Abstract
Vaccine delivery is an essential element for the development of mucosal vaccine, but it remains to be investigated how physical barriers such as mucus and cilia affect vaccine delivery efficacy. Previously, we reported that C-terminal fragment of Clostridium perfringens enterotoxin (C-CPE) targeted claudin-4, which is expressed by the epithelium associated with nasopharynx-associated lymphoid tissue (NALT), and could be effective as a nasal vaccine delivery. Mice lacking tubulin tyrosine ligase-like family, member 1 (Ttll1-KO mice) showed mucus accumulation in nasal cavity due to the impaired motility of respiratory cilia. Ttll1-KO mice nasally immunized with C-CPE fused to pneumococcal surface protein A (PspA-C-CPE) showed reduced PspA-specific nasal IgA responses, impaired germinal center formation, and decreased germinal center B-cells and follicular helper T cells in the NALT. Although there was no change in the expression of claudin-4 in the NALT epithelium in Ttll1-KO mice, the epithelium was covered by a dense mucus that prevented the binding of PspA-C-CPE to NALT. However, administration of expectorant N-acetylcysteine removed the mucus and rescued the PspA-specific nasal IgA response. These results show that the accumulation of mucus caused by impaired respiratory cilia function is an interfering factor in the C-CPE-based claudin-4-targeting nasal vaccine.
Introduction
Mucosal vaccines are used clinically to induce antigen-specific immune responses in mucosal tissue as the first line of defense against pathogens1,2. Secretory IgA is an effector molecule that prevents pathogenic invasion and neutralizes toxins2; therefore, mucosal vaccines must efficiently induce secretory IgA.
Mucosa-associated lymphoid tissues (MALTs) play a key role in the induction of antigen-specific secretory IgA responses in mucosal tissues. Nasopharynx-associated lymphoid tissue (NALT) is a representative MALT in the nose3. NALT has efferent, but not afferent, lymph as the conventional site of entry for antigens delivered by antigen-capturing dendritic cells. In addition, M cells exist at the NALT epithelium and act as antigen uptake cells from the nasal cavity to the NALT3. After processing, the antigens are presented to T cells and B cells located in specialized sites within the NALT called germinal centers (GCs), and B cells undergo IgA class switching with the help of follicular helper T cells (Tfh cells)4. Therefore, the delivery of antigens to the NALT is an important means of inducing antigen-specific secretory IgA responses.
The mucosal epithelium acts as a physical barrier to the uptake of antigen into MALT. This barrier function of epithelial cells is established by cell–cell connections called tight junctions, which are specialized connections between adjacent epithelial cells that hold the cells together and regulate the passage of materials across the epithelial membrane5. Tight junctions contain a variety of proteins, including claudins, occludin, tricellulin, angulins, and zonula occludens5,6. Although these tight junction molecules would at first appear to be preventive factors for vaccine delivery, they are actually prospective targets for the delivery of nasal vaccines. Indeed, we previously used Clostridium perfringens enterotoxin (CPE) to target the tight junctions in the mucosal epithelium associated with NALT. CPE binds to claudins in tight junctions through its C-terminus and forms a pore by polymerization through its N-terminus, which disrupts the barrier function of the epithelial layer and causes cytotoxicity7. Because claudin-4 is preferentially expressed in the mucosal epithelium associated with the NALT, including the M cells8–10, we used recombinant C-terminus of CPE (C-CPE) to deliver an antigen to the epithelium without inducing cytotoxicity8,9. In another study, we found that nasally administered pneumococcal surface protein A (PspA), a surface protein expressed by Streptococcus pneumoniae, fused to C-CPE (PspA-C-CPE) preferentially bound to NALT, including to M cells, and induced PspA-specific immune responses in the systemic and respiratory compartments11. We also confirmed that these immune responses were sufficient to protect against respiratory pneumococcal infection11.
In addition to the tight junction, mucus is another physical barrier to effective mucosal vaccination. Mucus is a slippery secretion composed of mucins, serum proteins, inorganic salts, and lipids suspended in water that is produced by goblet cells in the epithelium of the respiratory tract12. Disulfide bonds crosslink the secreted mucins to produce a viscoelastic gel that covers the epithelium and prevents attachment of exogenous materials. The amount of mucus on the surface of the respiratory epithelium is controlled by the beating of the mucocilia, which are hair-like, tubulin-based structures that project from the body of epithelial cells in the respiratory tract13. Several post-translational modifications of the tubulin subunits are necessary for the mucocilia to assume the correct curved morphology and to beat asymmetrically14–17. For example, tubulin glutamylation, which is catalyzed by tubulin tyrosine ligase-like protein 1 (Ttll1)18, adds several glutamic acids to the tubulin C-terminal tail domain, which is essential for ciliary function. We previously demonstrated that knockout of Ttll1 in mice resulted in impaired tubulin glutamylation and a change in mucociliary morphology from the usual curved form to a straight form, which resulted in mucus accumulation in the nasal cavity due to a lack of asymmetry in the mucocilia beating cycle14.
