Abstract
Molecular diffusion measured with diffusion weighted MRI (DWI) offers a probe for tissue microstructure. However, inferring microstructural properties from conventional DWI data is a complex inverse problem and has to account for heterogeneity in sizes, shapes and orientations of the tissue compartments contained within an imaging voxel. Alternative experimental means for disentangling the signal signatures of such features could provide a stronger link between the data and its interpretation. Double diffusion encoding (DDE) offers the possibility to factor out variation in compartment shapes from orientational dispersion of anisotropic domains by measuring the correlation between diffusivity in multiple directions. Time dependence of the diffusion is another effect reflecting the dimensions and distributions of barriers. In this paper we extend on DDE with a modified version of the oscillating gradient spin echo (OGSE) experiment, giving a basic contrast mechanism closely linked to both the temporal diffusion spectrum and the compartment anisotropy. We demonstrate our new method on post mortem brain tissue and show that we retrieve the correct temporal diffusion tensor spectrum in synthetic data from Monte Carlo simulations of random walks in a range of disordered geometries of different sizes and shapes.
Introduction
Diffusion weighted imaging (DWI) probes the mobility of water molecules and provides a high sensitivity to tissue composition on length scales far below the image resolution of conventional magnetic resonance imaging (MRI). Since its introduction in the 1980’s it has become a popular imaging biomarker for detection of subtle differences between healthy and diseased tissue with important clinical application in ischemic stroke and tumor classification1. DWI data from conventional pulsed field gradient spin echo (PGSE) experiments is commonly approximated by a diffusion tensor, i.e. diffusion tensor imaging (DTI), to provide rotationally invariant metrics characterizing the diffusion in anisotropic tissue compartments2. Besides mean diffusivity reflecting cellular packing and size, DTI provides anisotropy estimates quantified by fractional anisotropy (FA) which is a characteristic feature of anisotropic cell types such as axons in white matter and muscle fibers3. An interpreter of DTI data must consider a number of effects operating on microscopic to macroscopic length scales. These effects range from direct influences on the water mobility such as cell sizes and shapes, to ensemble effects from orientational dispersion or partial volumes of differing tissue types and cerebrospinal fluid (CSF). Multiple relaxation rates or exchange between compartments can also affect the signal depending on the time frame of the experiment4.
Model based approaches
A number of biophysical models for extracting microscopic tissue properties from conventional DWI data acquired with pulsed field gradient spin echo (PGSE) experiments have been proposed and can guide this interpretation5,6. Brain tissue is often modelled using thin tubes or 1D sticks to capture the mainly fibrous intracellular component of axons, dendrites or astrocytes embedded in an extracellullar space described by a time independent diffusion tensor7,8. Popular models like NODDI (Neurite Orientation Dispersion and Density Imaging) provide estimates of neurite density and orientational dispersion that may also be extracted by model guided interpretation of DTI metrics9,10. The DWI signal is in such models usually assumed to arise from separate compartments with each its diffusion tensor, making the DWI signal a sum of mono-exponential decays with respect to diffusion weighting. In reality, non-monoexponential effects arise in the signal either from compartment mixtures, time dependent diffusion or dispersion of anisotropic compartments. This poses a complex inverse problem where many different qualities provide similar features in the measured data resulting in a broad variety of plausible interpretations11,12. Alternative models describing exchange across the heterogeneity is a different approach for describing the complexity of real tissue6. In this context, basic model parameters such as the single compartment diffusion coefficients are yet pending to be quantitatively verified and understood.
Need for data driven specificity and model independence
As important as understanding the biophysical interpretation of the model parameters is to understand how they vary over a healthy population or in specific pathologies and how such variations may affect the model predictions and their interpretations. Model free approaches like kurtosis imaging offer an alternative approach to representing non-monoexponential features in DWI data unbiased by model assumptions, but does not solve the problem of low specificity13,14. Sensitizing the data acquisition itself to correlations between multiple parameters is one way to achieve a richer information content from the sample and could be a way forward to improve specificity to individual signal components.
Double Diffusion Encoding (DDE)
Double diffusion encoding (DDE) or multidimensional diffusion encoding (MDE) are approaches where the signal is weighted to the correlation in diffusivity between multiple independent directions within the same excitation15. This allows differentiation of the signal from a distribution of disperse anisotropic compartments from that of a distribution of isotropic compartments with varying (isotropic) diffusivities15–17. Different implementations of this principle have recently been developed suitable for imaging and the derived microscopic FA (μFA) is equivalent to FA but insensitive to global effects of dispersion and thus proposed as a more specific marker of tissue microstructure18–20.
Oscilating Gradient Spin Echo (OGSE) and time dependent diffusion
Diffusion time is an additional conceptually interesting parameter for probing the microstructure at different length scales21–24. Time dependent diffusivity has been shown to arise in biophysical models that include distributions of permeable barriers25, from general random perturbations to the local diffusivity22 and from diffusion between impermeable barriers26. In neuronal tissue, diffusion time is a weak contrast parameter on the time scales reachable with conventional PGSE in a clinical setting27–29 but shorter time scales can be investigated with oscillating gradient spin echo (OGSE)30–32. Time dependence measured with short diffusion times with PGSE or high frequencies with OGSE are associated6,22. However, OGSE may provide a higher diffusion weighting for given gradient hardware limitations. Moreover, exchange and mixing during a longer OGSE gradient duration will provide a closer to mono-exponential signal attenuation as the individual spins have probed a larger portion of the whole geometry33,34. A cosine modulated diffusion weighting gradient train is a frequency specific filter for the temporal diffusion spectrum (D(ω)) which provides a stringent description of a restricted diffusion compartment that in turn can be used to describe the signal from arbitrary gradient schemes26,35. D(ω), formally the Fourier transform of the molecular velocity autocorrelation function26,36, describes the transition from low frequencies, where reflections at barriers cause temporal correlations in molecular displacement, to high frequences where the diffusion is free and Brownian. OGSE measurements can be used to eg. characterize different tumors and ischaemic tissue32,37,38, and provides a strong tissue contrast in brain regions with large cell densities such as hippocampus and cerebellum39–41.
Combining DDE and OGSE: Circularly Polarised OGSE (CP-OGSE)
Requiring strong gradient amplitudes, OGSE is mainly explored in post mortem settings on powerful preclinical MRI systems, but practical implementations have also been demonstrated for human in vivo use38,42. We recently introduced circularly polarised OGSE (CP-OGSE) with the main benefit of improving the contrast to noise by increasing the diffusion weighting of the conventional OGSE experiment with a factor of two41. CP-OGSE applies two orthogonal and dephased OGSE gradient encoding trains providing independent time dependent diffusion weighting in two directions simultaneously, displaying both the qualities of a DDE experiment as earlier proposed41.
