Abstract
The aim of our study is to investigate the protective effect of Spirulina fusiformis against streptozotocin-induced diabetes in Wistar albino rats. Rats were divided into five groups: group I was normal control, group II was diabetic control (50 mg/kg b.w. of streptozotocin, i.p.), group III was Spirulina fusiformis (400 mg/kg b.w., p.o.) treated diabetic rats; group IV was Glibenclamide (0. 6 mg/kg b.w., p.o.) treated diabetic rats and group V was treated with Spirulina fusiformis (400 mg/kg b.w., p.o.) alone. There was significant elevation in the levels of blood glucose, serum lipid profile and serum renal markers (total protein, urea, creatinine and uric acid) in the diabetic rats. Also, diabetic rats showed significantly (P < 0.05) reduced antioxidant status (reduced levels of superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferase and reduced glutathione; increased levels of TBARS), impaired oral glucose tolerance and elevated HbA1C. Spirulina fusiformis was able to normalize the above mentioned parameters. Significant histopathological changes were found in the pancreas, liver and kidney sections of the diabetic control group while treatment with Spirulina fusiformis was able to minimize the extent of tissue damage. Current study shows that Spirulina fusiformis possesses significant antidiabetic and antihyperlipidemic effects in streptozotocin-induced diabetic rats by effectively reducing the rise in blood glucose levels and lipid profile.
Keywords: Spirulina fusiformis, Antidiabetic activity, Streptozotocin, Glibenclamide
Introduction
Diabetes mellitus (DM) is due to metabolic imbalance which is non-physiological (Machha et al. 2007). It is a worldwide health problem (Eliza et al. 2009) where 7% of the world’s adult population is affected by DM (Babu et al. 2013). DM is one of the leading causes of death in the global scenario (Devi et al. 2012). It is classified as defects in insulin secretion which is termed as type-1 (or) resistance to insulin action which is termed as type-2 (Sirasanagandla et al. 2013). DM is associated with hyperglycemia which would cause damage to the blood vessels, eyes, nerves and kidneys. DM will affect social, psychological and physical activities of the life. Some of the clinical manifestations of DM are polyuria, weight loss, blurring of vision and thirst. Chronic hyperglycemia may cause ketoacidosis or non-hyperosmolar coma which occurs in severe DM due to the absence of treatments (Devi et al. 2012). DM is characterized by an increase in triglyceride and low-density lipoprotein (LDL), but a decrease in high-density lipoprotein (Sadek et al. 2013). Glibenclamide (GLB) has the property of antihyperglycemic activity, which belongs to the sulphonyl urea class of antidiabetic medication. GLB is administrated along with the adjunct, which lowers the blood glucose level in DM patient (Sharma and Kar 2014). GLB binds and activates sulphonyl urea receptor 1, which regulates ATP-sensitive potassium channels in pancreatic β-cell. This results in membrane depolarization to open voltage-gated calcium channels, which increases intracellular calcium and stimulates insulin release. This also reduces glucose from the liver, which blocks the conversion of glycogen to glucose (Sharma and Kar 2014). DM patients are reported to have higher incidence of myocardial infarction than non-DM patients (Keidan et al. 2002). There are many adverse effects which are associated with the continuous use of such synthetic drugs.
Researchers around the globe currently focus on herbal and natural products for antidiabetic effects. Drugs from natural sources are more economical to the patients, which could be bought in their affordable rate (Eliza et al. 2009). Around 45,000 Indian plant species are known to have significant medicinal properties. Antidiabetic activity is one of the main medicinal properties of these herbal plants (Zheng et al. 2011). Spirulina fusiformis is a Cyanobacterium which has been used as a protein-rich food supplement with ethno pharmacological significance in both humans and animals (Sadek et al. 2017). This blue-green alga grows in a warm climate and is a rich source of minerals, vitamins and antioxidant pigments (Sabina et al. 2009). Spirulina is being investigated through various studies for its antidiabetic, immunomodulatory and antioxidant activities (Finamore et al. 2017; Gargouri et al. 2017).
Streptozotocin (STZ) is being used to induce both type I and type II DM. Multiple low doses of STZ induce type I DM while single dosage of STZ induces type II DM (Deeds et al. 2011). The current study involves the use of a single dose of STZ to achieve establish type II DM in the experimental rats. The aim of our investigation is to study the effects of Spirulina fusiformis on streptozotocin (STZ)-induced diabetes in rats.
Materials and methods
Experimental animals
Female Wistar albino rats with mean body weight 130 g were used in this study. The rats were obtained from VIT Animal house, VIT University, Vellore, Tamil Nadu, India. The rats were provided with pelleted commercial feed which was obtained from Hindustan Lever Ltd., Bangalore, India. Water and rat feed were provided ad libitum. The rats were cared and treated properly treated according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Chennai, India. The ethical clearance approval number for current study is VIT/IAEC/11th/October 10th/No. 25.
Dosage
Rats were kept in fasting condition for 12 h, after which diabetes was induced by intraperitoneal injection. STZ (50 mg/kg b.w.) was used to induce diabetes in rats. The progression of the disease was confirmed by blood glucose test. GLB (0. 6 mg/kg b.w.) was administrated orally and was used as the standard drug. Spirulina fusiformis (400 mg/kg b.w.) was administrated orally for 28 days (Chen et al. 2005; Erejuwa et al. 2011b; Martin and Sabina 2016).
