Abstract
A simple scheme to determine charge distribution in endohedral complexes is suggested. It is based on comparison of inner-shell atomic orbital energies of the encapsulated species to the corresponding energies in reference systems with unambiguously defined charges on X. This robust approach is applied to endohedral borospherenes X@B39, for which the conventional schemes provide in some cases quite different results. Efficiency of proposed scheme also has been proven for typical fullerene based Sc3N@C80 endohedral complex.
Introduction
Atomic charge is one of the most widely used concepts in chemistry. But, it can unambiguously be defined only in some trivial cases. For instance, in homogeneous diatomic molecules with the total charge Q, the charge on each atom is equal to Q/2. In general, however, some assumptions should be made in order to quantify the charge distribution in a molecular system. Many different schemes to define atomic charges have been developed. Most popular of them are schemes based on the distribution of the electron density obtained from quantum-mechanical calculation and its subsequent transformation into atomic charges –Mulliken1, Lowdin2, Hirshfeld3, CM54, Weinhold5,6 (natural population analysis, NPA), and Bader7,8 (quantum theory of atoms in molecules, QTAIM) population analysis.
Discovery of buckminsterfullerene C609, and its La encapsulated derivative10–12 made a new milestone in chemistry. Fullerenes containing confined atoms or small molecules are called endohedral fullerenes (EFs). Most often the incarcerated species is a metal atom or metallic cluster, and such systems are called endohedral metallofullerenes (EMFs)13–22. Charge transfer between the encapsulated species and the cage in EMFs is important because it determines the most suitable cage among the many possible isomers. For instance, in Sc2C2@C68 with a formal charge transfer of 4 electrons, the cage corresponds to the 6073 isomer23, whereas the cage that encapsulates Sc3N@C68 with a formal charge of 6 electrons is the 614024. Although many EMFs have been intensively studied they continue to attract much attention of researchers from various fields of natural sciences. Recent discovery by Zhai et al. of all-boron fullerenes B40− and B400 (referred in literature as borospherenes)25 has given a new impetus to the chemistry of endohedral compounds and stimulated a large number of experimental and theoretical studies. A few months later Bai et al. studied computationally the viability of endohedral metalloborospherenes M@B40 (M = Ca, Sr). DFT calculations revealed great stability due to the almost perfect match in size of encapsulated fragment and cage. It was found that all these metalloborospherenes are formally charge-transfer M2+B402− complexes26. Other endohedral complexes of borospherenes based on B36− 27, B382− 28, and B39− 29, cages demonstrate similar behaviour. A number of works predicting the existence of endohedral borospherenes have been published very recently30–34. Making an analogy with carbon fullerenes we can expect that endohedral borospherenes will be synthetized at an early date.
Results and Discussions
Focusing our attention on endohedral Cl@B39 borospherene complex based on B39 cage subunit, we have encountered the problem of the reliability of charge determination on central fragment. The population analysis carried out within various schemes demonstrates significantly different distribution of charges. However, a reliable definition of the charge localized on the encapsulated fragment as well as its changes is important for the understanding of chemical reactivity, and, in particular, for photo-induced charge transfer (CT) process in endohedral compounds35–38.
In this work, we present a new scheme for assessment of charge distribution on endohedral complexes. Proposed method is based on straightforward physical model and could be used as a convenient tool for the culling of inadequate charge schemes. All calculations were performed with the PBE0/Def2-TZVP (with ECP-28 and ECP-60 for Ag and Au atoms39 correspondently) method40–42 using Gaussian 09 program43.
Numerous proofs that metal-encapsulating borospherenes could demonstrate remarkable charge transfer properties have been recently reported. Among them, the work published by Chen et al.29 demonstrates at first-principles level the viability of the axially chiral metalloborospherenes Ca@B39+. At the same time there are no examples of halogen encapsulated borospherenes, despite the fact that such complex is a unique system of halogen encapsulate into superhalogen unit. The uniqueness of the Cl@B39 complex is that both fragments exhibit high electron affinity (Cl – 3.612 eV44, B39 – 3.845 eV45). Thus it seems extremely difficult to predict charge distribution in such system. With a certain degree of confidence it can be assumed that charge separation should be observed, however it is completely unclear which of the fragments will be negatively charged and which positively.
Charge distribution analysis for B39 boroshperene complexes
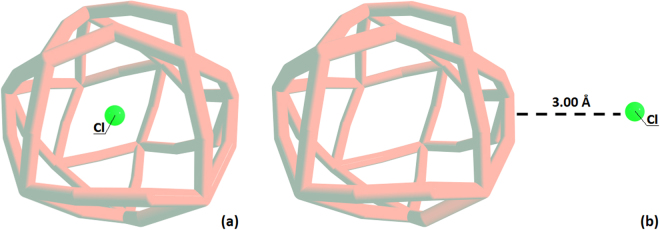
We focused our attention on complexes such as Cl@B39 (Fig. 1a), in particular on the description of charge states in such systems using various approaches. We found that charges on internal fragment, as well as on the cage, depend dramatically on the population scheme used.
