Abstract
Hikikomori, a severe form of social withdrawal syndrome, is a growing social issue in Japan and internationally. The pathophysiology of hikikomori has not yet been elucidated and an effective treatment remains to be established. Recently, we revealed that avoidant personality disorder is the most common comorbidity of hikikomori. Thus, we have postulated that avoidant personality is the personality underpinning hikikomori. First, we herein show relationships between avoidant personality traits, blood biomarkers, hikikomori-related psychological features, and behavioural characteristics assessed by a trust game in non-hikikomori volunteers. Avoidant personality traits were negatively associated with high-density lipoprotein cholesterol (HDL-C) and uric acid (UA) in men, and positively associated with fibrin degeneration products (FDP) and high sensitivity C-reactive protein (hsCRP) in women. Next, we recruited actual individuals with hikikomori, and compared avoidant personality traits, blood biomarkers, and psychological features between individuals with hikikomori and age-matched healthy controls. Individuals with hikikomori had higher avoidant personality scores in both sexes, and showed lower serum UA levels in men and lower HDL-C levels in women compared with healthy controls. This is the first report showing possible blood biomarkers for hikikomori, and opens the door to clarify the underlying biological pathophysiology of hikikomori.
Introduction
“Hikikomori”, a severe form of social withdrawal seen more often in individuals of relatively young age, has become a growing social issue since around 1990 in Japan1–4. Hikikomori is usually defined as follows: (i) spending most of the day and nearly every day at home, (ii) avoiding social situations such as attending school or workplace, (iii) avoiding social relationships such as friendships or contact with family members, and (iv) significant distress or impairment due to social isolation (i to iii require duration of at least 6 months)5. An epidemiological study of hikikomori showed a lifetime prevalence of more than 1% in adults in Japan2. Although hikikomori had been previously thought to be proper only to Japanese society/culture6, recent international studies have revealed that hikikomori is found in various races and areas around the world including Hong Kong, urban areas of China, India, South Korea, Spain, and the United States5–10. Our recent international survey has shown that the most common comorbidity of hikikomori is avoidant personality disorder11. Hikikomori and avoidant personality disorder seem to have many psychological and behavioural features in common: shyness; ambivalent attachment styles and life experiences including rejection by peers and parents12; high loneliness and impaired social networks; apparent inability to maintain meaningful social ties5; social withdrawal and avoidance of real-world human interactions; and tendency toward indirect interpersonal exchanges via the Internet13. Urgent development of effective prevention and treatment methods for hikikomori is required. However, there has been neither preventive nor treatment measures of hikikomori, because the pathophysiology of hikikomori has not been fully elucidated yet.
Herein, we preliminary explored the underlying psychological and biological pathophysiology of hikikomori. First, we hypothesized that avoidant personality traits would be associated with hikikomori, and investigated the association between avoidant personality traits, blood biomarkers, behavioural characteristics, and psychological aspects in student-age non-clinical samples (Study 1). Interpersonal relationships in the real world are at least partially based on behaviours derived from unconscious decision-making14–17. To assess behavioural characteristics, we applied self-rated questionnaires and also a personal computer (PC)-based trust game, which can accurately evaluate unconscious decision-making and consequently estimate interpersonal relationships which participants have in the real world16. Second, we compared avoidant personality traits and candidate blood biomarkers for avoidant personality traits identified in Study 1 between actual individuals with hikikomori and age-matched healthy control subjects (Study 2).
In Study 1, we recruited a total of 101 young non-clinical volunteers in a university campus and collected their socio-demographic information. Forty-six participants were male, and no participant was excluded from this study. Participants’ demographic details are shown in Table 1. Each participant completed a series of self-rated questionnaires, played a PC based trust game, and provided a non-fasting venous blood sample.
Table 1.
Demographic data.
| Study 1. | |||
|---|---|---|---|
| male | female | p value | |
| n | 46 | 55 | |
| age, median (IQR) | 21.0 (4.0) | 21.0 (2.0) | 0.43b |
| nationality | |||
| Japanese | 44 | 55 | 0.12a |
| not Japanese (Asians) | 2 | 0 | |
| cohabitation | |||
| living alone | 33 | 35 | 0.39a |
| not living alone | 13 | 20 | |
| status | |||
| current student | 44 | 55 | 0.37a |
| worker | 2 | 0 | |
| Study 2. | |||
| hikikomori | healthy control | p value | |
| n, male/female | 29/26 | 34/44 | |
| age, median (IQR) | |||
| male | 38.0 (19.0) | 35.0 (8.0) | 0.61b |
| female | 34.0 (12.0) | 39.0 (17.0) | 0.092b |
aChi-squared test, bWelch’s t test or Mann-Whitney U test.