In the present study, we used Ttll1-KO mice to evaluate how the mucocilia and mucus in the nasal cavity affect the antigen-specific immune responses induced by immunization with a C-CPE-based, claudin-4-targeting nasal vaccine.
Results and Discussion
Antigen-specific nasal IgA response was decreased in Ttll1-KO mice nasally immunized with PspA-C-CPE
To examine whether airway mucociliary function affected the efficacy of the claudin-4-targeting nasal vaccine, we nasally immunized Ttll1 mice with PspA-C-CPE once a week for three weeks. One week after the last immunization, we measured the concentration of PspA-specific antibodies with comparing Ttll1-hetero (He) to -KO mice. We first measured the concentration of PspA-specific antibodies in the nasal fluid. PspA-specific nasal IgA prevents colonization, or at least the initial stages of colonization, by S. pneumoniae2. The concentration of PspA-specific IgA antibody in the nasal fluid of Ttll1-KO mice was decreased compared with that of Ttll1-He mice (Fig. 1). In addition to PspA-specific nasal IgA, it is known that PspA-specific serum IgG eliminates S. pneumoniae19. Therefore, we also checked PspA-specific serum IgG. Unexpectedly, we found that the concentration of PspA-specific serum IgG was comparable between Ttll1-He and -KO mice (Supplementary Figure 1).
Figure 1.
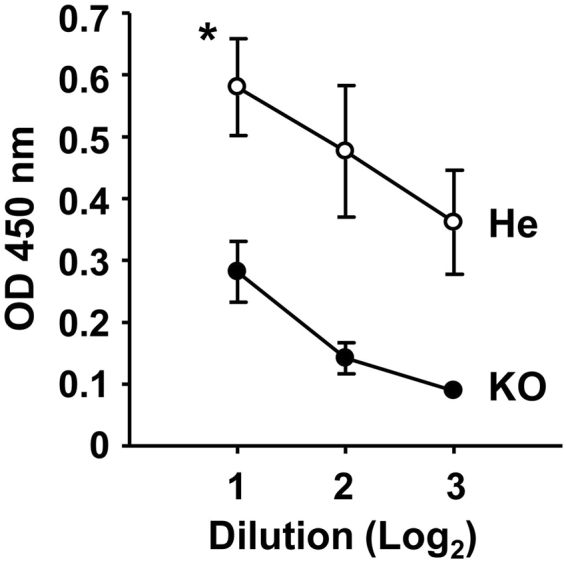
Antigen-specific nasal immune response was decreased in Ttll1-KO mice nasally immunized with PspA-C-CPE. Ttll1-hetero (He) and -knockout (KO) mice were nasally immunized with PspA-C-CPE once a week for three weeks. One week after the final immunization, PspA-specific nasal IgA was measured by means of an enzyme-linked immunosorbent assay. Ttll1-He mice, n = 4; Ttll1-KO mice, n = 3. Data are presented as mean ± SEM and are representative of two independent experiments. Values were compared by using Welch’s t-test. *P < 0.05. OD, optical density.
In addition to NALT, there are several alternative pathways through which immune responses can be induced. For instance, inducible bronchus-associated lymphoid tissue (iBALT) is induced by virus-based vaccine delivery (e.g., vaccinia virus vector), inflammation and infection20–22. The immunological structure and functions of iBALT are similar to those of other MALTs with regard to the initiation of antigen-specific immune responses23–25. Therefore, it is possible that nasal immunization with PspA-C-CPE induced the formation of iBALT as an inductive site for the systemic immune response in Ttll1-KO mice. Another possibility is the involvement of M cells in the respiratory epithelium26. The morphologic and immunologic functions of respiratory M cells, such as their short microvilli and the ability to take up vaccine antigens and pathogens (e.g., Salmonella spp.), are the same as those of the M cells in the NALT26. Thus, respiratory M cells appear to be an alternative pathway for the induction of systemic immune responses in Ttll1-KO mice.
We also checked the mice’s protective immunity against pneumococcal infection. Although PspA-specific nasal IgA was impaired in Ttll1-KO mice, survival rate was comparable between Ttll1-He and -KO mice (Supplementary Figure 2). In general, physical barriers such as mucus prevent the attachment of pathogens to epithelium. Therefore, it is likely that the dense nasal mucus in Ttll1-KO mice prevented the attachment of S. pneumoniae to epithelial cells and thus they showed low susceptibility to pneumococcal infection.
Together, these findings show that impaired airway mucociliary function prevented the induction of the nasal immune response.
Immune responses in the GCs of NALT were impaired in Ttll1-KO mice immunized with PspA-C-CPE
Nasal vaccines are generally designed to deliver antigen to the NALT, which is the lymphoid tissue responsible for the induction of antigen-specific immune responses in the nasal tissues3,27–29. PspA-C-CPE also binds to NALT epithelium, and leads to the induction of PspA-specific immune responses11. To determine how the PspA-specific nasal IgA response was impaired in Ttll1-KO mice nasally immunized with PspA-C-CPE, we determined the frequencies and percentages of different types of cell in the NALT. Flow cytometric analysis revealed that the frequencies and percentages of B220+ B cells, CD11c+ dendritic cells, CD3+ T cells, CD3+CD4+ T cells, and CD3+CD8α+ T cells were comparable in the NALT of Ttll1-He and -KO mice (Supplementary Figure 3).