Figure 1.
Graphical representation of endohedral boroshperene complex Cl@B39 (a) and (B39 + Cl) vdW complex (b).
In contrast, for van der Waals complex (Fig. 1b) composed of B39 and Cl separated by 3 Å, (B39 + Cl) vdW, the predicted charges demonstrate a significant similarity (Table 1).
Table 1.
Charge density analysis (in electrons) performed with Mulliken, Löwdin, Hirshfeld, CM5, QTAIM, and Natural Population Analysis (NPA) schemes for endohedral Cl@B39 and (B39 + Cl) van der Waals (vdW) complex obtained at PBE0/Def2-TZVP level of theory. Total charge is in all cases zero.
| Mulliken | Löwdin | Hirshfeld | CM5 | QTAIM | NPA | |
|---|---|---|---|---|---|---|
| Cl@B39 | ||||||
| B39 | 0.111 | −0.758 | −0.002 | −0.114 | 0.658 | 0.560 |
| Cl | −0.111 | 0.758 | 0.002 | 0.114 | −0.618 | −0.560 |
| (B39 + Cl) vdW | ||||||
| B39 | 0.410 | 0.239 | 0.393 | 0.385 | 0.580 | 0.480 |
| Cl | −0.410 | −0.239 | −0.393 | −0.385 | −0.547 | −0.480 |
As can be seen from Table 1, for Cl@B39 complex, charge on the Cl fragment can vary from −0.62 e (QTAIM) to 0.76 e (Löwdin) depending on the used scheme. At the same time, charge on the cage ranges from −0.76 e (Löwdin) to 0.66 e (QTAIM). Presuming that behaviour of these fragments in endohedral complex does not change significantly, the reliability of the charges obtained based on Löwdin population analysis (QCl = 0.76), as well as on Hirshfeld (QCl = 0.00) and CM5 (QCl = 0.14) schemes should be called into question. For the van de Waals complex, the obtained charge values on the Cl atom are much closer to each other and vary from −0.24 e (Löwdin) to −0.58 e (QTAIM).
The main problem in this case is the selection of the most reliable method/scheme of charge assessment, which is almost impossible without resorting to chemical intuition.
Model description
It is well known that the ionization potential (binding energy) of the inner-shell electrons of an atom in a molecular system depends on the charge state and chemical surroundings of the atom46. A linear relationship of the binding energy (Eb) measured by X-ray photoelectron spectroscopy (XPS) and an atomic charge q computed with quantum mechanical methods (Eq. (1)) was established for different classes of chemical compounds
| 1 |
The XPS shift ΔEb is determined with respect to the binding energy Eb0 found for a reference. Further studies showed, however, that the electrostatic potential φ created by chemical surroundings has to be accounted for47
| 2 |
There are inherent physical limitations that prevent accurate determination of the atomic charges. In particular, the parameters q and φ in Eq. (2) refer to the ground electronic state of the system, while the measured XPS shift, ΔEb, reflects both the ground and the final excited state (with a hole in the inner shell). To separate the contributions Aq and φ, some additional assumptions should be used. In spite of these and some other restrictions, XPS has been widely employed for chemical analysis48.
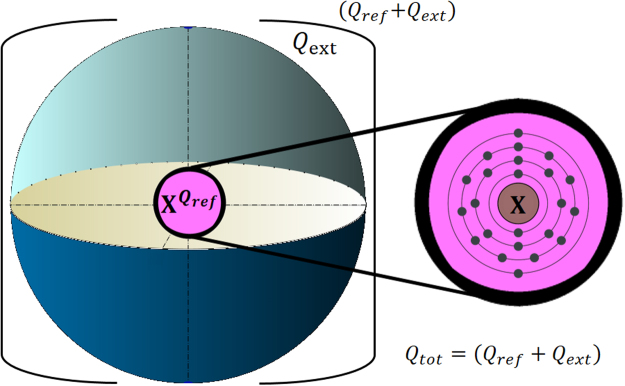
Let’s consider a model system consisting of a multi-electron fragment X with partial charge Qref incorporated into a cage constructed of N atoms with partial charge Qext (Fig. 2).
Figure 2.
Physical model used in this work.
Similar to Eq. (2) we assume that the orbital energy of core electrons of an atom of fragment X (X consists of either a single atom or several atoms) can be approximately presented as a simple function of the charge QX on fragment X, corrected by the external electrostatic potential φex on atom due to the cage
| 3 |
The non-trivial point here is how to calibrate so that the charge QX in the reference systems is uniquely defined.