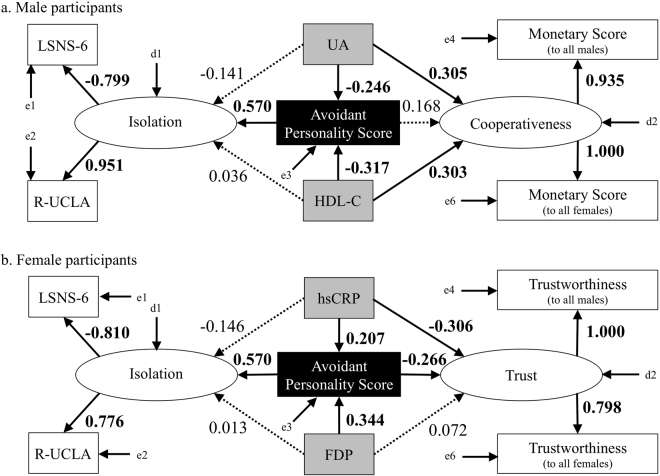
First, we assessed causal relationships between avoidant personality score, blood biomarkers, behavioural characteristics, and psychometrics using structural equation modeling (SEM). Next, we examined the association between avoidant personality score and blood biomarkers using multiple regression analyses with stepwise method for efficient biomarkers detection. Finally, correlation analyses were performed between avoidant personality score, blood biomarkers, behavioural characteristics, and psychometrics. The best-fit models by gender are shown in Fig. 1. Good fits of each model were obtained as follows: χ2 = 8.143 (p = 0.62; statistically insignificance means a good fit), goodness of fit index (GFI) = 0.954, comparative fit index (CFI) = 1.000, Tucker–Lewis index (TLI) = 1.025, and the root mean square error of approximation (RMSEA) <0.001 in men; χ2 = 6.569 (p = 0.77), GFI = 0.968, CFI = 1.000, TLI = 1.070, RMSEA <0.001 in women. The SEM suggested that higher avoidant personality scores led to stronger isolation that increased loneliness and decreased social support and networks (i.e., high scores of the Revised University of California, Los Angeles Loneliness Scale (R-UCLA) and the Lubben Social Network Scale-6 (LSNS-6), respectively) in both genders. In men, lower serum high-density lipoprotein cholesterol (HDL-C) and serum uric acid (UA) were proposed to lead to higher avoidant personality scores (standardized path coefficient (β) = −0.317, p = 0.020; and β = −0.246, p = 0.072, respectively), and less cooperativeness (β = 0.303, p = 0.038; and β = 0.305, p = 0.033, respectively). However, the direct causality between avoidant personality score and cooperativeness was not shown (β = 0.168, p = 0.26). In women, higher plasma fibrin degeneration products (FDP) and serum high sensitivity C-reactive protein (hsCRP) are suggested to lead to higher avoidant personality score (β = 0.344, p = 0.006; and β = 0.207, p = 0.097, respectively). Furthermore, higher avoidant personality score and hsCRP tended to make female participants estimate others to be untrustworthy (β = −0.266, p = 0.051; and β = −0.306, p = 0.018, respectively).
Figure 1.
Structure equation models showing the connections between avoidant personality score, blood biomarkers, psychological features, and behavioural characteristics in non-hikikomori volunteers. The arrows with bold lines have a statistically significance or a trend, and broken lines have neither a statistically significance nor a trend. The numbers above the arrows show standardized path coefficients. e: error terms, d: disturbance terms.
Next, we examined the association between avoidant personality score and blood biomarkers using multiple regression analyses with stepwise method for efficient biomarkers detection. Regression analyses with stepwise method revealed that avoidant personality scores in men and women were predicted by HDL-C (β = −0.323, p = 0.028, Condition Index = 10.383) and FDP (β = 0.329, p = 0.014, Condition Index = 12.252), respectively.
Finally, correlation analyses were performed between avoidant personality score, blood biomarkers, behavioural characteristics, and psychometrics. Correlation analyses showed negative correlations between avoidant personality score and HDL-C, total bilirubin, and UA in men; and positive correlations between avoidant personality score and FDP and hsCRP in women (Supplementary Table 1). Although correlation analyses showed no significant associations between avoidant personality score and results of the trust game in men, significant negative correlations were found between avoidant personality score and extensive results of the trust game in women (Supplementary Table 2), suggesting that higher avoidant personality traits appeared to make female participants more mistrustful of and less attracted to others. Moreover, trust games showed that men with higher HDL-C or UA levels tend to be more cooperative (i.e., give more money to their partners). Women with higher hsCRP levels showed weaker trust in their partners, especially in male partners (Supplementary Tables 3–4). In both genders, participants with higher avoidant personality score were more likely to be lonely (i.e., higher scores of the Preference for Solitude Scale (PSS) and R-UCLA), socially anxious (i.e., higher score of the Mini Social Phobia Inventory (MINI-SPIN)), lacking social support (i.e., lower scores of LSNS-6 and the Multidimensional Scale of Perceived Social Support (MSPSS)), and mistrustful of others (i.e., lower score of the Yamagishi and Yamagishi’s trust scale (YYS)). Interestingly, avoidant personality score had a positive correlation with the Internet Addiction Test (IAT) only in male participants (Supplementary Table 1). In men, significant positive correlations were found between FDP and YYS. Negative correlations were shown between total bilirubin and PSS; and between UA and MINI-SPIN. In women, significant positive correlations were found between serum low-density lipoprotein cholesterol (LDL-C) and LSNS-6. A negative correlation was shown between total bilirubin and YYS or MSPSS (Supplementary Tables 3–4).
In accord with the results of Study 1, we recruited 55 individuals with hikikomori and 78 age-matched non-clinical volunteers as a healthy control group in Study 2. Twenty-nine individuals and 34 volunteers were male; and one individual with hikikomori was excluded from this study because of a history of traumatic brain injury. Participants’ demographic details are shown in Table 1. Each participant completed a series of self-rated questionnaires that were the same as in Study 1, underwent a structured interview for hikikomori with an experienced psychiatrist or a psychologist, and provided a non-fasting venous blood sample. The blood biomarkers measured were the same as in Study 1. First, we assessed normality of data, and then compared avoidant personality score and blood biomarkers between the two groups. Next, we assessed classifying efficiencies and potentials of blood biomarkers using canonical discriminant analysis and receiver-operating characteristic (ROC) curves. Finally, correlation analyses were performed between avoidant personality score, blood biomarkers, and psychometrics.