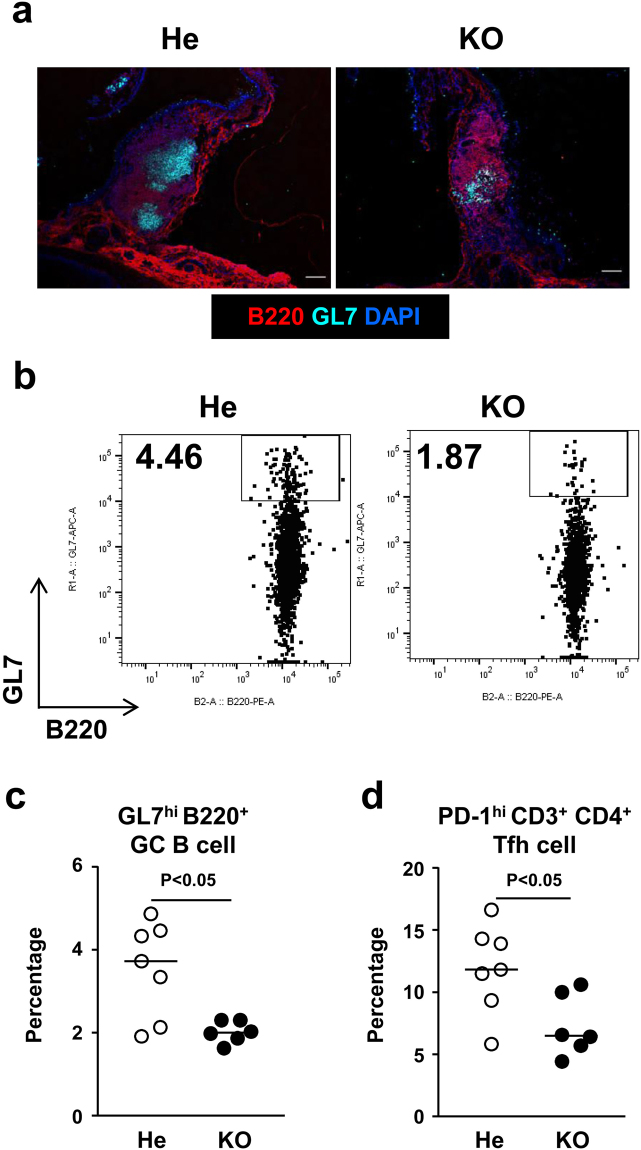
We next examined the cellular composition and formation of GCs in the NALT, where naïve B cells undergo IgA class switching upon antigen stimulation30. Nasal immunization with PspA-C-CPE induced GC formation and induced GL7highB220+ GC B cell proliferation in the NALT of Ttll1-He mice (Fig. 2a–c). However, GCs were smaller and had fewer B cells in the NALT of Ttll1-KO mice compared with Ttll1-He mice (Fig. 2a–c). Furthermore, the percentage of follicular helper T cells (Tfh cells), which play an important role in GC formation and IgA class switching4, was significantly lower in the NALT of Ttll1-KO mice compared with in the NALT of Ttll1-He mice (Fig. 2d).
Figure 2.
Immune responses in the germinal center of nasopharynx-associated lymphoid tissue were impaired in Ttll1-KO mice immunized with PspA-C-CPE. Ttll1-hetero (He) and -knockout (KO) mice were nasally immunized with PspA-C-CPE once a week for three weeks. (a) One week after the final immunization, sections of nasopharynx-associated lymphoid tissue (NALT) were stained with B220 (red), GL7 (light blue), and DAPI (blue). Scale bars, 100 µm. Ttll1-He, n = 5; Ttll1-KO, n = 4. (b–d). Frequency and numbers of germinal center (GC) B cells (b,c) and follicular helper T (Tfh) cells (d) in the NALT were determined by means of flow cytometry. Bars indicate the median value. Data were collected from two separate experiments. Values were compared by using the non-parametric Mann–Whitney U test.
These results show that impaired GC formation in the NALT was associated with the attenuation of the nasal IgA antibody response to nasal immunization with PspA-C-CPE in Ttll1-KO mice.
Binding of PspA-C-CPE to the mucosal epithelium associated with the NALT was impaired in Ttll1-KO mice
We then examined the mechanisms underlying the impaired PspA-specific nasal IgA response that arose in Ttll1-KO mice nasally immunized with PspA-C-CPE. Immunofluorescence staining was used to examine the expression of claudin-4, the target molecule of C-CPE, in the mucosal epithelium associated with the NALT. Claudin-4 was highly expressed on the mucosal epithelium associated with the NALT in both Ttll1-He and -KO mice (Supplementary Figure 4), suggesting that the impaired antigen-specific nasal IgA response observed in the Ttll1-KO mice was not a result of reduced claudin-4 expression.