We propose the following step-by-step scheme to derive the charge on X in X@Cage species:
- One constructs reference systems where charge on X is unambiguously defined. The reference systems for such a situation include the fragment X carrying the total charge Qref (e.g. Qref = −1, 0, + 1 etc) embedded into a sphere having the charge Qext. The total charge Qtot of the reference system, Qtot = Qref + Qext, is equal to the charge of X@Cage. In most cases X@Cage is neutral (Qtot = 0) and thus for any reference system Qext = −Qref. If X@Cage is charged, Qext = Qtot − QX. The radius of the sphere R is determined by the cage geometry. For a cage comprising N atoms, R can be defined as
where ri is the distance from atom i in the cage to the center of the cage determined by the non-weighted mean of the coordinates of the atoms in the cage. In this approach:4 5 -
Quantum mechanical calculation with molecular charge Qref (e.g. Qref = −1, 0, + 1, etc.) are carried out. The computed orbital energy of inner shell K, , together with the electrostatic potential created by the sphere provides :
6 The found values are used to obtain a quadratic interpolation of the dependence. Note that the encapsulated species X comprise usually only several atoms and its calculation is very fast.
Quantum mechanical calculations of X@Cage provide the value in the “real” system. Using the interpolated function derived in the previous step we could directly obtain the charge QX on the encapsulated fragment.
These three steps represent the simplest scheme to estimate charge separation between encapsulated species X and the cage in endohedral fullerenes and in similar structures. As shown below, a more elaborated treatment of the electrostatic embedding in the reference systems does not provide significantly different results. Also, as we will see, the proposed scheme is quite robust to choosing the inner shell K in X when constructing the interpolated function .
The classical point charge model was used to evaluate the electrostatic correction. This model implies the replacement of cage atoms by array of point charges placed at the nuclei of the constituted atoms. This approach has proven itself well in various fields of chemical science and computational biology49,50.
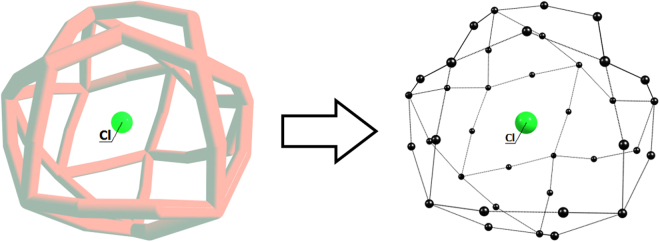
To verify the applicability of the proposed model to the charge assessment of the central fragment in endohedral complexes, the borospherene species Cl@B39 was considered in detail (Fig. 3).
Figure 3.
Transformation of Cl@B39 complex within the point charge model framework necessary for calibration curve construction. Left part of figure corresponds to Cl@B39 complex, right part represents central fragment (Cl) surrounded by compensation charges (black spheres) allocated at former boron nuclei places.
Primary, it is necessary to perform a series of calculations for Cl fragment in various charge states. For this we have selected 5 states – Cl2−, Cl1−, Cl0, Cl1+, and Cl2+ in their ground states. Despite the fact that Cl2+ as well as other halogen cations goes beyond conventional chemical principles and its existence is apparently impossible under ordinary conditions, we will take it into account as a model point to have a wider range of calibration. Including a large set of charge states into calibration makes a proposed scheme more robust and universal due to the fact that even in difficult cases a result can be found within the interpolation grid. Of course, in case of Cl fragment, data about its possible charge states is well known, but in case of more sophisticated encapsulated unit a necessary information can simply not be available. Moreover, it was found that inclusion of 1s orbital energy of Cl2+ unit does not lead to physically meaningless result, therefore we recommend to use greater number of charge states for constructing a calibration curve.
To take into account correction to electrostatic potential, boron atoms forming cage have to be replaced with point charges (Fig. 3) in such way that total compensation charge has to be equal to the charge on Cl fragment in current charge state. For example, for Cl1+ cation compensation charge have to be equal to −1, while for Cl1− fragment the compensation charge have to be equal to +1. It seems obvious that there are more than one way of the compensation charge distribution over cage nodes when the neutrality condition is satisfied.
To investigate the effect of compensation charge distribution on 1s orbital energy we performed calculations for 4 different models (Table S1, ESI). To construct first of them, even charge distribution over the cage were used, while for Cl0 charge state no compensation point charges have been applied (CCS1, Table S1). In next three schemes, the charges on cage were generated randomly to compensate the charge −1 and then be linearly scaled to the charge state −2. In the case of Cl1+ and Cl2+, compensation charges on the cage were obtained from previously generated only with opposite sign (CCS2, CCS3, and CCS4 in Table S1). Last scheme is characterized by random generation of charges on all cage nodes for each state of Cl fragment. Only equality of the charge on Cl fragment and sum of compensation charges was monitored (CCS5, Table S1). Calibration curves and equations of approximate functions are shown in Fig. S1. Detailed analysis of given charge distribution schemes revealed that electrostatic correction for 1 s orbital energy value depends only very weakly on particular compensation charge scheme. For the studied charge distributions, the range of the correction values is about one order of magnitude less than the correction values themselves (Table S2). At the same time, central fragment charges predicted on the basis of the mentioned charge compensation schemes are in the range from −0.44 e to −0.51 e. Thus, taking into account small differences in charge prediction caused by particular charge compensation method, we will use the even charge distribution as a compensation method.
