Abstract
Colloidal gold nanoparticles (AuNPs) are of interest as non-toxic carriers for drug delivery owing to their advanced properties, such as extensive surface-to-volume ratio and possibilities for tailoring their charge, hydrophilicity and functionality through surface chemistries. To date, various biocompatible polymers have been used for surface decoration of AuNPs to enhance their stability, payloads capacity and cellular uptake. This study describes a facile one-step method to synthesize stable AuNPs loaded with combination of two anticancer therapeutics, -bleomycin and doxorubicin. Anticancer activities, cytotoxicity, uptake and intracellular localization of the AuNPs were demonstrated in HeLa cells. We show that the therapeutic efficacy of the nanohybrid drug was strongly enhanced by the active targeting by the nanoscale delivery system to HeLa cells with a significant decrease of the half-maximal effective drug concentration, through blockage of HeLa cancer cell cycle. These results provide rationale for further progress of AuNPs-assisted combination chemotherapy using two drugs at optimized effective concentrations which act via different mechanisms thus decreasing possibilities of development of the cancer drug resistance, reduction of systemic drug toxicity and improvement of outcomes of chemotherapy.
Subject terms: Chemotherapy, Drug delivery
Introduction
In 2017, approximately 600,920 people were expected to die1 because of cancer only in United States. Ovarian cancer, a one of the deadliest gynaecological malignancy characterized by highest mortality rates, was identified by the National Institutes of Health (NIH) as a target disease for innovative nanotechnology2 solutions. Human papillomavirus (HPV) infections are attributed to ~70% of cervical cancers3. Gardasil®, Gardasil®-9, and Cervarix® are clinically approved vaccines for cervical cancer and provide protection against all known HPV infections3–5. However a lack of vaccination and the fact that vaccines are not distributed worldwide4,5 mean cervical cancer management remains problematic. Compared to the prophylactic vaccines, surgical resection followed by chemotherapy is an ineffective treatment for ovarian cancer originating from HPV. Administration of chemotherapy agents and multidrug resistance primarily attribute to severe side effects6 including negative gynaecological and obstetric outcomes7.
The absence of an anatomical barrier around ovaries8 can cause tumor cells to metastasize through the lymphatic systems and blood vessels. Surgical resection and systemic chemotherapy affects the drug’s effectiveness. Hence high chemotherapeutic doses are required to target the epithelial and mucosal membranes of the affected cells. New treatment strategies against the disease are urgently required to overcome the high toxicity induced by systemic chemotherapy. For clinical applications the optimizing of appropriate dose of drug is essential in order to minimize possible side toxicity effects. Gold nanoparticles (AuNPs), as a kind of versatile and generally nontoxic material9,10, have attracted widespread interest for therapeutic applications in humans11. On the other hand, few research groups12,13 have reported of the AuNPs toxicity which is dependent on various factors, such as the particles size and morphology and effects of environments and, therefore, challenging to predict14–18.
Gold nanoparticles (AuNPs), have become highly feasible materials for treating malignant tumors19 owing to their unique quenching efficiencies20,21, biocompatibility22,23 and significant surface modifiability24–26. For example, Astra Zeneca in partnership with Cytimmune, focused on AuNPs-based nanomedicine in cancer treatment11. The PEGylated (polyethylene glycol) colloid gold particles based product, Aurimune (CYT-6091)11 whose nontoxicity has been declared27 is under current clinical trials. It can efficiently deliver the tumor necrosis factor alpha (TNFα) to tumor sites. Additionally, numerous other AuNPs-based chemotherapy mediators such as Auroshell28,29 are striving for success in clinical trials11,28–30. AuNPs have promising biocompatibility22,23 along with tuneable physicochemical properties that is very attractive for design of advanced systems for cancer treatment. In fact AuNPs due to their easy surface modification have been widely studied as drug delivery vehicles31–33 and for targeting34 specific cells or tissues. Researchers have developed modified drug delivery systems based on AuNPs with enhanced in vivo stability and biodistribution26. Biopolymer-conjugated AuNPs are used in cosmetic products35, delivery systems36, bio marker detection37, biosensing38, diagnostics39 and theranostics35,40. Colloidal AuNPs do not accumulate in the liver, resulting in no hepatic dysfunction in in vivo/in vitro models41. Furthermore, AuNPs shielded from phagocytes uptake via coating with PEG brush gradually clear from the body41 and can be degraded within the cells22. As multifunctional agents, AuNPs can be utilized for combined therapy and diagnostics (theranostics) such as for photo thermal ablation of tumors42, radiation sensitizers43 and in vivo/in vitro real time imaging.
Recently hybrid AuNPs have been synthesized using dicarboxylic-terminated PEG by using a one-step technique26,44. Herein, we adopted similar robust approach to synthesize high quality stable bleomycin (BLM) and doxorubicin (DOX) capped AuNPs. BLM is one of the most effective and readily available anticancer drugs. While details of BLM mechanism of activity are not completely resolved, it has been demonstrated that it acts via metal ions-dependent production of reactive oxygen species (ROS) and DNA strand breaks45. Among the most serious complications of BLM is lung injury46. Surface-modified anticancer nanocarriers which cause no or minimal side effects and at the same time provide enhanced drug loading capacity and enhanced blood circulation time are of great interest for cancer therapy. Chemotherapeutic drug efficacy at the tumor site is hindered either by poor bioavailability or dose-limiting side effects. Therefore, to further overcome these limits, we use a combination-chemotherapy strategy through adding another drug doxorubicin (DOX) within the same drug delivery nanocarrier to control the dose-limiting severe side effects. Mechanism of DOX anticancer activity is believed to include intercalation of its chromophore part between two bases of a cancer DNA, arresting the process of replication and therefore inhibition of macromolecules biosynthesis47.
The usage of BLM- and -DOX-decorated AuNPs could be prolonged if controlled and low dosage drug could be delivered specifically to the target. Moreover, combination of two chemotherapeutics which act via different mechanisms can reduce risk of evolving drug resistant cancer cells and, in addition, to overcome tumor heterogeneity, or “preferable” drug responsiveness of various cells within the same cancer type. We show that BLM- and DOX- decorated AuNPs exhibit excellent colloidal stability, high drug loading capacity and enhanced cellular uptake against HeLa cells. The toxicity and therapeutic effectiveness of the nanocarrier were evaluated by flow cytometry, while cellular internalization was evaluated by laser scanning confocal microscopy (LSCM). Synthesis of AuNPs decorated with two chemotherapy agents and study of their efficiency to carry anti-cancer drug to the tumor site and sustainably cure diseases, was the main goal of this study.