Extending CP-OGSE: Eliptically Polarised OGSE (EP-OGSE)
In this work we demonstrate the possibility of mapping the diffusion spectrum in disordered samples with a modified version of CP-OGSE we call elliptically polarised OGSE (EP-OGSE) by modulating the relative strengths and phases of two orthogonal oscillating gradients. We show that this approach gives a direct contrast to microscopic and time dependent anisotropy in disordered samples with an isotropic orientational distribution of separate tissue compartments and how the frequency dependent microscopic (single compartment) DTI metrics can be retrieved. We demonstrate this effect in EP-OGSE data acquired from an ex vivo monkey brain, quantifying the frequency dependent anisotropic diffusion tensors in gray and white matter. Monte Carlo simulations confirm our theoretical predictions, and resolve single compartment diffusion tensor spectra in disordered samples.
Theory
Gradient waveform and signal attenuation
The diffusion weighting gradient vector in conventional PGSE assumes a parallel and anti-parallel configuration in the gradient train. In OGSE, it follows a cosine trajectory. In CP-OGSE, two perpendicular out of phase gradients simultaneously follow a cosine trajectory, making the gradient trajectory circular. The EP-OGSE waveform used in this study alters the eccentricity of this circular trajectory via the ellipticity angle χ. An idealised representation of this gradient waveform is:
| 1 |
where G is the maximum gradient amplitude, ωm is the angular modulation frequency, T is the duration of each gradient. and are unit vectors. Under the Gaussian phase approximation (GPA)1,36,43 absent bulk motion and in a single tissue compartment, the DWI signal E from a single gradient trajectory at readout time follows:
| 2 |
| 3 |
where β is the spin phase variance over the ensemble of water molecules, here related to the frequency dependent diffusivity tensor D(ω) and γ is the gyromagnetic ratio26,36. The signal is effectively characterized by D(ω) filtered by F(ω)FΤ(−ω). Under GPA and encoding spectra with their weight dominated by a single peak at the oscillation frequency ωm we can probe D(ω) with the signal attenuation factor β = ΣBij(ωm)Dij(ωm) in Eqn. 2 where Dij and Bij are the respective elements of D(ω) and the frequency specific diffusion weighting B(ωm) matrix2,39. The latter is for the gradient trajectory in Eqn. 1 then given by:
| 4 |
The full D(ω) tensor can be estimated with a DTI approach by acquiring a range of measurements with different B-matrices realized with different gradient strengths and orientations and this carries over to EP-OGSE where χ, G, T, ωm, and are the free parameters. This allows probing of the diffusion spectrum, leading to curves like on Fig. 1d, with information about restrictions in the tissue. The off diagonal terms in B(ωm) both rotate the elliptical polarization in the xy-plane and shift the eigenvalues, corresponding to unwanted gradient interactions. For the idealized gradient representation in Eqn. (1), their magnitude relative to the diagonals however vanishes as ((4)), implying that their influence is constrained to low frequencies. They are further proportional to the assymmetric function cos(χ)sin(χ), which is a feature of the χ-modulation that carries over to practical EP-OGSE implementations. The off diagonal terms can thus be eliminated by applying the first and second gradient trajectories in a spin echo sequence with opposed rotations, i.e. χ1 = −χ2. For the diagonalized B(ωm)-matrix, the attenuation can now be written as:
| 5 |
where b is the total diffusion weighting determined by the trace of Eqn. 4, .
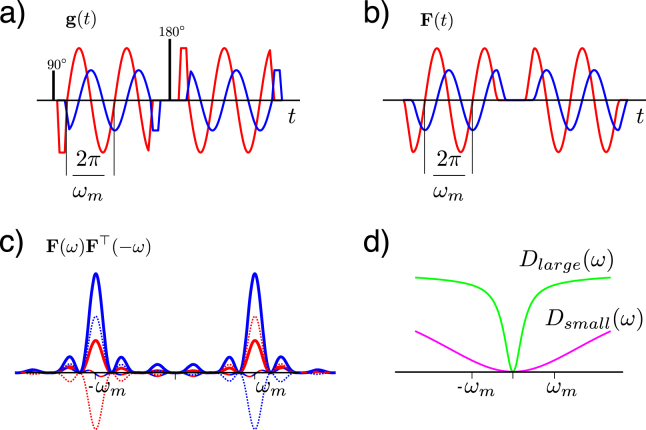
Figure 1.
(a) An example of two orthogonal gradient components of an elliptically oscillating gradient vector with ellipticity angle χ = 30° and modulation frequency ωm. The waveforms shown are the effective gradients with inverted polarity after the 180°-pulse in this spin echo experiment. (b) The dephasing vector components calculated as the time integral of the gradients in a). (c) The weighting filter of the diffusion spectrum tensor is given by the elements of F(ω)FΤ(−ω). The diagonal elements are real with the same distinct peak at the modulation frequency in both axes but with modulated strengths (solid lines). The off diagonal elements are complex (real (dashed), imaginary (dotted)) but integrate to zero. (d) Examples of temporal diffusion spectra of large and small restrictions. The gradient trajectory in (a) would mainly filter out the diffusivity around ωm.
Modulating the polarisation and parctical EP-OGSE implementation
The polarisation angle χ alters the shape of the elliptical polarisation. χ = 0° corresponds to linear polarisation in the x-direction, χ = 90° corresponds to linear polarisation in the y-direction and χ = 45° corresponds to circular polarisation (CP-OGSE)41. Several practical considerations come into the design of OGSE gradients, in particular the initiation of the gradient trains and the polarity of the gradient after the refocusing pulse35,42. We will in the following simulations and experiments use orthogonal gradient pairs as previously implemented in ref.41 but independently scaled by varying χ. Figure 1a shows the actual gradients used for ωm = 100 Hz parametrized with respect to an intermediate χ = 30°. The filters acting on D(ωm) are from Eqn. 3 given by the elements of F(ω)FΤ(−ω) and shown in Fig. 1c. Note that the diagonal elements (solid lines) have identical shape with two symmetric peaks at ω and −ω but scaled according to χ. The sharpness of those peaks increases with the number of oscillations. The off diagonal terms are complex with a negligible real part (dashed lines), a consequence of the spin echo sequence with opposed rotations, and an imaginary part (dotted lines) with odd symmetry which thus integrate to zero in Eqn. 3. Since the b-value for fixed T decreases quickly with frequency as an inverse square, the gradient strength is typically adjusted to match a constant b-value over a range of ωm.
EP-OGSE signal in ensembles of disordered axially symmetric compartments
From the signal attenuation of a single compartment we now consider ensembles of non-exchanging microscopic compartments. With EP-OGSE under the GPA, the attenuation from a single non-exchanging compartment is monoexponential in b at fixed ωm (Eqns (2) and (5)). A parametrization of the signal that satisfies this and is both appropriate for cylindrically symmetric geometries and amenable to spherical averaging is considered in ref.44. The attenuation with a gradient along an axis for a compartment oriented along axis n is here modelled as:
| 6 |
where DL and DT are the frequency specific diffusivities in respectively the longitudinal and transversal axes of the compartment and ba is diffusion weighting along . For a gradient train in the xy-plane it becomes:
| 7 |
| 8 |
| 9 |
Here, θ and φ denote the azimuthal and polar angles of the compartment orientation vector n in spherical coordinates.