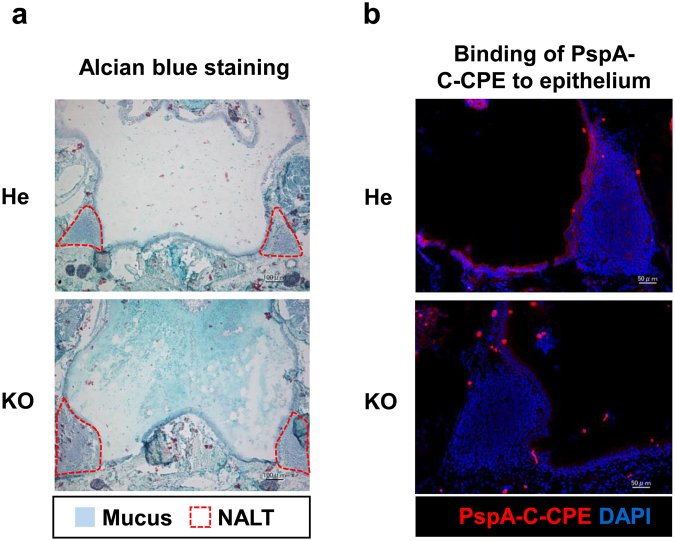
In a previous study, we found that impaired airway mucociliary motility caused mucus to accumulate in the nasal cavity of Ttll1-KO mice14, which led us to hypothesize that excessive amounts of mucus in the nasal cavity may prevent the binding of PspA-C-CPE to the mucosal epithelium associated with the NALT. Consistent with our previous findings14, in the present study we found that a dense mucus covered the NALT epithelium in Ttll1-KO mice but not in Ttll1-He mice (Fig. 3a). In addition, when we examined the intranasal distribution of PspA-C-CPE, we found that the binding of PspA-C-CPE to the mucosal epithelium associated with the NALT was attenuated in Ttll1-KO mice (Fig. 3b).
Figure 3.
A dense mucus and reduced binding of PspA-C-CPE to the nasopharynx-associated lymphoid tissue epithelium was found in Ttll1-KO mice. (a) The mucus in sections of nasopharynx-associated lymphoid tissue (NALT) was stained with Alcian blue. Ttll1-He, n = 5; Ttll1-KO, n = 5. (b) Ttll1-hetero (He) or -knockout (KO) mice were nasally administered biotinylated PspA-C-CPE. Sections of NALT were stained with Alexa Fluor 546-conjugated streptavidin (red) and DAPI (blue). Ttll1-He, n = 3; Ttll1-KO, n = 3.
These findings indicate that accumulation of a dense mucus prevented the binding of PspA-C-CPE to the mucosal epithelium associated with the NALT, and therefore that the nasal vaccine was unable to induce PspA-specific nasal IgA response in Ttll1-KO mice.
PspA-specific nasal immune IgA response was improved by removal of the nasal mucus in Ttll1-KO mice
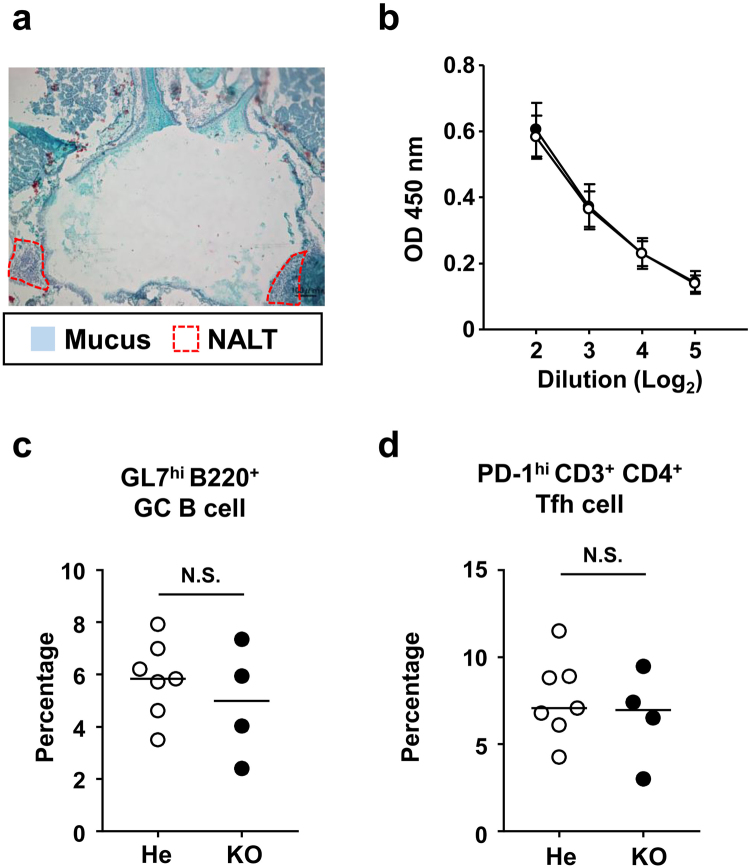
We hypothesized that the dense mucus covering the mucosal epithelium associated with the NALT in Ttll1-KO mice prevented the binding of PspA-C-CPE to the NALT epithelium, preventing the induction of the nasal IgA immune responses. Previous studies have demonstrated that removal of nasal mucus improves drug absorption in the nose31. We therefore removed the mucus by using N-acetylcysteine, which is a clinical expectorant that acts by cleaving the disulfide bonds between the mucin molecules in mucus31. We confirmed that the mucus was cleared from the NALT epithelium at 30 to 60 min after nasal administration of N-acetylcysteine in Ttll1-KO mice (Fig. 4a and Supplementary Figure 5). We also found that nasally administered PspA-C-CPE was retained at the mucosal epithelium associated with the NALT for 30 to 60 minutes after administration in C57BL/6 mice (Supplementary Figure 6). It is possible that reduced thiol could be reoxidized by air if longer extension time; therefore, based on these results, we nasally administered N-acetylcysteine to Ttll1-He or -KO mice followed 30 min later by nasal immunization with PspA-C-CPE. Our current findings suggested that Ttll1-KO mice without N-acetylcysteine treatment showed a decrease in PspA-specific nasal IgA together with impaired GC formation in the NALT because PspA-C-CPE was trapped by the dense nasal mucus. In contrast, the PspA-specific nasal IgA responses were comparable between Ttll1-He and -KO mice with N-acetylcysteine treatment (Fig. 4b). Furthermore, the percentages of GC B cells and Tfh cells were also comparable between Ttll1-He and -KO mice with N-acetylcysteine treatment (Fig. 4c,d). These results indicate that the dense mucus produced by the Ttll1-KO mice impaired the nasal immune responses induced by PspA-C-CPE, and that the removal of the mucus by administration of an expectorant rescued the impaired nasal immune response.