Results and Discussion
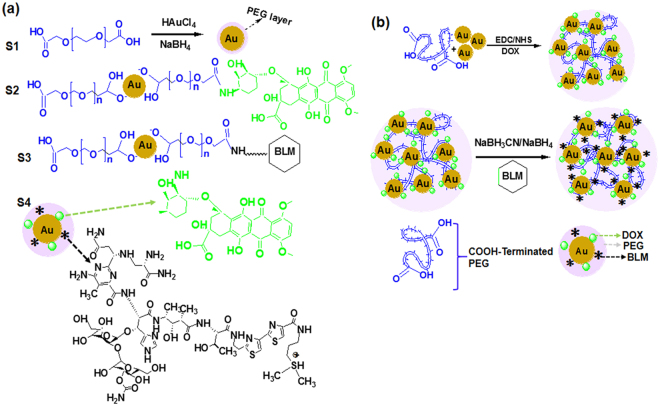
Here we focused on the development of the following gold nanoparticles-based drug delivery systems: AuNPs covered by PEG carrying carboxylic groups, PEG-AuNPs linked to DOX, PEG-AuNPs linked to BLM, and, finally PEG-AuNPs linked to both DOX and BLM (referred to as S1, S2, S3 and S4, correspondingly). PEG-capped Au NPs (S1) and their functionalization with DOX (S2) were synthesized with some modification to the previously described procedure48. Next, the S1 and S2 samples were used for conjugation with BLM through carbodiimide chemistry. As shown in Fig. 1(a,b), BLM was immobilized on AuNPs surface through an amide bond between the carboxylic and amino- groups of the PEG residue and BLM, respectively. Further, the S1 were conjugated with both of the therapeutic payloads DOX and BLM to enable active targeting of HeLa cells. Moreover, cellular uptake of two chemotherapeutics is observed significantly decreases in cancer cell’s acidic environments (at pH 6.0 uptake is 50% less to that observed at pH 7.0) which is significantly limits the drug impact in the cell nuclei. As result, direct release of drug into the cytoplasm could decrease drug the half-maximal effective drug concentration (EC50) values. Anticancer therapy efficiency can be significantly enhanced by using S1 as nanocarriers.
Figure 1.
Synthesis of PEG-AuNPs NPs. (a) Chemical structure of DOX and BLM and steps involved in conjugation to S1 surface; (b) Schematic illustration of S2, S3 and S4 NPs formation.
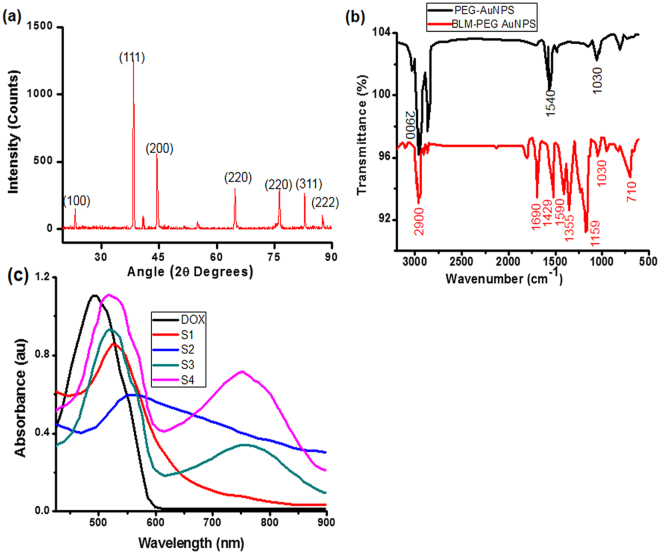
The structure of as synthesized AuNPs was confirmed by using X-ray Diffraction (XRD) technique. The XRD pattern can be seen in Fig. 2(a) with observed diffraction peaks at 2θ = 22.68°, 38.41°, 44.86°, 64.80°, 77.90° and 82.65° with corresponding lattice planes (110), (111), (200), (220), (220), (311) and (222). The observed diffraction peaks were compared with JCPDS cards (JCPDS # 04–0784) which revealed the composition of crystalline gold with predominant orientation plane (111) that could be assigned to spherical AuNPs49. Figure 2(b) shows the FTIR spectra and confirms the surface modification of Au NPs. Characteristic peaks (Fig. 2b) at around 2900, 1690 and 1355 cm−1 with a slight shift in wave numbers were observed which are assigned to –CH, amide I and III bonds respectively, extra peaks at around 1560 and 1429 cm−1, showing the presence of primary –COO− and CH2 groups. However the peaks at 1159, 1030 and 710 cm−1 were also observed and corresponding assigned to C-O-C, ether and C-H bending respectively. The relative intensity decline, characteristic signals from presence of functional groups and slight shift in wave numbers indicates the drug conjugation on the Au NPs surface.
Figure 2.
(a) XRD spectra of PEG-AuNPs; (b) FTIR spectra of PEG-AuNPs and Bleomycin conjugated PEG-AuNPs; (c) Recorded UV–Vis spectra of all PEG-AuNPs.
The successful conjugation and interaction between BLM and AuNPs were also verified by UV–visible spectroscopy as summarized in Fig. 2(c) and Table 1. The 517 nm peak was observed for S1 AuNPs which is similar to nearly ~10 nm in core diameter of AuNPs50,51. A strong absorption band at around 550 nm was observed after coating the S1 surface with BLM which could be associated with refractive index change of the AuNPs. The absorption intensity and peak position of localized surface plasmon (LSP) spectra strongly depends on the morphology, size, shape and accumulation of AuNPs52. The spectral data on S4 clearly show that the nanoparticles surface modification had taken place with characteristic red-shift red-shift in LSP position52,53.
Table 1.