Avoidant personality score in individuals with hikikomori was significantly higher than in healthy controls in both genders (p < 0.001). Male individuals with hikikomori had significantly lower UA (p = 0.001), and female individuals with hikikomori had significantly lower HDL-C (p = 0.011) (Tables 2–3). We performed discriminant analysis using the diagnosis of hikikomori as the dependent variable, and UA in male participants and HDL-C in female participants as predictor variables based on the results of comparison of blood biomarkers between individuals with hikikomori and healthy controls. Moreover, to evaluate the diagnostic potential of the biomarkers, we constructed a ROC curve and then calculated the area under the curve (AUC) and 95% confidence interval (CI). The percentages of correct classification between hikikomori and healthy control groups were 72.4% with a sensitivity of 66.7% and a specificity of 76.5% in male participants, and 61.3% with a sensitivity of 61.1% and a specificity of 61.4% in female participants. AUC (95% CI) was 0.772 (0.647–0.897) with a sensitivity of 70.6% and a specificity of 75.0% (p < 0.001) in male participants, and 0.693 (0.550–0.835) with a sensitivity of 65.9% and a specificity of 61.1% (p = 0.018) in female participants (Supplementary Table 5). Although not significant, correlation analyses showed that UA was positively correlated with PSS (r = 0.379, p = 0.068) and MINI-SPIN (ρ = 0.404, p = 0.050) in male individuals with hikikomori. HDL-C was not correlated with avoidant personality score and any psychometrics in female individuals with hikikomori (Supplementary Tables 6–7).
Table 2.
Comparison of avoidant personality score and blood biomarkers between male individuals with hikikomori and healthy control.
| hikikomori | healthy control | p value | |||||
|---|---|---|---|---|---|---|---|
| n | median/mean | IQR/SD | n | median/mean | IQR/SD | ||
| avoidant personality score biomarkers | 28 | 5.0 | 2.0 | 34 | 2.0 | 2.0 | <0.001a |
| HDL-C | 24 | 54.1 | 11.8 | 34 | 58.7 | 14.6 | 0.21a |
| LDL-C | 24 | 106.0 | 48.0 | 34 | 118.5 | 38.0 | 0.64a |
| FDP | 24 | 0 | 0 | 34 | 0 | 0 | 0.36a |
| total bilirubin | 24 | 0.5 | 0.3 | 34 | 0.6 | 0.2 | 0.20a |
| UA | 24 | 5.0 | 1.3 | 34 | 6.2 | 1.3 | 0.001b |
| hsCRP | 24 | 342.0 | 620.0 | 34 | 387.5 | 828.0 | 0.99a |
aMann-Whitney U test, bStudent’s or Welch’s t test.
Table 3.
Comparison of avoidant personality score and blood biomarkers between female individuals with hikikomori and healthy control.
| hikikomori | healthy control | p value | |||||
|---|---|---|---|---|---|---|---|
| n | median/mean | IQR/SD | n | median/mean | IQR/SD | ||
| avoidant personality score | 24 | 5.0 | 3.0 | 44 | 1.5 | 3.0 | <0.001a |
| biomarkers | |||||||
| HDL-C | 18 | 64.7 | 11.1 | 44 | 73.3 | 12.2 | 0.011b |
| LDL-C | 18 | 123.8 | 33.7 | 44 | 114.0 | 31.0 | 0.27b |
| FDP | 14 | 0 | 0 | 43 | 0 | 0 | 0.14a |
| total bilirubin | 18 | 0.50 | 0.25 | 44 | 0.55 | 0.30 | 0.42a |
| UA | 18 | 4.50 | 1.70 | 44 | 4.00 | 1.45 | 0.53a |
| hsCRP | 18 | 215.5 | 2137.0 | 44 | 213.5 | 572.0 | 0.14a |
aMann-Whitney U test, bStudent’s or Welch’s t test.
In sum, we herein investigated the biological pathophysiology of hikikomori by focusing on avoidant personality traits. Study 1 showed significant relationships between avoidant personality traits, blood biomarkers, and behavioural characteristics assessed by the trust game, and psychological features (loneliness, social anxiety, trust, and Internet addiction) in healthy volunteers. Candidate blood biomarkers of avoidant personality traits were identified as follows: HDL-C and UA in men, and FDP and hsCRP in women. The SEM analysis suggests that avoidant personality traits exacerbating psychological isolation in both sexes were indirectly associated with behavioural uncooperativeness in men, and directly induced distrust of others in women. Study 2 revealed that individuals with hikikomori had higher avoidant personality scores in both sexes, lower serum UA levels in men, and lower serum HDL-C levels in women.
Social relationships cannot be assessed only by self-rated questionnaires and interviews as social relationships in the real world are based on unconscious behavioural and emotional patterns, and usually have deviations from self-rated sociability itself. Therein, experimental economic games, developed in the fields of social psychology and economics, help offer insight into real-world behaviour and personality16. Economic games have been proposed as a novel candidate for an assessment tool of real-world interpersonal problems in patients with psychiatric disorders including personality disorders those who tend to have difficulties in appropriate decision-making18. Trust game is such an economic game and has been used to evaluate a person’s trust toward others19. Trust is a basis of cooperation in social interactions, leading to socially supportive and harmonious interpersonal relationships. Using the trust game, we previously reported sex differences in behaviour and alterations of trust behaviour, but our previous study was limited by lack of assessing biomarkers16. To our knowledge, this is the first report to show that interpersonal behaviour is significantly related with blood biomarkers, supplementing our previous study16.
Sex differences were consistently observed in the present cross-sectional study. Our study indicates that blood substances are likely to change behaviour (i.e., Cooperative) in males, and emotions (i.e., Trust) in females. There are gender differences in how people seek social support. Men tend to seek instrumental and tangible support, whereas women often seek emotional support20,21. Sex differences in the way people seek social support are possibly associated with psychosocial features of avoidant personality traits, leading to sex differences in our study. It would be effective to seek gender segregated treatment approaches for avoidant personality. For example, exercise or diet interventions, which affect UA and HDL-C levels, may be effective for men with avoidant personality traits. In contrast, women with avoidant personality traits may need psychotherapeutic approaches focusing on personality pathologies and trust. Differences between sexes are also possibly caused by multiple factors such as sex hormones and estrous cycles which influence mental functions including mood and emotion22, lifestyle differences, and cultures which accept sex differences in thought, cognition, behaviour, intrapersonal masculinity and femininity.
