. The initial LVADs were pulsatile and extracorporeal, but current contemporary devices have continuous flow and are internally implanted. As such, they are smaller, more durable, and less invasive [2,3]. However, there are still significant complications during long-term LVAD therapy [4] with multifactorial etiologies including both patient physiology and pump performance.
LVAD therapy improves outcomes in HF patients by improving hemodynamics, unloading the left ventricle and augmenting cardiac output (CO) [5]. As a result, LVADs enhance peripheral circulation, improve end-organ dysfunction [6], increase exercise capacity, and relieve HF symptoms [7]. Hemodynamic assessment during LVAD support may clarify the role of hemodynamic derangements in the development of LVAD complications. Our group has developed echocardiographic and hemodynamic ramp tests as a tool to facilitate hemodynamic optimization by adjusting LVAD speed and medical therapy [8]. Such procedures may be key to overcoming adverse events and improving clinical outcomes.
In this review, we will discuss how to measure hemodynamics, changes in hemodynamics after LVAD implantation, hemodynamic profiles during complications, and clinical implications and prognostic impact of hemodynamic optimization with ramp testing.
LVAD types
A variety of LVADs is clinically available, and thus far, most of the LVADs currently used are implantable continuous-flow devices [1]. In Japan, paracorporeal, pulsatile-flow LVADs are still used as bridge to decision, since continuous-flow LVADs are only available as bridge to transplantation [9].
The current continuous-flow LVADs available in Japan include: EVAHEART (Sun Medical, Nagano, Japan) [10], Jarvik 2000 (Jarvik Heart, Inc., New York, NY, USA) [11], and HeartMate II (Abbott, Abbott Park, IL, USA) (Fig. 1) [2]. In the USA, the only devices that are approved for commercial use are the HeartMate II, HeartMate 3 (Abbott) [12] and HVAD (Medtronic, Minneapolis, MN, USA). The HeartAssist5 (ReliantHeart Inc., Houston, TX, USA) [13] and Jarvik 2000 are currently under investigation [14]. HVAD, HeartMate 3, and HeartAssist5 may be available in Japan shortly. In this article, we will focus on the continuous-flow LVADs.
Fig. 1.
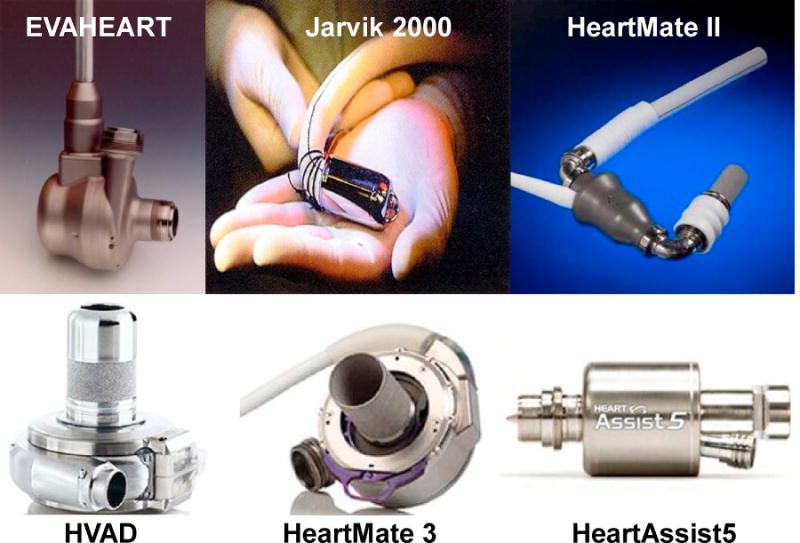
Continuous-flow left ventricular assist device available in Japan and the USA.
Measuring hemodynamics during LVAD support
The hemodynamic assessment of LVAD patients starts with blood pressure measurement. Higher blood pressure has been associated with increases in intracranial hemorrhage, thromboembolic events, and progressive aortic insufficiency [15]. Unfortunately, the reduced pulse pressure during continuous-flow LVAD support limits our ability to accurately measure blood pressure with traditional oscillometric blood pressure cuffs, and Doppler opening blood pressure is commonly used as a surrogate of mean arterial pressure. Arterial lines are the gold standard for monitoring blood pressure, but are invasive and not practical for ambulatory use.
Physical examination is the most common tool to assess hemodynamics in patients with HF [16]. However, preliminary data from a prospective trial at our institution show that physical examination has low sensitivity in assessing hemodynamics compared to right heart catheterization (RHC), including central venous pressure (CVP), pulmonary capillary wedge pressure (PCWP), and cardiac index (CI) [17]. Invasive RHC remains the gold standard to assess hemodynamics in LVAD patients.
