Abstract
Objective
To examine trends in use of systemic disease-modifying antirheumatic drugs (DMARD) among patients with psoriatic arthritis (PsA) in the U.S.
Methods
Using claims data (2004–2015) from a large U.S. commercial healthplan, we identified patients with PsA who initiated DMARDs. We examined baseline patient characteristics and initial treatment patterns. We then assessed changes in the DMARD regimen over the 12-month period after the 1st DMARD initiation date. Poisson regression estimated age- and sex-adjusted incidence rates of treatment changes in each calendar year.
Results
We identified 9,222 PsA patients who initiated DMARDs (42.8% biologic and 57.2% conventional synthetic). Biologic DMARD (bDMARD) initiators were younger than conventional synthetic DMARD (csDMARD) initiators (mean age±sd: 48±13 vs. 52±14 years) and had generally fewer comorbidities, but a higher proportion of bDMARD initiators received non-systemic treatments for psoriasis at baseline. Methotrexate was the most frequently used DMARD, constituting 80.6% of csDMARD initiation. Etanercept (49.1%) was the most commonly prescribed bDMARD followed by adalimumab (34.4%). During the 12-month followup after the 1st DMARD initiation, 20.1% bDMARD and 31.1% csDMARD initiators had their initial DMARD regimen modified, with an increasing trend in treatment modifications over the 11-year study period (p=0.03). Overall, 5.3% of patients had treatment discontinuation, but the rates decreased over time (p<0.001).
Conclusions
In this large cohort of PsA patients initiated on DMARDs, over 40% were treated with a bDMARD. We found an increasing trend in treatment modification after the initial DMARD use and a decreasing trend in complete DMARD discontinuation over the past decade.
Keywords: psoriatic arthritis, DMARD, biological therapy
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis primarily occurring in patients with psoriasis (1, 2). With an annual incidence of approximately 3.6 –7.2 cases per 100,000, about 0.25% of the U.S. population suffer from PsA (3). It primarily affects patients with psoriasis with a prevalence of up to 36% (4). PsA is a complex disease affecting several musculoskeletal and extraarticular organs, and the commonly defined clinical domains include peripheral arthritis, axial disease, enthesitis, dactylitis, skin disease, and nail disease. Treatment choices for PsA depend on the clinical domains involved, disease severity, and co-morbidities present (1, 2).
Patients with PsA suffer significant functional impairment with progressive joint damage, and treatment is aimed at controlling disease activity to improve patient outcomes and prevent further disease progression (2). Several studies report a benefit of early and intensive treatment on the long-term outcome of PsA including a recent trial attends to a tight control approach with a target of remission or minimal disease activity (2, 5, 6, 7). The recent and significant developments in PsA treatment allowed an array of pharmacotherapy options from conventional synthetic DMARDs (csDMARD) such as methotrexate to biological DMARDs (bDMARD) including TNF-α inhibitors, an interleukin-12 and 23 antagonist, and interleukin-17 monoclonal antibodies to be available in managing PsA (1, 2, 8). These agents have shown to exert differential effects on treating PsA affecting varying clinical domains involved (1, 2). Despite these developments, patients are undertreated for their psoriatic diseases with 22% of patients with severe psoriasis being treated with topical agents alone (9–11). The persisting perception of PsA following a relatively benign course, potential side effects, patients’ tolerance to medications, and patient affordability may attribute to such under-treatment (5, 10). Furthermore, with a wide spectrum and heterogeneous nature of clinical manifestations of PsA as well as limited evidence of differential efficacy of various treatments available, making an informed treatment choice continues to remain challenging (2).
With expanding therapeutic options and shifting treatment recommendations, it is critical to evaluate the utilization patterns of these medications and treatment trends among patients with PsA in routine care settings over the past decade to assess the extent of use of available treatments. Thus, in this study, we sought to elucidate the patterns and time trends in utilization of systemic therapy in a contemporary cohort of commercially insured patients with PsA in the US. We aimed to determine the trends in systemic medication use and assess any trending changes over time while providing comprehensive descriptions of pharmacotherapy use patterns in patients with PsA.