The signal from an ensemble of compartments with an isotropic orientational distribution becomes:
| 10 |
The integral in Eqn. 10 can for linear and circular polarizations be written in forms independent of the azimuth φ and performed:
| 11 |
| 12 |
A uniform distribution can be emulated in data from samples with unknown orientational distributions by a powder average, i.e. averaging measurements with uniformly distributed gradient directions19,45. Variations of Eqn. 11 have been used in several applications in diffusion weighted imaging and spectroscopy8,44,46,47. A general solution to these equations in the special case of axially symmetric diffusion weighting and diffusion tensors is derived in ref.48 From those estimates of DL and DT the single compartment μFA can be calculated as:
| 13 |
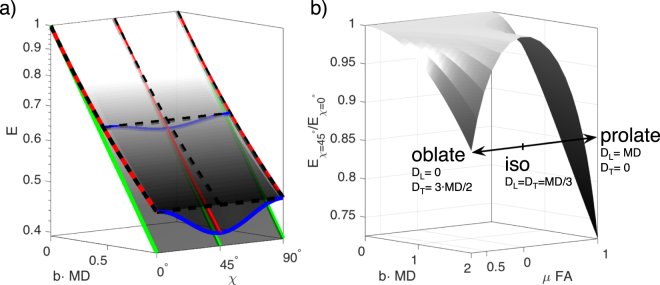
Figure 2a shows an example of signal attenuations described by Eqs. 10, 11 and 12. Underlying microscopic anisotropy gives a modulation over χ with the lowest signal at 45° similar to a DDE experiment with an orthogonal gradient pair. The depth of this χ-modulation via the ratio depends under our assumptions solely on the difference DT − DL. Figure 2b shows this ratio for different tensor geometries with different μFA. Oblate geometry corresponds to DT > DL, prolate to DT < DL.
Figure 2.
(a) An example of signal surfaces for three different distributions of diffusion tensors calculated from Eqn. 10 over a range of b-values and ellipticity angles χ. MD is the mean diffusivity of the underlying diffusion tensor. The lowest plane highlighted with green lines is a single isotropic domain resulting in a mono-exponential attenuation and no χ-modulation. The curved middle plane highlighted with solid red lines over different b and solid blue lines over different χ was generated from an ensemble of uniformly oriented anisotropic tensors. The top plane highlighted with dashed black lines comes from a distribution of isotropic diffusivities and shows no χ-modulation as expected for isotropic compartments. The three planes share the same mean diffusivity indicated by their common initial slope. (b) The ratio Eχ=45/Eχ=0 for DT – DL varied to realize different tensor geometries from oblate to prolate with varying μFA. The ratio, corresponding to the depth of the modulation, clearly depends on μFA and tensor geometry.
Results
EP-OGSE on ex-vivo brain tissue
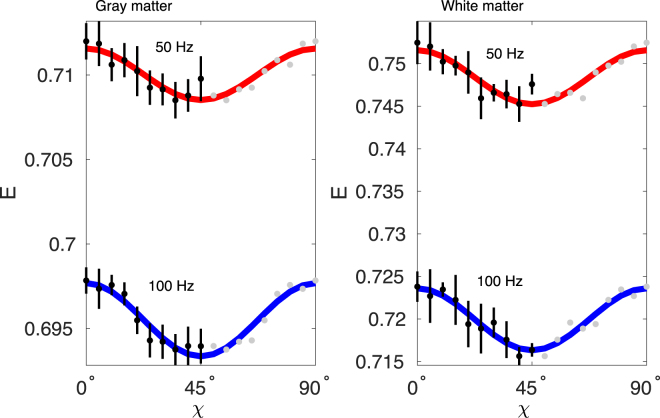
Figure 3 shows a b = 0 coronal slice from a vervet monkey used in the experiment and derived masks for gray and white matter. Repetition averaged data normalised to b = 0 from the respective regions over different polarization angles and fixed b = 0.8 ms/μm2 are shown in Fig. 4 and reflect the theoretical predictions for anisotropic substrates (blue curves in Fig. 2a). The baseline signal decreases with frequency as expected from the sigmoidal shape of the diffusion spectrum for restricted diffusion illustrated in Fig. 1d. The fitted model (solid lines in Fig. 4) corresponds well to the measured data with fitted model parameters stated in Table 1, suggesting an increase in both DL and DT with frequency with a net decrease in μFA in both gray and white matter. The time dependence in this frequency span is most pronounced in white matter compared to gray matter. Note that a voxelwise map was not possible as the measurements where performed with fixed gradient directions and analyzed over large ROIs to provide rotationally invariance and high SNR.
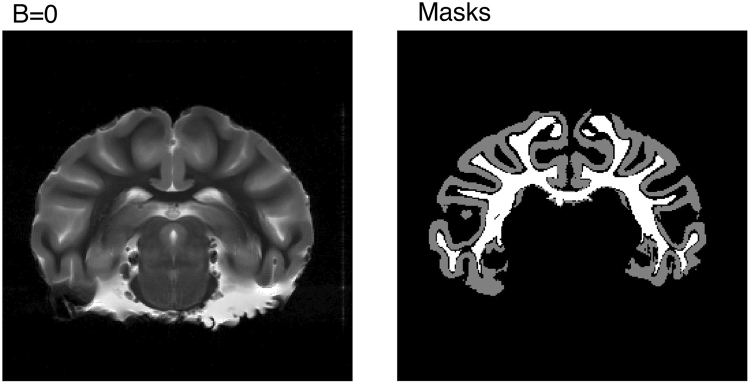
Figure 3.
A coronal b = 0 image of the monkey brain and gray and white matter ROIs created from thresholding the b = 0 image. Subcortical structures were excluded by manual delineation.
Figure 4.
Signal (dots) and fitted model (solid lines) over ellipticity angle χ in the gray and white matter ROIs at 50 Hz and 100 Hz. Data were averaged over waveforms with the same eccentricity to achieve a more robust estimate of a uniform distribution and points over 45° are mirrored and redundant and thus plotted in gray. The standard deviation over 6 averages are shown as error bar for each data point. Note that the y-scales are different and that the χ-modulation is larger in white matter.
Table 1.
Fitted longitudinal and transversal diffusivities DL and DT (μm2/ms) and microscopic fractional diffusivity μFA from the gray and white matter ROI data shown in Fig. 4. The indicated errors are standard deviations on the parameters calculated from the least squares fit.