Zeta-potential and hydrodynamic diameter of synthesized PEG-AuNPs dispersed in different media.
| Sample | Effective Diameter (nm) | Zeta-potential (mV) | Hydrodynamic Diameter (nm) in PBS | Hydrodynamic Diameter (nm) in FBS | Hydrodynamic Diameter (nm) in DMEM |
|---|---|---|---|---|---|
| S1 | 13.0 ± 0.3 | −23.7 ± 2.1 | 14.6 ± 0.1 | 13.2 ± 0.4 | 11.3 ± 0.1 |
| S2 | 14.2 ± 0.1 | −7.2 ± 3.0 | 15.3 ± 0.3 | 13.7 ± 0.2 | 10.1 ± 0.3 |
| S3 | 13.5 ± 0.2 | −6.0 ± 0.6 | 11.6 ± 0.3 | 13.3 ± 0.2 | 11.4 ± 0.3 |
| S4 | 13.7 ± 0.1 | 5.0 ± 0.7 | 11.9 ± 0.3 | 11.8 ± 0.3 | 11.7 ± 0.3 |
A strong absorption band at around 528 nm was observed when S1 were conjugated with DOX (S2, shifted for bound DOX comparing to 480 nm for the free DOX). Subsequently similar to longitudinal plasmon oscillations48, an intense resonance band was observed at approximately 750 nm. However, in the case of the S3 sample the resulting solution was transparent and therefore a significant decrease in spectral intensity with red-shifted peak was observed. The red shift from 750 to 764 nm indicated the presence of BLM. The UV–Vis spectrum clearly proved successful conjugation of S1 with anticancer drugs. The quantitation of drug-loading efficiency was estimated from UV–Vis absorption (see Supplementary Fig. S1). For this, an excess of DOX (9.0 µg) was added to various concentrations of the S1 to prepare S2 and S4 products. After filtration, the residual amounts of DOX were determined to be around ~ 7.5 µg in S2 and S4 products. The drug loading AuNPs calculated using eq. (1) was found to be 33% with the drug entrapment efficiency was about 83%. The efficient DOX loading indicates potential use of BLM decorated AuNPs as nanocarriers.
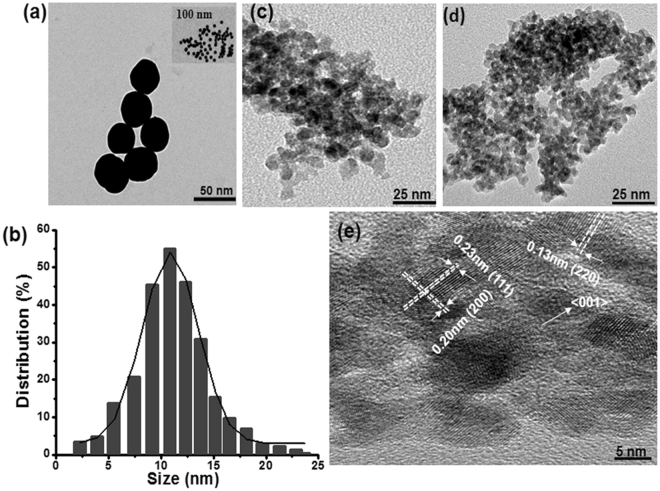
The morphology of as synthesised NPs as well as the presence of aggregation was inspected by TEM analysis (Fig. 3). Figure 3(a) shows that AuNPs were well crystallized, spherical in shape, without particle accumulation and having an average particle diameter of 13 nm. Inset (Fig. 3a) depicts that the Au NPs were highly dispersed. Figure 3(b) represents the particle size histograms of synthesised Au NPs in the absence of any thiol capping ligand. After DOX conjugation, the nanostructures exhibited spherical shape (Fig. 3c) with slight particles aggregation. When BLM was added to the DOX conjugated S2 product solution, a similar trend was observed as shown in Fig. 3(d). Figure 3(c,d) also shows the effect of ligands addition in Au NPs solution. In the presence of ligands, dispersity as well as size of the Au NPs were decreased54. The crystal structure of the synthesised was also inspected with high-resolution transmission electron microscopy (HRTEM). Figure 3(e) shows that Au {111} planes were prominent with a lattice spacing of ~0.23 nm whereas Au {200} and Au {220} crystallographic planes were also present with d-spacing of ~0.20 and 0.13 nm, respectively55. Dominant Au {200} crystallographic planes were found with a growth direction of <001> (Fig. 3e). It is possible that some cluster accumulation was caused by steric effects and electrostatic forces between BLM and PEG-AuNPs surface. However, during TEM sample preparation, solvent evaporation may possibly have also led to cluster accumulation. BLM-particle interactions can influence drug release owing to the PEG chains presence and/or electrostatic interactions. In general, colloidal solution was stabilized by DOX/ BLM loading onto the S1 (Fig. 3c,d).
Figure 3.
TEM images to verify the morphologies of the synthesized Au NPs. (a,b) TEM image and particle size distribution of plain Au NPs; (d,e) TEM images of DOX and BLM conjugated PEG-AuNPs; (e) HRTEM image with crystalline lattices of Au{111}, Au{200} and Au{220}.
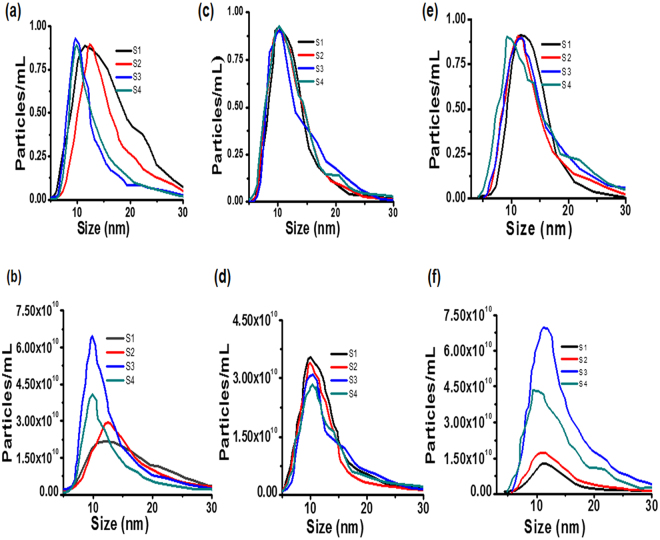
Table 1 and Fig. 4 summarize the effective diameter, Zeta-potential and hydrodynamic diameters of all the products after 24 hours dispersion in three different media: PBS, FBS and DMEM. Overall hydrodynamic diameters measured by Nanoparticles Tracking Analysis (NTA) in cell culture media were slightly larger than the ones measured by TEM (Fig. 3). This increase in hydrodynamic diameter was associated with PEG coating on the AuNP surface or high salt environment of cell culture media. NTA results revealed that all products were stable with no aggregation observed after 24 hours of incubation at room temperature in typical cell culture media. As observed in Fig. 4, no significant changes in diameter were recorded in the case of FBS and DMEM supplemented with 10% FBS. Hydrodynamic diameter and surface potential on all NPs samples were also measured using DLS and Zeta potential. After conjugation of BLM or DOX, it was found that hydrodynamic diameter increased slightly and these results are in good agreement with previously reported work56. S1 have a negative potential charge of −23.70 ± 2.11 mV and hence confirm the existence of carboxylic group on the AuNPs surface. The zeta potentials on various concentrations of AuNPs are summarized in Table 1, and on the basis of these data it is possible to conclude that all samples were stable. Thus, we can hypothesize that in future in-vivo studies these nanoparticles will be stable after intravenous administration. Therefore, our synthesized PEG-AuNPs have the physicochemical properties suitable for enhanced accumulation and active targeting against HeLa cells.
