Abstract
Background and Purpose
Histamine modulates several behaviours and physiological functions, and its deficiency is associated with neuropsychiatric disorders. Gestational intake of valproic acid (VPA) is linked to autism spectrum disorder (ASD), characterized by impaired sociability and stereotypies. VPA effects on the neurochemistry and functional morphology of the histaminergic system in ASD are unclear. Zebrafish are highly social, and given the similarities between zebrafish and human neurotransmitter systems, we have studied the effects of VPA on histamine in zebrafish.
Experimental Approach
Histaminergic, dopaminergic and noradrenergic systems of larval and adult zebrafish exposed to VPA from the end of gastrulation until neural tube formation were studied using HPLC, quantitative PCR, immunocytochemistry and in situ hybridization. Sociability, dark‐flash response and locomotion were also studied.
Key Results
Zebrafish larvae exposed to VPA showed decreased locomotion and an abnormal dark‐flash response. Additionally, a reduced number of histaminergic neurons, low histamine and altered mRNA expression of key genes of the monoaminergic systems were also detected. The reduced mRNA expression of genes of the studied systems persisted until adulthood. Furthermore, adult VPA‐exposed animals presented lower brain levels of noradrenaline and 3,4‐dihydroxyphenylacetic acid, along with impaired sociability.
Conclusions and Implications
VPA exposure in early development causes molecular and neurochemical alterations in zebrafish, which persist into adulthood and accompany impaired sociability. These findings will highlight the possible involvement of the histaminergic system in outcomes related to neuropsychiatric disorders. Furthermore, it supports zebrafish as a tool to investigate mechanisms underlying these disorders.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- ASD
autism spectrum disorder
- DBH
dopamine β‐hydroxylase
- dpf
days post fertilization
- HDC
histidine decarboxylase
- hpf
hours post fertilization
- mpf
months post fertilization
- rpl13a
ribosomal protein large subunit 13a
- VPA
valproic acid
Introduction
Valproic acid (VPA) is a simple branched‐chain fatty acid widely used to treat epilepsy and bipolar disorder (Phiel et al., 2001). As a histone deacetylase inhibitor, VPA induces acetylation of histones and non‐histone proteins, which are associated with nucleosome remodelling and gene transcription (Phiel et al., 2001). A Danish study that followed children born between 1996 and 2006 associated prenatal exposure to VPA with autism spectrum disorder (ASD), concluding that for individuals born to mothers exposed to VPA during pregnancy, the risk of ASD increased fivefold over the general population risk (Christensen et al., 2013). Thus, exposure to VPA has been used to reproduce the core symptoms of ASD in animal studies (Bambini‐Junior et al., 2014).
ASD is a neurodevelopmental disorder characterized by deficits in sociability and communication, and by a limited repertoire of interests and behaviours (Happé and Ronald, 2008). Alterations in different neurotransmitter systems, such as serotonergic, dopaminergic, GABAergic and glutamatergic systems have been reported in ASD patients and in animal models of the disorder (McDougle et al., 2005; Bambini‐Junior et al., 2014). Surprisingly, the histaminergic system has received less attention in ASD studies.
Histamine modulates a series of behaviours and physiological functions, such as the sleep–wake cycle (Haas and Panula, 2003), motivation and goal‐directed behaviours (Burgess, 2010), feeding behaviour (Gotoh et al., 2013) and short‐ and long‐term memory acquisition (Kraus et al., 2013). Its deficiency has been associated with different neuropsychiatric disorders (Panula and Nuutinen, 2013). Recently, altered expression of histamine receptors and other histaminergic genes in post mortem dorsolateral prefrontal cortex (DLPFC) samples of individuals with ASD was reported (Wright et al., 2017). Also ciproxifan, an inverse agonist of histamine H3 receptor, attenuated the impaired sociability presented by mice prenatally exposed to VPA (Baronio et al., 2015). As these receptor regulates the release of histamine and some other neurotransmitters (Panula et al., 2015), it is possible that the augmented release of histamine could be beneficial in the disorder.
Zebrafish (Danio rerio) provide important tools in neuroscience and have been successfully used to study basic mechanisms of different brain disorders (Kalueff et al., 2014). These vertebrates display similarities to humans and rodents regarding neurochemistry, anatomy and pharmacology (Kaslin and Panula, 2001; Sundvik and Panula, 2012). More specifically, the neuronal histaminergic system of the zebrafish in the CNS is similar not only to humans and rodents (Eriksson et al., 1998) but also to several different phylogenetic levels of vertebrates, including reptiles (Inagaki et al., 1990), amphibians (Airaksinen and Panula, 1990) and other teleost fish (Inagaki et al., 1991). Briefly, the histaminergic neurons appear early in the developing larval zebrafish and are located in the ventral hypothalamus and innervate the rostral dorsal telencephalon (Eriksson et al., 1998). This neurotransmitter system appears during the period when the larva starts to actively search for food (Eriksson et al., 1998), supporting the proposed role for the histaminergic system in alertness modulation.
Zebrafish are highly social and prefer to swim in groups for different reasons, such as mating, foraging or avoiding predators (Pitcher, 1986). Given the high homology between the zebrafish and human genomes (Barbazuk et al., 2000) as well as the similarities displayed between these species in neurotransmitter systems (Eriksson et al., 1998; Kaslin and Panula, 2001; Sundvik and Panula, 2012), it seemed reasonable to use zebrafish to study the effect of VPA, an ASD‐associated drug, on the histaminergic system.
The main goal of our study was to evaluate the behaviour and the status of the histaminergic system of larval and adult zebrafish exposed to VPA during neural tube formation. Therefore, the level of histamine, histamine receptors and histidine decarboxylase (HDC), the enzyme controlling the formation of histamine, were studied. Our group demonstrated previously that dopamine regulates the differentiation of histaminergic neurons and that these cells surround L‐tyrosine hydroxylase 2 (TH2)‐expressing neurons in the hypothalamus (Chen et al., 2016). Due to the close relationship between these two systems, we analysed TH1 and TH2, the enzymes responsible for catalysing the conversion of the amino acid L‐tyrosine to L‐DOPA, the rate limiting step in dopamine biosynthesis. We also included in the analysis, dopamine β‐hydroxylase (DBH), which converts dopamine to noradrenaline, as well as the levels of catecholamines and their metabolites. Basic locomotor activity, dark‐flash response and social interaction were the relevant behaviours analysed.