| Gray matter | White matter | ||||
|---|---|---|---|---|---|
| 50 Hz | 100 Hz | 50 Hz | 100 Hz | ||
| D L | 0.73 ± 0.01 | 0.83 ± 0.01 | D L | 0.81 ± 0.02 | 0.89 ± 0.02 |
| D T | 0.28 ± 0.007 | 0.28 ± 0.005 | D T | 0.16 ± 0.01 | 0.19 ± 0.007 |
| μFA | 0.54 ± 0.02 | 0.60 ± 0.01 | μFA | 0.78 ± 0.03 | 0.76 ± 0.02 |
EP-OGSE Monte Carlo simulations with ellipsoidal restrictions
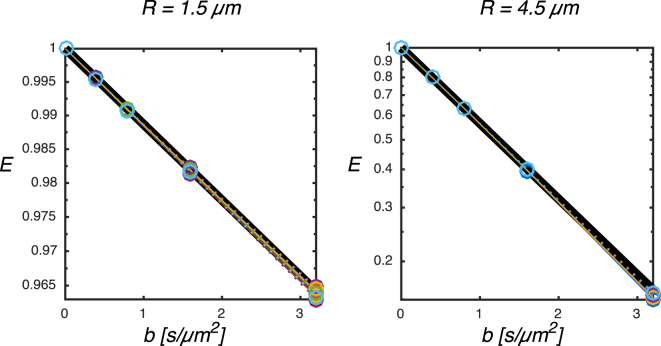
Monte Carlo simulations were performed in ellipsoidal restrictions with 24 uniformly distributed gradient orientations. Biases could be introduced by non-monoexponential signal decay in the individual compartments. To investigate this we plot the log transformed simulated signals in two spherical domains and compare these to the linear attenuation calculated from the lowest b-value (Fig. 5). We observe good linearity up to a b-value of 1.6 s/μm2 with less than 0.2% deviation but up to 1% at 3.2 s/μm2. These effects are larger at χ = 0° compared to χ = 45°. The signal is expected to be rotationally invariant in the spherical restrictions but signal deviations up to 0.4 % at 3.2 s/μm2 were observed across the 24 different gradient orientations reflecting the numerical noise levels in our simulation. Only b-values up to 1.6 s/μm2 where included in the following analysis.
Figure 5.
Attenuation curves for spherical restrictions with radii 1.5 and 4.5 μm at 50 Hz for the EP-OGSE measurements in 24 uniformly distributed orientations performed with χ = 0° (circles, solid colored lines) and χ = 45° (circles, dotted colored lines). The black line indicates the initial monoexponential decay and deviations from this is observed at high b-values/gradient strengths as the diffraction limit is approached.
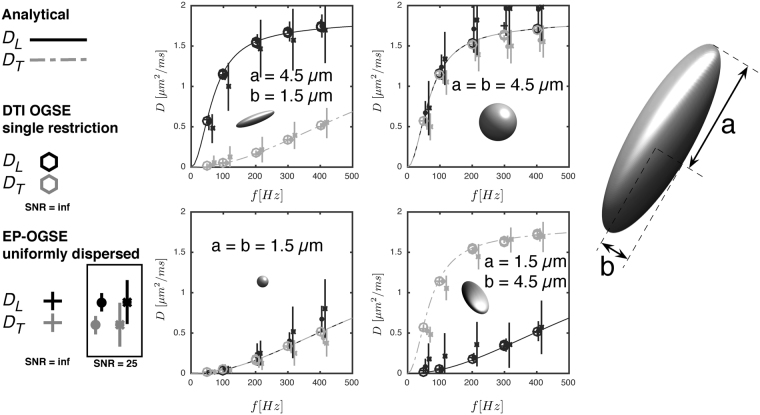
The temporal diffusion spectra from four restrictions with different sizes and shapes are shown in Fig. 6. We here see good agreement between the DL and DT calculated from conventional OGSE in aligned compartments and those found by fitting Eqn. 10 to a uniformly distributed powder average (squares vs. crosses in Fig. 6). To investigate the influence of noise, the 96 Monte Carlo datapoints (4 b-values and 24 directions) were diluted with random noise from a Gaussian distribution reflecting an SNR of 25 (solid dots with error bars). An additional noise analysis was performed in a simulation with a lower maximum b-value of 0.6 s/μm2 (asterices with error bars). The results demonstrate that the precision of the estimates improves with b-value and the maximum gradient amplitude available. We also see that the diffusion spectra along the individual ellipsoid axes can be modelled well by interpreting DT and DL as the frequency dependent diffusivities in spherical restrictions with the same radius26,49, but with some deviations for the most eccentric restrictions (lines in the same figure).
Figure 6.
Longitudinal (black) and transversal (gray) temporal diffusion spectra for ellipsoidal restrictions with longitudinal and transversal axes (a and b) estimated from EP-OGSE Monte Carlo simulations of fully dispersed samples (crosses) compared to estimates from conventional DTI OGSE of single compartments (hexagons). The EP-OGSE estimates were also estimated from noisy data with SNR = 25 using both a maximum b-value of 1.6 s/μm2 and 0.6 s/μm2 shown as filled dots and asterices. The latter b-value is in the range possible up to 100 Hz at a maximum gradient strength of 300 mT/m. The error bars indicate the standard deviation of each estimate. The solid lines are the analytical solutions for spheres with radii a (solid black) and b (gray dashed).
Discussion
We present an experimentally feasible method for detecting the microscopic temporal diffusion spectrum in disordered microscopically anisotropic tissue. DDE benefits from a direct contrast to the microscopic anisotropy as for instance parametrized by μFA16. EP-OGSE combines the individual specificities of OGSE with those of DDE and provides specificity to the microscopic temporal diffusion tensor in disperse samples and its derived metrics such as μFA.
Experiment Frequency dependent diffusion in tissue
Applying EP-OGSE to a fixated monkey brain, we found increases in the modelled DT in white matter but not in gray matter. Interestingly, we also found increases in DL both in gray and white matter not predicted by a cylindrical model with free diffusivity along its length axis commonly assumed in biophysical models. This effect could be due to undulations on the length scale of the free diffusion path length of the encoding gradient or structures in the cytoplasm along the axon or in the extracellullar matrix acting as barriers21,23,50,51. The data at hand with only two frequency points does not reveal the curvature of the diffusion spectrum but the prospect of isolating the time dependent anisotropic components of a complex tissue environment could potentially isolate interesting features in future experiments. The effective medium theory (EMT) describes the low frequency (long diffusion time) behavior when diffusing spins approach the tortuosity limit6,22. The ω-dependence predicted by EMT relates to the dimensionality and correlation lengths of a complex geometry. In a disordered 2D lattice, such as the extracellullar space perpendicular to irregularly packed white matter axons, a linear dependence in frequency or 1/t is predicted and observed with both OGSE and PGSE23,24. In contrast, disorder along 1D structures that may be caused by different varicosities along axons or dendrites relates to a -dependence21,23. Even though we were not able to investigate broader frequency ranges our results do support time dependence along and perpendicular to white matter axons, but only along neurites in gray matter. Individual restrictions show a weaker ω2-dependence which may be a less dominant effect in neuronal tissue with small restriction sizes and low frequencies. This effect could play a larger role in characterization of other tissues as we recently demonstrated in OGSE measurements of myocardial tissue in the heart52,53.
Imperfect orientational distribution
The current experiment was designed to demonstrate the χ modulation but has limitations that could be improved in future implementations. Our data do not represent a perfect spherical average of orientations as only one plane was measured (see methods). We believe that this problem was partially mitigated by averaging over a large ROI with a wide distribution of directions but this prevents calculations of voxelwise maps. In a future implementation for voxelwise quantification, the modulation could be estimated from the linear and circular polarisations (χ = 0° and χ = 45°) acquired in sufficient number of orientations to achieve a robust spherical average similar to previous work using parallel and perpendicular DDE19. Interaction with unwanted diffusion weighting from imaging gradients, in particular crusher and slice gradients, might be considered in high resolution settings. Their contributions in the current setting is negligible and would provide only a frequency independent term Bzz in diffusion encoding matrix, perpendicular to the plane of the oscillating gradients54.