Figure 4.
Nanoparticle tracking analysis of AuNPs after 24 hour’s exposure and dispersed in PBS (a,b); FBS (c,d); DMEM (e,f); (a,c,e) control experiments’ data were deducted and then curves were normalized from 0 to 1; (b,d,f) variances of sample maximal concentration.
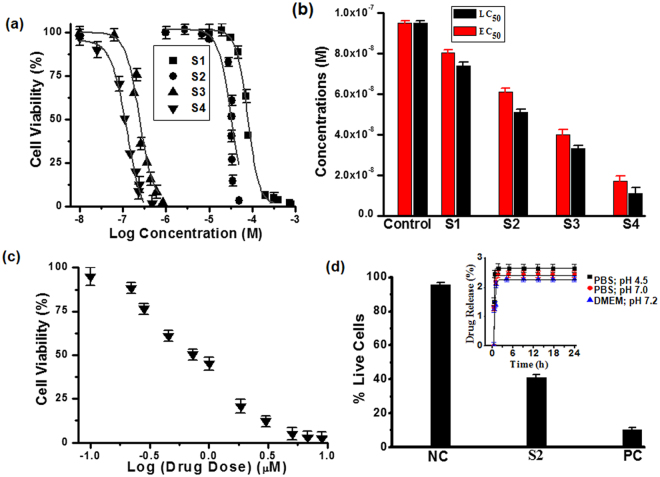
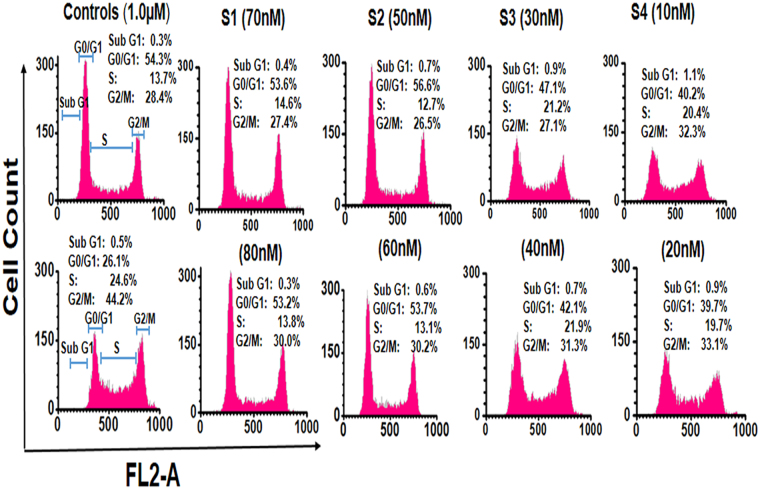
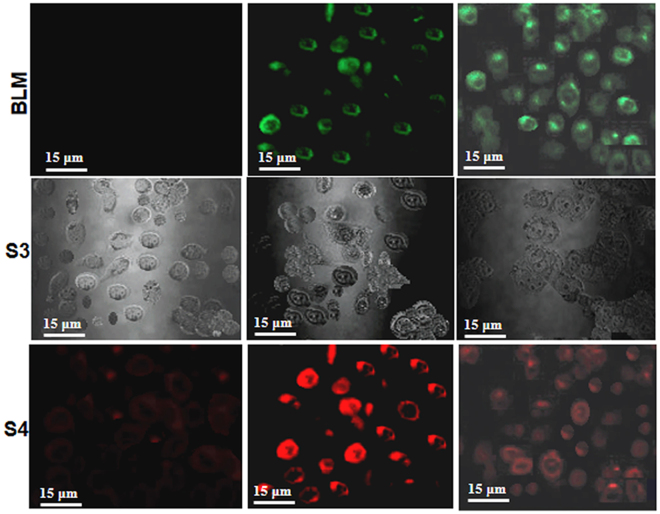
Figures (5 and 6) illustrate the flow-cytometric analysis of HeLa cell following 24 hours exposure against various concentration of the NPs. The dose-response relationship in Fig. 5(a) shows that the half-maximal lethal concentration (LC50) values decreased when BLM was loaded into S1. Additionally, while HeLa cells were actively targeted with S4, the decrease in LC50 values was obvious. Hence targeting with BLM capped AuNPs (S4) have shown increase in cytotoxicity as compared to S1, S2 and S3 products (Fig. 5a,b). However, the half-maximal effective drug concentration (EC50) values for BLM, S3 and S4 were also measured for comparison. The individual effect of BLM drug was also tested (Fig. 5c) and its EC50 values were compared with effects of the drug delivered from S3 and S4. Based on this comparative analysis, it is feasible to conclude that the combination of the two anticancer therapeutics in the S4 delivery nanovehicle resulted in improved cytotoxic effect at relatively lower concentrations. Figure 5(d), shows that the chemotherapeutic effect of actively targeted BLM-modified PEG-AuNPs was significantly higher against HeLa cells. Cytotoxicity and percentage of live cells after 24 hours expose to S1and S2 were also assessed (Fig. 5d). As presented in Fig. 5(d), no significant cytotoxicity was observed, which reflects the non-toxic nature of the nanocarrier. The inset Fig. 5(d), provides an additional experimental data on time-dependence of the drug release from concentrations and pHs (4.5, 7.0) Thus, the acidic environment at pH 4.5, typical for a caner tissue environment, stimulated drug release, while neutral and higher pH values showed negligible effect on the drug release. However, 2.3% drug release was calculated after three hours and after that it remained constant, as shown in inset of Fig. 5(d). HeLa cells exposed to various concentrations of PEG-AuNPs exhibited significant evidence of apoptosis (Fig. 6 and Table 2). As it presented in Fig. 6, at remarkably low concentrations, cell population shifts towards G2/M-phase upon exposure of HeLa cells to the S4 product. Thus, G2/M-phase cell cycle arrest and cell apoptosis were ultimately associated with combined anticancer drugs activity. A slight change in cell population towards the G2/M phase was also observed in case of the S3. On the contrary, negligible cell apoptosis and change in cell population were observed at relatively higher drugs concentrations when cells were treated with the S1 and the S2. These findings are in agreement with the LC50/ EC50 values and verify advantages of Au-nanoparticles-assisted combined anticancer therapy approach. Cell proliferation and drug release were observed using laser scanning confocal microscopy (LSCM). Figure 7 shows the proliferation of HeLa cells and their interactions with the as synthesized AuNPs. Figure 7 also illustrates the presence of S2, S3 and S4 drug delivery constructs in HeLa cells when incubated with their various concentrations for 24 hours. Images were captured subsequently. Cells interactions with AuNPs and cells densities were diminished in a dose-dependent manner. The positive zeta potential on S4 product stimulates them in binding with cell membrane thus allowing endocytosis. Image overlapping demonstrates AuNPs attachment to the cancer cells. In Fig. 7, red fluoresce also validates the presence of DOX molecules within the HeLa cells, confirming its effective release. The red fluorescence of DOX molecules appeared to be scattered over the entire nucleus. Figure 7 demonstrates how the S2, S3 and S4 NPs were internalized into the cell membrane which is in good agreement with delivery of BLM by multifunctional core-shell NPs57. Additionally, LSCM results (Fig. 7) proved that the drug release was possible due to enzymatic cleavage of the amide bond and these findings are in line with previous studies58,59. Interestingly, although we have observed that the NPs are stable and do not agglomerate as aqueous colloid dispersion, they appeared to cluster in cell nucleus after the internalization, possibly owing to the combined effect of an intra-cellular spatial confinement and intrinsic biochemistries (e.g. reduction condition, enzymes, etc. that could affect the surface PEG ligands stability). In addition, this process depends on the particles concentration and incubation time.