Oxidative stress and inflammation have recently been highlighted to understand not only pathological mechanisms of various psychiatric disorders but also socio-culture-based human behavior22,23. In Study 1, candidate blood biomarkers of avoidant personality traits were identified as follows: HDL-C and UA in men, and FDP and hsCRP in women. Interestingly, both HDL-C and UA are known as antioxidants. HDL-C has antioxidant and also anti-inflammatory functions24, and UA is a representative endogenous antioxidant widely erasing reactive oxygen species25. Oxidative stress decreases serotonin levels in the brain26 and has been highlighted as a candidate in the etiology of psychiatric diseases including depression27. For example, patients with major depressive and anxiety disorders have lower plasma UA levels28. Considering the association between dysregulation of the serotonergic system and avoidant personality disorder29, the decrease of antioxidants may induce social anxiety and avoidance behaviour via oxidative stress in men. Furthermore, previous studies have reported that both HDL-C and UA were associated with psychological and behavioural features. Low HDL-C was associated with specific personality traits characterized by self-centeredness, emotionality, and erratic behaviour30. Men with high serum UA levels are likely to have a hyperthymic temperament31. Economic generosity of males with higher HDL-C or UA found in the present study was possibly a result of altruistic traits and hyperthymic temperament. Both FDP and hsCRP are associated with inflammation. FDP has pro-inflammatory effects32, and hsCRP is widely used in clinical settings as a non-specific marker of acute inflammation. Inflammation has been recently gathering attention in the research field of various psychiatric problems including anxiety and depression15,33. The possible link between inflammation and avoidant personality traits is obesity. Obesity is positively correlated with inflammation33 and also avoidant personality disorder34. These findings suggest that avoidant personality traits may be due to inflammation, which influences emotions in women. The trust game conducted in the present study showed that women possibly judged whether somebody is trustworthy or not under the direct or indirect (i.e., through avoidant personality traits) influence of inflammation, proposing that inflammation affects the social decision-making system. We have previously reported that minocycline, an antibiotic drug that has an anti-inflammatory action via suppressing microglial activation, affected behaviour in the trust game35, suggesting the possibility that unconsciousness-based actions are modulated by brain inflammation15. These minocycline trials and the findings in the present study indicate that anti-inflammatory therapy is possibly important for treatment and prevention of avoidant personality-related problems including social withdrawal and its severe form, hikikomori. Further investigations on the association between inflammation and avoidant personality-related problems are required.
In Study 2, individuals with hikikomori had higher avoidant personality scores in both sexes. This trend suggests symptomatic similarities between hikikomori and avoidant personality disorder, and is congruent with the previous report that the most common comorbidity of hikikomori was avoidant personality disorder11. Our proposed blood biomarkers associated with hikikomori are UA in males and HDL-C in females. As discussed above, both UA and HDL-C have an antioxidative activity in common, suggesting that the phenomenon of hikikomori is possible associated with oxidative stress rather than inflammation in both genders contrary to expectations. The reason remains unclear, but we prospect that active social interaction may induce inflammation, and withdrawal from social interaction may ameliorate inflammation, causing individuals with hikikomori to have little inflammation.
The present study has several limitations. First, our study is based on relatively small samples recruited only in the same region, consequently resulting in selection bias. However, no reports exist comparing individuals with hikikomori and healthy controls, and analyzing multifaceted aspects of avoidant personality traits including behavioural features of more than 100 non-clinical persons. Therefore, we believe in the importance of our study. Second, we cannot exclude the possibility that some participants in Study 1 had psychiatric disorders, because structured diagnostic interviews for psychiatric disorders were not conducted for each healthy volunteer. In the present study, the possibility cannot be denied that some individuals with hikikomori have comorbidity with other psychiatric conditions such as depression and/or neurodevelopmental disorders. In future studies, structured diagnostic interviews such as the Mini-International Neuropsychiatric Interview (MINI)36 and the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2)37–39 should be conducted in individuals with hikikomori, and comparison studies are warranted to clarify the similarity and differences of blood biomarkers between hikikomori and other psychiatric disorders. Third, we did not measure direct biomarkers regarding oxidative stress such as the total oxidant status and the total antioxidant status. Evaluating these direct oxidative stress-evaluating biomarkers might provide further strong evidence. Fourth, although individuals with hikikomori had lower serum levels of UA and HDL-C in the present study, their levels were all within normal limits. Recently, multidimensional evaluation systems using large data samples have been regarded to be important in developing useful biomarkers for diagnosis of psychiatric disorders40,41. Thus, the development of an evaluation method combining blood biomarkers with other biomarkers including brain imaging and behavioural characteristics is strongly needed for diagnosis and/or prediction of hikikomori. Fifth, some individuals with hikikomori in the present study took psychiatric medications, which may have influenced the present outcomes. Further investigation should be conducted considering the influence of medication in clinical samples. Sixth, this is a cross-sectional study not allowing any causal relationships between avoidant personality traits, blood biomarkers, and behavioural and psychological features. Therefore, we compensated this limitation by using SEMs. Finally, there might be some confounding factors such as tobacco use and eating/fitness habits. Hikikomori is suggested to be a phenomenon caused due to bio-psycho-social, cultural, or environmental etiologies6, all of which could be potential confounding factors. Hence, further studies are needed to validate our findings and elucidate the role of biomarkers in avoidant personality and hikikomori.