Figure 4.
PspA-specific nasal immune IgA response was improved by removal of the nasal mucus in Ttll1-KO mice. (a) Ttll1-knockout (KO) mice were nasally administrated N-acetylcysteine. After 30 min, mucus in sections of nasopharynx-associated lymphoid tissue (NALT) was visualized by staining with Alcian blue. (b–d) Thirty minutes after N-acetylcysteine administration, Ttll1-hetero (He) (○) or -KO mice (●) were nasally immunized with PspA-C-CPE (once a week for three weeks). One week after the final immunization, the level of PspA-specific nasal IgA was measured by means of an enzyme-linked immunosorbent assay (b). Data are presented as mean ± SEM. Mononuclear cells were isolated from NALT and flow cytometric analysis was used to determine the percentages of germinal center (GC) B cells (c) and follicular helper T (Tfh) cells (d). Bars indicate the median value. The data are representative of two independent experiments. Values were compared by using the non-parametric Mann–Whitney U test.
It is noteworthy that although the mucus was removed, the function of the mucocilia would have remained impaired, suggesting that the function of the mucocilia does not affect the efficacy of nasal vaccines. Allergies such as hay fever also cause mucus to accumulate in the nose. Therefore, in patients with allergies, removal of the nasal mucus either by using expectorants (e.g., N-acetylcysteine) or simply by blowing the nose immediately prior to immunization may ensure the complete induction of immune responses by nasal vaccines.
In summary, we elucidated the immunological role of airway mucociliary function with respect to delivery of a claudin-4-targeting nasal vaccine in Ttll1-KO mice, which possess straight rather than normal curved airway mucocilia due to impaired tubulin glutamylation, resulting in the loss of beating asymmetry and accumulation of a dense nasal mucus14. This dense nasal mucus prevented the binding of PspA-C-CPE to NALT epithelium, leading to reduced PspA-specific nasal IgA responses together with impaired GC formation in the NALT. Removal of the nasal mucus by using an expectorant rescued the nasal immune response. In addition to claudins, other tight junction proteins (e.g., occludin, tricellulin, angulins) are considered as targets for the delivery of nasal vaccines. For example, Clostridium perfringens iota-toxin binds to angulin-1, which is expressed by respiratory epithelium32,33. Since the present results indicate that vaccine delivery to NALT epithelium is affected by the accumulation of a dense nasal mucus, we conclude that nasal vaccines targeting occludin, tricellulin, and angulins may be possible but would similarly be affected by this accumulation of dense nasal mucus.
In this study, we used Ttll1-He mice as the controls for Ttll1-KO mice. We confirmed that the binding of PspA-C-CPE to NALT epithelium was identical between Ttll1-He and wild-type (WT) mice11. We also confirmed that Ttll1-WT and Ttll1-He mice showed comparable PspA-specific immune responses and GC formation in the NALT (Supplementary Figure 7a–d). In addition, we found that mucus removal had no effect on immune response induction in Ttll1-WT mice because Ttll1-WT mice did not show any accumulation of nasal mucus, which is consistent with the findings in Ttll1-He mice (Supplementary Figure 7e,f). These findings further suggest that mucus is a preventive factor for claudin-4-targeting nasal vaccine delivery.