Figure 5.
LC50 and EC50 determination. (a) Flow cytometric analysis of HeLa cells after 24 hours exposure at tested concentrations of NPs; (b) LC50 values (black) of S1, S2, S3 and S4 NPs, EC50 (red) assessment of individual BLM, BLM loaded onto S3, S4; (c) HeLa cell exposed to various concentrations of BLM; (d) Percentage of live cells analysis after HeLa cell exposed for 24 hours. Inset: drug release percentage (%) overtime in various dispersing media at 37 °C.
Figure 6.
FL2-A histograms, illustrating the nanoparticles (S1, S2, S3 & S4) effects on HeLa cells cycle. Cell cycle analysis for different stages of cells were carried out by manual grating on FL2-A histograms after 24 hours exposure.
Table 2.
HeLa cells’ 24 hours exposure against tested concentrations of PEG-AuNPs and NPs-effects on cell cycle along with untreated and BLM-treated value.
| Sample | Concentration (M) | Sub G1 average | G0/G1 phase average | S phase average | G2/M phase average |
|---|---|---|---|---|---|
| Untreated | 0 | 0.3 | 54.3 | 13.7 | 28.4 |
| BLM-treated | 1.5 µM | 0.5 | 26.1 | 24.6 | 44.2 |
| S1 | 70 nM | 0.4 | 53.6 | 14.6 | 27.4 |
| 80 nM | 0.3 | 53.2 | 13.8 | 30.0 | |
| S2 | 50 nM | 0.7 | 56.6 | 12.7 | 26.5 |
| 60 nM | 0.6 | 53.7 | 13.1 | 30.2 | |
| S3 | 30 nM | 0.9 | 47.1 | 21.2 | 27.1 |
| 40 nM | 0.7 | 42.1 | 21.9 | 31.3 | |
| S4 | 10 nM | 1.1 | 40.2 | 20.4 | 32.3 |
| 20 nM | 0.9 | 39.7 | 19.7 | 33.1 |
Figure 7.
Confocal microscopic images data of HeLa cells. Cells were treated with different concentrations of S2, S3 and S4 NPs for 24 hours. First column S2, second column S3 and third column S4. The first row represents cells stained with BLM alone, the second row signifies for S3 and the third row represents an overlay of S4 product.
Additionally, the red fluorescence images confirm the presence of DOX molecules which therefore, endorses this hypothesis. The inset Fig. 5(d) explains the drug release behaviour of S3 and S4 for both acidic and neutral pHs. At both pHs, the AuNPs exhibited a lower drug release profile confirming the stability of the amide bond at neutral pH that allows for triggered drug release at the acidified environment of a cancerous site. Moreover, amide bond stability can improve drug pharmacokinetic profiles as well as its circulation time in the blood60. However, in more complex realistic situation in vivo a tumor environment is characterized not only by acidification, but also by presence of trypsins, enzymes capable of amid bond scission. Stability of amid bond allows for longer circulation of the pro-drug in blood. Once the nanocarrier reaches the cancer site, the bond can be cleaved allowing for on-demand tumour environment-mediated drug release. Moreover, drug release can be further enhanced via application of an external stimulus, such as laser light (see Supplementary Fig. S2).
The hydrodynamic diameters (Table 1 & Fig. 4) did not impact the drug release when HeLa cells were incubated with PEG-AuNPs at 37 °C. The drug release performance was also assessed in DMEM (Fig. 5d) in order to further prove this hypothesis. Once again these results confirmed that enzymatic cleavage inside of cancer cells is required to attain significant drug release. Thus, an additional benefit of our designed surface-modified AuNPs as drug carriers, is that they can be used for drug release specifically to cancer cells while the drug interaction probability with healthy cells is limited. The BLM-capped AuNPs can be effectively used to treat highly resistant cancer cells61. Nevertheless, our aim was to use low concentration of AuNPs as well as S3 and S4 in this study. The promising low concentrations of anticancer drug based AuNPs would lead this approach to early phase preclinical application in the near future. The minimization of drug side effects will be the most rewarding factor in terms of improving the quality of life for cancer patients.
Conclusion
In conclusion, in the present paper we report on the designing of stable BLM-capped DOX complex on PEG-AuNPs for efficient drug delivery. PEG-AuNPs (S1) nanocarriers can be efficiently applied for combined anticancer drugs delivery to HeLa cells via an active targeting approach, at lower doses, while maintaining drug cytotoxic activity. This study suggests the following important advantages: first, synthesized BLM-capped DOX conjugation onto gold nanostructure have excellent stability, drug loading capacity and specific cancer cell environment-mediated release behaviour; second, enhanced EC50 values for BLM capped-DOX complex as compared to that of free BLM drug as well as the carrier loaded with individual drugs against HeLa cells was observed. In light of these results and the “two-punch” approach, we believe that our proposed work offers an excellent opportunity to design nanocarriers with active targeting against ovarian cancer. While improving their bio-distribution and bioavailability profiles in vivo, further studies are envisaged to progress ovarian cancer treatments.
Materials and Methods
Analytical grade reagents gold chloride trihydrate (HAuCl4·3H2O), N-hydroxysuccinimide (C4H5NO3), sodium tetrahydroborate (NaBH4), polyethylene glycol (PEG), bleomycin (BLM) and cysteamine (~95%) were obtained from (Alfa Aesar, China), (1-ethyl-3-(3-dimethylaminopropyl)-carbodimide hydrochloride) (EDC) and ethanol were purchased from (Aladdin Reagent, China). Doxorubicin was purchased from the Iranian Red Crescent Society. All stock solutions were prepared in purified water (Milli-Q Plus 185 Water Purifier, USA) having a resistivity close to 18 MΩ.cm. Experiments were conducted at room temperature. All reagents were used as provided, unless otherwise stated.