To conclude, we herein revealed the following aspects: inflammatory and antioxidant markers of HDL-C, FDP, UA and hsCRP were correlated with avoidant personality traits, cooperative behaviour and/or trust, and psychological features of isolation by gender. Individuals with hikikomori had higher avoidant personality traits in both genders, lower UA in men, and lower HDL-C in women. Based on the present findings, we suggest a novel hypothesis: avoidant personality may be formed by interactive relationships between oxidative stress and inflammation, and affects behavioural and psychological features, leading to the phenomenon of hikikomori. Therefore, we believe that the present study will shed new light on clarifying the underlying biological basis of hikikomori.
Methods
The present study was carried out in accordance with the latest version of the Declaration of Helsinki and approved by the ethics committee of Kyushu University. All participants signed written informed consent to participate after a complete instruction of the study.
Study 1
Participants
A university campus was selected as a place to advertise our study for recruiting young non-clinical volunteers. We used posters and flyers on the Kyushu University campus targeting students and staff as healthy volunteers. A total of 101 volunteers were recruited, and most of them were university students. After obtaining informed consent, their socio-demographic information was collected. A medical doctor carefully asked all participants regarding their physical condition. Exclusion criteria included age < 17, not proficient in Japanese, a self-reported history of psychiatric disorders including schizophrenia, traumatic brain injury, or severe heart, liver, or kidney disease.
Procedure
All participants completed a series of self-rated questionnaires, played a trust game16,42, and provided a non-fasting venous blood sample. Measured blood biomarkers were as follows: serum HDL-C, LDL-C, serum total bilirubin, UA, hsCRP, and plasma FDP. These biomarkers were adopted because all items could be easily measured in normal clinical settings and have been gathering attention in the research field of various psychiatric diseases including schizophrenia43–47. Some biomarkers were selected for future comparison with the large cohorts, the Midlife in the United States and the Midlife in Japan48. Although tested samples were non-fasting venous blood, effects of meal on serum LDL-C and HDL-C were thought to be negligible49.
Self-rated Questionnaires
All participants completed the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) Personality Disorders Personality Questionnaire (SCID-II/PQ), a 119-item questionnaire version of the SCID-II, on which the items are answered using a “yes (=1 point)” and “no (=0 point)” response format50. The items relevant to each personality disorder were summed for a total score defined as an index of each personality trait. We evaluated avoidant personality scores in participants with SCID-II/PQ (Max. 7 points for the personality trait). Moreover, considering common psychological and behavioural features of avoidant personality disorder and hikikomori, we administered the following questionnaires: PSS, assessing one’s preference for solitude51; R-UCLA, assessing one’s degree of loneliness52,53; YYS, measuring respondents’ estimation of others’ trustworthiness54; IAT, assessing the degree to which Internet use affects daily routine, social life, productivity, sleeping pattern and feelings55; LSNS-6, gauging social isolation by assessing the number of people in one’s social network with whom one has social contact and social support56,57; MSPSS, measuring the perceived adequacy of support from family, friends and significant others58; and MINI-SPIN, having good efficiency in distinguishing individuals with generalized social anxiety in a health care setting59,60.
PC-based Trust Game
We evaluated trusting behaviour of participants towards others by a trust game, conducted on PCs based on our previous study16. In this two-player game, each player initially receives an explanation of the game rules and is given 1300 yen (JPY) [about 12 USD]. The first player then decides how much of the 1300 JPY to give to the second player (partner) and rates the partner’s attractiveness and trustworthiness based on their photographs presented on a computer screen (both of the score ranges: 0–9). The amount of money given to the partner by the first player (i.e., Monetary Scores) is tripled. The second player then decides whether to split the money equally with the first player or take away the entire amount of money. In this PC experiment, all the participants were actually assigned to be the first player, and the second players were virtual players on a computer screen. The participants had no information about their partners except for the facial photographs including the head and shoulders with a neutral facial expression. Photos of second players were selected from professional fashion models (i.e., “high attractive partners”) or lay individuals (i.e., “ordinary attractive partners”). We randomly selected 40 pictures (10 each of professional male fashion models, professional female fashion models, lay males, and lay females) for the trust game. The first player was not aware of the partner’s decision. The first player’s decision as to how much money to give to the second player is thought to reflect their level of trust in the second player. After the experiments, each participant was actually paid the amount of money corresponding to the result of a randomly selected game from all games as a reward.
Statistics
The path analysis and the other analyses were performed by SPSS AMOS version 24.0 and SPSS version 22 (SPSS, Inc., Chicago, IL, USA), respectively. All analyses were stratified by participant gender. Differences in the demographic data between both genders were analyzed using chi-squared test, Welch’s t test, or Mann-Whitney U test. First, we assessed causal relationships between avoidant personality score, blood biomarkers, psychometrics, and behavioural characteristics by using SEM. The latent variables consisted of (i) “Isolation”: PSS, R-UCLA, IAT, LSNS-6, and MSPSS; (ii) “Interpersonal Psychology”: YYS and MINI-SPIN; (iii) “Cooperativeness”: Monetary Scores; (iv) “Trust”: Trustworthiness; (v) “Affection”: Attractiveness. Various models were generated and assessed using the following fitting criterion: χ2, GFI, CFI, TLI, and RMSEA. χ2 is so-called ‘a badness of fit’ (we obtain a good fit model if p value is greater than 0.05). GFI greater than 0.90, CFI greater than 0.95, TLI greater than 0.95, and RMSEA less than 0.08 indicate an acceptable fit. GFI greater than 0.95, CFI greater than 0.97, TLI greater than 0.97, and RMSEA less than 0.05 indicate a good fit61. We compared the models to obtain the best-fit model using the parameter of Akaike’s Information Criterion. Second, multiple regression analyses were performed with stepwise method for efficient biomarkers detection. All blood biomarkers were the independent variables, and avoidant personality score was the dependent variable. To avoid the issue of multicollinearity, we chose the stepwise option and confirmed that the scores of condition indices were below fifteen for each final model. Finally, to calculate Pearson’s correlation coefficients (r) or Spearman’s correlation coefficients (ρ), correlation analyses were performed between avoidant personality score, blood biomarkers, psychometrics, and behavioural characteristics. We used a two-tailed significance level of p = 0.05 and a trend level of p = 0.1 throughout the study.