METHODS
Study Design and data source
We conducted a cohort study using de-identified claims data from the Clinformatics™ Datamart (OptumInsight, Eden Prairie, MN) between July 1, 2004 and September 30, 2015. The Clinformatics™ Datamart (OptumInsight, Eden Prairie, MN) contains demographic data and longitudinal claims information including hospitalization, outpatient visits, procedures, and pharmacy dispensing, and plan information for all United Healthcare beneficiaries. The United Healthcare insures primarily working adults and their family members across the U.S. The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital. Patient informed consent was not required as the database was de-identified to protect subject confidentiality.
Study cohort
Patients aged ≥18 years who had at least one visit coded for PsA (ICD-9-CM 696.0) were eligible for the study cohort. To minimize potential misclassification of PsA, an additional visit coded for psoriasis (ICD-9-CM 696.1) preceding or on the date PsA diagnosis was required. Of these patients with PsA, we identified initiators of csDMARD (methotrexate, sulfasalazine, leflunomide, and cyclosporine) or bDMARD (etanercept, adalimumab, infliximab, certolizumab, golimumab, ustekinumab, and alefacept). To ensure that we only include new users of csDMARD or bDMARD, patients were required to have continuous enrollment for at least 6 months prior to the 1st dispensing of either csDMARD or bDMARD (i.e., index date). Follow-up started the day after the index date and ended on the first occurrence of any of the following censoring events: disenrollment from the health plan, death or the end of the 12-month follow-up or the end of study database (i.e., September 30, 2015).
Baseline characteristics and treatment initiation patterns
We assessed patient characteristics related to demographics, comorbidities, initial DMARD regimens and prescriber during the 6-month baseline period. We assumed that treatment was initiated by a specialist if patients’ most recent visit coded for PsA or psoriasis was to a rheumatologist or dermatologist prior to the index date.
Changes in the initial DMARD regimen
We examined the extent of modifications in their initial DMARD regimen during follow-up after the index date. Any changes in the initial DMARD regimen was defined as treatment modification. We estimated the time elapsed between the index date and treatment modification. Treatment discontinuation was defined as no more dispensing of any systemic DMARDs in 180 days following the end of days supply after the last DMARD prescription during follow-up. To minimize right censoring and allow for sufficient follow-up time, we assessed treatment modifications and trends in patients with index dates between July 1, 2004 and September 30, 2014. Also, to further assess any potential impact of right censoring on our results, these outcomes were assessed among patients who completed the follow-up as part of a sensitivity analysis.
Statistical analysis
Descriptive statistics illustrated patient characteristics and treatment patterns. Continuous variables were presented as means (SD) or medians (25th, 75th percentiles), and categorical variables were presented as frequencies and percentages. Baseline characteristics and initial treatment patterns were presented separately for bDMARD and csDMARD initiators. A generalized linear model with Poisson distribution was used to assess the rates of treatment changes and yearly trends. Age- and sex-adjusted rates (aIR) were estimated with an offset of log-transformed person-year accounting for the varying amounts of time observed. The aIR of treatment modification and discontinuation were reported for the overall cohort as well as stratified by bDMARDs and csDMARDs. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary NC).