Treatment design
The rats were divided into five groups of six rats each. Group 1 rats were considered as normal control. Group 2 rats served as diabetic control, which were administrated with a single dose of STZ (50 mg/kg b.w.). Group 3 includes Spirulina fusiformis (400 mg/kg b.w.; p.o.) treated STZ-induced diabetic rats. Group 4 was GLB (0. 6 mg/kg b.w.) treated diabetic rats. Spirulina fusiformis alone treated rats represented Group 5. The treatment duration was 28 days.
Estimation of glucose
Blood glucose was tested with the help of a glucometer (One Touch Ultra-2, India) at regular intervals of 7 days by tail vein puncture (Fujiwara et al. 1988).
Sacrifice and storage of sample
After killing, blood was collected by cardiac puncture technique and serum was separated to be used for various analyses. Liver, kidney and pancreas were stored in 10% formalin solution after washing it in a phosphate-buffered saline (PBS) solution.
Biochemical analysis
Liver and kidney samples were homogenized in PBS and the supernatant on centrifugation was used for the estimation of thiobarbituric acid reactive substances (TBARS) (Ohkawa et al. 1979), activities of superoxide dismutase (SOD) (Marklund and Marklund 1974) and catalase (CAT) (Sinha 1972), total protein (Classics Lowry et al. 1951), glutathione-S-transferase (GST) (Habig et al. 1974), glutathione peroxidase (Gpx) (Zachara et al. 2004) and reduced glutathione (GSH) (Cigremis et al. 2009). Serum biochemical parameters such as creatinine, uric acid, urea, total protein, total cholesterol, high-density lipoprotein (HDL) and triglycerides (TGL) were estimated using commercial diagnostic kits purchased from AutoSpan diagnostics Ltd., India and the assays were carried out as per the manufacturer’s instructions. Whole blood samples (ethylene diamine tetra-acetic acid [EDTA] anticoagulated) were used for the estimation of HbA1C using Bio-Rad D-10 HPLC.
Histopathological examination
Portions of liver, kidney and pancreas were processed by routine histopathological techniques and the sections thus obtained were stained with hematoxylin and eosin. They are used to the histopathological changes in the experimental rats.
Statistical analysis
ANOVA was performed for the statistical analysis of obtained data and values are expressed as mean ± SD. The significant differences between the groups were determined by Student Newman–Keul’s test. P < 0.05 implied significance.
Results and discussion
Effect of Spirulina fusiformis on body weight of STZ-treated rats
There was significant (P < 0.05) decrease in the body weight of the diabetic rats compared to the control group (Table 1). There are various studies which prove the significant (P < 0.05) decrease in body weight of STZ-induced rats (Erejuwa et al. 2011a). The body weight of standard group (GLB-treated) was almost similar to the body weight of the diabetic rats. It has been observed that GLB was not able to normalize the body weight of diabetic rats (Erejuwa et al. 2011a). The diabetic rats treated with Spirulina fusiformis showed increase in body weight, which is similar to the normal group.
Table 1.
Effect of Spirulina fusiformis on body weight of STZ-treated rats
| Week | Group I Normal |
Group II STZ (50 mg/kg b.w.) |
Group III STZ + SPI (400 mg/kg. b.w.) |
Group IV STZ + GLB (0.6 mg/kg b.w.) |
Group V SPI (400 mg/kg. b.w.) |
|---|---|---|---|---|---|
| 0 | 218.92 ± 4.41 | 180.49 ± 3.39a* | 181.73 ± 1.87a* | 185.57 ± 0.56a* | 240.15 ± 1.26a*b*c*d* |
| 1 | 233.86 ± 2.03 | 176.35 ± 1.41a* | 180.56 ± 1.26a*b* | 200.78 ± 1.40a*b*c* | 255.18 ± 3.21a*b*c*d* |
| 2 | 241.15 ± 0.66 | 170. 28 ± 3.16a* | 175.78 ± 3.16a*b* | 208.84 ± 2.00a*b*c* | 260.75 ± 1.26a*b*c*d* |
| 3 | 250.75 ± 0.63 | 165.25 ± 0.63a* | 179.84 ± 0.63a*b* | 220.75 ± 0.63a*b*c* | 268.48 ± 1.89a*b*c*d* |
| 4 | 256.28 ± 0.63 | 152.95 ± 1.42a* | 178.94 ± 2.01a*b* | 235.84 ± 3.25a*b*c* | 273.55 ± 2.02a*b*c*d* |
| 5 | 220.42 ± 3.41 | 180.52 ± 1.42a* | 182.06 ± 1.42a* | 185.37 ± 3.16a* | 240.15 ± 1.38a*b*c*d* |
Each value represents the mean ± SD of six rats. Comparisons were made as follows: a-group I vs groups II, III, IV, V; b-group II vs group III, IV,V; c-group III vs group IV, V; d-group IV vs group V. The symbols represent statistical significance at *P < 0. 05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Blood glucose levels
STZ-treated rats show significant (P < 0.05) increase in the level of glucose (Fig. 1), which lies in the diabetic range (≥ 200 mg/dl). This result was found to be similar to several studies on STZ-induced models (Eidi et al. 2006; Erejuwa et al. 2011a; Sadek and Shaheen 2014). The normal range of glucose level must be less than 140 mg/dl. GLB was able to bring back increased glucose level to near normal range which is similar to several studies (Erejuwa et al. 2011a; Devi et al. 2012), whereas Spirulina fusiformis-treated group was able to control the level in normal range (Sadek et al. 2017). The partial destruction of the beta cells by streptozotocin had led to increase in blood glucose levels which were brought to near normal levels by Spirulina administration. The blue green algae being studied extensively for its antioxidative activity might have reduced the extent of oxidative damage to the pancreatic beta cells.