Methods
Experimental animals and VPA exposure
All animal care and experimental procedures complied with the ethical guidelines of the European convention (ESAVI/6100/04.10.07/2015) and were approved by the Office of the Regional Government of Southern Finland. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
A total of 420 larval (5 days post fertilization, dpf) and 22 adult male (6 months post fertilization, mpf) zebrafish of the wild‐type Turku strain were used in the experiments. This strain has been maintained for more than 15 years and has been used in previous publications (Kaslin and Panula, 2001; Sundvik et al., 2011). For breeding, adult zebrafish were placed in tanks overnight and were separated by a transparent barrier that was removed on the following morning. After collection, embryos were transferred to a Petri dish containing 1 × E3 (zebrafish embryonic medium; 5.00 mM NaCl, 0.44 mM CaCl2, 0.33 mM MgSO4 and 0.17 mM KCl). At 10 hpf, embryos were randomly distributed in wells (30 embryos per well) of a 6‐well plate containing 1 × E3 (controls) or 25 μM VPA (Sigma P4543, Darmstadt, Germany) diluted in 1 × E3. The solution containing VPA was removed at 24 hpf, replaced by 1 × E3, and embryos were maintained in an incubator at 28.5°C. Embryos exposed to VPA or the control solution were observed for morphological abnormalities every day until 5 dpf. Of the embryos exposed to VPA, 27% died or were excluded due to obvious malformations (e.g. spinal curvature). In pilot studies based on previous publications (Zimmermann et al., 2015), we exposed zebrafish to 50 μM of VPA, and this dose led to a high mortality rate at 5 dpf (more than 50%). Later, we reduced the dose to 35 and 25 μM. Exposing embryos to 35 μM of VPA led to clearly arrested development, high rates of deformities at 5 dpf and mortality after 10 dpf. We then defined 25 μM as the optimal concentration of VPA. At 5 dpf, Some of the larvae were used for the analyses and the remaining animals were grown until 6 mpf in 3 L transparent acrylic tanks with system water. A timeline of the experiments is shown in Figure 1.
Figure 1.
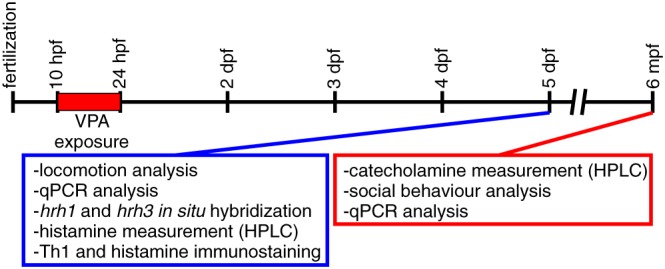
Timeline of the experiments. Exposure to 25 μM VPA was applied from 10 to 24 hpf. Behavioural tests and the study of histaminergic and catecholaminergic systems of zebrafish were performed at 5 dpf and 6 mpf.
Larval locomotion assay and dark‐flash response
Zebrafish locomotor activity is controlled by light, and larvae display characteristic behaviours during switches between light and dark states (Burgess and Granato, 2007). The larvae were tracked individually on a 48‐well plate using the DanioVision system and EthoVision XT software (Noldus Information Technology, Wageningen, The Netherlands). During light conditions, the illuminance was set at approximately 330 lux, in accordance with our previous studies (Sundvik et al., 2011). The larvae were habituated to the system for 10 min, followed by a 15 min basal locomotion assay in light. This was followed by alternating 2 min periods of light and darkness, with three periods of darkness in total. The transition between different illumination conditions was carried out instantaneously.
Adult locomotion assay and social interaction
Adult fish were individually tracked in separate cylindrical observation tanks (inner diameter 22 cm and water depth 8 cm), as described previously (Peitsaro et al., 2003). The fish had 1 min of habituation time in the tank before the tracking started. The swimming performance of the animals was automatically detected and tracked for 10 min by a digital video camera connected to a standard PC computer system running the EthoVision 3.1 software (Noldus Information Technology, Wageningen, The Netherlands).
Zebrafish are visually attracted to conspecifics and instinctively aggregate into shoals. We utilized this innate tendency to develop a fish visual choice test of preference for social interaction, based on the rodent three‐chamber sociability test (Baronio et al., 2015) and similar approaches that have been utilized with zebrafish (Zimmermann et al., 2015; Liu et al., 2016). In this assay, the social interaction of adult animals was evaluated in an acrylic apparatus (29 cm length × 19 cm height × 29 cm width) divided by a transparent wall into two chambers, one of which was subdivided in two smaller compartments. A group of eight fish, serving as stimulus, was placed in one of the compartments, and the other compartment was filled with stones and plant imitations. The tested fish was put in the other chamber, and the time spent next to the stimulus (Zone 2), the object (Zone 3) and in the distal part of the chamber (Zone 1) was measured with EthoVision 3.1 software (Noldus Information Technology, Wageningen, The Netherlands).
Quantitative PCR (qPCR)
Larval and adult zebrafish were killed in ice‐cold water prior to RNA extraction. Total RNA was extracted using the RNeasy mini Kit (Qiagen, Hilden, Germany) according to the instructions of the manufacturer. Larval samples (15 pooled larvae per sample) and the whole brains of adult fish were collected, and 1.0 μg of total RNA was reverse‐transcribed using SuperScriptTM III reverse transcriptase (Invitrogen, Carlsbad, USA). qPCR was performed with the LightCycler 480 real‐time PCR system and the LightCycler 480 SYBR green I master kit (Roche Applied Science, Mannheim, Germany). Primers for amplification were designed by Primer‐BLAST (NCBI), and sequences are shown in Table 1. Cycling parameters were as follows: 95°C for 30 s and 45 cycles of the following, 95°C for 10 s and 62°C for 45 s. Fluorescence changes were monitored with SYBR Green after every cycle. Dissociation curve analysis was performed (0.2°C per s increase from 60 to 95°C with continuous fluorescence readings) at the end of cycles to ensure that only a single amplicon was obtained. All reactions were performed in duplicate to ensure the reliability of single values. Results were evaluated with the LightCycler 480 Software version 1.5. Quantification was done by Ct value comparison (Livak and Schmittgen, 2001), using the Ct value of ribosomal protein large subunit 13a (rpl13a) as the reference control. rpl13a has been considered the most reliable reference gene in a study which evaluated a large number of reference genes for data from different tissues of zebrafish, during different developmental stages and after different chemical treatments (Xu et al., 2016).
Table 1.
List of the primers for qPCR analysis
| Gene | Forward primer | Reverse primer |
|---|---|---|
| hdc | TTCATGCGTCCTCTCCTGC | CCCCAGGCATGATGATGTTC |
| th1 | GACGGAAGATGATCGGAGACA | CCGCCATGTTCCGATTTCT |
| th2 | CTCCAGAAGAGAATGCCACATG | ACGTTCACTCTCCAGCTGAGTG |
| dbh | TGCAACCAGTCCACAGCGCA | GCTGTCCGCTCGCACCTCTG |
| rpl13a | AGAGAAAGCGCATGGTTGTCC | GCCTGGTACTTCCAGCCAACTT |
| hrh3 | CGCCACCGTCCTTGGGAACG | GGGGATGCAAAACCCGCCGA |
| hrh2 | GGCCACTAGGGGCGCACTTC | AGCGGAGCAGTGACCGCAAA |
| hrh1 | TCCTGATCCCGTCCGCACCA | CCCGACGGTATGCAGCGTCC |
| pcna | ACGCCTTGGCACTGGTCTT | CTCTGGAATGCCAAGCTGCT |
Histamine and catecholamines measurement by HPLC
Histamine (Yamatodani et al., 1985; Rozov et al., 2014) and catecholamines (Puttonen et al., 2013) were assayed by HPLC as described previously. Briefly, groups of 15 whole larvae were homogenized by sonication in 2% perchloric acid for each sample, centrifuged for 30 min at 15 000 g at 4°C, and filtered through a 0.45‐μm PVDF filter (Pall Life Sciences, Ann Arbor, USA) before loading onto the HPLC system. All analyses were done in duplicate to ensure the reliability of single values. The analytical HPLC system consisted of four Shimadzu LC20AD pumps, a Shimadzu SIL‐20AC autosampler, a Shimadzu RF‐10Axl fluorescence detector, a Shimazdu CBM‐20A controller and LCSOLUTION 1.21 software (Shimadzu, Kyoto, Japan). In order to normalize the results from HPLC measurement, we utilized the bicinchoninic acid assay kit (Thermo Fisher Scientific, Waltham, USA) to measure protein concentration.