RESULTS
Baseline characteristics and treatment initiation patterns
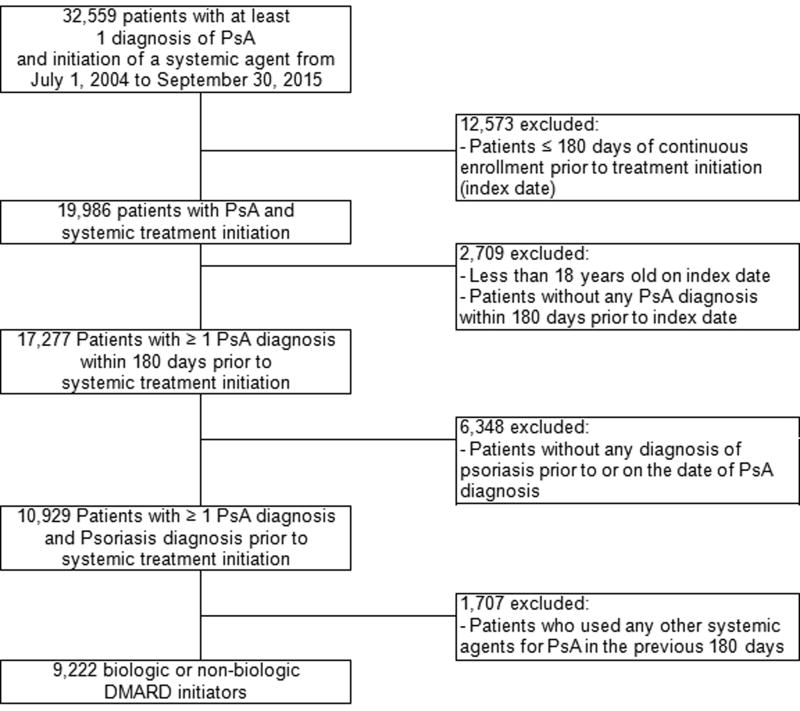
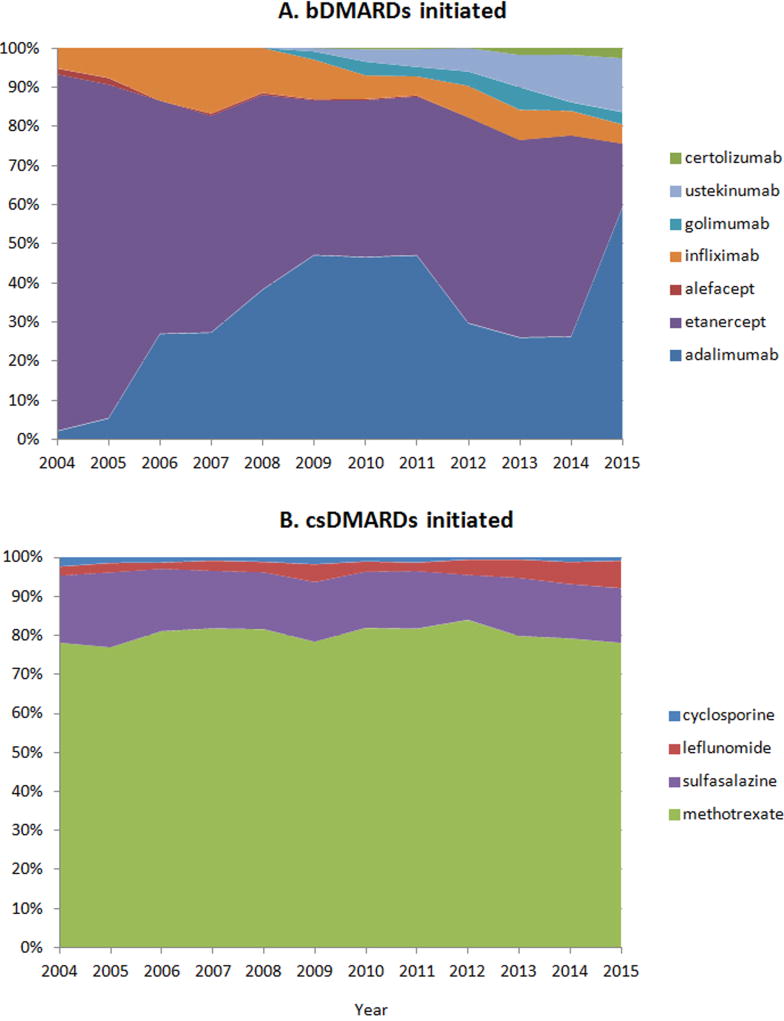
A total of 9,222 patients with PsA treated with a systemic DMARD were included in the analyses (Figure 1). Among these patients, csDMARDs were used as the first-line treatment more frequently than bDMARDs, with 5,276 (57.2%) initiating csDMARDs and 3,946 (42.8%) initiating bDMARDs. The proportion of patients initiating bDMARDs fluctuated throughout the study period, ranging from 37.8 to 51.3%. Patients initiating bDMARDs were younger with the mean age (SD) of 48 (13) years compared to patients initiating csDMARDs with the mean age (SD) of 52 (14) years and had overall less comorbidity burden. However, bDMARD initiators received more extensive treatment for psoriasis at baseline including topical vitamin D analogs and phototherapy (Table 1). Systemic DMARDs were initiated mostly by specialists; 80.2% of bDMARD and 72.8% of csDMARD initiation were prescribed by specialists (Table 1 presents further breakdowns of prescribing physicians’ specialties). Methotrexate was the most commonly initiated DMARD comprising more than 80% of all csDMARD initiations throughout the study period. Among bDMARDs, adalimumab and etanercept were most frequently used (34.4% and 49.1% of all biologic initiation, respectively) with adalimumab use increasing substantially over time (Figure 2). The use of etanercept appears to decrease since the approval of adalimumab in 2005 with the trends in use of these two agents varying between 2012 and 2015. The proportion of patients with PsA initiating ustekinumab gradually increased to 13.7% of all bDMARD initiations in 2015 since its approval for psoriasis in 2009 and further expanded after its approval for PsA in 2013.
Figure 1.
Table 1.