Fig. 1.
Effect of Spirulina fusiformis on blood glucose levels in Streptozotocin-induced diabetic rats. Each value represents the mean ± SD of six rats. Comparisons were made as follows: a group I vs groups II, III, IV, V; b group II vs group III, IV, V; c group III vs group IV, V; d group IV vs group V. The symbols represent statistical significance at *P < 0.05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Nephroprotective effect of Spirulina fusiformis in STZ-treated rats
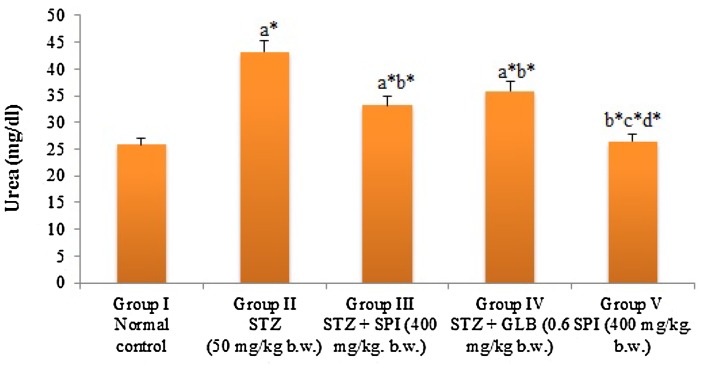
STZ-treated rats show alterations in renal functional markers. There was significant (P < 0.05) increase in the renal functional markers of the STZ-treated group (Figs. 2, 3). This alternation is similar to several studies (Eidi et al. 2006; Erejuwa et al. 2011a). GLB was observed to reduce the elevated levels of renal functional markers, which has also been reported in several studies (Erejuwa et al. 2011a; Devi et al. 2012). Spirulina fusiformis was able to significantly (P < 0.05) decrease the renal functional markers level, which was almost similar to standard group.
Fig. 2.
Effect of Spirulina fusiformis on serum renal function markers (creatinine and uric acid) in STZ-induced diabetic rats. Each value represents the mean ± SD of six rats. Comparisons were made as follows: a group I vs groups II, III, IV, V; b group II vs group III, IV, V; c group III vs group IV, V; d group IV vs group V. The symbols represent statistical significance at *P < 0.05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Fig. 3.
Effect of Spirulina fusiformis on the levels of serum urea in STZ-induced diabetic rats. Each value represents the mean ± SD of six rats. Comparisons were made as follows: a group I vs groups II, III, IV, V; b group II vs group III, IV, V; c group III vs group IV, V; d group IV vs group V. The symbols represent statistical significance at *P < 0.05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Effect of Spirulina fusiformis on antioxidant status of STZ-treated diabetic rats
Antioxidant parameters such as SOD, CAT and TBARS and also liver glycogen were observed to show a significant (P < 0.05) decrease in the level of the diabetic group (Table 2). Similar to the results of previously published studies, our study also shows that the STZ was able to reduce the antioxidant enzyme levels (Erejuwa et al. 2011a; Devi et al. 2012). In standard group, the level of SOD, CAT and liver glycogen was high compared to the diabetic group. Drug-treated group possessed significant (P < 0.05) increase in the levels of SOD, CAT and liver glycogen which was found to be similar to standard group. Whereas the level of TBARS was high in the diabetic group but the Spirulina fusiformis was able to revert back the elevation of the TBARS (Table 2). Liver and kidney of STZ-induced rats showed a significant (P < 0.05) reduction in the level GST, Gpx and GSH (Table 3). Decrease in the level of GST, Gpx and GSH was also observed in similar studies (Nagmoti et al. 2015; Lekshmi et al. 2015; Abdel-Daim et al. 2016). Decreased GSH level was due to the formation of hyperglycemia. Spirulina fusiformis-treated rats were able to reduce the formation of hyperglycemia which was observed by GSH, GST and Gpx level. GSH has the ability of scavenging free radicals. Increased GSH level indicates the reduction of free radicals that was predominant in Spirulina fusiformis-treated rats. The antioxidant effects of Spirulina fusiformis in current study are comparable with that of similar studies carried out in diabetic rat model involving natural compounds such as saponarin (Simeonova et al. 2016).
Table 2.
Effect of Spirulina fusiformis on SOD, catalase, TBARS, liver glycogen against STZ-induced rats
| Parameters | SOD (Units/min/mg protein) | Catalase (Units/min/mg protein) | TBARS (mM/TBARS/100 g of wet tissue) | Liver glycogen (mg glycogen/g liver tissue) | |||
|---|---|---|---|---|---|---|---|
| Liver | Kidney | Liver | Kidney | Liver | Kidney | Liver | |
| Group I | 74.50 ± 0.85 | 71.41 ± 0.89 | 94.33 ± 1.59 | 89.51 ± 1.27 | 1.08 ± 0.09 | 0.99 ± 0.03 | 9.65 ± 0.70 |
| Group II | 38.23 ± 0.80a* | 36.82 ± 0.99a* | 50.58 ± 1.55a* | 48.84 ± 1.05a* | 2.07 ± 0.15a* | 1.95 ± 0.12a* | 5.97 ± 0.63a* |
| Group III | 57.16 ± 1.35a*b* | 55.25 ± 1.41a*b* | 75.12 ± 1.91a*b* | 60.48 ± 1.74a*b* | 1.24 ± 0.10b* | 1.06 ± 0.10b* | 8.12 ± 0.02a*b* |
| Group IV | 58.25 ± 0.51a*b* | 56.20 ± 0.63a*b* | 76.31 ± 1.44a*b* | 63.76 ± 0.94a*b*c* | 1.31 ± 0.12b* | 1.13 ± 0.07b* | 7.25 ± 0.38a*b* |
| Group V | 75.23 ± 1.40b*c*d* | 73.12 ± 1.38b*c*d* | 97.51 ± 1.45b*c*d* | 93.42 ± 0.81a*b*c*d* | 0.97 ± 0.08b*c*d* | 1.01 ± 0.12b* | 10.19 ± 0.31b*c*d* |
Each value represents the mean ± SD of six rats. Comparisons were made as follows: a-group I vs groups II, III, IV, V; b-group II vs group III, IV,V; c-group III vs group IV, V; d-group IV vs group V. The symbols represent statistical significance at *P < 0. 05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Table 3.