Extensions to the single tensor picture
Our analysis includes a single type of disordered local frequency dependent diffusion tensors which may be overly simplistic for many tissue types. Different flavors of the standard model models often relates the multi-exponential signal from individual components with Gaussian or at least mono-exponential behavior. A true biological substrate may be expected to contain a distribution of compartments of varying shape, size and orientations. Given the independent sensitivities of EP-OGSE to variations related to anisotropy and distributions of isotropic diffusivities, we may be able to improve our modelling by adapting approaches in the DDE literature based on for instance cumulant expansion representations of the signal, model based assumptions of the underlying distributions or multidimensional Laplace transforms19,55,56. This may also yield other correlation experiments exploring joint distributions of diffusion, diffusion time, chemical shift, exchange, relaxation and magnetization transfer effects57–61. Time dependent diffusion implies a non-Gaussian diffusion propagator with non-monoexponential attenuation with respect to the b-value22. This would violate the simplistic view of the signal as a sum of mono-exponential signal components in Eqn. 12. However, the OGSE signal attenuation is built up by repeated phase encodings with low dephasing amplitude which to a larger degree satisfies the GPA with a mono-exponential decay compared to a PGSE with the same b-value62. The longer gradient duration of the OGSE experiment also allows for exchange effects to attenuate which could describe the lower kurtosis observed in OGSE data compared to PGSE acquired with the same b-value and settings reflecting similar diffusion times34. It is yet an open question to what extent the residual non-monoexponential decay in OGSE can be viewed as a mixture of non-exchanging compartments with individual mono-exponential decays. Such effects could arise from either a spread in isotropic diffusivities or an orientational distribution of anisotropic diffusivities. An interesting extension to the current EP-OGSE experiment would be to explore exchange effects at constant ωm with a varying number of oscillations.
Simulations
Agreement with single-compartment OGSE
Our Monte Carlo simulations confirm, under the assumption of axial symmetry, that the EP-OGSE retrieves the same frequency dependent diffusion tensor metrics in samples with isotropic orientational distributions as a DTI analysis of OGSE data on a single substrate. This demonstrates disentanglement of the orientational distribution, a global feature, from the microscopic single compartment diffusivity. We found a good correspondence between the analytical solution for the diffusion spectrum in a sphere and DL and DT derived from both EP-OGSE and DTI OGSE in ellipsoidal geometries with the same axial radii. This could be useful for analytical calculation of the signal from arbitrary waveforms without the need of computationally expensive simulations. Estimates from noisy data showed that some bias in DL and DT can be expected from data with an experimentally feasible SNR of 25 especially for larger restrictions. Noise sensitivity decreased for the dataset with the highest maximum b-value.
Disentangling global effects
Under the assumption of a single local compartment, linear encoding and Eqn. 11 are sufficient to estimate the underlying anisotropic diffusion spectrum as proposed in previous studies8,44. The benefit of multidimensional encoding lies in disambiguating the non-monoexponential attenuation arising from distributions of isotropic diffusivities from that arising from disperse anisotropic diffusion tensors, as illustrated on Fig. 2a: While linear encoding provides the same surface for both kinds of tissue, EP-OGSE provides a χ-modulation with anisotropic compartments. Additionally, the depth of this modulation depends directly on μFA as illustrated in Fig. 2b.
Gradient interactions
The Monte Carlo simulations demonstrated mono-exponentiality up to moderate b-values shown in Fig. 5. For the largest b-value and large restrictions we indeed observe deviations that can bias the measurement which relies on signal deviations on the order of a few percents. This indicates that the wavelength of the phase encoding wave vector 1/F(t) approaches the restriction size as can explored in the diffraction patterns at high q-values in conventional PGSE36. Further, this effect was as expected largest for χ = 0° where the gradient amplitude is concentrated to one axis and would thus underestimate the χ-modulation in this geometry. Due to the good agreement between the Monte Carlo data and the analytical description of the diffusion spectra we surmise that the dominating non-monoexponential decay can be attributed to anisotropy under the conditions of the simulations. Further spin displacement correlations between the axes may occur in the presence of bulk flow or if the single compartment signals are not well described by the Gaussian phase approximation, for instance due to more complex shapes like curvatures on the length scale of the free diffusion path length over the time frame of the encoding gradients63,64.
EP-OGSE in perspective
Similarity with angular DDE
The EP-OGSE sequence has some similarities with the classic angular DDE experiment. However, rather than changing the angle of a second encoding gradient, the relative strengths of two dephased orthogonal gradients are modulated, thus varying the weights of the diffusion encoding axes while fixing their orientations. The off diagonal terms of B vanish, excluding signal modulations from non-uniform orientational distributions, while symmetries of the signal over the modulation angle χ are introduced.
A similar approach was also recently presented in an angular DDE experiment by Paulsen et al. where the relative strengths of two subsequent pulsed field gradient blocks were modulated43. This leads to a similar modulation over 90° in anisotropic samples. However, the diffusion is here probed at different (but interdependent) time scales along each axis. A benefit of EP-OGSE is that the probed time scale is constant over χ and can be independently modulated by ωm.
Under the assumption of time independent diffusion, fixed encoding axes can also be achieved if multiple encoding gradients deviate from the same symmetry axis with the same but opposed angles or by numerically optimized gradient waveforms48,65.
Trapezoidal instead of harmonic waveforms
Ianus et al. suggested in a simulation study to use an angular DDE experiment with trapezoidal OGSE waveforms for model based estimation of axonal diameters66. Our gradient waveform layout with simultaneous gradients has the benefit of maximizing the available time for diffusion weighting on both axes which both increases the maximum diffusion weighting with a factor of two as well as balances the gradient usage over the sequence and corrects for possible gradient imperfections. The encoding gradient waveforms and thus the encoding power spectra, i.e. the filters to the temporal diffusion spectrum, are also maintained along each axis with this configuration at all mixing times deliberately chosen or dictated by the length of the inversion pulses. However, at the expense of lower b-value and longer sequence length, separated gradient pairs with constant amplitude decouples the individual encodings and eases the requirement of mono-exponential decay with analytical descriptions of the b2-term67,68.
Trapezoidal oscillating gradients in single directions have been proposed as more efficient than harmonic oscillations used in this study as they provide higher relative spectral encoding amplitude than cosine gradients of equal duration38,66, but could in principle also be applied simultaneously. This would however with maximum gradient amplitude be limited to gradient trajectories on a cube and not with uniformly distributed orientations. Experimentally, trapezoidal gradients can also be difficult to achieve in practice at higher frequencies due to hardware limitations68. OGSE is in general challenging to perform on clinical systems with limited gradient amplitude and the EP-OGSE method will initially be more suitable for preclinical MRI or diffusion NMR experiments of phantoms or post mortem tissue. With gradient strengths of 80 mT/m or 300 mT/m, reflecting state-of-the-art clinical and experimental human MRI gradient systems the available b-value at 100 Hz would according to the trace of Eqn. 4 be on the order of 0.045 and 0.63 s/μm2 for two 40 ms gradients in a spin echo sequence. The latter approaches the b-value of 0.8 s/μm2μm2 used in the experiments of this study and was thus included in the simulations. For applications in non-neuronal tissue, for instance in muscle or tumor tissue, larger restriction sizes could be investigated with lower frequency demands and potentially higher b-values. Optimal frequency range and SNR requirements should be considered with respect to the expected intrinsic diffusivity and geometry of the restriction of interest.