Preparation of Au-PEG NPs and nanodrugs
Four steps (S1, S2, S3 and S4) with slight modification were involved in synthesis of PEGylation AuNPs48. Initially, 100 mL of chloroauric acid (HAuCl4.3H2O) was dissolved into 1.25 mL of dicarboxylic PEG and was stirred for 1 h at room temperature. While stirring, 30 mL aqueous solution of sodium tetrahydroborate (0.01 M, NaBH4) was added to this mixture and the solution colour transformed from yellow into bright red. The bright red shade of gold precursor confirmed the presence of PEGylated gold nanoparticles in the solution. Unconjugated PEG was then removed by centrifugation62. Supernatant was then discarded and residue was resuspended in phosphate-buffered saline (PBS) for future usage.
DOX loading on AuNPs
For DOX conjugation onto the S1 surface, a reported procedure48,63 with slight modification was adopted. In brief, S1 was dispersed into an aqueous solution of 60 μL of 1-(3-dimethylaminopropyl)-N′-Ethyl carbodiimide hydrochloride/ N-hydroxysuccinimide (EDC/C4H5NO3) (80/20 mg ratio). Then 3 mL of DOX (18.0 mM) was added to this solution and stirred for three hours. Ultrafiltration (Ultrafree-MC VV Filter, Merck, Germany) was used for separation of DOX conjugated nanoparticles. The DOX loading efficiency was calculated by UV-Vis measurements at 480 nm from the difference between the DOX conjugated to the S1 and the amount of unbound DOX in the supernatant using following equation:
| 1 |
BLM loading onto S1 and S2 surface
The BLM conjugation onto S1 and S2 surfaces was performed in PBS at pH 7. Briefly, 200 µL (0.1 µg/mL) of BLM in PBS was dissolved into 5 mL of S1 or S2 and stirred for three hours. The chemical processes involved in surface immobilization of PEG-AuNPs are shown in Fig. 1(a). In brief, gold substrates were soaked in 2-aminoethanethiol solution in the dark for 4 hours. The extra thiol was removed by twice washing the gold substrates with milli-Q water and ethyl alcohol. Synthesized PEG-AuNPs were then mixed in 2 mL of phosphate buffered saline containing 0.5MEQ of N-hydroxysuccinimide (NHS) and stirred at 25 °C for three hours. This solution was released for drying overnight. Figure 1(a) represents the synthesis steps involved in this study and Fig. 1(b) shows conjugation of BLM onto the surface of PEG-AuNPs. The successful conjugations were monitored by zeta potential and dynamic light scattering (DLS).
Material characterization
The XRD pattern of the PEG-AuNP samples was recorded by a Jordan Valley’s D1 (Jordan Valley Semiconductors Ltd) equipped with a Cu Kα (λ = 1.5406 A°) radiation filter. The obtained PEG-AuNP XRD patterns were then compared with JCPDS cards (JCPDS # 04–0784) present in the XRD software. The chemical structure and functional groups of the PEG-AuNPs were analysed by Fourier transform infrared spectroscopy (FT-IR, Bruker Optics IFS 66 V/S, Germany). The spectrum was measured in transmission mode ranging from 4000–600 cm−1 wave numbers by KBr pellet method. UV–visible spectroscopy was performed with a double-beam Lambda 850 UV/VIS spectrophotometer (Perkin-Elmer Inc., USA). Conjugated and unconjugated samples were analysed in the 400–900 nm spectral range. All spectra were recorded using a 0.0125 mg/mL solution of the PEG-AuNPs. The assembly and morphology of PEG-AuNPs was studied with TEM (JEOL, JEM 2100F). TEM samples were prepared onto carbon-coated copper grids (Ted Pella, USA) and were dried at room temperature for microscopic observation. Electrophoretic mode of zetasizer (Malvern, UK) was used to measure the zeta potential (ζ) on PEG-AuNPs surface. The PEG-AuNPs hydrodynamic diameters were measured by using nanoparticle tracking analysis (NTA). NTA was carried out at room temperature by using a NanoSight system (Malvern, UK).
Cell culture
HeLa cells were obtained from the School of Life Sciences and Technology, UESTC, China. The cells cultivated in DMEM (SH30081, HyClone, USA) supplemented with 10% fetal bovine serum (FB15011 Clark Bioscince, USA) and 45 U/mL of penicillin-G. Cells were maintained at 37 °C under 95% moisturized air with 5% CO2. Prior to experiment, cells were trypsinized (0.25% Trypsin plus EDTA, HyClone, USA) and stored in canted neck flasks (Corning Inc., USA) under completely aseptic conditions.
Flow cytometric analysis and LC50 and EC50 determination
5000 HeLa cells/well were seeded into a 24-well plate and exposed for 24 hours to various concentrations (ranging from 10 to 100_nM) of S1, S2, S3 and S4 products. Negative controls (untreated cells) and positive control (treated cells) were also studied in our experiment for comparison. The most effective concentrations of PEG-AuNPs that resulted in sub-cytotoxic effects were as follows: (70, 80) nM for S1, (50, 60) nM for S2, (30, 40) nM for S3 and (10, 20) nM for S4 products. After incubation, HeLa cells were washed twice with PBS and transferred to Eppendorf tubes containing fresh media. Cells were then centrifuged for ten minutes at 6000 rpm. Supernatants were then discarded and lysis buffer was added. A flow cytometer (Accuri™ C6, Becton Dickinson, UK) and DNA Reagent Kit (Becton Dickinson, UK) were used for cell counting and cell cycle analysis. Count times were kept constant for all samples and cells populations were investigated. FSC-A versus FSC-H graphs were used to analyze the cell nuclei, with debris being excluded by applying a gate (P1). 10,000 events were acquired in the gate and a pulse geometry gate (such as FSC-H x FSC-A) was applied at low scatter intensities to eliminate cell debris. Cell aggregates were excluded from analysis using gating (P2 in P1) and doublet elimination. All measurements were repeated three times and data is reported as dose-response curves. From the obtained curves the LC50 data for PEG-AuNPs and EC50 of BLM were extrapolated. Analysis of cell cycle for four distinct phases (Sub G1, G0/G1, S, and G2/M) was carried out through manual gating on FL2-A histogram64.