Effect of Spirulina fusiformis on GST, Gpx, GSH against STZ-induced rats
| Parameters | Glutathione-S-transferase (nmol of CDNB–GSH conjugate formed/min/mg protein) | Glutathione peroxidase (μg of GSH utilized/min/mg protein) | Reduced glutathione (nmol/mg protein) | |||
|---|---|---|---|---|---|---|
| Liver | Kidney | Liver | Kidney | Liver | Kidney | |
| Group I | 93.80 ± 0.31 | 88.78 ± 0.21 | 67.10 ± 0.59 | 70.41 ± 0.65 | 51.53 ± 0.59 | 48.76 ± 0.67 |
| Group II | 47.79 ± 0.04a* | 50.13 ± 0.94a* | 29.08 ± 0.14a* | 33.65 ± 0.72a* | 25.22 ± 0.20a* | 26.40 ± 0.53a* |
| Group III | 86.67 ± 0.09a*b* | 87.09 ± 0.01b* | 65.90 ± 0.65a*b* | 69.19 ± 0.91b* | 53.43 ± 0.10b* | 42.08 ± 0.76b* |
| Group IV | 92.21 ± 0.24a*b*c* | 85.81 ± 0.43a*b* | 61.45 ± 0.23b*c* | 64.05 ± 0.76b* | 48.51 ± 0.46b*c* | 45.89 ± 0.10a*c* |
| Group V | 90.23 ± 0.11b*d* | 90.02 ± 0.71a*d* | 66.82 ± 0.70a*b*c*d* | 71.32 ± 0.18b*c*d* | 54.83 ± 0.32a*c*d* | 40.45 ± 0.05b*d* |
Each value represents the mean ± SD of six rats. Comparisons were made as follows: a-group I vs groups II, III, IV, V; b-group II vs group III, IV,V; c-group III vs group IV, V; d-group IV vs group V. The symbols represent statistical significance at *P < 0. 05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Effect of Spirulina fusiformis on total protein, total cholesterol, HDL and triglyceride
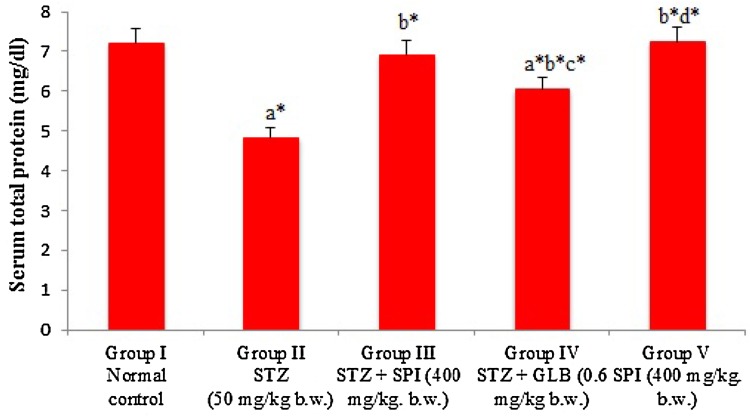
Total protein (Fig. 4) and HDL (Fig. 5) level of diabetic rats showed a significant (P < 0.05) decrease in its level. Spirulina fusiformis-treated diabetic group was observed to normalize the level of total protein and HDL. Elevation in total cholesterol (Fig. 5), triglyceride (Fig. 5) and Hb A1C (Fig. 6) was observed in the serum of diabetic rats; this is also similar to several STZ-induced studies (Eidi et al. 2006; Erejuwa et al. 2011a). The Spirulina fusiformis groups were observed to reduce the significant (P < 0.05) elevated levels of total cholesterol and triglyceride, which lies in the normal levels. The levels of drug-treated group were similar to the levels of the standard group. Spirulina being a rich protein supplement and antioxidant it might have antihyperlipidemic activities, thereby reducing the rise in serum cholesterol.
Fig. 4.
Effect of Spirulina fusiformis on serum total protein levels in STZ-induced diabetic rats. Each value represents the mean ± SD of six rats. Comparisons were made as follows: a group I vs groups II, III, IV, V; b group II vs group III, IV, V; c group III vs group IV, V; d group IV vs group V. The symbols represent statistical significance at *P < 0.05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Fig. 5.
Effect of Spirulina fusiformis on serum total cholesterol, HDL and triglyceride levels in STZ-induced diabetic rats. Each value represents the mean ± SD of six rats. Comparisons were made as follows: a group I vs groups II, III, IV, V; b group II vs group III, IV, V; c group III vs group IV, V; d group IV vs group V. The symbols represent statistical significance at *P < 0.05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
Fig. 6.