Conclusion
EP-OGSE combines OGSE with DDE, offering sensitivity to microscopic anisotropy and the frequency dependent μFA in disperse media of uniformly oriented, axially symmetric diffusion compartments. Monte Carlo simulations show that EP-OGSE retrieves the same single compartment frequency dependent diffusion tensor in disperse media as OGSE in a single aligned compartment. Measurements on ex-vivo monkey gray and white matter imply an increase with frequency of transverse as well as longitudinal diffusivity and a net decrease in μFA. The proposed method demonstrates the ability to characterize the temporal diffusion tensor spectrum and provides input for understanding the signal from more general gradient waveforms.
Methods
Experiment
EP-OGSE data were acquired using an Agilent 4.7 T preclinical scanner using a quadrature transmit/recieve coil. EP-OGSE gradient waveforms were designed using the CP-OGSE gradient designs described earlier but scaled as described in the theory section41. The gradient amplitudes were adjusted numerically to realize a b-value of 0.8 ms/μm2 at oscillation frequencies of 50 Hz and 100 Hz with a waveform length of 25 ms repeated on each side of a refocusing pulse in a spin echo sequence. The ellipticity angle χ was incremented in 19 steps from 0° to 90° and applied in one plane parallel with the image plane. Maximum gradient strength applied was 500 mT/m. 4 coronal slices were acquired with a 2D spin-echo sequence with a voxel size of 0.23 × 0.23 × 2.5 mm3, matrix size of 256 × 256, TE = 71.7 ms and TR = 2.5 s. The protocol was repeated 6 times and every measurement was interleaved with a b = 0 image resulting in 760 image volumes and a total imaging time of 82 hours. To ensure stable sample and RF-coil temperature during different gradient loads, the sample was heated using a conditioned air flow through the bore controlled by a small animal monitoring system (SA Instruments). The temperature was measured on the coil wall 5 cm from the sample and kept at (23 ± 0.1)°C throughout data acquisition. An excised perfusion fixated brain from a 3.5 year old vervet monkey was used for the experiments. Residual formalin from the fixation process was rinsed in phosphate buffered saline (PBS) to enhance T2-relaxation and the brain was submerged in a sealed plastic bag with new PBS using a setup optimised for ex vivo DWI described earlier69. The post mortem monkey brain specimen was obtained from the Behavioral Science Foundation, St. Kitts and the live animal was socially housed in enriched environments. All procedures for handling and sacrificing the live animal prior to our post mortem experiment were reviewed and approved by the Institutional Review Board of the Behavioral Science Foundation acting under the auspices of the Canadian Council on Animal Care.
Simulation
Monte Carlo random walk simulations were performed using in house software implemented in Matlab (Mathworks, Natick, MA, USA) optimized with regards to step length and number of walkers70. Random walks were performed in axially symmetric ellipsoidal restrictions with all 16 combinations of longitudinal and transversal radii a and b between 1.5 and 4.5 μm with increments of 1 μm. Time step was 1 μs and the free diffusivity was set to D0 = 2 μm2/ms. The waveform layout was identical to the experiment, but performed with χ = 0° and 45° only, 5 frequencies between 50 and 400 Hz and b-values for b = [0 0.4 0.8 1.6 3.2] ms/μm2 and b = [0 0.15 0.3 0.45 0.6] with 24 uniformly distributed orientations over the sphere relative to the substrates symmetry axes.
Analysis
The analyses were performed with in house software implemented in Matlab (Mathworks, Natick, MA, USA). Masks for gray and white matter ROIs were created by thresholding the b = 0 images. All diffusion weighted images were normalized to its subsequent b = 0 image and the data were averaged over the two ROIs. To eliminate effects of global anisotropy, the data were also averaged symmetrically around χ = 45°. Subcortical structures were excluded using a manually drawn mask. A slight evaporation of PBS was observed during the experiment resulting in a shift in intensity in partial volume voxels. To only include data with consistent tissue fractions we excluded voxels with more than 5% signal variation in the b = 0 images over the experiment. B-matrices were calibrated for each gradient waveform to achieve a constant signal attenuation on a water ROI corresponding to a free diffusivity of D0 = 2 μm2/ms reflecting the free diffusion coefficient at room temperature.
Simulation data were analysed in two ways. First, the compartment diffusion tensor spectrum for each substrate was estimated from data with linear polarisation using linear regression. Second, data were averaged over all gradient directions for each frequency, substrate and polarisation to emulate an isotropic orientational distribution.
Eqn. 10 was fitted to both the averaged experimental and simulated data with respect to DL and DT using non-linear least squares fitting. Simulated data were compared with the analytical descriptions for the diffusion spectrum in spherical restrictions26. The error intervals of the experimentally determined DL and DT were calculated as the diagonals of where J is the estimated Jacobian provided by Matlab in the least squares fit and σs is the variance of the residuals. Error propagation was further assessed in the Monte Carlo data with random Gaussian noise added to the EP-OGSE data reflecting an SNR of 25 in the individual datapoints. Mean and standard deviations of the parameter fits were calculated over 100 repetitions.
Data availabillity
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
Henrik Lundell is supported by a Sapere Aude award from the Danish Research Council for Independent Research, Production and Technology (4093-00280 A and 4093-00280B).