Taken together, the present findings indicate that nasal mucus acts as a barrier against the delivery of nasal vaccines, and, therefore, that removal of nasal mucus is one approach to improve the efficacy of nasal vaccines.
Methods
Mice
Ttll1-KO mice (C57BL/6 background) were generated as previously described14. C57BL/6 mice were purchased from SLC, Inc. (Shizuoka, Japan). In the infection experiment, we killed the mice if their body weight was reduced by 20% or more. All experiments were approved by the Animal Care and Use Committee of the National Institutes of Biomedical Innovation, Health, and Nutrition (Approval Nos. DS27-47R1 and DS27-48R1) and were conducted in accordance with their guidelines.
Preparation of the PspA-C-CPE fusion protein
pET16b plasmids encoding PspA or PspA-C-CPE were prepared as previously described11. To obtain recombinant protein, plasmids were transformed into Escherichia coli strain BL21 (DE3) (TOYOBO, Osaka, Japan). To induce the production of PspA or PspA-C-CPE, isopropyl-D-thiogalactopyranoside (Nacalai Tesque, Kyoto, Japan) was added to the culture medium. The culture pellet was sonicated in buffer A (10 mM Tris–HCl [pH 8.0], 400 mM NaCl2, 5 mM MgCl2, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM 2-mercaptoethanol, and 10% glycerol). The supernatant was loaded onto a HiTrap HP column (GE Healthcare, Pittsburgh, Pennsylvania, USA). PspA or PspA-C-CPE protein was eluted with buffer A containing 100 to 500 mM imidazole. The solvent was exchanged with phosphate-buffered saline (PBS) by using a PD-10 column (GE Healthcare). The concentration of recombinant protein was measured by using a BCA Protein Assay Kit (Life Technologies, Carlsbad, California, USA). PspA-C-CPE was biotinylated by using a biotinylation kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Immunization and mucus removal
Mice were nasally immunized with PspA-C-CPE (PspA: 5 µg, C-CPE: 2 µg) once a week for three weeks. One week after the final immunization, nasal fluid and serum were collected as previously reported8.
To remove nasal mucus, mice were nasally administered 15 µg of N-acetylcysteine (Sigma-Aldrich, St Louis, Missouri, USA). After 30 min, the mice were nasally immunized with PspA-C-CPE as described above.
Enzyme-linked immunosorbent assay of PspA-specific production
The levels of PspA-specific IgA in nasal fluid and PspA-specific IgG in serum were measured by means of an enzyme-linked immune sorbent assay11. Ninety-six-well immunoassay plates were coated with PspA (0.05 µg/well) and incubated at 4 °C overnight. To prevent nonspecific binding, the plates were treated with 1% bovine serum albumin in PBS for 2 h at room temperature. After washing the plates with 0.05% Tween 20 in PBS, 2-fold serially diluted serum and nasal fluid were added to the wells and the plates were incubated for 2 h at room temperature. After washing the plates with 0.05% Tween 20 in PBS, goat anti-mouse IgA or IgG-conjugated with horseradish peroxidase (SouthernBiotech, Birmingham, Alabama, USA) was added to the wells and the plates were incubated for 1 h at room temperature. PspA-specific antibodies were detected by using 3,3′,5,5′-tetramethylbenzidine peroxide substrate. Optical density (wavelength 450 nm) was used an index of the progression of the color reaction.
S. pneumoniae culture and infection
S. pneumoniae Xen10 (parental strain, A66.1 serotype 3; Caliper Life Sciences) were growth in brain–heart infusion broth at 37 °C under a 5% CO2 atmosphere with no aeration. S. pneumoniae Xen10 were washed and diluted with PBS. One week after the final immunization, mice were nasally challenged with 1.5 × 107 CFU of S. pneumoniae Xen10. The survival of mice was monitored for 14 days.
Cell isolation and flow cytometric analysis
To isolate mononuclear cells from NALT, NALT was first obtained from the upper jaw of the mice. NALT cells were isolated by gently rubbing the NALT sample with a needle under a stereoscopic microscope. After washing with PBS, the collected cells were treated with anti-mouse CD16/32 (clone 93; BioLegend, San Diego, California, USA) for 15 min at room temperature. After washing with PBS containing 2% newborn calf serum, the cells were stained with fluorescein isothiocyanate-conjugated hamster anti-mouse CD3ε (clone 145-2C11, BD Biosciences, San Diego, California, USA), phycoerythrin (PE)-conjugated rat anti-mouse B220 (clone RA3-6B2, BD Biosciences), PE-conjugated rat anti-mouse PD-1 (clone 29F1.A12, BioLegend), Alexa Fluor 647-conjugated rat anti-mouse GL7 (clone GL7, BioLegend), PE-Cy7-conjugated rat anti-mouse CD4 (clone RM4-5, BD Biosciences), PE-Cy7-conjugated Armenian hamster anti-mouse CD11c (clone N418, BioLegend), APC-Cy7-conjugated rat anti-mouse CD8α (clone 53-6.7, BD Biosciences), and Brilliant Violet 421-conjugated rat anti-mouse CD45 (clone 30-F11, BioLegend) for 30 min at 4 °C. After washing with PBS containing 2% newborn calf serum, cells were treated with 7-Amino-Actinomycin D (BioLegend) for 10 min at 4 °C and analyzed by means of flow cytometry (MACSQuant) (Miltenyi Biotec, Auburn, California, USA).
