Abstract
Background
Appropriate immunosuppressive therapy for patients with idiopathic membranous nephropathy (MN) remains controversial. The effect of mycophenolate mofetil (MMF) versus cyclosporine (CsA) combined with low-dose corticosteroids was evaluated in patients with idiopathic MN in a multi-center randomized trial (NCT01282073).
Methods
A total of 39 biopsy-proven idiopathic MN patients with severe proteinuria were randomly assigned to receive MMF combined with low-dose corticosteroids (MMF group) versus CsA combined with low-dose corticosteroids (CsA group), respectively, and followed up for 48 weeks. Complete or partial remission rate of proteinuria and estimated glomerular filtration rate (eGFR) at 48 weeks were compared.
Results
The level of proteinuria at baseline and at 48 weeks was 8.9 ± 5.9 and 2.1 ± 3.1 g/day, respectively, in the MMF group compared to 8.4 ± 3.5 and 3.2 ± 5.7 g/day, respectively, in the CsA group. In total, 76.1% of the MMF group and 66.7% of the CsA group achieved remission at 48 weeks (95% confidence interval, −0.18 to 0.38). There was no difference in eGFR between the two groups. Anti-phospholipase A2 receptor Ab levels at baseline decreased at 48 weeks in the complete or partial remission group (P = 0.001), but were unchanged in the no-response group. There were no significant differences between the two groups in changes in the Gastrointestinal Symptom Rating Scale and Gastrointestinal Quality of Life Index scores from baseline to 48 weeks.
Conclusion
In combination with low-dose corticosteroids, the effect of MMF may not be inferior to that of CsA in patients with idiopathic MN, with similar adverse effects including gastrointestinal symptoms.
Trial Registration
ClinicalTrials.gov Identifier: NCT01282073
Keywords: Membranous Nephropathy, Cyclosporine, Mycophenolate Mofetil, Corticosteroids
Graphical Abstract
INTRODUCTION
Idiopathic membranous nephropathy (MN) is one of the most common types of nephrotic syndrome in adults worldwide.1,2 Previous reports of the natural history of idiopathic MN showed that 5%–30% and 40% of patients had spontaneous complete or partial remission of proteinuria at 5 years, respectively, whereas 30%–40% progressed to end-stage renal disease within 5–15 years.3,4
Given the slowly progressive natural course and substantial spontaneous remission rate of this disorder, immunosuppressive agents are recommended only for patients who are at high risk of disease progression or developing complications of nephrotic syndrome.5 Sustained proteinuria for at least 3 months and decreased estimated glomerular filtration rate (eGFR) were reported risk factors for progression of idiopathic MN.6 The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that immunosuppressive agents for idiopathic MN should be initiated selectively in patients at risk of disease progression, including those with persistent proteinuria exceeding 4 g/day and remaining at over 50% of baseline value despite 6 months of conservative treatment with renin-angiotensin-aldosterone system blockade.7
Although various immunosuppressive agents have been used for treatment of idiopathic MN, their use remains controversial. Oral alkylating agents, such as cyclophosphamide and chlorambucil in conjunction with corticosteroids, are effective in inducing remission and preventing end-stage renal disease.8,9 However, the toxicities of alkylating agents are of concern.10 While calcineurin inhibitors including cyclosporine (CsA) or tacrolimus are usually recommended as alternatives to alkylating agents, the nephrotoxicity of calcineurin inhibitors can be a challenge in the presence of pre-existing deterioration of kidney function. Moreover, CsA-based therapy is recommended as an alternative to alkylating agents in patients who choose not to receive or have contraindications for an alkylating agent regimen because of low to moderate quality of evidence in the KDIGO guidelines.7
Mycophenolate mofetil (MMF), an immunosuppressive agent, inhibits antibody formation and the proliferation of both T and B cells; it also downregulates the expression of adhesion molecules on lymphocytes, thereby impairing binding to endothelial cells.11,12 MMF has been used to treat idiopathic MN as well as lupus nephritis and kidney transplantation recipients.13,14,15,16
Previous small randomized studies have shown a similar efficacy of MMF with corticosteroids compared to traditional cytotoxic agents combined with corticosteroids in patients with idiopathic MN.17,18 However, there are no data comparing the efficacy of MMF versus CsA in combination with corticosteroids for treatment of idiopathic MN. Therefore, this multi-center, randomized controlled trial aimed to evaluate the effect of MMF versus CsA in combination with low-dose corticosteroids in patients with idiopathic MN (MMFPRIMER, www.ClinicalTrials.gov NCT01282073).