Baseline characteristics of the study population
| Biologic DMARD initiators (n=3,946) |
Conventional synthetic DMARD initiators (n=5,276) |
|
|---|---|---|
|
| ||
| mean ± SD or % | mean ± SD or % | |
| Demographics | ||
| Age, years | 47.8 ± 12.6 | 52.1 ± 13.8 |
| Female | 47.6 | 55.3 |
| Healthcare Utilization | ||
| Number of physician office visits | 5.8 ± 4.1 | 6.3 ± 4.0 |
| Number of hospitalization | 0.08 ± 0.3 | 0.07 ± 0.3 |
| Number of ER visits | 0.2 ± 0.6 | 0.2 ± 0.6 |
| Comorbidities | ||
| Cancer | 10.0 | 11.3 |
| Hypertension | 30.0 | 36.0 |
| Diabetes | 15.2 | 16.8 |
| Coronary artery disease | 4.4 | 6.6 |
| Ischemic stroke | 0.8 | 1.0 |
| COPD | 2.5 | 3.2 |
| Asthma | 4.5 | 4.8 |
| Chronic liver disease | 5.7 | 3.2 |
| Smoking | 7.8 | 8.9 |
| Obesity | 7.3 | 8.6 |
| Depression | 10.3 | 10.8 |
| Chronic kidney disease | 0.2 | 0.3 |
| Combined comorbidity score* | − 0.07 ± 0.6 | − 0.1 ± 0.7 |
| Medication Use | ||
| ACE inhibitors | 13.8 | 16.7 |
| Angiotensin II receptor blockers | 8.7 | 9.4 |
| Beta-blockers | 11.2 | 13.0 |
| Calcium channel blockers | 8.0 | 9.4 |
| Thiazides | 12.8 | 15.8 |
| Loop diuretics | 3.4 | 5.3 |
| Other diuretics | 2.6 | 3.8 |
| Oral anticoagulants | 1.4 | 2.2 |
| Statins | 18.0 | 22.8 |
| Antidepressants | 22.2 | 24.4 |
| Benzodiazepines | 13.5 | 11.7 |
| Non-selective NSAIDs | 34.2 | 48.6 |
| Coxibs | 6.5 | 8.3 |
| Opioids | 29.8 | 32.5 |
| Oral glucocorticoids | 23.0 | 37.5 |
| Topical steroids | 58.6 | 59.6 |
| Topical vitamin D | 22.3 | 17.2 |
| Phototherapy | 5.0 | 3.3 |
| Treatment Initiation by Specialists | 80.2 | 72.8 |
| Rheumatologists | 46.8 | 61.1 |
| Dermatologists | 33.4 | 11.7 |
The range of combined comorbidity score is −2 to 26
ER=emergency room, COPD= chronic obstructive pulmonary disease, ACE= angiotensin converting enzyme, NSAID= non-steroid antiinflammatory drug
Figure 2.