Histochemical analysis of tissue specimens
To examine the expression of claudin-4 in NALT, NALT was embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek Japan, Tokyo, Japan) and cut into 6-µm sections by using a cryostat. Sections were fixed in 100% acetone for 1 min at 4 °C. To prevent non-specific binding, sections were treated with 2% fetal calf serum in PBS for 30 min at room temperature. The sections were then washed with PBS and stained with anti-claudin-4 antibody34 at 4 °C overnight. After the sections were again washed with PBS, they were stained with Cy3-goat anti-rat IgG for 30 min at room temperature, washed again with PBS, and stained with 4′,6-diamidino-2-phenylindole (DAPI). After a final wash with PBS, the sections were mounted in Fluoromount (Diagnostic BioSystems, Pleasanton, California, USA) and observed by means of fluorescence microscopy (BZ-9000, Keyence, Osaka, Japan).
To stain mucus, skin and excess soft tissue was removed from the head of the mice, embedded in Super Cryo Embedding Medium (Section-lab, Hiroshima, Japan), and cut into 6-µm sections by using a cryostat. The sections were treated with 3% CH3COOH solution for 3 min at room temperature, stained with Alcian Blue Solution (Sigma-Aldrich), washed again with 3% CH3COOH solution, and stained with Nuclear Fast Red Solution (Sigma-Aldrich) for 1 min at room temperature. The sections were then washed with running water, mounted in Fluoromount (Diagnostic BioSystems), and observed by using an optical microscope.
To examine the binding of PspA-C-CPE to NALT epithelium, mice were nasally administered with biotinylated PspA-C-CPE (PspA: 5 µg, C-CPE: 2 µg). After 30 min, skin and excess soft tissue was removed from the head of the mice, embedded in Super Cryo Embedding Medium (Section-lab), and cut into 6-µm sections by using a cryostat. The sections were fixed with 100% acetone for 1 min at 4 °C, followed by treatment with 2% fetal calf serum in PBS for 30 min at room temperature to prevent non-specific binding. After washing with PBS, the sections were stained with Alexa Fluor 546-conjugated streptavidin and incubated at 4 °C overnight to detect biotinylated PspA-C-CPE, washed again with PBS, and stained with DAPI. The sections were then washed with PBS, mounted in Fluoromount (Diagnostic BioSystems), and observed by means of fluorescence microscopy (BZ-9000, Keyence).
Data analysis
Data are presented as mean ± SEM. Statistical analyses were performed by using Welch’s t-test or the non-parametric Mann–Whitney U test (GraphPad Software, San Diego, California).
Electronic supplementary material
Acknowledgements
We thank Dr. Y. Horiguchi (Osaka University) for providing the C-CPE cDNA, and all the members of our laboratory for their useful comments and discussions. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Japan Society for the Promotion of Science [grant numbers JP26293111, JP16H01373, JP23229004,JP 15K18950, JP15H05790, JP17K08301, JP17H04134, JP15H05898B1], the Ministry of Health, Labour and Welfare of Japan and the Research on Development of New Drugs program of the Japan Agency for Medical Research and Development [grant numbers JP17fk0108223h0002, JP17ek0410032s0102, JP17fk0108207h0002, JP17ek0210078h0002, JP17ak0101068h0001, JP17gm1010006s0101, JP17gm0910004, JPek0410032h0002, the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry, the Astellas Foundation for Research on Metabolic Disorders, the Terumo Foundation for Life Sciences and Arts, The Canon foundation, and the Suzuken Memorial Foundation.