Estep et al. found that Doppler echocardiography provides an estimate of invasive hemodynamics. They demonstrated good correlation between Doppler echocardiographic and invasive measurements in mean right atrial pressure (r = 0.863; p < 0.001), systolic pulmonary artery pressure (PAP) (r = 0.880; p < 0.001), and pulmonary vascular resistance (PVR) (r = 0.643; p < 0.001) in 50 consecutive patients with HeartMate II, although optimal results require expert technique [18].
Estimation of the PCWP in patients with centrifugal continuous-flow LVAD may be achieved by analyzing the flow wave derived from the pump power. Recently, we reported that the early filling phase slope measured from the HVAD waveform (as displayed on the HVAD clinical screen) is directly correlated to the measured PCWP [19]. Our findings were corroborated by the report from Lai et al., which also demonstrated that the HVAD waveform had an excellent predictive value [20]. More studies are required to demonstrate whether waveform analysis can be routinely used as a clinical tool.
Innovative monitoring devices, such as the CardioMEMS Heart Failure Monitoring System (Abbott) [21] and Remote Dielectric Sensing (Sensible Medical Innovations Ltd., Kfar Neter, Israel) [22], are currently under investigation (Fig. 2).
Fig. 2.
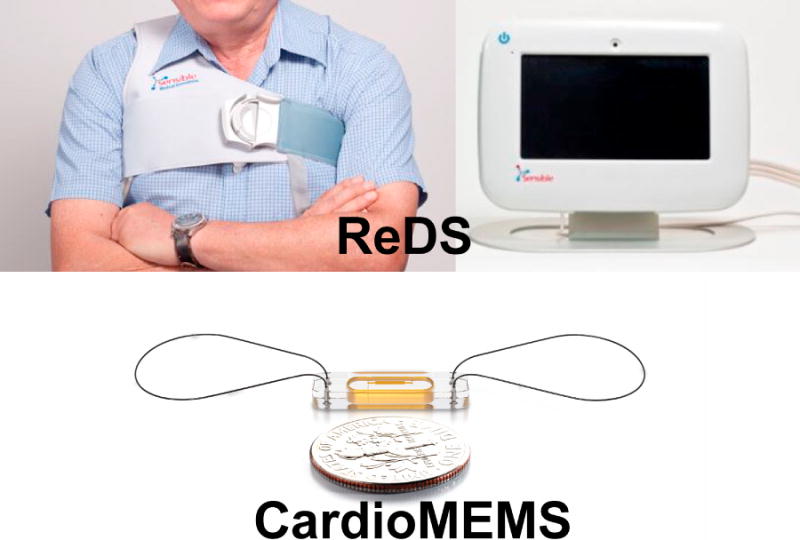
Non-invasive devices to monitor hemodynamics.
Ramp test and optimization of hemodynamics
The International Society of Heart and Lung Transplantation guidelines recommend echocardiogram as an integral part of determining optimal LVAD speed, with goals including adequate LV unloading with midline LV septum and minimal mitral valve regurgitation (MR) (class I) [23]. Adjusting LVAD speed to allow intermittent aortic valve opening is currently in the guidelines as a class IIb recommendation. However, these recommendations are vague and not standardized. RHC is recommended in specific situations such as recurrent HF symptoms, pulmonary hypertension (PH), and right ventricular failure (RVF) (class I), or when LVAD explantation is considered (class IIa) [23]. Routine RHC is not recommended in the guidelines.
We recently showed in clinically stable outpatients that 57% of patients had abnormally elevated CVP and PCWP at baseline LVAD speed [8]. This finding suggests that current approaches to speed optimization are inadequate, and that measurement of hemodynamics provides significant additional information above clinical assessment. All patients may benefit from a hemodynamic-guided optimization of LVAD speed and medical therapy.
To develop a standardized approach to hemodynamic assessment and optimization, we modified our previously described echocardiographic ramp test to create an invasive hemodynamic ramp protocol [24,25]. In this protocol, LV end-systolic dimension, LV end-diastolic dimension, the frequency of aortic valve (AV) opening, and the degree of MR and AV regurgitation are measured at the baseline LVAD speed, along with hemodynamic parameters including CVP, PAP, PCWP, and CO and CI by Fick measured by invasive RHC. After these measurements, the LVAD speed is turned down to 8000 RPM in HeartMate II and 2300 RPM in HVAD. The LVAD speed is subsequently increased stepwise every 2 min by 400 RPM in HeartMate II (8000–12,000 RPM) and 100 RPM in HVAD (2300–3200 RPM). The aforementioned echocardiographic and hemodynamic parameters are measured at each LVAD speed. The study is terminated when LV end-diastolic dimension is less than 3.0 cm or a significant suction event occurs. At the conclusion, LVAD speed is set targeting CVP <12 mmHg and PCWP <18 mmHg with the secondary goal of allowing intermittent AV opening and minimal MR.