Effect of Spirulina fusiformis on HbA1C levels in STZ-induced diabetic rats. Each value represents the mean ± SD of six rats. Comparisons were made as follows: a group I vs groups II, III, IV, V; b group II vs group III, IV, V; c group III vs group IV, V; d group IV vs group V. The symbols represent statistical significance at *P < 0.05. Statistical analysis was calculated by one-way ANOVA followed by the Student Newman–Keul’s test
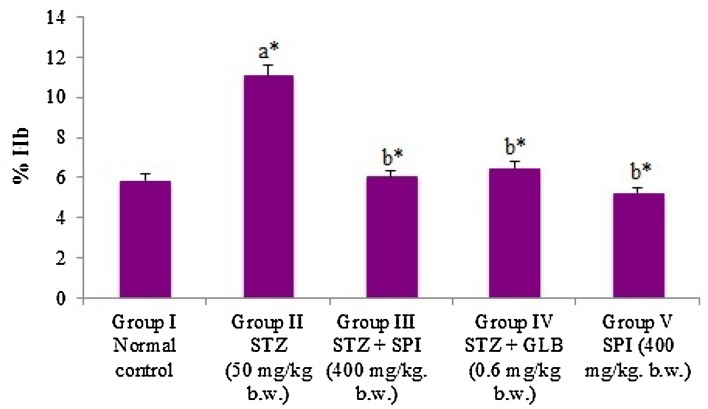
Effect of Spirulina fusiformis on HbA1C levels
HbA1C levels were found to be significantly (P < 0.05) elevated in the diabetic rats. The administration of Spirulina fusiformis was able to reduce the elevated levels of HbA1C significantly (P < 0.05), thereby providing evidence for its effectiveness in glycemic control. Glycated hemoglobin levels reflect the extent of exposure of the red blood cells to glucose (Beltran del Rio et al. 2016). Studies have shown that several plant extracts and phytocompounds with significant antioxidant and antidiabetic potential are effective in reducing the HbA1C levels in diabetic rat models (Kamalakkannan and Prince 2006; Kotha et al. 2017). Our finding regarding the effect of Spirulina fusiformis in maintaining glycemic control is well correlated with such similar studies.
Effect of Spirulina fusiformis on pancreas, liver and kidney tissue histopathology
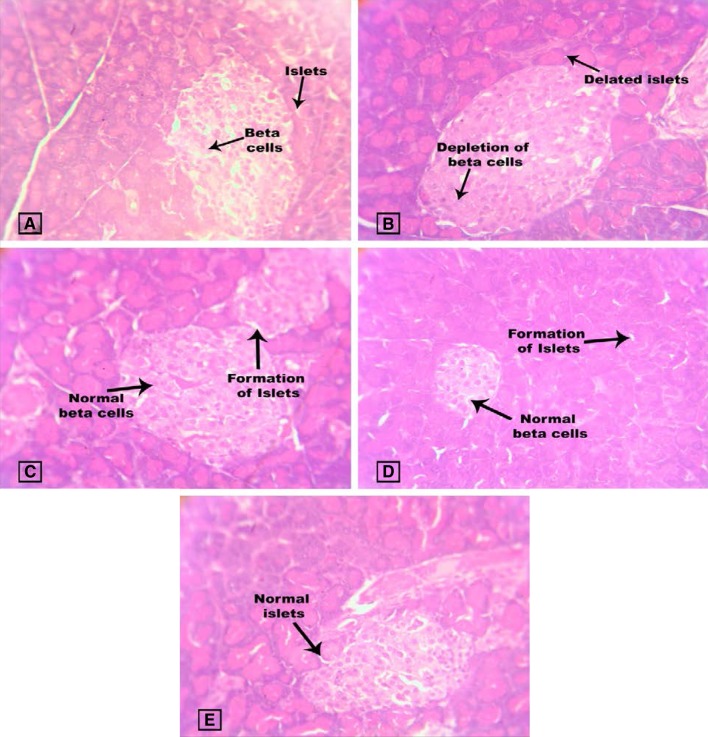
The normal pancreas was observed with exocrine acini and their intra lobular ducts, which was in small, round and light-staining the islets of Langerhans. The centre of islet cells consists of small β-cell and the periphery consists of large alpha cells. The diabetic pancreas was observed with the presence of connective tissues with separate lobules. The β-cell comprises basophilic granules and alpha cells comprise eosinophilic granules. It is also observed with necrosis, severe damage in islet of langerhans, reduced number of cells and islets size. This was also observed in various studies (Erejuwa et al. 2011b). Some degenerated β-cell and lymphocytic infiltration were observed in the diabetic pancreas. In treated group, the pancreatic lobules were separated by thin connecting tissues, which was also similar to various studies (Erejuwa et al. 2011b; Devi et al. 2012). Pancreas was also reported to be damaged by alloxan (Ragavan and Krishnakumari 2006). In diabetic control rats, pancreas islets cells were reduced in number, which were restored to near normal in Spirulina fusiformis-treated group. Spirulina fusiformis is observed to restore the islets size and damages of cells (Fig. 7).
Fig. 7.