Author Contributions
H.S. planned and performed the immunologic experiments, analyzed the data, and wrote the paper; T.N., A.N., and H.L. performed the immunologic experiments and analyzed the data; K.I. and M.S. provided the mice and discussed the data; Y.H. provided the antibody and discussed the data; H.K., K.Y., and M.K. were involved in discussions related to the study; J.K. planned the experiments and wrote the paper; all authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21120-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lamichhane A, Azegami T, Kiyono H. The mucosal immune system for vaccine development. Vaccine. 2014;32:6711–6723. doi: 10.1016/j.vaccine.2014.08.089. [DOI] [PubMed] [Google Scholar]
- 2.Fukuyama Y, et al. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. Journal of Immunology. 2010;185:1755–1762. doi: 10.4049/jimmunol.1000831. [DOI] [PubMed] [Google Scholar]
- 3.Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends in Immunology. 2008;29:505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014;2:e28960. doi: 10.4161/tisb.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010;2:a002907. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smedley JG, 3rd, Uzal FA, McClane BA. Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin. Infection and Immunity. 2007;75:2381–2390. doi: 10.1128/IAI.01737-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakutani H, et al. Mucosal vaccination using claudin-4-targeting. Biomaterials. 2010;31:5463–5471. doi: 10.1016/j.biomaterials.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Kakutani H, Kondoh M, Watari A, Yagi K. The safety of a mucosal vaccine using the C-terminal fragment of Clostridium perfringens enterotoxin. Die Pharmazie. 2010;65:766–769. [PubMed] [Google Scholar]
- 10.Rajapaksa TE, Stover-Hamer M, Fernandez X, Eckelhoefer HA, Lo DD. Claudin 4-targeted protein incorporated into PLGA nanoparticles can mediate M cell targeted delivery. Journal of Controlled Release. 2010;142:196–205. doi: 10.1016/j.jconrel.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, et al. C-terminal Clostridium perfringens enterotoxin-mediated antigen delivery for nasal pneumococcal vaccine. PLoS One. 2015;10:e0126352. doi: 10.1371/journal.pone.0126352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdugo P. Goblet cells secretion and mucogenesis. Annu. Rev. Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- 13.Gizurarson S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015;38:497–506. doi: 10.1248/bpb.b14-00398. [DOI] [PubMed] [Google Scholar]
- 14.Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10490–10495. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung AT, Jahn TL. High speed cinemicrographic studies on rabbit tracheal (ciliated) epithelia: determination of the beat pattern of tracheal cilia. Pediatr. Res. 1976;10:140–144. doi: 10.1203/00006450-197602000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Konno A, Setou M, Ikegami K. Ciliary and flagellar structure and function–their regulations by posttranslational modifications of axonemal tubulin. Int. Rev. Cell Mol. Biol. 2012;294:133–170. doi: 10.1016/B978-0-12-394305-7.00003-3. [DOI] [PubMed] [Google Scholar]
- 17.Gaertig J, Wloga D. Ciliary tubulin and its post-translational modifications. Curr. Top. Dev. Biol. 2008;85:83–113. doi: 10.1016/S0070-2153(08)00804-1. [DOI] [PubMed] [Google Scholar]
- 18.Janke C, et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science. 2005;308:1758–1762. doi: 10.1126/science.1113010. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, et al. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. Journal of Immunology. 1998;161:4115–4121. [PubMed] [Google Scholar]
- 20.Guest IC, Sell S. Bronchial lesions of mouse model of asthma are preceded by immune complex vasculitis and induced bronchial associated lymphoid tissue (iBALT) Lab. Invest. 2015;95:886–902. doi: 10.1038/labinvest.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyron-Quiroz JE, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 22.Halle S, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tango M, Suzuki E, Gejyo F, Ushiki T. The presence of specialized epithelial cells on the bronchus-associated lymphoid tissue (BALT) in the mouse. Arch. Histol. Cytol. 2000;63:81–89. doi: 10.1679/aohc.63.81. [DOI] [PubMed] [Google Scholar]
- 24.Morin MJ, Warner A, Fields BN. A pathway for entry of reoviruses into the host through M cells of the respiratory tract. The Journal of Experimental Medicine. 1994;180:1523–1527. doi: 10.1084/jem.180.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teitelbaum R, et al. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/S1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- 26.Kim DY, et al. The airway antigen sampling system: respiratory M cells as an alternative gateway for inhaled antigens. Journal of Immunology. 2011;186:4253–4262. doi: 10.4049/jimmunol.0903794. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, et al. Transgene vaccination using Ulex europaeus agglutinin I (UEA-1) for targeted mucosal immunization against HIV-1 envelope. Vaccine. 2005;23:3836–3842. doi: 10.1016/j.vaccine.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Nochi T, et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nature Materials. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 29.Jia Y, Krishnan L, Omri A. Nasal and pulmonary vaccine delivery using particulate carriers. Expert Opinion on Drug Delivery. 2015;12:993–1008. doi: 10.1517/17425247.2015.1044435. [DOI] [PubMed] [Google Scholar]
- 30.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nature Reviews. Immunology. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuyama T, Morita T, Horikiri Y, Yamahara H, Yoshino H. Influence of fillers in powder formulations containing N-acetyl-L-cysteine on nasal peptide absorption. Journal of Controlled Release. 2007;120:88–94. doi: 10.1016/j.jconrel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Higashi T, et al. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. Journal of Cell Science. 2013;126:966–977. doi: 10.1242/jcs.116442. [DOI] [PubMed] [Google Scholar]
- 33.Krug SM, et al. Angubindin-1, a novel paracellular absorption enhancer acting at the tricellular tight junction. Journal of Controlled Release. 2017;260:1–11. doi: 10.1016/j.jconrel.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Kawai Y, et al. Claudin-4 induction by E-protein activity in later stages of CD4/8 double-positive thymocytes to increase positive selection efficiency. Proc. Natl. Acad. Sci. USA. 2011;108:4075–4080. doi: 10.1073/pnas.1014178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.