In our initial trial, 35 LVAD patients (21 HeartMate II and 14 HVAD) underwent hemodynamic ramp test, during which variables were measured at 9 speed settings [8]. Only 43% of patients had normal CVP and PCWP at baseline LVAD speed. Hemodynamic normalization was achieved in 56% of patients after speed adjustment (Fig. 3). Hemodynamic ramp testing was performed with therapeutic anticoagulation and no adverse events related to the RHC occurred. The utility of invasive ramp test is applicable to many devices and we recently reported ramp tests modified to HeartMate 3 and HeartAssist 5 patients [26,27]. Of note, the majority of patients supported with HeartMate 3 (62.5%) had normal CVP and PCWP at baseline speed, and the number with normal hemodynamics increased up to 81.3% after speed adjustment.
Fig. 3.
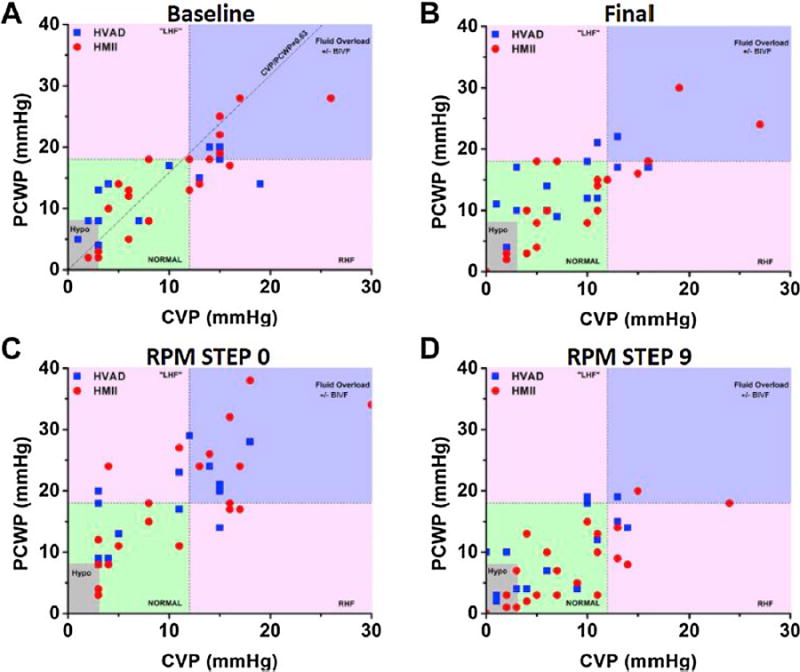
Relationship of CVP and PCWP in each patient at baseline, final, step 0 (lowest speed), and step 9 (highest speed). Reprinted from [8] with permission from Elsevier. CVP, central venous pressure; PCWP, pulmonary capillary wedge pressure.
Hemodynamics during LVAD therapy
Hemodynamics following LVAD implantation
Significant hemodynamic derangement is the hallmark of advanced HF, particularly reduced CO and elevated PCWP and CVP. In the MOMENTUM 3 trial, CVP was 10.3 ± 5.8 mmHg, PCWP was 23.4 ± 8.5 mmHg, and 2 CI was 1.9 ± 0.5 L/min/m just before HeartMate 3 implantation [12]. Following LVAD implantation, PCWP decreases and CO increases dramatically. Comparison of hemodynamics in the clinical setting among different devices is difficult [28,29], as hemodynamics are also dependent on patient background characteristics, device setting, and patient management protocol [30]. In general, LV unloading leads to a decrease in mean PAP, increase in right ventricular stroke work index (RVSWI), and improvement in tricuspid regurgitation [31]. Furthermore, Goodwin et al. showed that LVAD implantation corrected functional MR across all severity levels without any concomitant perioperative mitral valve intervention [32].
Continuous LV unloading leads to reverse remodeling with a decrease in LV dimension and increase in LV ejection fraction after several months following LVAD implantation, although the degree of change varies among patients [33,34]. Increase in peripheral circulation improves end-organ dysfunction [6], which can further improve patient hemodynamics. We previously reported that preoperative use of intra-aortic balloon pump improved end-organ function (assessed by total bilirubin and creatinine levels) in the month following LVAD implantation compared with background-matched patients without balloon pump [35]. Preoperative efforts to improve hemodynamics may be crucial to better outcomes following LVAD implantation.
Long-term hemodynamics
As overall duration of LVAD support continues to grow, assessment of long-term hemodynamics is of increasing importance. There are few studies reporting long-term trends in hemodynamics during LVAD support. Kalathiya et al. showed in a longitudinal study that CI declined in patients with HeartMate II after 2 years of continuous support, possibly due to an increase in afterload [36].
Our team recently showed that the hemodynamic profiles at set speed and in response to speed changes were preserved at a second test performed within 2 years following the first test in stable LVAD outpatients [37]. Each patient may thus have a “hemodynamic fingerprint,” and deviations from their baseline values may aid in diagnosis at times of clinical deterioration or device malfunction, as described in the following section. The further development of non-invasive or implantable hemodynamic monitoring devices may provide more information about changes in hemodynamics during long-term LVAD support.