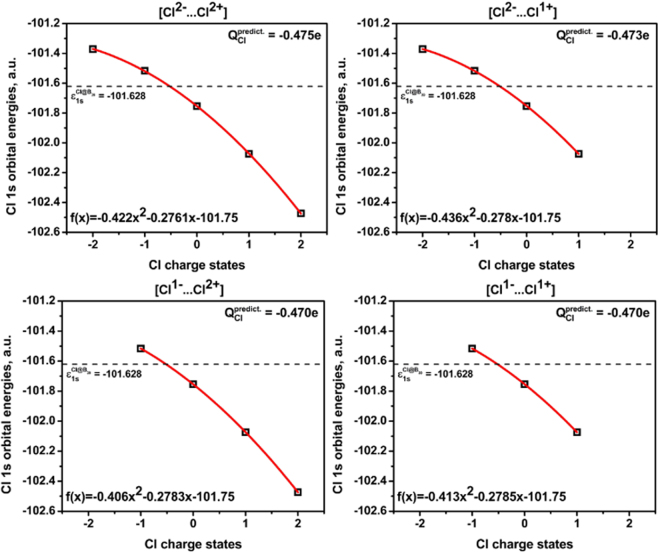
At the next stage of our work, we studied the effect of the charge states taken into consideration on the predicted charge values of the Cl@B39 complex. It was found that the charge predicted within the proposed method depends weakly on charge states of Cl fragments that were taken into account in calibration. Four different calibration curves were built using various Cl charge states – 1 st: [Cl2−…Cl2+] with 5 reference points (QCl = −0.475), 2nd: [Cl2−…Cl1+] with 4 points (QCl = −0.473), 3rd: [Cl1−…Cl2+] with 4 points (QCl = −0.470), and 4th: [Cl1−…Cl1+] with 3 points (QCl = −0.470) (Fig. 4).
Figure 4.
Calibration curves constructed using various number of reference points (top left – [Cl2−…Cl2+] – 5 points, top right – [Cl2−…Cl1+] – 4 points, bottom left – [Cl1−…Cl2+] – 4 points and bottom right – [Cl1−…Cl1+] – 3 points) for charge prediction on central Cl fragment in Cl@B39 complex (Cl 1s orbital energy in complex of interest = −101.628 a.u.).
As can be seen in Fig. 4, minor differences (of less than 0.01 e) in the predicted charge on the Cl fragment obtained from different calibration curves are found. This error is one order of magnitude smaller than the error caused by the use of different charge compensation schemes.
Thus, we can conclude that for studied Cl@B39 complex proposed method has shown its best side. However, we were wondering if proposed method can equally work well with other K-type orbitals. To check this, Cu@B39 complex has been considered. Cu atom has 3 inner s-orbitals. Calibrations curves construct for 1s, 2s, and 3s orbitals based on five Cu charge states [Cu2−…Cu2+] reference points predict charge on central fragment 0.416 e, 0.391 e, and 0.398 e correspondently (Fig. S2, ESI). Thus, one can assume that any inner orbital, as far as it not involved in any orbital interaction with the cage orbitals, could be used for construction of the calibration. The latest suggests that the proposed scheme can be extended to “heavy” elements, for which pseudopotentials are used.
Model verification
Finally, to verify the proposed approach several systems with general formula X@B39, where X represents halogens (F, Cl, Br), small radicals (NO, CN, FO, CF) or metals of 1B group of periodic chart (Cu, Ag, Au) were investigated (Fig. S3). The summary results are presented in Table 2.
Table 2.
Charge density analysis performed with Mulliken, Löwdin, Hirshfeld, CM5, QTAIM, and NPA schemes for endohedral X@B39 complex and the predicted charge of X (Qx predicted)a obtained within the method proposed in this work. Units are electrons.