Immunohistochemistry
Larvae were killed in ice‐cold water and collected for overnight fixation in 4% 1‐ethyl‐3,3(dimethyl‐aminopropyl)carbodiimide (EDAC; Carbosynth, Berkshire, UK) for staining. The detailed protocol for immunohistochemistry has been described previously (Sallinen et al., 2009). Rabbit polyclonal anti‐histamine 19C antibody (1:10 000) (Panula et al., 1990), mouse monoclonal proliferating cell nuclear antigen (PCNA) antibody (lot GR201287, 1:1000, Abcam, Cambridge, UK) (Thummel et al., 2008) and mouse monoclonal L‐tyrosine hydroxylase (TH) antibody (lot 907001, 1:1000, Immunostar, Hudson, USA) (Semenova et al., 2014) were used. For detection, the samples were incubated with Alexa‐conjugated antibodies (Alexa Anti‐rabbit 488 and Anti‐mouse 568, Invitrogen, Carlsbad, USA) diluted 1:1000.
Fluorescent in situ hybridization
Larvae were killed in ice‐cold water and collected for overnight fixation in 4% paraformaldehyde diluted in PBS at 4°C. Following fixation, the larvae were washed in PBS, followed by dissection of the brain. The brains were dehydrated in methanol. In situ hybridization was carried out as described previously (Lauter et al., 2014), with minor modifications as described (Sundvik et al., 2011; Chen et al., 2016). Digoxigenin‐ and fluorescein‐labelled riboprobes against histamine H1 and H3 receptors specified earlier (Sundvik et al., 2011) were used. DY‐647P1 (Dyomics 647P1‐01, Jena, Germany) and 5(6)‐TAMRA (Thermo Fisher Scientific C1171, Waltham, USA) conjugated tyramides were synthesized as described (Speel et al., 1998). The tyramides were diluted 1:250 in amplification buffer, and amplification was carried out for 15 min. Samples were mounted in 75% glycerol diluted with PBS for imaging.
Microscopy and imaging
Immunohistochemistry and in situ hybridization samples were imaged using a Leica SP2 AOBS confocal microscope. For whole brain images, an HC PL APO 20/0.70 CS objective was used. The Alexa 488‐ and 568‐labelled secondary antibodies were detected using a 488 nm argon laser and a 568 nm diode laser respectively. The emission wavelength areas were adjusted to 500–550 and 590–650 nm, respectively, to avoid bleedthrough. The TAMRA‐ and DY647P1‐conjugated tyramides were detected using a 568 nm diode and 633 nm Helium/Neon laser respectively. The emission wavelength areas were adjusted to 570–622 and 650–700 nm, respectively, to avoid bleedthrough. Immunohistochemistry stacks were acquired using the software defined optimized step size between focal planes. Image stacks were analysed in Fiji (Schindelin et al., 2012), and cell numbers were quantified manually, without knowledge of the cell treatments.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Sample sizes were based on a pilot study and other toxicological investigations conducted in our group. Fish were randomly distributed in arenas and automatically analysed simultaneously. Cell counts, qPCR analyses and HPLC were carried out blindly, using numbered samples. All primary data in this paper will be freely available for reanalysis. Results are shown as means ± SEM. Data were analysed by Student's t‐test or two‐way repeated measure ANOVA followed by Sidak's post hoc test, and P < 0.05 was considered statistically significant. Data analysis was performed by GraphPad Prism version 7 software (San Diego, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Results
Behaviour of the larvae
The basal locomotor activity of the VPA‐exposed larvae was significantly decreased by approximately 75% under normal light conditions (Figure 2A). When the lights were switched off for a 2 min period, all larvae responded with an increase in locomotion, with the VPA‐exposed larvae displaying significantly increased locomotion during the first period of darkness, when compared with control larvae. In the subsequent periods of darkness, VPA‐exposed and control larvae showed an equal level of locomotor activity. When the light was switched back on, the VPA‐exposed larvae returned to a lower level of activity in comparison with control animals. We further analysed the light–dark transitions at a 1 s resolution, in order to see if the dark‐induced flash response observed during the first second of darkness (time point 11) (Burgess and Granato, 2007) was intact. This dark‐flash response was present in the VPA‐exposed larvae during the first transition (Figure 2B). In the second (Figure 2C) and third transition (Figure 2D), the response was significantly weaker in VPA‐exposed larvae in comparison with the control group.
Figure 2.
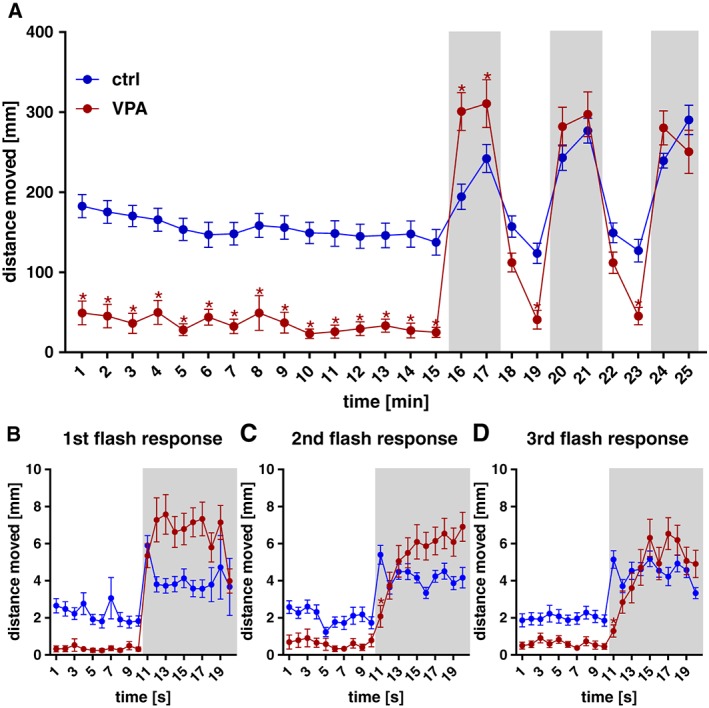
Locomotor activity of VPA‐exposed and control zebrafish larvae at 5 dpf. Basal locomotion was evaluated in light during a 15 min time period. VPA‐exposed larvae displayed a significantly decreased locomotor activity when compared with the control group. After that, larvae were exposed to alternating 2 min periods of light and darkness (grey shaded areas), with three periods of darkness in total. VPA‐exposed larvae were significantly more active than the control group during the first period of darkness, no difference between groups was detected in the other two periods of darkness and the VPA group moved significantly less during the light periods (A). Analysing the light–dark transitions at a 1 s resolution (B–D), the dark‐induced flash response in the VPA group was present in the first transition (B) but significantly weaker when compared to control group in the second (C) and third (D) transitions. Data are expressed as mean ± SEM, n = 32 per group. * P < 0.05, significantly different from control; two‐way repeated measures ANOVA, with Sidak's post test.