Study 2
Participant
This case-control study was conducted in Fukuoka, a major metropolitan area in southern Japan. Study recruitment occurred in six psychiatric hospitals and clinics affiliated with the Department of Neuropsychiatry of Kyushu University, and two community mental health centers. We included all individuals from the eight clinical sites who met criteria for currently having hikikomori based on a structured diagnostic interview designed for this project. Exclusion criteria were age <15 or >50, a self-reported history of schizophrenia, traumatic brain injury, or severe heart, liver, or kidney disease. For healthy control group recruitment, we used posters and flyers on the Kyushu University campus. Healthy controls were who: (i) did not have a history of current hikikomori or past physical isolation; and (ii) did not meet criteria for any current psychiatric disorder as per the Structured Clinical Interview for the DSM–IV Axis I Disorders. Exclusion criteria were age <15 or >50, a self-reported history of any psychiatric disorders, traumatic brain injury, or severe heart, liver, or kidney disease.
Procedure
All participants completed a series of self-rated questionnaires same as conducted in Study 1, underwent a structured interview for hikikomori with a study psychiatrist or psychologist, and provided a non-fasting venous blood sample. The structured interview had been developed for hikikomori in our prior theoretical work and empirical research11,62. Criteria for hikikomori were as follows: (i) spending most of the time at one’s home or residence at least for six months; (ii) significant distress or impairment associated with the social withdrawal; and (iii) a physical or medical etiology is not the primary reason for the social withdrawal. Measured blood biomarkers were same as in Study 1 for the same reasons.
Statistics
All analyses were performed by SPSS version 22 (SPSS, Inc., Chicago, IL, USA) and stratified by participant gender. Differences in age between two groups were analyzed using Welch’s t test or Mann-Whitney U test. First, we assessed normality of data using Shapiro-Wilk test, and then performed Mann-Whitney U test or Student’s/Welch’s t test to compare avoidant personality score and blood biomarkers between the two groups. Second, to assess the classifying efficiency of each blood biomarker, we performed canonical discriminant analysis using hikikomori diagnosis as the dependent variable, and candidate biomarkers based on the comparisons between two groups as the predictor variables. Cross-validation procedure by leave-one out classification was performed in this discriminant analysis. Moreover, ROC curves were constructed to assess the potential of candidate biomarkers. Finally, to calculate Pearson’s correlation coefficients (r) or Spearman’s correlation coefficients (ρ), correlation analyses were performed between avoidant personality score, blood biomarkers, and psychometrics. We used a two-tailed significance level of p = 0.05 and a trend level of p = 0.1 throughout the study.
Electronic supplementary material
Acknowledgements
The authors would like to thank Ms. Ryoko Katsuki, Ms. Sakumi Kakimoto, Ms. Yoko Zushi and Ms. Haruna Doi for their technical assistance. This work was supported by a Grant-in-Aid for Scientific Research on (1) Innovative Areas “Will Dynamics” of The Ministry of Education, Culture, Sports, Science, and Technology, Japan (JP16H06403 to T.A.K.), (2) The Japan Agency for Medical Research and Development (AMED) (“Yugo-no” and “Syogaisya-Taisaku-Sogo-Kenkyu-Kaihatsu-Jigyo” to T.A.K. & S.K.), (3) The Japan Society for the Promotion of Science (JSPS) - KAKENHI (JP26713039 and JP15K15431 to T.A.K., and JP16H02666 to S.K.), (4) The JSPS Bilateral Joint Research Project between USA-Japan (to T.A.K. and A.R.T.), (5) Young Principal Investigators’ Research Grant of Innovation Center for Medical Redox Navigation, Kyushu University (to T.A.K.), (6) the SENSHIN Medical Research Foundation (to T.A.K. and S.K.). Dr. Teo is supported by the Department of Veterans Affairs, and the views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author Contributions
All authors contributed substantially to the scientific process leading up to the writing of the present manuscript. T.A.K., the principal investigator of the present research, and K.H., the first author, created the conception and design of the project and wrote the protocol. The performance of experiments and data analyses/interpretation was performed by K.H., T.A.K., M.W., A.R.T., H.H., N.K., N. Shim., H.T., J.K., M.T., M.O., N.Sa., M.S.-K., and H.K. K.H. wrote the first draft of the manuscript. Critical revisions of the manuscript were made by T.A.K., K.H., M.W., A.R.T., N. Shin, and S.K. All authors approved this submission in its current form.
Competing Interests
The authors declare no competing interests.