| X | Mulliken | Löwdin | Hirshfeld | CM5 | NPA | AIM | predict.a |
|---|---|---|---|---|---|---|---|
| F | −0.603 | −0.300 | −0.334 | −0.305 | −0.842 | −0.822 | −0.585 (1s F) |
| Cl | −0.111 | 0.758 | 0.002 | 0.114 | −0.560 | −0.618 | −0.475 (1s Cl) |
| Br | −0.326 | 1.142 | 0.209 | 0.417 | −0.258 | −0.357 | −0.251 (1s Br) |
| NO | 0.444 | 0.842 | 0.458 | 0.473 | 0.127 | 0.030 | 0.212 (1s O) 0.253 (1s N) |
| CN | −0.793 | 0.162 | −0.177 | −0.117 | −1.070 | −0.899 | −0.763 (1s N) −0.841 (1s C) |
| FO | −0.267 | 0.297 | 0.027 | 0.085 | −0.532 | −0.619 | −0.252 (1s F) −0.356 (1s O) |
| CF | 0.014 | 0.865 | 0.472 | 0.582 | 0.001 | 0.086 | 0.176 (1s F) 0.257 (1s C) |
| Cu | 0.187 | 0.306 | 0.487 | 0.825 | 0.754 | 0.719 | 0.416 (1s Cu) 0.391 (2s Cu) 0.398 (3s Cu) |
| Ag | 0.142 | 0.620 | 0.506 | 0.984 | 0.738 | 0.586 | 0.443 (4s Ag) |
| Au | −0.589 | 0.705 | 0.560 | 0.972 | 0.799 | 0.417 | 0.441 (5s Au) |
aInformation in brackets refer to the atoms and orbitals used for calibration.
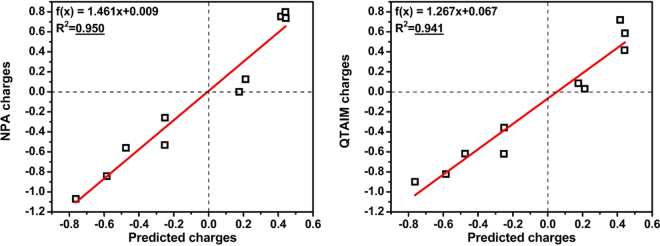
As can be seen from Table 2, the proposed charge assessment approach provides the physically adequate results in all studied cases. For each cases, Mulliken and Löwdin charge assessment schemes provide the worst performance with correlations with the predicted charges of R2 = 0.388 for Mulliken and R2 = 0.172 for Löwdin, respectively. Moreover, sometimes they provide physically meaningless results, such as in case of Au@B39 (Mulliken) or Cl@B39, Br@B39, FO@B39 and CN@B39 (Löwdin) complexes. Hirshfeld charges are quite well correlated with the predicted charges (R2 = 0.909) but the charges are noticeably underestimated. CM5 method yields comparable to Hirshfeld correlation with the predicted results with R2 = 0.896. Equally to Hirshfeld scheme, significant charge underestimation for CM5 is observed. In some cases Hirshfeld and CM5 schemes does not reproduce the sign of charge on the central fragment (Fig. S4, Table S3, ESI). Finally, the best correlations between calculated and predicted charges were found for NPA and QTAIM schemes (Fig. 5).
Figure 5.
Correlations of the NPA and QTAIM charges with the results obtained by the proposed method. Units are electrons.
Thus, we can conclude that the predicted charge assessment method provide results comparable with well-known methods and can be used as a fast and convenient selection tool that avoids assignment of unphysical charges to encapsulated species.
To demonstrate that the proposed method can be applied not only to boron cages but also to a wide range of endohedral compounds, one of the most studied (both synthetically51–53 and theoretically54,55) Sc3N@Ih-C80 cluster has been investigated. Detailed charge distribution analysis within Mulliken, Löwdin, Hirshfeld, CM5, QTAIM, and NPA schemes has been performed. For Sc3N@Ih-C80 complex, calculated charge values on the Sc3N fragment vary from −0.090 e obtained with Mulliken scheme to 0.851 e and 0.969 e for Löwdin and Hirshfeld ones, correspondently. CM5 scheme provides 2.062 e, while NPA and Bader analysis result in 3.426 e and 3.772 e (for more details see ESI). Scheme proposed in current paper predicts the charge on central fragment of 1.502 e based on 1s N orbitals calibration. Thus, with this latter example we show that encapsulated fragment charge estimation scheme described above can be universally used for any endohedral system.
The remarkable feature of this approach is that for fragment X consisting of more than one atom, calibration curve can be constructed using the 1s orbital energy of any of the atoms of the incarcerated molecule. For example, calibration curves constructed using O 1s orbital energies and N 1s orbital energies for NO@B39 complex predict 0.212 e and 0.253 e charge on NO species, respectively (in detail, the procedure for constructing the calibration curves and the charges prediction for denoted NO species and all others considered in this paper is presented in supporting information). An agreement between the results obtained from different calibration curves is a good indicator of reliability of the result. However, it should be emphasized that this charge assessment method does not allow determination of charge on the individual atoms of the encapsulated fragment X, but only provides the charge on the whole fragment. On the other hand, taking into account physical model underlying the method described the following limitations must be noted. First, the proposed model does not take into account orbital interactions. Thus, if orbitals of fragment X are involved in strong orbital interactions, a significant error could be observed. Second, because the compensation charge in our method were treated within the framework of point charge model, a small underestimation of the predicted charge could take a place. The reason for this is that charge associated with particular node of cell is not point charge in fact, but only distributed electron density located somewhat closer to the fragment X, which in turn, will certainly affect the accuracy of the electrostatic correction.