Changes in the initial DMARD regimen
A total of 8,101 patients, 3,476 bDMARD and 4,625 csDMARD initiators, were included in the final analyses of assessing treatment changes; 2,137 patients – 20.1% of bDMARD and 31.1% of csDMARD initiators – had their initial DMARD treatment modified during the 1-year after the index date. The median (IQR) time to treatment modification after initiating a bDMARD was 148 days (76–229) while it occurred more rapidly among patients initiating csDMARDs with the median (IQR) time of 102 days (42–184). Initiating a bDMARD, etanercept or adalimumab in particular, following the initial treatment of methotrexate was the most commonly observed modification pattern occurring in 15.5% of all csDMARD initiators. Among patients with initial treatment with bDMARDs, the most frequent therapy modification involved the addition of methotrexate, which was observed in 6.7% of bDMARD initiators, followed by the switches between etanercept and adalimumab (5.9%). An overall crude incidence rate (95% CI) of treatment modification was 37.1 cases (35.6–38.7) per 100 person-years. Treatment modification after initiating systemic therapy occurred more frequently after initiating a csDMARD with the aIR (95% CI) of 39.3 cases (36.9–41.9) per 100 person-years compared to the aIR (95% CI) of 21.1 (19.4–23.0) per 100 person-years among bDMARD initiators with an overall increasing yearly trend in therapy modifications (p-value for trend =0.03). Further details on the trend analyses are provided in Supplementary Table 1 and Supplementary Figure 1.
Treatment discontinuation occurred in 5.3% of all patients with an overall crude incidence rate (95% CI) of 6.2 cases (5.7–6.9) per 100 person-years. A higher rate of discontinuation after initiating a csDMARD was observed compared to a bDMARD with the aIR (95% CI) of 6.9 (6.1–7.8) per 100 person-years and 5.6 (4.7–6.6) per 100 person-years, respectively. Rates of complete DMARD discontinuation in the first 12 month following the initial DMARD dispensing decreased (p-value for trend <0.001) over the 11-year study period (Supplementary Table 1 and Supplementary Figure 1). The results from a sensitivity analysis restricted to patients who completed the 1-year follow-up demonstrated consistent patterns (results not shown).
DISCUSSIONS
This cohort study evaluated pharmacologic treatment patterns involving systemic DMARD agents for PsA management over the past decade using a large U.S. commercial healthcare claims database. We observed that csDMARDs remained the preferred first-line systemic treatment initiated among patients with PsA while over 40% of treatment initiation involved bDMARDs during the 11-year study period. Several studies also examined PsA treatment patterns, and trends of initial systemic treatment use observed in our study were similar to the trends reported by the previous studies while our data extended to more recent years (9, 12–13). However, the majority of these studies focused on the overall medication utilization patterns in this patient population unheeding of any changes occurring over time. In the last decade, substantial therapeutic advances have been made in the field of PsA management including many emerging treatment options with biologic agents, a better understanding of pathogenesis and accumulating clinical evidence defining a role of existing and newer compounds as well as evolving evidence- and consensus-based recommendations to attain the target of remission or low disease activity in treating PsA (1, 5, 8, 14). Also, there is growing evidence of differential effectiveness of csDMARDs and bDMARDs especially involving patient-centered and patient-reported outcomes aimed to reflect all dimensions of disease activity (2, 15). With the considerable clinical advancements, dynamic changes in treatment patterns were evident among patients with PsA over the past decade. Although methotrexate remained the mainstay of initial systemic DMARD, relatively rapid treatment modifications involving other treatment mostly including TNF-α inhibitors were apparent with such modification in treatment becoming more prevalent in recent years. We also observed overall improved persistence of systemic DMARDs in this patient population during the study period with consistently higher rates of abrupt treatment discontinuation observed in patients initiating csDMARDs compared to patients initiating bDMARDs.