Adult behaviour
We saw no difference in the basal locomotor activity between VPA‐exposed and control adult zebrafish (Figure 3F). Figure 3A, B shows the traces of swimming pattern of control and VPA‐exposed fish during the social behaviour test. When compared with the control group, VPA‐exposed fish spent less time in the zone closest to the compartment with the stimulus fish (Figure 3D). In addition, VPA exposure induced an increase in the time spent in the Zone 3, which is closest to the compartment with the object (Figure 3E). No difference was detected in the time spent in zone 1 (distal zone) between the VPA and control fish (Figure 3C).
Figure 3.
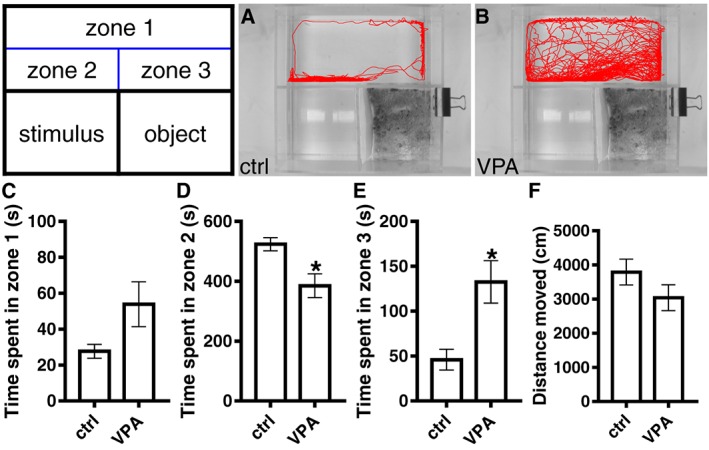
Social interaction and locomotion assays of adult zebrafish. Social interaction of control (A) and VPA (B) fish was determined during a 10 min of video recording session in a home‐made social interaction apparatus. Zones 1–3 are digital zones, whereas the apparatus itself is physically divided into three chambers. The zone 1 is the distal area, zone 2 corresponds to the segment closest to the stimulus (group of eight fish) and the zone 3 is the segment closest to the object. The time spent in each zone was measured using EthoVision 3.1 software (C–E). In a different assay, a cylindrical arena, the locomotor activity of both groups was recorded, and no difference in the total distance moved was detected (F). Data are expressed as mean ± SEM; n = 6 per group. * P < 0.05, significantly different from control; Student's t‐test.
Quantitative PCR
The expression levels of the transcripts of the genes for the H1, H2 and H3 receptors, HDC, PCNA TH1, TH2 and DBH in 5 dpf larvae and whole brain of adult fish from both groups were measured using qPCR (Figure 4). The expression levels of mRNA for H1, H2 and H3 receptors, HDC, TH1, and DBH at 5 dpf were significantly decreased in the VPA‐exposed group in comparison to controls. The decreases seen in H3 receptor, HDC, TH1, and DBH mRNA were also seen in adulthood (Figures 4 and 5).
Figure 4.
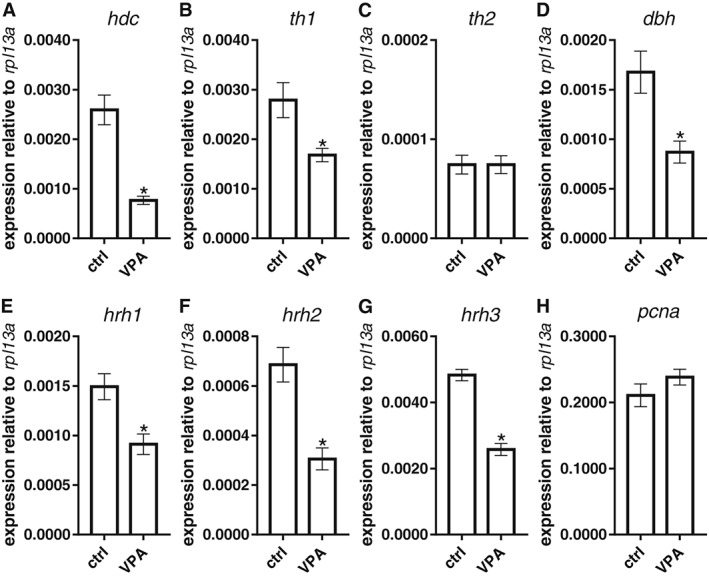
mRNA expression of key genes of the histaminergic and dopaminergic systems in VPA‐exposed zebrafish larvae. The histaminergic system was affected by VPA exposure, with reduced levels of mRNA for HDC (hdc; A), H1(hrh1;E), H2 (hrh2; F) and H3 receptors (hrh3; G) . Changes were also detected in the dopaminergic and noradrenergic systems with a decrease in TH1 (th1;B) and DBH (dbh; D) mRNA levels respectively. Expression levels of TH2 (th2; C) and PCNA (pcna; H) were similar in both groups. Data are expressed as mean ± SEM; n = 5 per group. * P < 0.05, significantly different from control; Student's t‐test.
Figure 5.
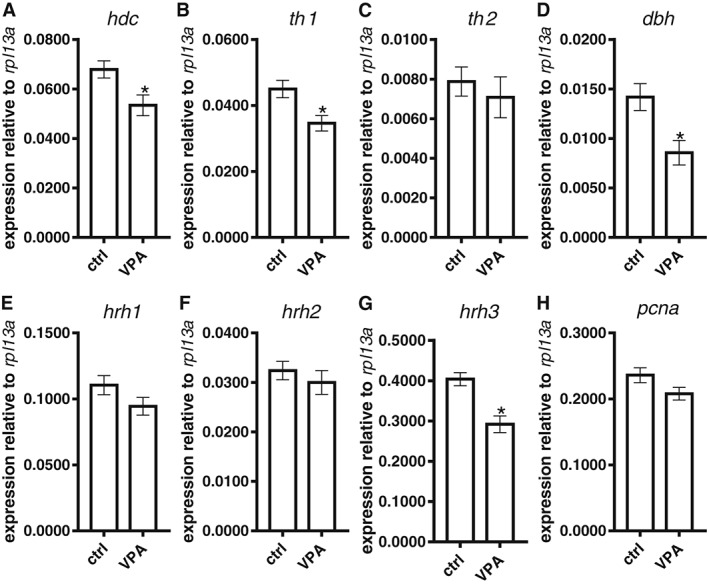
mRNA expression of key genes of the histaminergic and dopaminergic systems in the brain of adult VPA‐exposed zebrafish. The reductions caused by VPA exposure in the larval stage in mRNA for HDC (hdc; A), TH1 (th1; B), DBH (dbh; D) and H3 receptors (hrh3; G) persisted until adulthood. However, no significant changes in the expression of mRNA for H1 (hrh1; E) or H2 receptors (hrh2; F) were seen in adult VPA‐exposed brains in comparison to controls. As in larval fish, expression of mRNA for TH2 (th2; C) and PCNA (pcna; H) was unchanged. Data are expressed as mean ± SEM; n = 5 per group. * P < 0.05, significantly different from control; Student's t‐test.