Cellular internalization and imaging of HeLa cells with AuNPs
The cellular internalization of drug in HeLa cells was recorded by using an inverted laser scanning confocal microscope (Olympus, IX71, Japan) equipped with 633 nm optical system. The fluorescence images of AuNPs was excited by a 561 nm laser. Cells morphology were analysed by recording differential interference contrast (DIC) snapshot in a transmission mode. Drug delivery measurements were performed at λexc = 561 nm as previously described22. The AuNPs’s reflectance was higher than cellular organelles. Furthermore by removing the 630 nm optical filter, confocal reflectance mode was used to record highly reflected light from AuNPs. Snapshots were used as a qualitative technique.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors would like to acknowledge the Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China (UESTC) for all the support provided. Work at Argonne National Laboratory and use of the Centre for Nanoscale Materials was supported by the US. Department of Energy (DOE), Office of Sciences, Basic Energy Sciences (BES), under Contract No. DE-AC02-06CH11357. The support of the Ministry of Education and Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST «MISiS» (№K3-2017-070), implemented by a governmental decree dated 16th of March 2013, N 211 is gratefully acknowledged.
Author Contributions
M.U.F., and H.W. performed the experiments, nanoparticle synthesis, coating of the samples and drafted the manuscript. A.A.F. helped in cell culturing, UV-Vis and imaging, E.A.R., and V.N. assisted with data analysis and interpretation, and wrote the manuscript, A.N., A.A., P.B.N., and Z.W. discussed the results, and assisted with the manuscript revision.
Competing Interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-022-15966-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/5/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-022-15966-1
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21331-y.
References
- 1.American Association for Cancer Research (AACR), Cancer Progress Report. https://www.aacrfoundation.org/Pages/cancer-progress-report.aspx (2017).
- 2.National Cancer Institute at National Institutes of Health (NIH), Cancer Stat Facts: Ovarian Cancer, https://seer.cancer.gov/statfacts/html/ovary.html (2016).
- 3.Chasan, R. Cervical Cancer, Yesterday, Today and Tomorrow, NIH, Research Timelines. https://www.report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. (2013).
- 4.Schiller JT, Castellsagué X, Garland SM. A Review of Clinical Trials of Human Papillomavirus Prophylactic Vaccines. Vaccine. 2012;30:F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildesheim A, et al. Effect of Human Papillomavirus 16/18 L1 Viruslike Particle Vaccine Among Young Women With Preexisting Infection. Jama. 2007;298:743. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 6.Moss C, Kaye SB. Ovarian cancer. European Journal of Cancer. 2002;38:1701–1707. doi: 10.1016/s0959-8049(02)00161-2. [DOI] [PubMed] [Google Scholar]
- 7.Lin C-J, et al. Integrated self-assembling drug delivery system possessing dual responsive and active targeting for orthotopic ovarian cancer theranostics. Biomaterials. 2016;90:12–26. doi: 10.1016/j.biomaterials.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nature Reviews Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng G, et al. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature nanotechnology. 2009;4:669–673. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 10.Bessar H, et al. Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids and surfaces. B, Biointerfaces. 2016;141:141–147. doi: 10.1016/j.colsurfb.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Cythlmmune and Aurimune: A nanomedicine platform. http://cytimmune.com/#pipeline. (2015).
- 12.Pan Y, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 13.Sung JH, et al. Subchronic inhalation toxicity of gold nanoparticles. Particle and fibre toxicology. 2011;8:16. doi: 10.1186/1743-8977-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber A, Bundschuh M, Klingelhofer D, Groneberg DA. Gold nanoparticles: recent aspects for human toxicology. Journal of occupational medicine and toxicology. 2013;8:32. doi: 10.1186/1745-6673-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate chemistry. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 16.Bozich JS, et al. Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environ. Sci.: Nano. 2014;1:260–270. doi: 10.1039/c4en00006d. [DOI] [Google Scholar]
- 17.Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL. Toxicity of engineered nanoparticles in the environment. Analytical chemistry. 2013;85:3036–3049. doi: 10.1021/ac303636s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? Journal of nanoparticle research: an interdisciplinary forum for nanoscale science and technology. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muddineti OS, Ghosh B, Biswas S. Current trends in using polymer coated gold nanoparticles for cancer therapy. Int J Pharm. 2015;484:252–267. doi: 10.1016/j.ijpharm.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y-W, Huang C-C, Chang H-T. Gold nanoparticle probes for the detection of mercury, lead and copper ions. The Analyst. 2011;136:863–871. doi: 10.1039/c0an00652a. [DOI] [PubMed] [Google Scholar]
- 21.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Movia D, et al. A safe-by-design approach to the development of gold nanoboxes as carriers for internalization into cancer cells. Biomaterials. 2014;35:2543–2557. doi: 10.1016/j.biomaterials.2013.12.057. [DOI] [PubMed] [Google Scholar]
- 23.Fratoddi I, Venditti I, Cametti C, Russo MV. The puzzle of toxicity of gold nanoparticles. The case-study of HeLa cells. Toxicology Research. 2015;4:796–800. doi: 10.1039/c4tx00168k. [DOI] [Google Scholar]
- 24.Liu D, Wang Z, Jiang X. Gold nanoparticles for the colorimetric and fluorescent detection of ions and small organic molecules. Nanoscale. 2011;3:1421. doi: 10.1039/c0nr00887g. [DOI] [PubMed] [Google Scholar]
- 25.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chemical Society Reviews. 2009;38:1759. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 26.Spadavecchia J, et al. Targeted polyethylene glycol gold nanoparticles for the treatment of pancreatic cancer: from synthesis to proof-of-concept in vitro studies. International journal of nanomedicine. 2016;11:791–822. doi: 10.2147/IJN.S97476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Molecular pharmaceutics. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 28.Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP. Evaluation of the toxicity of intravenous delivery of auroshell particles (gold-silica nanoshells) International journal of toxicology. 2012;31:584–594. doi: 10.1177/1091581812465969. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JA, et al. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer research. 2009;69:1659–1667. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- 30.Libutti SK, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng F, Cheng R, Deng C, Zhong Z. Intracellular drug release nanosystems. Materials Today. 2012;15:436–442. doi: 10.1016/s1369-7021(12)70195-5. [DOI] [Google Scholar]