Footnotes
Motoki Watabe and Alan R. Teo contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21260-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kato TA, Shinfuku N, Sartorius N, Kanba S. Are Japan’s hikikomori and depression in young people spreading abroad? Lancet. 2011;378:1070. doi: 10.1016/S0140-6736(11)61475-X. [DOI] [PubMed] [Google Scholar]
- 2.Kato TA, Kanba S, Teo AR. A 39-Year-Old “Adultolescent”: Understanding Social Withdrawal in Japan. Am J Psychiatry. 2016;173:112–114. doi: 10.1176/appi.ajp.2015.15081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato TA, Kanba S, Teo AR. Hikikomori: experience in Japan and international relevance. World Psychiatry. 2018;17:105–106. doi: 10.1002/wps.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding C. Hikikomori. Lancet Psychiatry. 2018;5:28–29. doi: 10.1016/S2215-0366(17)30491-1. [DOI] [PubMed] [Google Scholar]
- 5.Teo AR, et al. Identification of the hikikomori syndrome of social withdrawal: Psychosocial features and treatment preferences in four countries. Int J Soc Psychiatry. 2015;61:64–72. doi: 10.1177/0020764014535758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato TA, et al. Does the ‘hikikomori’ syndrome of social withdrawal exist outside Japan? A preliminary international investigation. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1061–1075. doi: 10.1007/s00127-011-0411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong PWC, Liu LL, Li TMH, Kato TA, Teo AR. Does hikikomori (severe social withdrawal) exist among young people in urban areas of China? Asian J Psychiatr. 2017;30:175–176. doi: 10.1016/j.ajp.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Malagon-Amor A, Corcoles-Martinez D, Martin-Lopez LM, Perez-Sola V. Hikikomori in Spain: A descriptive study. Int J Soc Psychiatry. 2015;61:475–483. doi: 10.1177/0020764014553003. [DOI] [PubMed] [Google Scholar]
- 9.Wong PW, et al. The prevalence and correlates of severe social withdrawal (hikikomori) in Hong Kong: A cross-sectional telephone-based survey study. Int J Soc Psychiatry. 2015;61:330–342. doi: 10.1177/0020764014543711. [DOI] [PubMed] [Google Scholar]
- 10.Kato TA, Kanba S. Boundless syndromes in modern society: An interconnected world producing novel psychopathology in the 21st century. Psychiatry Clin Neurosci. 2016;70:1–2. doi: 10.1111/pcn.12368. [DOI] [PubMed] [Google Scholar]
- 11.Teo AR, et al. Psychopathology associated with social withdrawal: Idiopathic and comorbid presentations. Psychiatry Res. 2015;228:182–183. doi: 10.1016/j.psychres.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Krieg A, Dickie JR. Attachment and hikikomori: a psychosocial developmental model. Int J Soc Psychiatry. 2013;59:61–72. doi: 10.1177/0020764011423182. [DOI] [PubMed] [Google Scholar]
- 13.Lee YS, Lee JY, Choi TY, Choi JT. Home visitation program for detecting, evaluating and treating socially withdrawn youth in Korea. Psychiatry Clin Neurosci. 2013;67:193–202. doi: 10.1111/pcn.12043. [DOI] [PubMed] [Google Scholar]
- 14.Kato TA, Watabe M, Kanba S. Neuron-glia interaction as a possible glue to translate the mind-brain gap: a novel multi-dimensional approach toward psychology and psychiatry. Front Psychiatry. 2013;4:139. doi: 10.3389/fpsyt.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato TA, Kanba S. Are microglia minding us? Digging up the unconscious mind-brain relationship from a neuropsychoanalytic approach. Front Hum Neurosci. 2013;7:13. doi: 10.3389/fnhum.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watabe M, et al. Relationship between trusting behaviors and psychometrics associated with social network and depression among young generation: a pilot study. PLoS One. 2015;10:e0120183. doi: 10.1371/journal.pone.0120183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton-Chellew MN, West SA. Pseudocompetition among groups increases human cooperation in a public-goods game. Animal Behaviour. 2012;84:947–952. doi: 10.1016/j.anbehav.2012.07.019. [DOI] [Google Scholar]
- 18.King-Casas B, et al. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg J, Dickhaut J, McCabe K. Trust, reciprocity, and social history. Game Econ Behav. 1995;10:122–142. doi: 10.1006/game.1995.1027. [DOI] [Google Scholar]
- 20.Forbes A, Roger D. Stress, social support and fear of disclosure. British Journal of Health Psychology. 1999;4:165–179. doi: 10.1348/135910799168551. [DOI] [Google Scholar]
- 21.Ashton WA. Effects of Gender and Gender Role Identification of Participant and Type of Social Support Resource on Support Seeking. Sex Roles. 1993;28:461–476. doi: 10.1007/BF00289608. [DOI] [Google Scholar]
- 22.Kato TA, Hayakawa K, Monji A, Kanba S. Missing and Possible Link between Neuroendocrine Factors, Neuropsychiatric Disorders, and Microglia. Front Integr Neurosci. 2013;7:53. doi: 10.3389/fnint.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin F, Friedman E, Quinn J, Chen DG, Mapstone M. Effect of leisure activities on inflammation and cognitive function in an aging sample. Arch Gerontol Geriatr. 2012;54:e398–404. doi: 10.1016/j.archger.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora V, Chopra K. Possible involvement of oxido-nitrosative stress induced neuro-inflammatory cascade and monoaminergic pathway: underpinning the correlation between nociceptive and depressive behaviour in a rodent model. J Affect Disord. 2013;151:1041–1052. doi: 10.1016/j.jad.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Palta P, Samuel LJ, Miller ER, 3rd, Szanton SL. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med. 2014;76:12–19. doi: 10.1097/PSY.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black CN, Bot M, Scheffer PG, Snieder H, Penninx B. Uric acid in major depressive and anxiety disorders. J Affect Disord. 2017;225:684–690. doi: 10.1016/j.jad.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Blom RM, et al. Association between a serotonin transporter promoter polymorphism (5HTTLPR) and personality disorder traits in a community sample. J Psychiatr Res. 2011;45:1153–1159. doi: 10.1016/j.jpsychires.