Author Contributions
H.L. and T.D. conceived the experiment, T.D. prepared the brain tissue for the experiments, all authors contributed to the optimisation of the experimental setup, J.S.N. and H.L. conducted the experiments and simulations, J.S.N. and H.L. analysed the results. All authors interpreted the results and reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le Bihan D. Diffusion MRI: What water tells us about the brain. EMBO Mol Med. 2014;6:569–573. doi: 10.1002/emmm.201404055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basser PJ, Mattiello J, Le Bihan D. M{R} diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - A technical review. NMR Biomed. 2002;7:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 4.Kärger J. NMR self-diffusion studies in heterogeneous systems. Adv Colloid Interface Sci. 1985;23:129–148. doi: 10.1016/0001-8686(85)80018-X. [DOI] [Google Scholar]
- 5.Nilsson M, Van Westen D, Ståhlberg F, Sundgren PC, Lätt J. The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter. MAGMA. 2013;26:345–370. doi: 10.1007/s10334-013-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novikov, D. S., Jespersen, S. N., Kiselev, V. G. & Fieremans, E. Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation. arXiv:1612.02059 1–38 (2016). [DOI] [PMC free article] [PubMed]
- 7.SN J, CD K, L Ø, JJ A, DA Y. Modeling dendrite density from magnetic resonance diffusion measurements. Neuroimage. 2007;34:1473–86. doi: 10.1016/j.neuroimage.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Kroenke C, Ackerman J, Yablonskiy D. On the nature of the NAA diffusion attenuated MR signal in the central nervous system. Magn Reson Med. 2004;52:1052–1059. doi: 10.1002/mrm.20260. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 10.Jespersen SN, Leigland LA, Cornea A, Kroenke CD. Determination of axonal and dendritic orientation distributions within the developing cerebral cortex by diffusion tensor imaging. IEEE Trans Med Imag. 2012;31:16–32. doi: 10.1109/TMI.2011.2162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferizi U, et al. White matter compartment models for in vivo diffusion MRI at 300mT/m. NeuroImage. 2015;118:468–483. doi: 10.1016/j.neuroimage.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Panagiotaki E, et al. Compartment models of the diffusion MR signal in brain white matter: A taxonomy and comparison. NeuroImage. 2012;59:2241–2254. doi: 10.1016/j.neuroimage.2011.09.081. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 14.Hansen, B. & Jespersen, S. Kurtosis fractional anisotropy, its contrast and estimation by proxy. Sci Rep10.1038/srep23999 (2016). [DOI] [PMC free article] [PubMed]
- 15.Mitra, P. P. Multiple wave-vector extensions of the NMR pulsed-field-gradient spin-echo diffusion measurement. PHYSICAL REVIEW B51 (1995). [DOI] [PubMed]
- 16.Shemesh N, et al. Conventions and nomenclature for double diffusion encoding NMR and MRI. Magn Reson Med. 2016;75:82–87. doi: 10.1002/mrm.25901. [DOI] [PubMed] [Google Scholar]
- 17.Topgaard D. Multidimensional diffusion MRI. JMR. 2017;275:98–113. doi: 10.1016/j.jmr.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Lawrenz M, Finsterbusch J. Double-wave-vector diffusion-weighted imaging reveals microscopic diffusion anisotropy in the living human brain. Magn Reson Med. 2013;69:1072–1082. doi: 10.1002/mrm.24347. [DOI] [PubMed] [Google Scholar]
- 19.Jespersen SN, Lundell H, Sønderby CK, Dyrby TB. Orientationally invariant metrics of apparent compartment eccentricity from double pulsed field gradient diffusion experiments. NMR Biomed. 2013;26:1647–1662. doi: 10.1002/nbm.2999. [DOI] [PubMed] [Google Scholar]
- 20.Lasi S, Szczepankiewicz F, Eriksson S, Nilsson M, Topgaard D. Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector. Front Phys. 2014;2:1–14. [Google Scholar]
- 21.Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proceedings of the National Academy of Sciences. 2014;111:5088–5093. doi: 10.1073/pnas.1316944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novikov, D. S. & Kiselev, V. G. Effective medium theory of a diffusion-weighted signal. NMR in Biomedicine10.1002/nbm.1584 (2010). [DOI] [PubMed]
- 23.Fieremans E, et al. In vivo observation and biophysical interpretation of time- dependent diffusion in human white matter Els. Neuroimage. 2016;129:414–427. doi: 10.1016/j.neuroimage.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burcaw L, Fieremans E, Novikov DS. Mesoscopic structure of neuronal tracts from time-dependent diffusion. NeuroImage. 2015;114:18–37. doi: 10.1016/j.neuroimage.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novikov, D. S. & Kiselev, V. G. Surface-to-volume ratio with oscillating gradients. Journal of Magnetic Resonance10.1016/j.jmr.2011.02.011 (2011). [DOI] [PubMed]
- 26.Stepišnik J. Time-dependent self-diffusion by NMR spin-echo. Physica B. 1993;183:343–350. doi: 10.1016/0921-4526(93)90124-O. [DOI] [Google Scholar]
- 27.Nilsson M, et al. On the effects of a varied diffusion time in vivo: is the diffusion in white matter restricted? MRI. 2009;27:176–187. doi: 10.1016/j.mri.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Clark C, Hedehus M. & ME, M. Diffusion time dependence of the apparent diffusion tensor in healthy human brain and white matter disease. Magn Reson Med. 2001;45:1126–1129. doi: 10.1002/mrm.1149. [DOI] [PubMed] [Google Scholar]
- 29.De Santis S, Jones DK, Roebroeck A. Including diffusion time dependence in the extra-axonal space improves in vivo estimates of axonal diameter and density in human white matter. NeuroImage. 2016;130:91–103. doi: 10.1016/j.neuroimage.2016.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross B, Kosfeld R. Anwendung der spin-echo-methode der messung der selbstdiffusion. Messtechnik. 1969;77:171–177. [Google Scholar]
- 31.Tanner JE. Self diffusion of water in frog muscle. Biophysical journal. 1979;28:107–116. doi: 10.1016/S0006-3495(79)85162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Does MD, Parsons EC, Gore JC. Oscillating gradient measurements of water diffusion in normal and globally ischemic rat brain. Magnetic Resonance in Medicine. 2003;49:206–215. doi: 10.1002/mrm.10385. [DOI] [PubMed] [Google Scholar]
- 33.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in Biomedicine. 2010;23:698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portnoy S, Flint JJ, Blackband SJ, Stanisz GJ. Oscillating and pulsed gradient diffusion magnetic resonance microscopy over an extended b-value range: Implications for the characterization of tissue microstructure. Magnetic Resonance in Medicine. 2013;69:1131–1145. doi: 10.1002/mrm.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons EC, Does MD, Gore JC. Temporal diffusion spectroscopy: Theory and implementation in restricted systems using oscillating gradients. Magn Reson Med. 2006;55:75–84. doi: 10.1002/mrm.20732. [DOI] [PubMed] [Google Scholar]
- 36.Callaghan, P. Translational Dynamics and Magnetic Resonance Principles of Pulsed Gradient Spin Echo NMR (Oxford University Press, 2011).