Effect of Spirulina fusiformis on pancreases histopathology. Pancreas histopathology (H & E staining): a Group I shows normal pancreases histology of beta cells and islets; b Group II damaged islets (arrow). c Group III shows the recovery of islets. d Group IV shows the recovery of islets. e Group V shows normal islets (arrow). The lobular architecture is maintained
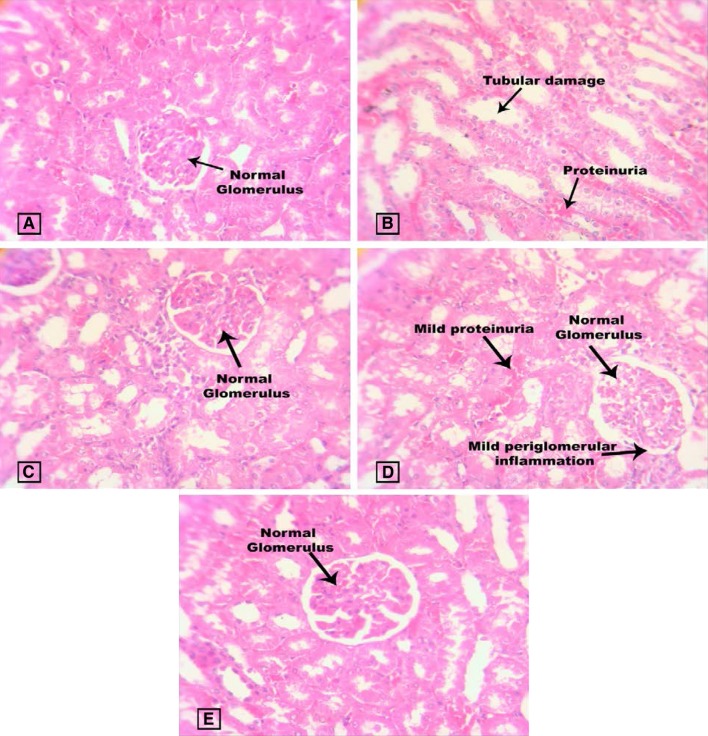
Figure 8 represents the kidney histopathology of study animals where normal control rats show normal morphological structure of the renal tubules, basement membrane and glomeruli. Diabetic control was characterized by vacuolation, expansion of mesangial cells, necrosis of epithelium, proteinuria and thickening of the glomerular basement membrane. This characterization was also observed in several other studies on STZ-induced diabetic rats (Erejuwa et al. 2011b). There was a restoration in renal cellularity with less damage in the renal tissue of GLB and Spirulina fusiformis-treated diabetic rats.
Fig. 8.
Effect of Spirulina fusiformis on kidney histopathology. Kidney histopathology (H & E staining): a Group I shows normal renal tissue morphology with normal glomerulus; b Group II shows tubular damage with protein urea; c Group III shows normal renal tissue morphology. d Group IV shows mild protein urea and mild periglomerulari inflammations; e Group V shows normal renal tissue morphology
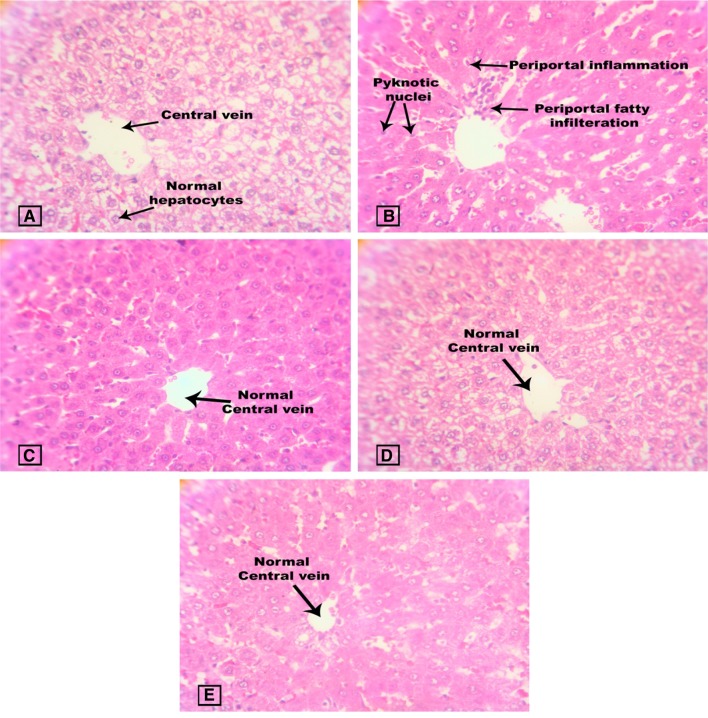
Figure 9 represents the liver histopathology of study animals where group I shows normal histopathology of the liver with central vein and hepatocytes. Histopathological changes in the diabetic liver were observed to show necrosis and periportal fatty infiltration. This is found to be in accordance with the previously published data (Ragavan and Krishnakumari 2006). Spirulina fusiformis alone treated group was observed to show normal hepatocytes with central vein and GLB-treated group also showed normal histology of liver.
Fig. 9.
Effect of Spirulina fusiformis on liver histopathology. Liver histopathology (H & E staining): a Group I shows normal liver histology with normal central vein and normal hepatocytes; b Group II liver section shows periportal inflammation (arrow), periportal infiltration and few pyknotic nuclei. The lobular architecture is maintained; c Group III shows normal central vein and normal hepatocyte morphology. d Group IV shows normal central vein and normal hepatocyte morphology; e Group V shows normal hepatocytes and central vein (arrow)
STZ has the activity of selective destruction of pancreatic islets β-cell cytotoxicity and it is frequently administrated for animal studies (Pari and Latha 2002). Administration of STZ is known to cause DM, with various complications like body weight loss, polyphagia, polydipsia, hyperglycaemia and glycosuria (Pari and Latha 2002).