Conclusions
In conclusion, we have developed a new approach for charge assessment of the encapsulated fragment based on the comparison of the low-lying orbital energies of atoms in central fragment in complex of interest with reference systems. The proposed approach demonstrates excellent performance on endohedral borospherenes X@B39 with encapsulated metal atoms, halogens or small radicals. Moreover, the workability of proposed approach has been demonstrated on the typical fullerene based Sc3N@Ih-C80 endohedral complex. In view of the physical and computational simplicity, the proposed method can be applied to very large systems. In cases when conventional schemes provide essentially different results, the proposed method could be used as a convenient and robust tool to exclude unreliable data from consideration. We are convinced that the proposed approach will find application in research of all kinds of endohedral complexes and related fields of chemical science.
Electronic supplementary material
Acknowledgements
We are grateful for financial support from the Spanish MINECO (CTQ2017-85341-P and CTQ2015-69363-P projects), the Catalan DIUE (2014SGR931, XRQTC, and ICREA Academia 2014 Award to M.S.), and the FEDER fund (UNGI10-4E-801). A. J. S. gratefully acknowledges The Interdisciplinary Centre for Mathematical and Molecular Modelling of the University of Warsaw (ICM) for computational facilities (grant no. G33-17).
Author Contributions
A.J.S., M.S. and A.A.V. conceived and designed research, analyzed data, interpreted results and wrote the paper. A.J.S. performed the calculations.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21240-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A. J. Stasyuk, Email: antony.stasuk@gmail.com
M. Solà, Email: miquel.sola@udg.edu
A. A. Voityuk, Email: alexander.voityuk@gmail.com
References
- 1.Mulliken RS. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955;23:1833–1840. doi: 10.1063/1.1740588. [DOI] [Google Scholar]
- 2.Löwdin PO. On the Non‐Orthogonality Problem Connected with the Use of Atomic Wave Functions in the Theory of Molecules and Crystals. J. Chem. Phys. 1950;18:365–375. doi: 10.1063/1.1747632. [DOI] [Google Scholar]
- 3.Hirshfeld FL. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta. 1977;44:129–138. doi: 10.1007/BF00549096. [DOI] [Google Scholar]
- 4.Marenich AV, Jerome SV, Cramer CJ, Truhlar DG. Charge Model 5: An Extension of Hirshfeld Population Analysis for the Accurate Description of Molecular Interactions in Gaseous and Condensed Phases. J. Chem. Theory Comput. 2012;8:527–541. doi: 10.1021/ct200866d. [DOI] [PubMed] [Google Scholar]
- 5.Foster JP, Weinhold F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980;102:7211–7218. doi: 10.1021/ja00544a007. [DOI] [Google Scholar]
- 6.Reed AE, Weinstock RB, Weinhold F. Natural population analysis. J. Chem. Phys. 1985;83:735–746. doi: 10.1063/1.449486. [DOI] [Google Scholar]
- 7.Bader, R. F. W. Atoms in Molecules: A Quantum Theory (Oxford, 1990).
- 8.Bader RFW. A quantum theory of molecular structure and its applications. Chem. Rev. 1991;91:893–928. doi: 10.1021/cr00005a013. [DOI] [Google Scholar]
- 9.Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. [DOI] [Google Scholar]
- 10.Heath JR, et al. Lanthanum complexes of spheroidal carbon shells. J. Am. Chem. Soc. 1985;107:7779–7780. doi: 10.1021/ja00311a102. [DOI] [Google Scholar]
- 11.Chai Y, et al. Fullerenes with metals inside. J. Phys. Chem. 1991;95:7564–7568. doi: 10.1021/j100173a002. [DOI] [Google Scholar]
- 12.Cox DM, Trevor DJ, Reichmann KC, Kaldor A. C60La: a deflated soccer ball? J. Am. Chem. Soc. 1986;108:2457–2458. doi: 10.1021/ja00269a060. [DOI] [PubMed] [Google Scholar]
- 13.Popov AA, Yang S, Dunsch L. Endohedral Fullerenes. Chem. Rev. 2013;113:5989–6113. doi: 10.1021/cr300297r. [DOI] [PubMed] [Google Scholar]