A major strength of our study results is the use of a large, representative sample of PsA patients at potentially various stages of PsA among the commercially insured population. The inclusion of such heterogeneous population of patients with PsA using systemic agents allows examining the broad dimensions of medication use in PsA. Our study provides comprehensive and contemporary systemic treatment patterns in this patient population, an area where evidence has been scarce. In addition to presenting an overview of real-world medication use in PsA patients, the patterns observed in our study further provide a more insight into various aspects that may need to be considered when conducting observational studies on PsA treatment including confounding factors potentially affecting the treatment choice as well as changing patterns involving specific pharmacotherapy over the treatment course.
Several limitations of data we used, however, may impede interpretations of our results including a short duration of follow-up as long-term treatment patterns may differ. Also, the baseline period of 6 months required to obtain pre-index characteristics and medication use patterns may have been insufficient to capture all prescription and disease history. More notably, one of the major limitations was the lack of PsA severity measures in claims database. Our attempt was to assess the extent of disease severity using approximated information including several healthcare utilization measures and medication use for various comorbidities including prior treatment for psoriasis. These proxies, however, may not accurately reflect the actual disease progression in this population, and lacking information on the PsA severity as well as the clinical domains involved precludes a more detailed assessment of treatment patterns among PsA patients, and the patterns might substantially differ based on the domains affected and disease severity. Comorbidities is another vital aspect of PsA treatment. While our data is limited by small sample sizes in subgroups of patients with comorbidities preventing any meaningful assessment of treatment patterns involving specific comorbidities, further studies with larger cohorts may add significant understandings of medication use pertained to various comorbidities. Also, treatment discontinuation used to reflect patients’ overall persistence to DMARD therapy in this population may not be suffice to reveal more complex adherence-related behaviors in patients with PsA, and several other relevant measures such as proportion days covered or medication possession ratio may be used to better depict these behaviors. Additionally, while we recognize that the plan’s formulary requirements may affect treatment decision, the present study was unable to further delineate and account for various formulary requirements available by different coverage plans of United HealthCare insurance. Furthermore, extending the data to more recent years to include novel agents such as secukinumab and ixekizumab may reveal varying treatment patterns among this patient population.
In conclusion, among patients with PsA in a commercially insured population, initiation of csDMARDs, mainly methotrexate, appears the mainstay of first-line systemic treatment over the past decade. We also noted the use of bDMARD as the initial DMARD regimen in over 40% of patients with PsA who initiated a systemic treatment. With a significant advancement in PsA treatment and expanding pharmacotherapy options in the past decade, the rate of treatment modification has increased while the rate of complete cessation of systemic DMARD treatment has decreased. In a rapidly evolving field of PsA treatment, further studies that elucidate more comprehensive real-world trends in the use of available treatment including more recent novel therapies for PsA may be needed.
Supplementary Material
Significance and Innovations.
The patterns and secular trends of systemic therapy with DMARDs were assessed among patients with psoriatic arthritis in real-world settings.
Among commercially insured patients with psoriatic arthritis who were initiated on DMARDs in the U.S., over 40% were with a bDMARD.