Spirulina fusiformis is reported to have hepatoprotective activity which is proved against galactosamine-induced rats (Sabina et al. 2013). Spirulina platensis was reported to protect against diabetic nephropathy (Zheng et al. 2013). A study on Spirulina has shown its protective role against apoptosis. Spirulina is an edible alga, which is used in improving antioxidant capacity (Chu et al. 2010). Hypolipidemic and hypoglycemic properties of Spirulina have been studied and it is reported to maintain the glucose concentration and maintain normal lipid profile against type-2 diabetes (Parikh et al. 2001). Study of Spirulina platensis on deltamethrin-induced rats has reported its antioxidant activity and ability to reduce the toxicity (Abdel-Daim et al. 2013). Spirulina has been reported to treat anemia and improve the immune function (Selmi et al. 2011). Spirulina is reported to have radio-protective activity which was experimented against ionizing-radiation induced oxidative stress and organ injury (Rehab 2012). A study on Spirulina maxima has reported to show protection against hepatotoxic, hyperglycaemic and hyperlilidaemic activity, which was analyzed on fructose-induced diabetic rats (Jarouliya et al. 2012).
Conclusion
The current study has demonstrated the effectiveness of Spirulina fusiformis in STZ-induced diabetic rats probably by its antioxidant activity. It is also evident that this blue green alga was able to maintain the serum renal function markers such as urea, creatinine and uric acid at near normal levels, thereby proving that it is effective in preventing renal damage due to diabetes. Our study also provides evidence on the antihyperlipidemic potential of Spirulina fusiformis in diabetic rats. Reduction in the elevated levels of HbA1C provides evidence on the potential of Spirulina in maintaining good glycemic control. Histopathological evidence shows that the algae prevent oxidative tissue damage, thereby preventing diabetic complications in the liver and pancreas. However, further studies at molecular level would aid explore the molecular mechanisms underlying the antidiabetic activity of Spirulina fusiformis against STZ-induced diabetes.
Acknowledgement
The authors are thankful to VIT University for providing the necessary facilities to carry out this research project.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Abdel-Daim MM, Abuzead SMM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS ONE. 2013;8:e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Daim M, El-Bialy BE, Rahman HGA, et al. Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed Pharmacother. 2016;77:79–85. doi: 10.1016/j.biopha.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Babu PVA, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24:1777–1789. doi: 10.1016/j.jnutbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran del Rio M, Tiwari M, Amodu LI, et al. Glycated hemoglobin, plasma glucose, and erythrocyte aging. J Diabetes Sci Technol. 2016;10:1303–1307. doi: 10.1177/1932296816659885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Brahmbhatt S, Gupta A, Sharma AC. Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phosphorylation in renal and vascular dysfunction. Cardiovasc Diabetol. 2005;4:3. doi: 10.1186/1475-2840-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W-L, Lim Y-W, Radhakrishnan AK, Lim P-E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement Altern Med. 2010;10:53. doi: 10.1186/1472-6882-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigremis Y, Turel H, Adiguzel K, et al. The effects of acute acetaminophen toxicity on hepatic mRNA expression of SOD, CAT, GSH-Px, and levels of peroxynitrite, nitric oxide, reduced glutathione, and malondialdehyde in rabbit. Mol Cell Biochem. 2009;323:31–38. doi: 10.1007/s11010-008-9961-8. [DOI] [PubMed] [Google Scholar]
- Classics Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Deeds M, Anderson J, Armstrong A, et al. Single dose streptozotocin induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45:131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi YA, Vrushabendra Swamy BM, Vishwanath Swamy KM, Ramu Ravi R. Antidiabetic activity of Echinochloa crusgalli (L.)P. Beauv grains extract in alloxan induced diabetic rats. Res J Pharmaceut Biol Chem Sci. 2012;3:1257. [Google Scholar]
- Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.)Sm., in STZ-induced diabetic rats. Chem Biol Interact. 2009;182:67–72. doi: 10.1016/j.cbi.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Erejuwa OO, Sulaiman SA, Wahab MS, et al. Effect of glibenclamide alone versus glibenclamide and honey on oxidative stress in pancreas of streptozotocin-induced diabetic rats. Int J Appl Res Nat Prod. 2011;4:1–10. [Google Scholar]
- Erejuwa OO, Sulaiman SA, Wahab MSA, et al. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int J Mol Sci. 2011;12:829–843. doi: 10.3390/ijms12010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finamore A, Palmery M, Bensehaila S, Peluso I (2017) Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly Spirulina. In: Oxidative medicine and cellular longevity. https://www.hindawi.com/journals/omcl/2017/3247528/cta/. Accessed 5 Jan 2018 [DOI] [PMC free article] [PubMed]
- Fujiwara T, Yoshioka S, Yoshioka T, et al. Characterization of new oral antidiabetic agent CS-045. Studies in KK and ob/ob mice and Zucker fatty rats. Diabetes. 1988;37:1549–1558. doi: 10.2337/diab.37.11.1549. [DOI] [PubMed] [Google Scholar]