Levels of histamine and other biogenic amines after VPA exposure
The HPLC measurements showed that the level of histamine in 5 dpf VPA‐exposed larvae was significantly reduced when compared with control larvae (Figure 7D). This effect did not persist into adulthood, as adult VPA‐exposed and control fish had similar levels of histamine in the brain (Figure 6H). However, adult VPA‐exposed fish had significantly decreased levels of noradrenaline in comparison to control fish (Figure 6B). Adult VPA‐exposed fish also had decreased levels of the dopamine metabolite, 3,4‐dihydroxyphenylacetic acid (Figure 6E). No significant changes were seen in the levels of dopamine (Figure 6A), the other dopamine metabolites (Figure 6F, G), 5‐HT (Figure 6C) and the 5‐HT metabolite, 5‐hydroxyindoleacetic acid (Figure 6D).
Figure 7.
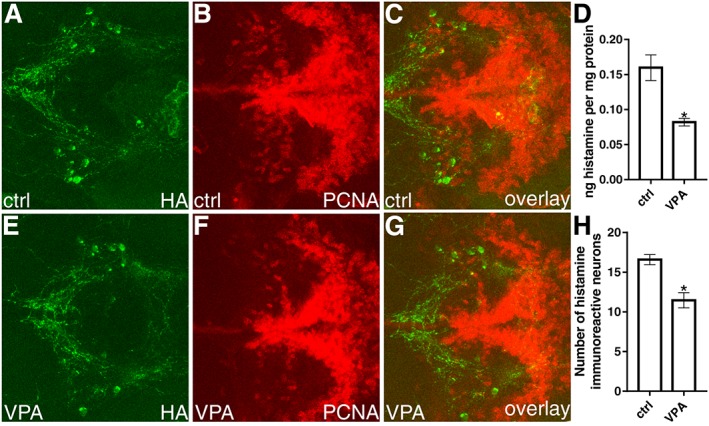
Histaminergic neurons and histamine in VPA‐exposed zebrafish larvae. Immunostainings were carried out on 5 dpf fish brains. A representative image for each group is shown. Images A and D show the histaminergic neurons of control and VPA‐exposed larvae respectively. When the cell numbers were quantified, a significant reduction of histamine immunoreactive neurons was detected in the VPA‐exposed larvae (G). VPA‐exposed larvae also had lower levels of histamine when compared to the control group (D). Comparison between the control larvae (B) and VPA group (E) on PCNA staining. Data are expressed as mean ± SEM; n = 12 per group. * P < 0.05, significantly different from control; Student's t‐test.
Figure 6.
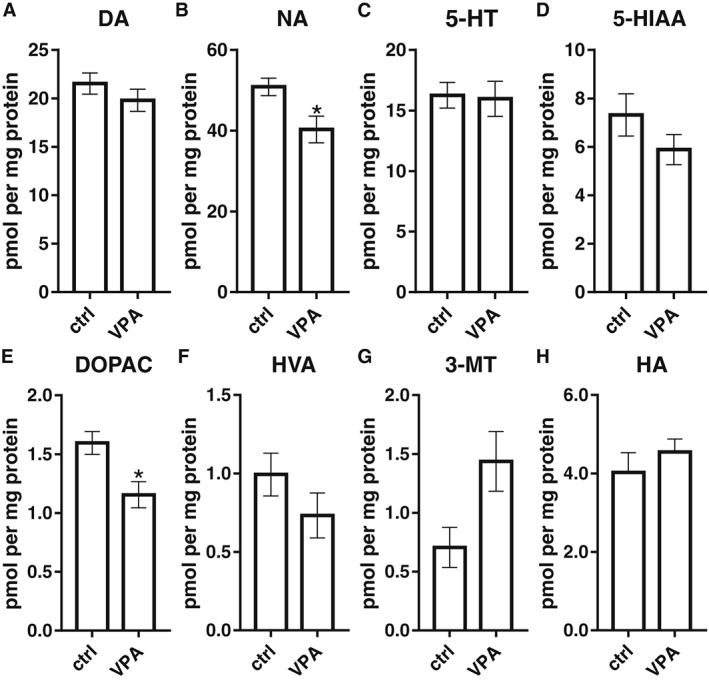
Measurements of neurotransmitters and metabolites by HPLC in the brain of adult zebrafish. Exposure to VPA during the embryonic period led to decreased levels of noradrenaline (NA; B) and 3,4‐dihydroxyphenylacetic acid (DOPAC; E) in the brains of adult zebrafish. No significant changes were detected in the levels of dopamine (DA; A), 5‐HT (C), 5‐hydroxyindoleacetic acid (5‐HIAA; D), homovanillic acid (HVA; F), 3‐methoxytyramine (3‐MT; G), histamine (HA; H). Data are expressed as mean ± SEM; n = 5 per group. * P < 0.05, significantly different from control; Student's t‐test.
Histamine and TH neuron numbers
The number of histamine immunoreactive neurons was significantly lower in 5 dpf VPA‐exposed larvae in comparison with the control group (Figure 7G). The shape of the caudal hypothalamus, which contains the histaminergic neurons of the zebrafish, appeared similar in both groups. The histaminergic neurons resided in an area with prominent expression of PCNA immunoreactivity in the posterior hypothalamus (Figure 7C, G). The VPA‐exposed larvae also showed a significantly decreased number of TH1‐immunoreactive neurons in the diencephalic (population 13, Figure 8G) and preoptic populations (populations 3 and 4, Figure 8D) in comparison to control larvae. However, no changes were seen in the number of neurons in the postoptic/thalamic/posterior tuberculum (populations 5,6 and 11, Figure 8E), posterior tuberal nucleus and anterior paraventricular organ (populations 8 and 12, Figure 8F). We also did not detect changes in the number of neurons in the noradrenergic locus coeruleus (Figure 8C). Cell populations were identified, named and numbered as described previously (Sallinen et al., 2009).
Figure 8.