- 14.Castro E, Hernandez Garcia A, Zavala G, Echegoyen L. Fullerenes in Biology and Medicine. J. Mater. Chem. B. 2017;5:6523–6536. doi: 10.1039/C7TB00855D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Wei T, Jin F. When metal clusters meet carbon cages: endohedral clusterfullerenes. Chem. Soc. Rev. 2017;46:5005–5058. doi: 10.1039/C6CS00498A. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Akasaka T, Nagase S. Chemistry of endohedral metallofullerenes: the role of metals. Chem. Commun. 2011;47:5942–5957. doi: 10.1039/c1cc10123d. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, Akasaka T, Nagase S. Endohedral Metal Atoms in Pristine and Functionalized Fullerene Cages. Acc. Chem. Res. 2010;43:92–102. doi: 10.1021/ar900140n. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Stevenson S, Dorn HC. Trimetallic Nitride Template Endohedral Metallofullerenes: Discovery, Structural Characterization, Reactivity, and Applications. Acc. Chem. Res. 2013;46:1548–1557. doi: 10.1021/ar300301v. [DOI] [PubMed] [Google Scholar]
- 19.Rudolf M, et al. Endohedral Metallofullerenes—Filled Fullerene Derivatives towards Multifunctional Reaction Center Mimics. Chem. Eur. J. 2012;18:5136–5148. doi: 10.1002/chem.201102844. [DOI] [PubMed] [Google Scholar]
- 20.Cerón MR, Li F-F, Echegoyen LA. Endohedral fullerenes: the importance of electronic, size and shape complementarity between the carbon cages and the corresponding encapsulated clusters. J. Phys. Org. Chem. 2014;27:258–264. doi: 10.1002/poc.3245. [DOI] [Google Scholar]
- 21.Garcia-Borràs M, Osuna S, Luis JM, Swart M, Solà M. The role of aromaticity in determining the molecular structure and reactivity of (endohedral metallo)fullerenes. Chem. Soc. Rev. 2014;43:5089–5105. doi: 10.1039/C4CS00040D. [DOI] [PubMed] [Google Scholar]
- 22.Popov, A. A. Endohedral Fullerenes: Electron Transfer and Spin (Cham, 2017).
- 23.Shi Z-Q, Wu X, Wang C-R, Lu X, Shinohara H. Isolation and Characterization of Sc2C2@C68: A Metal-Carbide Endofullerene with a Non-IPR Carbon Cage. Angew. Chem. Int. Ed. 2006;45:2107–2111. doi: 10.1002/anie.200503705. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson S, et al. Materials science: A stable non-classical metallofullerene family. Nature. 2000;408:427–428. doi: 10.1038/35044199. [DOI] [PubMed] [Google Scholar]
- 25.Zhai H-J, et al. Observation of an all-boron fullerene. Nat. Chem. 2014;6:727–731. doi: 10.1038/nchem.1999. [DOI] [PubMed] [Google Scholar]
- 26.Bai H, Chen Q, Zhai H-J, Li S-D. Endohedral and Exohedral Metalloborospherenes: M@B40 (M = Ca, Sr) and M&B40 (M = Be, Mg) Angew. Chem., Int. Ed. 2015;54:941–945. doi: 10.1002/anie.201408738. [DOI] [PubMed] [Google Scholar]
- 27.Tian W-J, et al. Saturn-like charge-transfer complexes Li4&B36, Li5&B36+, and Li6&B362+: exohedral metalloborospherenes with a perfect cage-like B364− core. Phys. Chem. Chem. Phys. 2016;18:9922–9926. doi: 10.1039/C6CP01279E. [DOI] [PubMed] [Google Scholar]
- 28.Chen, Q. et al. Endohedral Ca@B38: stabilization of a B382− borospherene dianion by metal encapsulation. Phys. Chem. Chem. Phys.18, 11610–11615, 10.1039/c5cp06169e (2016). [DOI] [PubMed]
- 29.Chen, Q. et al. Endohedral C3Ca@B39+ and C2Ca@B39+: axially chiral metalloborospherenes based on B39. Phys. Chem. Chem. Phys.17, 19690–19694, 10.1039/C5CP03178H (2015). [DOI] [PubMed]
- 30.Liu C, Yang L, Jin P, Hou QH, Li LL. Computational prediction of endohedral dimetalloborofullerenes M2@B80 (M = Sc, Y) Chem. Phys. Lett. 2017;676:89–94. doi: 10.1016/j.cplett.2017.03.054. [DOI] [Google Scholar]
- 31.Li SX, Zhang ZP, Long ZW, Qin SJ. Structures, stabilities and spectral properties of borospherene B44− and metalloborospherenes MB440/− (M = Li, Na, and K) Sci. Rep. 2017;7:40081. doi: 10.1038/srep40081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu QH, et al. Computational investigation on MBn (M = Li-Cs, Be-Ba, Sc-La and Ti; n = 28 and 38) J. Mol. Model. 2016;22:184. doi: 10.1007/s00894-016-3055-4. [DOI] [PubMed] [Google Scholar]
- 33.Bin L, et al. B40 fullerene as a highly sensitive molecular device for NH3 detection at low bias: a first-principles study. Nanotechnology. 2016;27:075501. doi: 10.1088/0957-4484/27/17/174001. [DOI] [PubMed] [Google Scholar]