With emerging treatment options for psoriatic arthritis over the past decade, an increasing trend in treatment modification after initiation of DMARDs was observed while a complete discontinuation of DMARDs decreased over the 11-year study period.
Acknowledgments
DHS is supported by the NIH grants K24 AR055989, P60 AR047782, U34 AR063911, and R01 HL119718.
Disclosures: This study has no specific funding. SC Kim has received research support to the Brigham and Women’s Hospital from Pfizer, Astra Zeneca, Bristol-Myers Squibb and Genentech.
Competing interests:
SCK has received research support from Lilly, Pfizer, Genentech, Bristol-Myers Squibb, Merck and AstraZeneca for unrelated studies.
DHS has received research support from Amgen, AstraZeneca, Genentech, Lilly and CORRONA, received royalties from UpToDate and served in unpaid roles in studies funded by Lilly, Novartis and Pfizer.
RJD has received research support from Merck.
JFM has received fees for his role as a consultant, advisor, and speaker for Biogen IDEC, AbbVie, Amgen, Eli Lilly, Novartis, Pfizer, Janssen, UCB, Kiniksa, Momenta and Mallinckrodt.
Footnotes
Authors’ contributions:
SCK and MPL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SCK is the guarantor for the study. All authors conceived and designed the study, analyzed and interpreted the data, and critically revised the manuscript for important intellectual content. MPL drafted the paper.
References
- 1.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–71. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 3.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:545–68. doi: 10.1016/j.rdc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaci D, Behrens F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69:729–35. doi: 10.1016/j.jaad.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Coates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O'Dwyer JL, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet. 2015;386:2489–98. doi: 10.1016/S0140-6736(15)00347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045–50. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]
- 7.Theander E, Husmark T, Alenius GM, Larsson PT, Teleman A, Geijer M, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA) Ann Rheum Dis. 2014;73:407–13. doi: 10.1136/annrheumdis-2012-201972. [DOI] [PubMed] [Google Scholar]
- 8.Ramiro S, Gaujoux-Viala C, Nam JL, Smolen JS, Buch M, Gossec L, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2014;73:529–35. doi: 10.1136/annrheumdis-2013-204575. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149:1180–5. doi: 10.1001/jamadermatol.2013.5264. [DOI] [PubMed] [Google Scholar]
- 10.Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46–54. doi: 10.1001/archdermatol.2009.319. [DOI] [PubMed] [Google Scholar]
- 11.Horn EJ, Fox KM, Patel V, Chiou CF, Dann F, Lebwohl M. Are patients with psoriasis undertreated? Results of National Psoriasis Foundation survey. J Am Acad Dermatol. 2007;57:957–62. doi: 10.1016/j.jaad.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Bonafede M, Fox KM, Watson C, Princic N, Gandra SR. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther. 2012;29:664–74. doi: 10.1007/s12325-012-0037-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HF, Gauthier G, Hiscock R, Curtis JR. Treatment patterns in psoriatic arthritis patients newly initiated on oral nonbiologic or biologic disease-modifying antirheumatic drugs. Arthritis Res Ther. 2014;16:420. doi: 10.1186/s13075-014-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarpa R, Peluso R, Atteno M, Manguso F, Spano A, Iervolino S, et al. The effectiveness of a traditional therapeutical approach in early psoriatic arthritis: results of a pilot randomised 6-month trial with methotrexate. Clin Rheumatol. 2008;27:823–6. doi: 10.1007/s10067-007-0787-7. [DOI] [PubMed] [Google Scholar]
- 15.Tillett W, Shaddick G, Jobling A, Askari A, Cooper A, Creamer P, et al. Effect of anti-TNF and conventional synthetic disease-modifying anti-rheumatic drug treatment on work disability and clinical outcome in a multicentre observational cohort study of psoriatic arthritis. Rheumatology. 2017;56:603–12. doi: 10.1093/rheumatology/kew433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.