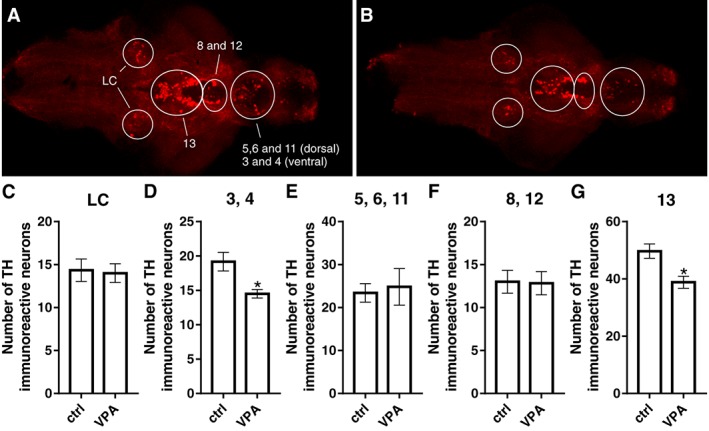
TH‐immunoreactive cell populations in the brain of zebrafish larvae. TH populations were identified in control (A) and VPA‐exposed (B) larval brains, which were imaged from the ventral side. The number of cells did not differ between groups in the LC (C) and in populations 5, 6 and 11 (E) and 8 and 12 (F). VPA‐exposed larval brains presented a reduction in the number of TH1‐immuoreactive cells in populations 3 and 4 (D) and 13 (G) when compared with the control group. A representative image for each group is shown. Data are expressed as mean ± SEM; n = 6 per group. *P < 0.05, significantly different from control; Student's t‐test.
Receptor expression by in situ hybridization
At 5 dpf, VPA‐exposed larvae (Figure 9B) showed decreased H3 receptor mRNA hybridization signal in the dorsal telencephalon, in comparison with control larvae (Figure 9A). Similarly, the H1 receptor hybridization signal of the VPA larvae (Figure 9D), in the same area, was clearly weaker when compared with the control group (Figure 9C). These results support the findings obtained by the qPCR analysis.
Figure 9.
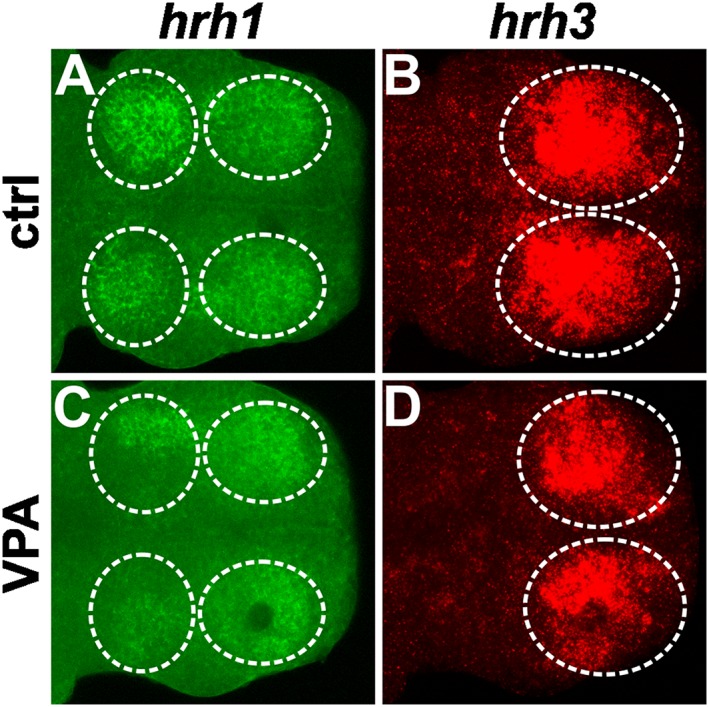
The expression pattern of genes for H1 (hrh1) and H3 receptors (hrh3) in zebrafish larvae detected by in situ hybridization. Samples are oriented with the anterior axis pointing to the right. Telencephalic hrh1 mRNA is localized to the dorsal pallium (anterior circles), and strong hrh1 expression is also seen in the habenula (posterior circles). Comparing the expression pattern of control (A) and VPA (C) larvae supports the qPCR finding, which indicates a reduction in the mRNA levels of this receptor in the VPA‐exposed group. A similar effect is seen for hrh3 expression in the dorsal pallium, showing a decrease in VPA‐exposed larvae (D) when compared with the control group (B) in accordance with the qPCR result. A representative image of each group (n = 6 per group) is shown.
Discussion
The most significant and surprising finding of the present study was the consistent decrease in the number of histaminergic neurons and histamine levels in VPA‐exposed fish. This significant reduction in histamine may have led to altered responses to sudden darkness, a phenomenon characteristic for larvae lacking histamine synthesizing capacity due to translation inhibition of the gene for HDC (Sundvik et al., 2011). Interestingly, an abnormal social behaviour was detected in VPA‐exposed fish as adults as well.
The criteria for VPA exposure used in our study was determined based on the developmental stage relevant data from other species. Clinical studies show that VPA exposure during the first trimester of pregnancy is associated with higher incidence of ASD. During this time window, the neural tube closes, and shortly after that, the neuroepithelium will generate neurons and glia in the CNS (Bayer et al., 1993). Based on that, prenatal exposure to VPA during the neural tube closure was used to develop a rat model of ASD (Rodier et al., 1996). The zebrafish embryos on the present study were exposed to VPA from the end of gastrulation to neural tube formation (Compagnon and Heisenberg, 2013).
At 5 dpf, larvae exposed to VPA presented reduced levels of histamine and mRNA for H1, H2 and H3 receptors and HDC. We also saw a reduction in the expression pattern of mRNA for H1 and H3 receptors in the telencephalon by in situ hybridization. VPA‐exposed larvae were generally hypoactive and also displayed an impaired dark‐flash response, suggesting a functional deficiency of the circuitry underlying this behaviour, which is known to be histamine‐dependent (Sundvik et al., 2011). Sudden changes in illumination lead to acute motor responses in zebrafish larvae (Burgess and Granato, 2007). It is hypothesized that the abrupt reduction in light mimics the shadow of a potential predator and that the large angle turns elicited and increased locomotor activity are a precursor to a visual startle response. Analysing the light–dark transitions at a 1 min resolution, VPA‐exposed larvae were significantly more active than the control group during the first period of darkness, which could indicate a larger visual startle response. Analysing the light–dark transitions at a 1 s resolution, the dark‐induced flash response in the VPA group was present in the first transition. However, the response was significantly weaker when compared to control group in the second and third transitions, which could be related to an impaired histaminergic system.
The number of histamine‐immunoreactive neurons was reduced in the larvae exposed to VPA. In zebrafish, VPA is known to reduce the proliferation of telencephalic cells without inducing apoptosis (Lee et al., 2013), but the levels of mRNA for PCNA were not different between control and VPA groups in our study. The qPCR may be unable to reflect a possible difference in the small area where the histaminergic neurons are found. It is noteworthy that although a reduction was also seen in the number of neurons in some TH populations, other TH populations were unaffected. These results indicate that the development of distinct monoaminergic populations is impaired by VPA exposure.
Although the brains of adult zebrafish exposed to VPA presented normal levels of histamine, we detected a decrease in the levels of mRNA for HDC and H3 receptors. The animals that displayed these abnormalities had impaired social behaviour. Others have reported that zebrafish embryonically exposed to VPA had a social interaction deficit (Zimmermann et al., 2015; Liu et al., 2016), one of the core symptoms of ASD. In addition, Zimmermann et al. later reported alterations in biochemical and molecular parameters of the purinergic system of zebrafish exposed to VPA (Zimmermann et al., 2017). The results corroborate the data available regarding purine metabolism in ASD patients (Stubbs et al., 1982) and the involvement of the adenosine A2A receptor on locomotion, anxiety and sleep regulation (Moreau and Huber, 1999), all relevant parameters in ASD.