Pulmonary hypertension
Patients with advanced HF often have World Health Organization class 2 PH with elevated PAP, high pulmonary vascular resistance (PVR), and wide transpulmonary artery pressure gradient (TPG) [38]. Several authors have shown that these parameters recover after LVAD implantation [39,40], although severely elevated PVR is a risk factor for RVF following LVAD implantation [41,42].
Zimpfer et al. reported that PVR recovered from 5.1 ± 2.8 Wood units to 2.0 ± 0.9 Wood units following LVAD implantation in 26 cardiac transplant candidates, and their peri-transplant mortality was comparable with 52 background-matched patients without PH [43]. In another study, PVR >3.0 Wood units normalized following LVAD implantation, and 1-year survival following cardiac transplantation was comparable to those without PH [44]. However, PVR was calculated as the TPG divided by the CO. Thus, decreases in PVR during LVAD support may reflect the enhanced CO more than changes in the pulmonary vasculature. Whether there is a reduction in the TPG following LVAD implantation remains unclear.
Data from Columbia University also showed a reduction in PVR following LVAD implantation. However, the in-hospital mortality was 3-fold higher in patients with elevated preoperative PVR (≥5 Wood units) compared to the low PVR group (<5 Wood units) [45]. The authors hypothesized that the reversal of pulmonary vascular remodeling was heterogeneous or incomplete despite normalization of PVR. Alternate parameters may be more useful to assess the severity of PH during LVAD support.
Hemodynamic changes at specific conditions
Hemodynamic assessment can also provide diagnostic information about the mechanisms of LVAD complications and assist with management decisions.
Right ventricular failure
RVF is a severe early complication following LVAD implantation, and is typically related to preoperative risk factors [46–50]. Acute RVF is characterized by the Interagency Registry for Mechanically Assisted Circulatory Support as elevation of CVP >16 mmHg, and its manifestations, including edema, ascites, and worsening hepatic or renal dysfunction [51]. Severity of RVF is further defined based on the duration of post-operative inotropes, use of inhaled nitric oxide or requirement for RV mechanical support.
Cordtz et al. observed less increase in CI and greater decrease in RVSWI immediately following HeartMate II implantation in patients who developed RVF compared to those who did not [52]. Repeat hemodynamic assessment can be used to identify RVF as a cause of persistent HF symptoms in patients on long-term support. Chronic RVF is associated with frequent hospitalizations, diuretic intolerance, renal and liver failure, need for inotropes, and increased mortality [53–56]. Precise hemodynamic mechanisms of chronic RVF warrant further investigation.
Aortic insufficiency
Aortic insufficiency (AI) is a common complication during LVAD support [57–60]. In the largest series to date, we reported that the freedom from greater than mild AI at 1 year was 77.6 ± 4.2%, and that at least moderate AI was to expected develop in 37.6 ± 13.3% of LVAD patients after 3 years [57]. The impact of AI on morbidity and mortality remains controversial [58–60], but understanding of the hemodynamic mechanism of AI may lead to improved clinical management.
In an animal model, CVP, mean left atrial pressure, and LV end-diastolic pressure were higher in animals with AI during EVAHEART support [61]. Systemic flow did not improve at incremental LVAD speeds, possibly due to progressive worsening of AI. We recently reported in patients with HeartMate II or HVAD LVADs that those with at least mild AI had higher CVP and PCWP and lower pulmonary artery pulsatility index (PAPi) compared to those without AI [62]. However, incremental increase of LVAD speeds led to normalization of filling pressures and improvement of CO at the expense of increased AI severity.
Implication of ramp test
Clinical benefit of ramp test
The clinical uses of ramp tests are manifold, ranging from troubleshooting of device malfunction to optimizing device settings.
One of the major indications for ramp testing is to diagnose LVAD flow obstruction and thrombosis [63]. An LV end-diastolic dimension slope less than an absolute value of 0.16 during ramp test is a strong predictor of thrombosis in HeartMate II patients [24], but this finding was not replicated in HVAD patients [25]. Whether earlier detection of obstruction to flow can be provided with addition of hemodynamic data remains uncertain.
The utility of ramp testing extends to clinically stable patients following LVAD implantation. Many patients have abnormal hemodynamics at set speed, despite reporting freedom from HF symptoms [8]. The hemodynamic profile obtained during ramp testing can be used to adjust medications with the goal of shifting hemodynamics into the normal range [37]. Although further studies are warranted, trends in hemodynamic parameters during speed changes may also have clinical implications. Jung et al. showed that a decrease in PCWP per ΔRPM during ramp test following HeartMate II implantation was related to lower New York Heart Association (NYHA) class. CO increase per ΔRPM was correlated with better quality of life [64].
Prognostic impact of hemodynamic optimization
At the conclusion of the ramp test, hemodynamic optimization is attempted. The optimization of LVAD speeds offers many theoretical benefits, although there are few published data thus far.