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosmond R, Baghei F, Holm G, Bjorntorp P. Relationships between personality disorders and anthropometry, hormones and metabolism in women. J Endocrinol Invest. 2001;24:159–165. doi: 10.1007/BF03343836. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzi TM, Borba DL, Dutra G, Lara DR. Association of serum uric acid levels with emotional and affective temperaments. J Affect Disord. 2010;121:161–164. doi: 10.1016/j.jad.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Lu PP, et al. Pro-inflammatory effect of fibrinogen and FDP on vascular smooth muscle cells by IL-6, TNF-alpha and iNOS. Life Sci. 2011;88:839–845. doi: 10.1016/j.lfs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Capuron L, et al. Relationship between adiposity, emotional status and eating behaviour in obese women: role of inflammation. Psychol Med. 2011;41:1517–1528. doi: 10.1017/S0033291710001984. [DOI] [PubMed] [Google Scholar]
- 34.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 35.Kato TA, et al. Minocycline modulates human social decision-making: possible impact of microglia on personality-oriented social behaviors. PLoS One. 2012;7:e40461. doi: 10.1371/journal.pone.0040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry59(Suppl 20), 22–33 quiz 34–57 (1998). [PubMed]
- 37.Brugha TS, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68:459–465. doi: 10.1001/archgenpsychiatry.2011.38. [DOI] [PubMed] [Google Scholar]
- 38.Brugha TS, et al. Epidemiology of autism in adults across age groups and ability levels. Br J Psychiatry. 2016;209:498–503. doi: 10.1192/bjp.bp.115.174649. [DOI] [PubMed] [Google Scholar]
- 39.Duda M, Kosmicki JA, Wall DP. Testing the accuracy of an observation-based classifier for rapid detection of autism risk. Transl Psychiatry. 2014;4:e424. doi: 10.1038/tp.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato TA, et al. Multidimensional anatomy of ‘modern type depression’ in Japan: A proposal for a different diagnostic approach to depression beyond the DSM-5. Psychiatry Clin Neurosci. 2016;70:7–23. doi: 10.1111/pcn.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thinking big in mental health. Nature Medicine24, 1, 10.1038/nm.4471 (2018). [DOI] [PubMed]
- 42.Watabe M, et al. Minocycline, a microglial inhibitor, reduces ‘honey trap’ risk in human economic exchange. Sci Rep. 2013;3:1685. doi: 10.1038/srep01685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alici D, et al. Evaluation of oxidative metabolism and oxidative DNA damage in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2016;70:109–115. doi: 10.1111/pcn.12362. [DOI] [PubMed] [Google Scholar]
- 44.Sarandol A, et al. First-episode psychosis is associated with oxidative stress: Effects of short-term antipsychotic treatment. Psychiatry Clin Neurosci. 2015;69:699–707. doi: 10.1111/pcn.12333. [DOI] [PubMed] [Google Scholar]
- 45.Kunugi H, Hori H, Ogawa S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci. 2015;69:597–608. doi: 10.1111/pcn.12299. [DOI] [PubMed] [Google Scholar]
- 46.Nishi D, et al. Omega-3 fatty acid supplementation for expectant mothers with depressive symptoms in Japan and Taiwan: An open-label trial. Psychiatry Clin Neurosci. 2016;70:253–254. doi: 10.1111/pcn.12388. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez Solano JJ, Gonzalez De Chavez M. Premorbid personality disorders in schizophrenia. Schizophr Res. 2000;44:137–144. doi: 10.1016/S0920-9964(99)00203-0. [DOI] [PubMed] [Google Scholar]
- 48.Ryff CD, et al. Culture, inequality, and health: evidence from the MIDUS and MIDJA comparison. Cult Brain. 2015;3:1–20. doi: 10.1007/s40167-015-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prentice RL, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 50.Miller JD, Bagby RM, Pilkonis PA, Reynolds SK, Lynam DR. A simplified technique for scoring DSM-IV personality disorders with the Five-Factor Model. Assessment. 2005;12:404–415. doi: 10.1177/1073191105280987. [DOI] [PubMed] [Google Scholar]
- 51.Burger JM. Individual differences in preference for solitude. Journal of Research in Personality. 1995;29:85–108. doi: 10.1006/jrpe.1995.1005. [DOI] [Google Scholar]
- 52.Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39:472–480. doi: 10.1037/0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- 53.Schumaker JF, Shea JD, Monfries MM, Groth-Marnat G. Loneliness and life satisfaction in Japan and Australia. J Psychol. 1993;127:65–71. doi: 10.1080/00223980.1993.9915543. [DOI] [PubMed] [Google Scholar]
- 54.Yamagishi T, Yamagishi M. Trust and commitment in the United States and Japan. Motivation and Emotion. 1994;18:129–166. doi: 10.1007/BF02249397. [DOI] [Google Scholar]
- 55.Widyanto L, McMurran M. The psychometric properties of the internet addiction test. Cyberpsychol Behav. 2004;7:443–450. doi: 10.1089/cpb.2004.7.443. [DOI] [PubMed] [Google Scholar]
- 56.Kurimoto A, et al. Reliability and validity of the Japanese version of the abbreviated Lubben Social Network Scale. Nihon Ronen Igakkai Zasshi. 2011;48:149–157. doi: 10.3143/geriatrics.48.149. [DOI] [PubMed] [Google Scholar]
- 57.Lubben J, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 58.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess. 1990;55:610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 59.Connor KM, Kobak KA, Churchill LE, Katzelnick D, Davidson JR. Mini-SPIN: A brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14:137–140. doi: 10.1002/da.1055. [DOI] [PubMed] [Google Scholar]
- 60.Nagata T, Nakajima T, Teo AR, Yamada H, Yoshimura C. Psychometric properties of the Japanese version of the Social Phobia Inventory. Psychiatry Clin Neurosci. 2013;67:160–166. doi: 10.1111/pcn.12037. [DOI] [PubMed] [Google Scholar]
- 61.Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods Psychol Res. 2003;8:23–74. [Google Scholar]
- 62.Teo AR, Gaw AC. Hikikomori, a Japanese culture-bound syndrome of social withdrawal?: A proposal for DSM-5. J Nerv Ment Dis. 2010;198:444–449. doi: 10.1097/NMD.0b013e3181e086b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.