- 32.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nature medicine. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 33.Ruan S, et al. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–435. doi: 10.1016/j.biomaterials.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Sosnik A, Carcaboso AM, Glisoni RJ, Moretton MA, Chiappetta DA. New old challenges in tuberculosis: potentially effective nanotechnologies in drug delivery. Adv Drug Deliv Rev. 2010;62:547–559. doi: 10.1016/j.addr.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Cui R, et al. Gold Nanoparticle-Colloidal Carbon Nanosphere Hybrid Material: Preparation, Characterization, and Application for an Amplified Electrochemical Immunoassay. Advanced Functional Materials. 2008;18:2197–2204. doi: 10.1002/adfm.200701340. [DOI] [Google Scholar]
- 36.Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Advanced Drug Delivery Reviews. 2010;62:346–361. doi: 10.1016/j.addr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eck W, et al. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS nano. 2008;2:2263–2272. doi: 10.1021/nn800429d. [DOI] [PubMed] [Google Scholar]
- 38.Cheng MM, et al. Nanotechnologies for biomolecular detection and medical diagnostics. Current opinion in chemical biology. 2006;10:11–19. doi: 10.1016/j.cbpa.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Baptista P, et al. Gold nanoparticles for the development of clinical diagnosis methods. Analytical and bioanalytical chemistry. 2008;391:943–950. doi: 10.1007/s00216-007-1768-z. [DOI] [PubMed] [Google Scholar]
- 40.Dong-Hyun K, Rozhkova EA, Rajh T, Bader SD, Novosad V. Synthesis of Hybrid Gold/Iron Oxide Nanoparticles in Block Copolymer Micelles for Imaging, Drug Delivery, and Magnetic Hyperthermia. IEEE Transactions on Magnetics. 2009;45:4821–4824. doi: 10.1109/tmag.2009.2024000. [DOI] [Google Scholar]
- 41.You J, et al. Pharmacokinetics, clearance, and biosafety of polyethylene glycol-coated hollow gold nanospheres. Particle and fibre toxicology. 2014;11:26. doi: 10.1186/1743-8977-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trachtenberg AJ, Filippini N, Mackay CE. The effects of APOE-epsilon4 on the BOLD response. Neurobiology of aging. 2012;33:323–334. doi: 10.1016/j.neurobiolaging.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Jablensky A, et al. Polymorphisms associated with normal memory variation also affect memory impairment in schizophrenia. Genes, brain, and behavior. 2011;10:410–417. doi: 10.1111/j.1601-183X.2011.00679.x. [DOI] [PubMed] [Google Scholar]
- 44.Spadavecchia J, Moreau J, Hottin J, Canva M. New cysteamine based functionalization for biochip applications. Sensors and Actuators B: Chemical. 2009;143:139–143. doi: 10.1016/j.snb.2009.09.007. [DOI] [Google Scholar]
- 45.Stubbe J, Kozarich JW. Mechanisms of bleomycin-induced DNA degradation. Chemical reviews. 1987;87:1107–1136. doi: 10.1021/cr00081a011. [DOI] [Google Scholar]
- 46.Leach HG, Chrobak I, Han R, Trojanowska M. Endothelial cells recruit macrophages and contribute to a fibrotic milieu in bleomycin lung injury. American journal of respiratory cell and molecular biology. 2013;49:1093–1101. doi: 10.1165/rcmb.2013-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. The Journal of pharmacy and pharmacology. 2013;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 48.Spadavecchia J, et al. Amplified plasmonic detection of DNA hybridization using doxorubicin-capped gold particles. The Analyst. 2014;139:157–164. doi: 10.1039/c3an01794j. [DOI] [PubMed] [Google Scholar]
- 49.Philip D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy. 2009;73:374–381. doi: 10.1016/j.saa.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 50.C. Martínez J, A. Chequer N, L. González J, Cordova T. Alternative Metodology for Gold Nanoparticles Diameter Characterization Using PCA Technique and UV-VIS Spectrophotometry. Nanoscience and Nanotechnology. 2013;2:184–189. doi: 10.5923/j.nn.20120206.06. [DOI] [Google Scholar]
- 51.Li C, et al. Au+-cetyltrimethylammonium bromide solution: A novel precursor for seed-mediated growth of gold nanoparticles in aqueous solution. Nano Research. 2012;6:29–37. doi: 10.1007/s12274-012-0278-2. [DOI] [Google Scholar]
- 52.Link S, El-Sayed MA. Optical properties and ultrafast dynamics of metallic nanocrystals. Annual review of physical chemistry. 2003;54:331–366. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- 53.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nature nanotechnology. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 54.Sahu P, Shimpi J, Lee HJ, Lee TR, Prasad BL. Digestive Ripening of Au Nanoparticles Using Multidentate Ligands. Langmuir: the ACS journal of surfaces and colloids. 2017;33:1943–1950. doi: 10.1021/acs.langmuir.6b03998. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, et al. Fabrication of highly sensitive gas sensor based on Au functionalized WO3 composite nanofibers by electrospinning. Sensors and Actuators B: Chemical. 2015;220:1112–1119. doi: 10.1016/j.snb.2015.05.121. [DOI] [Google Scholar]
- 56.Saulis G, Saule R. Size of the pores created by an electric pulse: microsecond vs millisecond pulses. Biochimica et biophysica acta. 2012;1818:3032–3039. doi: 10.1016/j.bbamem.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Georgelin T, Bombard S, Siaugue JM, Cabuil V. Nanoparticle-mediated delivery of bleomycin. Angewandte Chemie. 2010;49:8897–8901. doi: 10.1002/anie.201003316. [DOI] [PubMed] [Google Scholar]
- 58.Etrych T, Subr V, Laga R, Rihova B, Ulbrich K. Polymer conjugates of doxorubicin bound through an amide and hydrazone bond: Impact of the carrier structure onto synergistic action in the treatment of solid tumours. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2014;58:1–12. doi: 10.1016/j.ejps.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Ulbrich K, et al. Polymeric drugs based on conjugates of synthetic and natural macromolecules. Journal of Controlled Release. 2000;64:63–79. doi: 10.1016/s0168-3659(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 60.Wong PT, Choi SK. Mechanisms of drug release in nanotherapeutic delivery systems. Chemical reviews. 2015;115:3388–3432. doi: 10.1021/cr5004634. [DOI] [PubMed] [Google Scholar]
- 61.Jelveh S, Chithrani DB. Gold nanostructures as a platform for combinational therapy in future cancer therapeutics. Cancers. 2011;3:1081–1110. doi: 10.3390/cancers3011081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS nano. 2010;4:1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pippa, N., Pispas, S., Demetzos, C. & Sivolapenko, G. Advanced nanocarriers for an antitumor peptide. Journal of Nanoparticle Research15, 10.1007/s11051-013-2062-y (2013).
- 64.Saquib Q, et al. Phorate-induced oxidative stress, DNA damage and transcriptional activation of p53 and caspase genes in male Wistar rats. Toxicology and applied pharmacology. 2012;259:54–65. doi: 10.1016/j.taap.2011.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.