Data on long-term prognosis with speed optimization are emerging. We prospectively followed an observational cohort of 62 LVAD patients for 2 years after attempted hemodynamic optimization with an invasive ramp test. HF readmission rates were significantly lower in patients with optimized hemodynamics compared with those whose hemodynamics could not be optimized (0.22 events/year vs. 0.36 events/year) [65]. The efficacy of speed optimization on RV function was also reported in a small number of HVAD patients. At 3 months following speed optimization by echocardiographic ramp test, RV fractional area change and RV longitudinal peak systolic strain were improved, along with a significant decrease in N-terminal pro-brain natriuretic peptide level [66]. Whether hemodynamic optimization is associated with prevention of chronic RVF and better survival remains unknown.
We also showed that hemodynamic optimization was achieved irrespective of the existence of AI, whereas the degree of AI worsened at incremental LVAD speeds [62]. Future prospective studies should investigate whether hemodynamic normalization or intermittent AV opening should be the primary goal of ramp testing.
Decoupling between dPAP and PCWP in LVAD patients
Decoupling is defined as the difference between diastolic PAP and PCWP, and is an index of pulmonary vascular disease. We recently showed that decoupling of >5 mmHg was frequently observed following LVAD implantation, irrespective of the presence of preoperative PH. Excessive decoupling was a strong prognostic predictor of outcomes compared with other indices of PH such as PVR. Normalization of decoupling following a hemodynamic ramp test was associated with lower HF readmission rates [67]. The decoupling itself was not a target of optimization in this study, and the prognostic impact of aggressive normalization of decoupling during ramp test requires further study.
Future perspectives
Hemodynamic evaluation during stable conditions and in response to speed change with ramp tests remains a relatively understudied and underutilized tool in the management of LVAD patients. Investigation into the long-term benefits of ramp tests on survival, HF readmission, and other comorbidities is warranted.
A protocol for ramp testing should become widely established. The first trial comparing echocardiographic ramp test to invasive hemodynamic ramp test is underway. The Ramp-It-Up study is a multicenter trial that randomizes HVAD patients to LVAD speed optimization with echocardiographic ramp study alone or with an invasive hemodynamic ramp study. The patients are being followed for six months, and the primary outcomes are HF readmission, 6-min walk test distance, NYHA classification, and quality of life measures.
Finally, the clinical implications of ramp tests should be compared and validated between Japan and the USA, as there are many differences in the populations, such as genetics, body habits, and dietary habits that may affect study results [68].
Conclusions
Hemodynamic assessment is a vital component of the clinical assessment of patients on LVAD support. In particular, echocardiographic and hemodynamic ramp tests provide us with a useful tool to optimize hemodynamics in this population. Hemodynamic optimization may prove to be a crucial strategy in improving clinical outcomes during LVAD support.
Acknowledgments
Disclosures statement
Teruhiko Imamura receives Postdoctoral Fellowship for Research Abroad of Japan Society for the Promotion of Science. Nir Uriel receives grant support from Abbott and Medtronic.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 4.Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, et al. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95(4):1276–81. doi: 10.1016/j.athoracsur.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339(21):1522–33. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 6.Imamura T, Kinugawa K, Shiga T, Endo M, Kato N, Inaba T, et al. Preoperative levels of bilirubin or creatinine adjusted by age can predict their reversibility after implantation of left ventricular assist device. Circ J. 2013;77(1):96–104. doi: 10.1253/circj.cj-12-0686. [DOI] [PubMed] [Google Scholar]