- Gargouri M, Hamed H, Akrouti A, et al. Effects of Spirulina platensis on lipid peroxidation, antioxidant defenses and tissue damages in kidney of alloxan-induced diabetic rats. Appl Physiol Nutr Metab. 2017 doi: 10.1139/apnm-2017-0461. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Jarouliya U, Zacharia JA, Kumar P, et al. Alleviation of metabolic abnormalities induced by excessive fructose administration in Wistar rats by Spirulina maxima. Indian J Med Res. 2012;135:422. [PMC free article] [PubMed] [Google Scholar]
- Kamalakkannan N, Prince PSM. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol. 2006;98:97–103. doi: 10.1111/j.1742-7843.2006.pto_241.x. [DOI] [PubMed] [Google Scholar]
- Keidan B, Hsia J, Katz R. Plasma lipids and antidiabetic agents: a brief overview. Br J Diabetes Vasc Dis. 2002;2:40–43. doi: 10.1177/14746514020020011801. [DOI] [Google Scholar]
- Kotha P, Badri KR, Nagalapuram R, et al. Anti-diabetic potential of the leaves of Anisomeles malabarica in streptozotocin induced diabetic rats. Cell Physiol Biochem. 2017;43:1689–1702. doi: 10.1159/000484030. [DOI] [PubMed] [Google Scholar]
- Lekshmi RK, Rajesh R, Mini S. Ethyl acetate fraction of Cissus quadrangularis stem ameliorates hyperglycaemia-mediated oxidative stress and suppresses inflammatory response in nicotinamide/streptozotocin induced type 2 diabetic rats. Phytomedicine. 2015;22:952–960. doi: 10.1016/j.phymed.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Machha A, Achike FI, Mustafa AM, Mustafa MR. Quercetin, a flavonoid antioxidant, modulates endothelium-derived nitric oxide bioavailability in diabetic rat aortas. Nitric Oxide. 2007;16:442–447. doi: 10.1016/j.niox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Sabina EP. Amelioration of anti-tuberculosis drug induced oxidative stress in kidneys by Spirulina fusiformis in a rat model. Ren Fail. 2016;38:1115–1121. doi: 10.1080/0886022X.2016.1184940. [DOI] [PubMed] [Google Scholar]
- Nagmoti DM, Kothavade PS, Bulani VD, et al. Antidiabetic and antihyperlipidemic activity of Pithecellobium dulce (Roxb.) Benth seeds extract in streptozotocin-induced diabetic rats. Eur J Integr Med. 2015;7:263–273. doi: 10.1016/j.eujim.2015.01.001. [DOI] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pari L, Latha M. Effect of Cassia auriculata flowers on blood sugar levels, serum and tissue lipids in streptozotocin diabetic rats. Singap Med J. 2002;43:617–621. [PubMed] [Google Scholar]
- Parikh P, Mani U, Iyer U. Role of Spirulina in the control of glycemia and lipidemia in Type 2 diabetes mellitus. J Med Food. 2001;4:193–199. doi: 10.1089/10966200152744463. [DOI] [PubMed] [Google Scholar]
- Ragavan B, Krishnakumari S. Effect of T. arjuna stem bark extract on histopathology of liver, kidney and pancreas of alloxan-induced diabetic rats. Afr J Biomed Res. 2006 [Google Scholar]
- Rehab Evaluation of the effect of Spirulina against Gamma irradiation induced oxidative stress and tissue injury in rats. Int J Appl Sci Eng Res. 2012 [Google Scholar]
- Sabina E, Samuel J, RajappaRamya S, et al. Hepatoprotective and antioxidant potential of Spirulina fusiformis on acetaminophen-induced hepatotoxicity in mice. IJIB. 2009;6:1–5. [Google Scholar]
- Sabina P, Rasool M, Kalaiselvan S. Protective effects of blue green algae Spirulina fusiformis against galactosamine-induced hepatotoxicity in mice. Asian J Pharm Clin Res. 2013;6:150–154. [Google Scholar]
- Sadek KM, Shaheen H. Biochemical efficacy of vitamin D in ameliorating endocrine and metabolic disorders in diabetic rats. Pharm Biol. 2014;52:591–596. doi: 10.3109/13880209.2013.854812. [DOI] [PubMed] [Google Scholar]
- Sadek KM, Taha N, Korshom M, Mandour A. thiamine ameliorate hepatic, renal dysfunction and dyslipidaemia in diabetic rats. J Curr Res Sci. 2013;1:35–39. [Google Scholar]
- Sadek KM, Lebda MA, Nasr SM, Shoukry M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed Pharmacother. 2017;92:1085–1094. doi: 10.1016/j.biopha.2017.06.023. [DOI] [PubMed] [Google Scholar]
- Selmi C, Leung PS, Fischer L, et al. The effects of Spirulina on anemia and immune function in senior citizens. Cell Mol Immunol. 2011;8:248–254. doi: 10.1038/cmi.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Kar A. Combined effects Gymnema sylvestre and glibenclamide on alloxan induced diabetic mice. Int J App Pharm. 2014;6:11–14. [Google Scholar]
- Simeonova R, Vitcheva V, Krasteva I, et al. Antidiabetic and antioxidant effects of saponarin from Gypsophila trichotoma on streptozotocin-induced diabetic normotensive and hypertensive rats. Phytomedicine. 2016;23:483–490. doi: 10.1016/j.phymed.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Sirasanagandla S, Kasetti RB, Shaik AN, et al. Antihyperglycemic and antihyperlipidemic activities of 2-(4-[(2-hydroxybenzyl) amino]-phenyl amino-methyl)-phenol in STZ induced diabetic rats. Eur J Med Chem. 2013;66:400–406. doi: 10.1016/j.ejmech.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Zachara BA, Salak A, Koterska D, et al. Selenium and glutathione peroxidases in blood of patients with different stages of chronic renal failure. J Trace Elem Med Biol. 2004;17:291–299. doi: 10.1016/S0946-672X(04)80031-2. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Wang W, et al. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J Ethnopharmacol. 2011;137:662–668. doi: 10.1016/j.jep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Zheng J, Inoguchi T, Sasaki S, et al. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol. 2013;304:R110–R120. doi: 10.1152/ajpcell.00006.2012. [DOI] [PubMed] [Google Scholar]