- 34.Stasyuk AJ, Solà M. Does the endohedral borospherene supersalt FLi2@B39 maintain the “super” properties of its subunits? Phys. Chem. Chem. Phys. 2017;19:21276–21281. doi: 10.1039/C7CP02550E. [DOI] [PubMed] [Google Scholar]
- 35.Kawashima Y, Ohkubo K, Fukuzumi S. Efficient Charge Separation in Li+@C60 Supramolecular Complexes with Electron Donors. Chem. - Asian J. 2015;10:44–54. doi: 10.1002/asia.201403075. [DOI] [PubMed] [Google Scholar]
- 36.Supur M, et al. Graphene oxide- Li+@C60 donor-acceptor composites for photoenergy conversion. Phys. Chem. Chem. Phys. 2015;17:15732–15738. doi: 10.1039/C5CP01403D. [DOI] [PubMed] [Google Scholar]
- 37.Martinez JP, Solà M, Voityuk AA. The Driving Force of Photoinduced Charge Separation in Metal-Cluster-Encapsulated Triphenylamine-[80]fullerenes. Chem. - Eur. J. 2016;22:17305–17310. doi: 10.1002/chem.201603504. [DOI] [PubMed] [Google Scholar]
- 38.Voityuk AA, Solà M. Photoinduced Charge Separation in the Carbon Nano-Onion C60@C240. J. Phys. Chem. A. 2016;120:5798–5804. doi: 10.1021/acs.jpca.6b04127. [DOI] [PubMed] [Google Scholar]
- 39.Andrae D, Häußermann U, Dolg M, Stoll H, Preuß H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta. 1990;77:123–141. doi: 10.1007/BF01114537. [DOI] [Google Scholar]
- 40.Adamo C, Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999;110:6158–6170. doi: 10.1063/1.478522. [DOI] [Google Scholar]
- 41.Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005;7:3297–3305. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- 42.Weigend F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006;8:1057–1065. doi: 10.1039/b515623h. [DOI] [PubMed] [Google Scholar]
- 43.Gaussian 09 Rev. E.01. (full citation in ESI).
- 44.Berzinsh U, et al. Isotope shift in the electron affinity of chlorine. Phys. Rev. A. 1995;51:231–238. doi: 10.1103/PhysRevA.51.231. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q, et al. Experimental and Theoretical Evidence of an Axially Chiral Borospherene. ACS Nano. 2015;9:754–760. doi: 10.1021/nn506262c. [DOI] [PubMed] [Google Scholar]
- 46.Slegbahn, K. et al. ESCA Atomic, Molecular and Solid State Structure Studied by Means of Electron Spectroscopy (Uppsala, 1967).
- 47.Slegbahn, K. et al. ESCA Applied to Free Molecules (Amsterdam/London, 1969).
- 48.Bagus PS, Ilton ES, Nelin CJ. The interpretation of XPS spectra: Insights into materials properties. Surf. Sci. Rep. 2013;68:273–304. doi: 10.1016/j.surfrep.2013.03.001. [DOI] [Google Scholar]
- 49.Lopes PEM, Roux B, MacKerell AD. Molecular modeling and dynamics studies with explicit inclusion of electronic polarizability: theory and applications. Theor. Chem. Acc. 2009;124:11–28. doi: 10.1007/s00214-009-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsunekawa S, Sahara R, Kawazoe Y, Kasuya A. Origin of the blue shift in ultraviolet absorption spectra of nanocrystalline CeO2−x particles. Mater. Trans., JIM. 2000;41:1104–1107. doi: 10.2320/matertrans1989.41.1104. [DOI] [Google Scholar]
- 51.Stevenson S, et al. Small-bandgap endohedral metallofullerenes in high yield and purity. Nature. 1999;401:55–57. doi: 10.1038/43415. [DOI] [Google Scholar]
- 52.Iezzi EB, et al. A Symmetric Derivative of the Trimetallic Nitride Endohedral Metallofullerene, Sc3N@C80. J. Am. Chem. Soc. 2002;124:524–525. doi: 10.1021/ja0171005. [DOI] [PubMed] [Google Scholar]
- 53.Cardona CM, Elliott B, Echegoyen L. Unexpected Chemical and Electrochemical Properties of M3N@C80 (M = Sc, Y, Er) J. Am. Chem. Soc. 2006;128:6480–6485. doi: 10.1021/ja061035n. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Borràs M, Osuna S, Luis JM, Swart M, Solà M. A Complete Guide on the Influence of Metal Clusters in the Diels–Alder Regioselectivity of Ih-C80 Endohedral Metallofullerenes. Chem. - Eur. J. 2013;19:14931–14940. doi: 10.1002/chem.201302202. [DOI] [PubMed] [Google Scholar]
- 55.Aroua S, Garcia-Borràs M, Osuna S, Yamakoshi Y. Essential Factors for Control of the Equilibrium in the Reversible Rearrangement of M3N@Ih-C80 Fulleropyrrolidines: Exohedral Functional Groups versus Endohedral Metal Clusters. Chem. - Eur. J. 2014;20:14032–14039. doi: 10.1002/chem.201403743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.