- 7.Nassif ME, Spertus JA, Jones PG, Fendler TJ, Allen LA, Grady KL, et al. Changes in disease-specific versus generic health status measures after left ventricular assist device implantation: insights from INTERMACS. J Heart Lung Transplant. 2017;36:1243–9. doi: 10.1016/j.healun.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, et al. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016;4(3):208–17. doi: 10.1016/j.jchf.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Suwa H, Seguchi O, Fujita T, Murata Y, Hieda M, Watanabe T, et al. Paracorporeal ventricular assist device as a bridge to transplant candidacy in the era of implantable continuous-flow ventricular assist device. J Artif Organs. 2014;17(1):16–22. doi: 10.1007/s10047-013-0731-3. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Yamazaki K, Nishinaka T, Ichihara Y, Ono M, Kyo S, et al. Post-approval study of a highly pulsed, low-shear-rate, continuous-flow, left ventricular assist device, EVAHEART: a Japanese multicenter study using J-MACS. J Heart Lung Transplant. 2014;33(6):599–608. doi: 10.1016/j.healun.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Zucchetta F, Tarzia V, Bottio T, Gerosa G. The Jarvik-2000 ventricular assist device implantation: how we do it. Ann Cardiothorac Surg. 2014;3(5):525–31. doi: 10.3978/j.issn.2225-319X.2014.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC, Jr, Colombo PC, et al. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376(5):440–50. doi: 10.1056/NEJMoa1610426. [DOI] [PubMed] [Google Scholar]
- 13.Pektok E, Demirozu ZT, Arat N, Yildiz O, Oklu E, Eker D, et al. Remote monitoring of left ventricular assist device parameters after HeartAssist-5 implantation. Artif Organs. 2013;37(9):820–5. doi: 10.1111/aor.12144. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 15.Saeed O, Jermyn R, Kargoli F, Madan S, Mannem S, Gunda S, et al. Blood pressure and adverse events during continuous flow left ventricular assist device support. Circ Heart Fail. 2015;8(3):551–6. doi: 10.1161/CIRCHEARTFAILURE.114.002000. [DOI] [PubMed] [Google Scholar]
- 16.Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41(10):1797–804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 17.Anyanwu E, Bhatia A, Tehrani DM, Deshmukh A, Rodgers D, Adatya S, et al. The accuracy of physical exam compared to RHC in LVAD patients. J Heart Lung Transplant. 2017;36(4 Suppl):S341–2. [Google Scholar]
- 18.Estep JD, Vivo RP, Krim SR, Cordero-Reyes AM, Elias B, Loebe M, et al. Echocardiographic evaluation of hemodynamics in patients with systolic heart failure supported by a continuous-flow LVAD. J Am Coll Cardiol. 2014;64(12):1231–41. doi: 10.1016/j.jacc.2014.06.1188. [DOI] [PubMed] [Google Scholar]
- 19.Grinstein J, Rodgers D, Kalantari S, Sayer G, Kim GH, Sarswat N, et al. HVAD waveform analysis as a noninvasive marker of pulmonary capillary wedge pressure: a first step toward the development of a smart left ventricular assist device pump. ASAIO J. 2017 doi: 10.1097/MAT.0000000000000604. http://dx.doi.org/10.1097/MAT.0000000000000604 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 20.Lai JV, Muthiah K, Macdonald PS, Jansz P, Hayward CS. Estimation of left ventricular assist device pre-load using pump flow waveform analysis. J Heart Lung Transplant. 2017;36(2):240–2. doi: 10.1016/j.healun.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 22.Amir O, Azzam ZS, Gaspar T, Faranesh-Abboud S, Andria N, Burkhoff D, et al. Validation of remote dielectric sensing (ReDS) technology for quantification of lung fluid status: comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int J Cardiol. 2016;221:841–6. doi: 10.1016/j.ijcard.2016.06.323. [DOI] [PubMed] [Google Scholar]
- 23.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60(18):1764–75. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uriel N, Levin AP, Sayer GT, Mody KP, Thomas SS, Adatya S, et al. Left ventricular decompression during speed optimization rampsin patients supported by continuous-flow left ventricular assist devices: device-specific performance characteristics and impact on diagnostic algorithms. J Card Fail. 2015;21(10):785–91. doi: 10.1016/j.cardfail.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Uriel N, Adatya S, Maly J, Kruse E, Rodgers D, Heatley G, et al. Clinical hemodynamic evaluation of patients implanted with a fully magnetically levitated left ventricular assist device (HeartMate 3) J Heart Lung Transplant. 2017;36(1):28–35. doi: 10.1016/j.healun.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Sayer G, Jeevanandam V, Ota T, Uriel N. Invasive hemodynamic echocardiographic ramp test in the HeartAssist5 LVAD: insights into device performance. ASAIO J. 2017;63(2):e10–2. doi: 10.1097/MAT.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto Y, Fujita T, Fukushima S, Hata H, Shimahara Y, Kume Y, et al. Comparison of hemodynamic performance and clinical results with EVA-HEART versus HeartMate II. ASAIO J. 2017;63(5):562–7. doi: 10.1097/MAT.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 29.Giridharan GA, Koenig SC, Soucy KG, Choi Y, Pirbodaghi T, Bartoli CR, et al. Left ventricular volume unloading with axial and centrifugal rotary blood pumps. ASAIO J. 2015;61(3):292–300. doi: 10.1097/MAT.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 30.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Atluri P, Fairman AS, MacArthur JW, Goldstone AB, Cohen JE, Howard JL, et al. Continuous flow left ventricular assist device implant significantly improves pulmonary hypertension, right ventricular contractility, and tricuspid valve competence. J Card Surg. 2013;28(6):770–5. doi: 10.1111/jocs.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodwin M, Nemeh HW, Borgi J, Paone G, Morgan JA. Resolution of mitral regurgitation with left ventricular assist device support. Ann Thorac Surg. 2017;104(3):811–8. doi: 10.1016/j.athoracsur.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Morgan JA, Brewer RJ, Nemeh HW, Murthy R, Williams CT, Lanfear DE, et al. Left ventricular reverse remodeling with a continuous flow left ventricular assist device measured by left ventricular end-diastolic dimensions and severity of mitral regurgitation. ASAIO J. 2012;58(6):574–7. doi: 10.1097/MAT.0b013e31826e4267. [DOI] [PubMed] [Google Scholar]