- 37.Colvin DC, et al. Earlier detection of tumor treatment response using magnetic resonance diffusion imaging with oscillating gradients. MRI. 2011;29:315–323. doi: 10.1016/j.mri.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron C, Beaulieu C. Oscillating gradient spin-echo (OGSE) diffusion tensor imaging of the human brain. Magn Reson Med. 2014;72:726–736. doi: 10.1002/mrm.24987. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal M, Jones MV, Calabresi PA, Mori S, Zhang J. Probing mouse brain microstructure using oscillating gradient diffusion MRI. Magn Reson Med. 2012;67:98–109. doi: 10.1002/mrm.22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kershaw J, et al. Systematic changes to the apparent diffusion tensor of in vivo rat brain measured with an oscillating-gradient spin-echo sequence. NeuroImage. 2013;70:10–20. doi: 10.1016/j.neuroimage.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 41.Lundell H, Sønderby CK, Dyrby TB. Diffusion weighted imaging with circularly polarized oscillating gradients. Magn Reson Med. 2015;73:1171–1176. doi: 10.1002/mrm.25211. [DOI] [PubMed] [Google Scholar]
- 42.Van AT, Holdsworth SJ, Bammer R. In vivo investigation of restricted diffusion in the human brain with optimized oscillating diffusion gradient encoding. Magn Reson Med. 2014;71:83–94. doi: 10.1002/mrm.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulsen JL, Özarslan E, Komlosh ME, Basser PJ, Song YQ. Detecting compartmental non-Gaussian diffusion with symmetrized double-PFG MRI. NMR Biomedicine. 2015;28:1550–1556. doi: 10.1002/nbm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaden E, Kruggel F, Alexander D. Quantitative mapping of the per-axon diffusion coefficients in brain white matter. Magn Reson Med. 2016;75:1752–1763. doi: 10.1002/mrm.25734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bak M, Nielsen N. REPULSION, A novel approach to efficient powder averaging in solid-state NMR. J Magn Reson. 1997;125:132–139. doi: 10.1006/jmre.1996.1087. [DOI] [PubMed] [Google Scholar]
- 46.Callaghan PT, Soderman O. Examination of the lamellar phase of aerosol OT/water using pulsed field gradient nuclear magnetic resonance. J Phys Chem. 1983;87:1737–1744. doi: 10.1021/j100233a019. [DOI] [Google Scholar]
- 47.Palombo, M., Ligneul, C. & Valette, J. Modeling diffusion of intracellular metabolites in the mouse brain up to very high diffusion-weighting: Diffusion in long fibers (almost) accounts for non-monoexponential attenuation. Magn Reson Med 343–350 10.1002/mrm.26548 (2016). [DOI] [PMC free article] [PubMed]
- 48.Eriksson, S., Lasič, S., Nilsson, M., Westin, C. F. & Topgaard, D. NMR diffusion-encoding with axial symmetry and variable anisotropy: Distinguishing between prolate and oblate microscopic diffusion tensors with unknown orientation distribution. J Chem Phys142 (2015). [DOI] [PMC free article] [PubMed]
- 49.Xu, J., Does, M. D. & Gore, J. C. Quantitative characterization of tissue microstructure with temporal diffusion spectroscopy. Journal of Magnetic Resonance10.1016/j.jmr.2009.06.022 (2009). [DOI] [PMC free article] [PubMed]
- 50.Colvin DC, et al. Effects of Intracellular Organelles on the Apparent Diffusion Coefficient of Water Molecules in Cultured Human Embryonic Kidney Cells. Magn Reson Med. 2011;65:796–801. doi: 10.1002/mrm.22666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilsson M, Lätt J, Ståhlberg F, van Westen D, Hagslätt H. The importance of axonal undulation in diffusion MR measurements: A Monte Carlo simulation study. NMR Biomedicine. 2012;25:795–805. doi: 10.1002/nbm.1795. [DOI] [PubMed] [Google Scholar]
- 52.Teh I, Schneider JE, Whittington HJ, Dyrby TB, Lundell H. Temporal Diffusion Spectroscopy in the Heart with Oscillating Gradients. Proc. Intl. Soc. Mag. Reson. Med. 2017;25:3114. [Google Scholar]
- 53.Nilsson, M., Lasič, S., Drobnjak, I., Topgaard, D. & Westin, C.-F. Resolution limit of cylinder diameter estimation by diffusion MRI: The impact of gradient waveform and orientation dispersion. NMR in Biomedicine e3711, 10.1002/nbm.3711 (2017). [DOI] [PMC free article] [PubMed]
- 54.Lundell H, Alexander C, Daniel, Dyrby BT. Apparent exchange rate imaging in anisotropic systems. NMR Biomed. 2014;27:918–925. doi: 10.1002/nbm.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szczepankiewicz F, et al. The link between diffusion MRI and tumor heterogeneity: Mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE) NeuroImage. 2016;142:522–532. doi: 10.1016/j.neuroimage.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Martins J, Topgaard D. Two-Dimensional Correlation of Isotropic and Directional Diffusion Using NMR. Phys Rev Lett. 2016;116:1–6. doi: 10.1103/PhysRevLett.116.116801. [DOI] [PubMed] [Google Scholar]
- 57.Ronen I, Moeller S, Ugurbil K, Kim DS. Analysis of the distribution of diffusion coefficients in cat brain at 9.4T using the inverse Laplace transformation. MRI. 2006;24:61–68. doi: 10.1016/j.mri.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Ronen I, Ercan E, Webb A. Axonal and glial microstructural information obtained with diffusion-weighted magnetic resonance spectroscopy at 7T. Front Integr Neurosci. 2013;7:1–10. doi: 10.3389/fnint.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Branzoli F, Ercan E, Webb A, Ronen I. The interaction between apparent diffusion coefficients and transverse relaxation rates of human brain metabolites and water studied by diffusion-weighted spectroscopy at 7T. NMR Biomed. 2014;27:495–506. doi: 10.1002/nbm.3085. [DOI] [PubMed] [Google Scholar]
- 60.Sønderby K, Kasper, Lundell H, Søgaard V, Lise, Dyrby BT. Apparent exchange rate imaging in anisotropic systems. Magn Reson Med. 2013;72:756–762. doi: 10.1002/mrm.24957. [DOI] [PubMed] [Google Scholar]
- 61.Shemesh N, et al. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nature communications. 2014;5:4958. doi: 10.1038/ncomms5958. [DOI] [PubMed] [Google Scholar]
- 62.Ianus, A., Siow, B., Drobnjak, I., Zhang, H. & Alexander, D. C. Gaussian phase distribution approximations for oscillating gradient spin echo diffusion MRI. Journal of Magnetic Resonance, 10.1016/j.jmr.2012.11.021 (2013). [DOI] [PubMed]
- 63.Wu D, Zhang J. The Effect of Microcirculatory Flow on Oscillating Gradient Diffusion MRI and Diffusion Encoding with Dual-Frequency Orthogonal Gradients (DEFOG) Magn Reson Med. 2016;77:1583–1592. doi: 10.1002/mrm.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jespersen S, Buhl N. The displacement correlation tensor: Microstructure, ensemble anisotropy and curving fibers. J Magn Reson. 2011;208:34–43. doi: 10.1016/j.jmr.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Westin CF, et al. Q-space trajectory imaging for multidimensional diffusion MRI of the human brain. NeuroImage. 2016;135:345–362. doi: 10.1016/j.neuroimage.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ianus, A., Shemesh, N., Alexander, D. C. & Drobnjak, I. Double oscillating diffusion encoding and sensitivity to microscopic anisotropy. Magn Reson Med, 10.1002/mrm.26393 (2016). [DOI] [PMC free article] [PubMed]
- 67.Jespersen, S. N., Lundell, H., Sønderby, C. K. & Dyrby, T. B. Commentary on “Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientation of order parameter by diffusion MRI with magic-angle spinning of the q-vector”. Frontiers in Physics, 10.1002/nbm.2999 (2014).
- 68.Ligneul, C. & Valette, J. Probing metabolite diffusion at ultra-short time scales in the mouse brain using optimized oscillating gradients and “short”-echo-time diffusion-weighted MRS. NMR Biomed3010.1002/nbm.3671 (2017). [DOI] [PMC free article] [PubMed]
- 69.Dyrby TB, et al. An ex vivo imaging pipeline for producing high-quality and high-resolution diffusion-weighted imaging datasets. HBM. 2011;32:544–563. doi: 10.1002/hbm.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall MG, Alexander DC. Convergence and parameter choice for Monte-Carlo simulations of diffusion MRI. IEEE Trans Med Imag. 2009;28:1354–1364. doi: 10.1109/TMI.2009.2015756. [DOI] [PubMed] [Google Scholar]