The brains of VPA fish also had abnormally low levels of noradrenaline, a neurotransmitter relevant for attention and cognition. In fact, increasing cerebral cortical noradrenaline levels has been suggested in the therapeutic approaches for attention deficit hyperactivity disorder (ADHD) (del Campo et al., 2011), a disorder that shares symptoms with ASD, in terms of social and other behavioural impairments (de Boo and Prins, 2007). In BTBR mice, a strain that exhibits reduced play and social approach behaviour and is utilized as an animal model of ASD, noradrenaline is significantly decreased in the cortex, striatum and hippocampus when compared to C57BL/6J mice (Guo and Commons, 2016).
Alterations in the histaminergic system are present in the pathophysiology of different neuropsychiatric disorders, including schizophrenia (Iwabuchi et al., 2005), ADHD (Stevenson et al., 2010) and Gilles de la Tourette syndrome (Castellan Baldan et al., 2014). Only recently, a post mortem analysis of the expression of histamine receptors and other histamine signalling genes in the DLPFC of ASD patients was performed (Wright et al., 2017). None of the genes of interest were individually identified to be differentially expressed in the DLPFC. In contrast to the individual expression analyses, a significant overexpression was detected in ASD samples when the genes were analysed as a gene set. It is important to highlight that ASD is a highly heterogeneous disorder and that the sample size in this study was small (13 individuals diagnosed with ASD and 39 non‐psychiatric controls), which could explain the inconsistence between the two analyses. Moreover, only one area of the brain was analysed, and the expression of histamine receptors occurs in different structures of the CNS.
Translating VPA doses used in humans to the equivalent in organism models is challenging. When pregnant women are exposed to VPA, the therapeutic doses can range from 200 to 3600 mg per day (Roullet et al., 2013), making it difficult to select a representative dose. Furthermore, the mechanisms of VPA transport across the human placenta is not clear and the degree of fetal exposure is uncertain (Semczuk‐Sikora et al., 2010). It is noteworthy that the use of VPA to model ASD does not aim at a therapeutic effect but rather establish a mechanism that leads to an ASD‐like phenotype. For instance, in already well‐established ASD rodent models, the VPA dose utilized is 5–20 times higher than that used in humans (Bambini‐Junior et al., 2014). Based on pilot studies and published data (Zimmermann et al., 2015), we exposed zebrafish to 50 μM VPA, and this led to a high mortality rate at 5 dpf (more than 50%). Exposing embryos to 35 μM of VPA led to a clear arrested development and high rates of deformities at 5 dpf and mortality after 10 dpf. We then defined 25 μM as the optimal VPA concentration as only a small proportion of larvae had to be excluded due to malformations. The lack of an intrauterine environment during zebrafish development does not allow us to fully reproduce the mechanism involving VPA exposure and the onset of autistic‐like features. Nevertheless, zebrafish and mammals generally have high concordance in developmental toxicity (Nishimura et al., 2016) and it is possible to expose zebrafish to various environmental factors neonatally in a more experimentally controllable environment.
In summary, we report significant molecular and neurochemical changes in the histaminergic, noradrenergic and dopaminergic systems of zebrafish exposed to VPA during a short period in early development. Importantly, we show that some of these changes persist into adulthood, despite the remarkable regenerative capacity of zebrafish, and that VPA‐exposed adult fish have an impaired social behaviour. These findings will bring more attention to a possible involvement of the histaminergic system in the outcomes related to neuropsychiatric disorders. Furthermore, it supports the use of zebrafish as a useful model system to investigate mechanisms underlying these disorders.
Author contributions
D.B., M.S. and P.P. conceived and designed the experiments. D.B., H.A.J.P., S.S. and E.L. collected and analysed data. D.B., H.A.J.P., M.S. and P.P. wrote the manuscript. All authors revised the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
We thank Henri Koivula (BSc) and Riikka Pesonen for expert technical help in fish maintenance. We also thank the Brazilian National Research Council (CNPq) for funding D.B. and Sigrid Juselius Foundation for project funding to P.P.
Baronio, D. , Puttonen, H. A. J. , Sundvik, M. , Semenova, S. , Lehtonen, E. , and Panula, P. (2018) Embryonic exposure to valproic acid affects the histaminergic system and the social behaviour of adult zebrafish (Danio rerio). British Journal of Pharmacology, 175: 797–809. doi: 10.1111/bph.14124.
References
- Airaksinen MS, Panula P (1990). Comparative neuroanatomy of the histaminergic system in the brain of the frog Xenopus laevis . J Comp Neurol 292: 412–423. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambini‐Junior V, Baronio D, MacKenzie J, Zanatta G, dos Santos Riesgo R, Gottfried C (2014). Prenatal exposure to valproate in animals and autism In: Comprehensive Guide to Autism. Springer: New York, NY, pp. 1779–1793. [Google Scholar]
- Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E et al (2000). The syntenic relationship of the zebrafish and human genomes. Genome Res 10: 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronio D, Castro K, Gonchoroski T, de Melo GM, Nunes GDF, Bambini‐Junior V et al (2015). Effects of an H3R antagonist on the animal model of autism induced by prenatal exposure to valproic acid. PLoS One 10: e0116363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X (1993). Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14: 83–144. [PubMed] [Google Scholar]
- de Boo GM, Prins PJM (2007). Social incompetence in children with ADHD: possible moderators and mediators in social‐skills training. Clin Psychol Rev 27: 78–97. [DOI] [PubMed] [Google Scholar]
- Burgess CR (2010). Histamine and orexin in the control of arousal, locomotion, and motivation. J Neurosci 30: 2810–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M (2007). Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol 210: 2526–2539. [DOI] [PubMed] [Google Scholar]
- del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW (2011). The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention‐deficit/hyperactivity disorder. Biol Psychiatry 69: e145–e157. [DOI] [PubMed] [Google Scholar]
- Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M et al (2014). Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron 81: 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Semenova S, Rozov S, Sundvik M, Bonkowsky JL, Panula P (2016). A novel developmental role for dopaminergic signaling to specify hypothalamic neurotransmitter identity. J Biol Chem 291: 21880–21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH et al (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309: 1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnon J, Heisenberg C‐P (2013). Neurulation: coordinating cell polarisation and lumen formation. EMBO J 32: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Peitsaro N, Karlstedt K, Kaslin J, Panula P (1998). Development of the histaminergic neurons and expression of histidine decarboxylase mRNA in the zebrafish brain in the absence of all peripheral histaminergic systems. Eur J Neurosci 10: 3799–3812. [DOI] [PubMed] [Google Scholar]
- Gotoh K, Masaki T, Chiba S, Ando H, Fujiwara K, Shimasaki T et al (2013). Brain‐derived neurotrophic factor, corticotropin‐releasing factor, and hypothalamic neuronal histamine interact to regulate feeding behavior. J Neurochem 125: 588–598. [DOI] [PubMed] [Google Scholar]