- 34.Drakos SG, Wever-Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, et al. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol. 2013;61(19):1985–94. doi: 10.1016/j.jacc.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imamura T, Kinugawa K, Nitta D, Hatano M, Kinoshita O, Nawata K, et al. Prophylactic intra-aortic balloon pump before ventricular assist device implantation reduces perioperative medical expenses and improves postoperative clinical course in INTERMACS profile 2 patients. Circ J. 2015;79(9):1963–9. doi: 10.1253/circj.CJ-15-0122. [DOI] [PubMed] [Google Scholar]
- 36.Kalathiya RJ, Houston BA, Chaisson JM, Grimm JC, Stevens GR, Sciortino CM, et al. Cardiac index declines during long-term left ventricular device support. Artif Organs. 2016;40(12):1105–12. doi: 10.1111/aor.12733. [DOI] [PubMed] [Google Scholar]
- 37.Imamura T, Burkhoff D, Rodgers D, Atadya S, Sarswat N, Kim G, et al. Repeated ramp tests on stable LVAD patients reveal patient-specific hemodynamic fingerprint. ASAIO J. 2017 doi: 10.1097/MAT.0000000000000705. http://dx.doi.org/10.1097/MAT.0000000000000705 [Epub ahead of print] [DOI] [PubMed]
- 38.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 39.Pauwaa S, Bhat G, Tatooles AJ, Aggarwal A, Martin M, Kumar A, et al. How effective are continuous flow left ventricular assist devices in lowering high pulmonary artery pressures in heart transplant candidates? Cardiol J. 2012;19(2):153–8. doi: 10.5603/cj.2012.0027. [DOI] [PubMed] [Google Scholar]
- 40.Kutty RS, Parameshwar J, Lewis C, Catarino PA, Sudarshan CD, Jenkins DP, et al. Use of centrifugal left ventricular assist device as a bridge to candidacy in severe heart failure with secondary pulmonary hypertension. Eur J Cardiothorac Surg. 2013;43(6):1237–42. doi: 10.1093/ejcts/ezs678. [DOI] [PubMed] [Google Scholar]
- 41.Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010;105(7):1030–5. doi: 10.1016/j.amjcard.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Imamura T, Kinugawa K, Kinoshita O, Nawata K, Ono M. High pulmonary vascular resistance in addition to low right ventricular stroke work index effectively predicts biventricular assist device requirement. J Artif Organs. 2016;19(1):44–53. doi: 10.1007/s10047-015-0867-4. [DOI] [PubMed] [Google Scholar]
- 43.Zimpfer D, Zrunek P, Sandner S, Schima H, Grimm M, Zuckermann A, et al. Post-transplant survival after lowering fixed pulmonary hypertension using left ventricular assist devices. Eur J Cardiothorac Surg. 2007;31(4):698–702. doi: 10.1016/j.ejcts.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 44.Alba AC, Rao V, Ross HJ, Jensen AS, Sander K, Gustafsson F, et al. Impact of fixed pulmonary hypertension on post-heart transplant outcomes in bridge-to-transplant patients. J Heart Lung Transplant. 2010;29(11):1253–8. doi: 10.1016/j.healun.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Tsukashita M, Takayama H, Takeda K, Han J, Colombo PC, Yuzefpolskaya M, et al. Effect of pulmonary vascular resistance before left ventricular assist device implantation on short- and long-term post-transplant survival. J Thorac Cardiovasc Surg. 2015;150(5):1352–60. 61.e1–2. doi: 10.1016/j.jtcvs.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail. 2016;22(2):110–6. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2016;35(1):67–73. doi: 10.1016/j.healun.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Raina A, Seetha Rammohan HR, Gertz ZM, Rame JE, Woo YJ, Kirkpatrick JN. Postoperative right ventricular failure after left ventricular assist device placement is predicted by preoperative echocardiographic structural, hemodynamic, and functional parameters. J Card Fail. 2013;19(1):16–24. doi: 10.1016/j.cardfail.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Mohamedali B, Doukky R, Karavalos K, Avery E, Bhat G. Mean arterial pressure to central venous pressure ratio: a novel marker for right ventricular failure after left ventricular assist device placement. J Card Fail. 2017;23(6):446–52. doi: 10.1016/j.cardfail.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Kashiyama N, Toda K, Nakamura T, Miyagawa S, Nishi H, Yoshikawa Y, et al. Evaluation of right ventricular function using liver stiffness in patients with left ventricular assist device. Eur J Cardiothorac Surg. 2017;51(4):715–21. doi: 10.1093/ejcts/ezw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34(9):1123–30. doi: 10.1016/j.healun.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Cordtz J, Nilsson JC, Hansen PB, Sander K, Olesen PS, Boesgaard S, et al. Right ventricular failure after implantation of a continuous-flow left ventricular assist device: early haemodynamic predictors. Eur J Cardiothorac Surg. 2014;45(5):847–53. doi: 10.1093/ejcts/ezt519. [DOI] [PubMed] [Google Scholar]
- 53.Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, et al. Incidence and clinical significance of late right heart failure during continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2015;34(8):1024–32. doi: 10.1016/j.healun.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Baran DA, Mehra MR. Late-onset right heart failure after left ventricular assist device implant: quo vadis? J Heart Lung Transplant. 2017;36(1):26–7. doi: 10.1016/j.healun.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Rich JD, Gosev I, Patel CB, Joseph S, Katz JN, Eckman PM, et al. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J Heart Lung Transplant. 2017;36(1):50–8. doi: 10.1016/j.healun.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Imamura T, Kinugawa K, Kato N, Muraoka H, Fujino T, Inaba T, et al. Late-onset right ventricular failure in patients with preoperative small left ventricle after implantation of continuous flow left ventricular assist device. Circ J. 2014;78(3):625–33. doi: 10.1253/circj.cj-13-1201. [DOI] [PubMed] [Google Scholar]
- 57.Jorde UP, Uriel N, Nahumi N, Bejar D, Gonzalez-Costello J, Thomas SS, et al. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circ Heart Fail. 2014;7(2):310–9. doi: 10.1161/CIRCHEARTFAILURE.113.000878. [DOI] [PubMed] [Google Scholar]
- 58.Cowger JA, Aaronson KD, Romano MA, Haft J, Pagani FD. Consequences of aortic insufficiency during long-term axial continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2014;33(12):1233–40. doi: 10.1016/j.healun.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Toda K, Fujita T, Domae K, Shimahara Y, Kobayashi J, Nakatani T. Late aortic insufficiency related to poor prognosis during left ventricular assist device support. Ann Thorac Surg. 2011;92(3):929–34. doi: 10.1016/j.athoracsur.2011.04.115. [DOI] [PubMed] [Google Scholar]
- 60.Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, Hatano M, et al. Aortic insufficiency in patients with sustained left ventricular systolic dysfunction after axial flow assist device implantation. Circ J. 2015;79(1):104–11. doi: 10.1253/circj.CJ-14-0944. [DOI] [PubMed] [Google Scholar]
- 61.Iizuka K, Nishinaka T, Takewa Y, Yamazaki K, Tatsumi E. The influence of pump rotation speed on hemodynamics and myocardial oxygen metabolism in left ventricular assist device support with aortic valve regurgitation. J Artif Organs. 2017 doi: 10.1007/s10047-017-0960-y. http://dx.doi.org/10.1007/s10047-017-0960-y [Epub ahead of print] [DOI] [PubMed]
- 62.Sayer G, Sarswat N, Kim GH, Adatya S, Medvedofsky D, Rodgers D, et al. The hemodynamic effects of aortic insufficiency in patients supported with continuous-flow left ventricular assist devices. J Card Fail. 2017;23(7):545–51. doi: 10.1016/j.cardfail.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen AB, Uriel N, Adatya S. New challenges in the treatment of patients with left ventricular support: LVAD thrombosis. Curr Heart Fail Rep. 2016;13(6):302–9. doi: 10.1007/s11897-016-0310-z. [DOI] [PubMed] [Google Scholar]
- 64.Jung MH, Gustafsson F, Houston B, Russell SD. Ramp study hemodynamics, functional capacity, and outcome in heart failure patients with continuous-flow left ventricular assist devices. ASAIO J. 2016;62(4):442–6. doi: 10.1097/MAT.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 65.Sarswat N, Atadya S, Sayer G, Kim G, Ota T, Jeevanandam V, et al. Outcomes with implementation of algorithmic hemodynamic ramps in patients with continuous flow LVADs. J Heart Lung Transplant. 2016;35(4 Suppl):S389–90. [Google Scholar]
- 66.Couperus LE, Delgado V, Khidir MJH, Vester MPM, Palmen M, Fiocco M, et al. Pump speed optimization in stable patients with a left ventricular assist device. ASAIO J. 2017;63(3):266–72. doi: 10.1097/MAT.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 67.Imamra T, Chung B, Nguyen A, Rodgers D, Sayer G, Adatya S, et al. Decoupling between diastolic pulmonary artery pressure and pulmonary capillary wedge pressure as a prognostic factor after continuous flow ventricular assist device implantation. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.003882. pii:e003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ono M, Sawa Y, Nakatani T, Tominaga R, Matsui Y, Yamazaki K, et al. Japanese multicenter outcomes with the HeartMate II left ventricular assist device in patients with small body surface area. Circ J. 2016;80(9):1931–6. doi: 10.1253/circj.CJ-16-0203. [DOI] [PubMed] [Google Scholar]