- Guo YP, Commons KG (2016). Serotonin neuron abnormalities in the BTBR mouse model of autism. Autism Res : 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H, Panula P (2003). The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci 4: 121–130. [DOI] [PubMed] [Google Scholar]
- Happé F, Ronald A (2008). The ‘fractionable autism triad’: a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev 18: 287–304. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Panula P, Yamatodani A, Wada H (1990). Organization of the histaminergic system in the brain of the turtle Chinemys reevesii . J Comp Neurol 297: 132–144. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Panula P, Yamatodani A, Wada H (1991). Organization of the histaminergic system in the brain of the teleost, Trachurus trachurus . J Comp Neurol 310: 94–102. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Ito C, Tashiro M, Kato M, Kano M, Itoh M et al (2005). Histamine H1 receptors in schizophrenic patients measured by positron emission tomography. Eur Neuropsychopharmacol 15: 185–191. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Gerlai R (2014). Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin JAN, Panula P (2001). Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). 377: 342–377. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MM, Prast H, Philippu A (2013). Facilitation of short‐term memory by histaminergic neurons in the nucleus accumbens is independent of cholinergic and glutamatergic transmission. Br J Pharmacol 170: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter G, Söll I, Hauptmann G (2014). Sensitive whole‐mount fluorescent in situ hybridization in zebrafish using enhanced tyramide signal amplification. Methods Mol Biol 1082: 175–185. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim Y‐H, Yun J‐S, Lee C‐J (2013). Valproic acid decreases the proliferation of telencephalic cells in zebrafish larvae. Neurotoxicol Teratol 39: 91–99. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Lin J, Xia Q, Guo N, Li Q (2016). Social preference deficits in juvenile zebrafish induced by early chronic exposure to sodium valproate. Front Behav Neurosci 10: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Erickson CA, Stigler KA, Posey DJ (2005). Neurochemistry in the pathophysiology of autism. J Clin Psychiatry : 9–18. [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Huber G (1999). Central adenosine A(2A) receptors: an overview. Brain Res Brain Res Rev 31: 65–82. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Inoue A, Sasagawa S, Koiwa J, Kawaguchi K, Kawase R et al (2016). Using zebrafish in systems toxicology for developmental toxicity testing. Congenit Anom (Kyoto) 56: 18–27. [DOI] [PubMed] [Google Scholar]
- Panula P, Nuutinen S (2013). The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci 14: 472–487. [DOI] [PubMed] [Google Scholar]
- Panula P, Airaksinen MS, Pirvola U, Kotilainen E (1990). A histamine‐containing neuronal system in human brain. Neuroscience 34: 127–132. [DOI] [PubMed] [Google Scholar]
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WLS et al (2015). International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67: 601–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsaro N, Kaslin J, Anichtchik OV, Panula P (2003). Modulation of the histaminergic system and behaviour by alpha‐fluoromethylhistidine in zebrafish. J Neurochem 86: 432–441. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276: 36734–36741. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ (1986). Functions of shoaling behaviour in teleosts In: The Behaviour of Teleost Fishes. Springer US: Boston, MA, pp. 294–337. [Google Scholar]
- Puttonen HA, Sundvik M, Rozov S, Chen YC, Panula P (2013). Acute ethanol treatment upregulates th1, th2, and hdc in larval zebrafish in stable networks. Front Neural Circuits 7: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J (1996). Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370: 247–9861. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Lai JKY, Foster JA (2013). In utero exposure to valproic acid and autism – a current review of clinical and animal studies. Neurotoxicol Teratol 36: 47–56. [DOI] [PubMed] [Google Scholar]
- Rozov SV, Zant JC, Karlstedt K, Porkka‐Heiskanen T, Panula P (2014). Periodic properties of the histaminergic system of the mouse brain. Eur J Neurosci 39: 218–228. [DOI] [PubMed] [Google Scholar]
- Sallinen V, Torkko V, Sundvik M, Reenilä I, Khrustalyov D, Kaslin J et al (2009). MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J Neurochem 108: 719–731. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al (2012). Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semczuk‐Sikora A, Czuczwar S, Semczuk A, Kwasniewska A, Semczuk M (2010). Valproic acid transfer across human placental cotyledon during dual perfusion in vitro . Ann Agric Environ Med 17: 153–157. [PubMed] [Google Scholar]
- Semenova SA, Chen Y‐C, Zhao X, Rauvala H, Panula P (2014). The tyrosine hydroxylase 2 (TH2) system in zebrafish brain and stress activation of hypothalamic cells. Histochem Cell Biol 142: 619–633. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speel EJ, Saremaslani P, Roth J, Hopman AH, Komminoth P (1998). Improved mRNA in situ hybridization on formaldehyde‐fixed and paraffin‐embedded tissue using signal amplification with different haptenized tyramides. Histochem Cell Biol 110: 571–577. [DOI] [PubMed] [Google Scholar]
- Stevenson J, Sonuga‐Barke E, McCann D, Grimshaw K, Parker KM, Rose‐Zerilli MJ et al (2010). The role of histamine degradation gene polymorphisms in moderating the effects of food additives on children's ADHD symptoms. Am J Psychiatry 167: 1108–1115. [DOI] [PubMed] [Google Scholar]
- Stubbs G, Litt M, Lis E, Jackson R, Voth W, Lindberg A et al (1982). Adenosine deaminase activity decreased in autism. J Am Acad Child Psychiatry 21: 71–74. [DOI] [PubMed] [Google Scholar]
- Sundvik M, Panula P (2012). Organization of the histaminergic system in adult zebrafish (Danio rerio) brain: neuron number, location, and cotransmitters. J Comp Neurol 520: 3827–3845. [DOI] [PubMed] [Google Scholar]
- Sundvik M, Kudo H, Toivonen P, Rozov S, Chen YC, Panula P (2011). The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J 25: 4338–4347. [DOI] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR (2008). Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res 87: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Shin JH, Rajpurohit A, Deep‐Soboslay A, Collado‐Torres L, Brandon NJ et al (2017). Altered expression of histamine signaling genes in autism spectrum disorder. Transl Psychiatry 7: e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li C, Zeng Q, Agrawal I, Zhu X, Gong Z (2016). Genome‐wide identification of suitable zebrafish Danio rerio reference genes for normalization of gene expression data by RT‐qPCR. J Fish Biol 88: 2095–2110. [DOI] [PubMed] [Google Scholar]
- Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T (1985). High‐performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation‐exchange chromatography coupled with post‐column derivatization fluorometry. J Chromatogr 344: 115–123. [DOI] [PubMed] [Google Scholar]
- Zimmermann FF, Gaspary KV, Leite CE, De Paula Cognato G, Bonan CD (2015). Embryological exposure to valproic acid induces social interaction deficits in zebrafish (Danio rerio): a developmental behavior analysis. Neurotoxicol Teratol 52: 36–41. [DOI] [PubMed] [Google Scholar]
- Zimmermann FF, Gaspary KV, Siebel AM, Leite CE, Kist LW, Bogo MR et al (2017). Analysis of extracellular nucleotide metabolism in adult zebrafish after embryological exposure to valproic acid. Mol Neurobiol 54: 3542–3553. [DOI] [PubMed] [Google Scholar]