METHODS
Study design and population
This multi-center, randomized controlled trial was conducted from June 2013 to May 2016. Patients with biopsy-proven idiopathic MN were assessed for eligibility for this study at multiple centers in the Republic of Korea. We also included patients who underwent a biopsy within the preceding 12 months, experienced worsening proteinuria, and exhibited deteriorating renal function, but satisfied the following inclusion criteria. Patients who were ≥ 18 years old were enrolled in this study if they had proteinuria > 8 g/day. Patients with proteinuria < 8 g/day were also enrolled if they met 3 or more of the following criteria: 1) eGFR < 60 mL/min/1.73 m2, 2) hypertension (blood pressure ≥ 140/90 mmHg or ≥ 120/80 with anti-hypertensive drugs), 3) 24-hour urinary protein > 5.0 g/day or spot urine protein to creatinine ratio > 5.0 g/g, 4) serum albumin < 3.0 g/dL, and 5) selectivity index > 0.2. eGFR was calculated by the modification of diet in renal disease (MDRD) equation. The selectivity index was calculated using the following equation: urine IgG × serum albumin/serum IgG × urine albumin. Exclusion criteria included the presence of moderate to severe gastrointestinal disorder at screening; a history of allergy to mycophenolate mofetil or cyclosporine; acute or chronic allergy within 4 weeks; presence of serious life-limiting comorbid disorders such as malignancy or uncontrollable active infection; drug or alcohol addiction within 6 months; uncontrolled high blood pressure (≥ 160/100 mmHg); eGFR ≤ 30 mL/min/1.73 m2 at screening; absolute neutrophil count < 1,500/mm3 or white blood cell (WBC) < 2,500/mm3; platelets < 100,000/mm3; three-times greater than normal liver function test values; pregnancy; or lactation. Patients who had received immunosuppressive agents within 6 months for secondary MN with systemic disorder, or had a life expectancy of less than 1 year were also excluded.
Randomization and treatment protocol
All patients were assigned using a block randomization technique to either the MMF group or the CsA group. The table of random numbers was generated using the SAS randomization program, which was managed by the Department of Clinical Statistics in the clinical trial center at Kyungpook National University Hospital in Daegu, Korea. Oral MMF or CsA were provided as prepacked drugs in identical bottles. Allocation concealment was done by sealed sequentially numbered opaque envelopes. They were consecutively numbered and bottles were provided to the patients according to the number allocated. Treatment consisted of oral prednisolone 0.15 mg/kg up to a maximum dose of 15 mg/day for patients in both groups. Prednisolone was maintained at a minimum of 5 mg/day during the study period. In the MMF group, oral MMF (Myconol®, Hanmi Pharmaceutical, Seoul, Korea) was added to corticosteroids. The MMF treatment regimen consisted of 500 mg twice daily in patients weighing less than 50 kg, or 750–1,000 mg twice daily in patients weighing more than 50 kg. The dose of MMF was adjusted in a range of 500–1,000 mg twice daily based on laboratory findings, at the discretion of the attending physician. The dose of MMF was reduced by 25%–33% of the previous dose when a patient had moderate to severe diarrhea. MMF was withheld when the WBC count was less than 4,000/mm3 or the patient had intolerable gastrointestinal symptoms, and was restarted at a 50% dose at least 2 weeks after recovery. MMF was discontinued when the patient showed severe adverse events, more than doubled values in liver function tests, newly developed malignancy, or increased serum creatinine level by over 50%. In the CsA group, oral CsA (Implanta soft cap®, Hanmi Pharmaceutical) was added to corticosteroids. CsA was started at a dose of 4 mg/kg and the dose was adjusted to maintain a 100 ± 50 ng/mL trough blood level. The trough level of CsA was not an absolute target and the dose could be adjusted at the physician's discretion. The dose was decreased in cases in which serum CsA level ≥ 250 ng/mL, there was an increase in serum creatinine level of more than 0.3 mg/dL compared to baseline, elevated serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level, or increased serum bilirubin level ≥ 2 mg/dL. CsA was discontinued when serum creatinine level did not improve 4 weeks after the dose was reduced in cases of increased serum creatinine ≥ 30% over baseline. CsA was also discontinued when the patient had severe adverse events or uncontrolled high blood pressure despite the use of three or more anti-hypertensive drugs at maximally-tolerated doses. Concurrent treatment was not standardized; however, blood pressure and proteinuria were primarily treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Statins were used to decrease serum cholesterol levels. Patients were assessed 2 weeks after starting the treatment regimen, every 4 to 6 weeks for the next 24 weeks, and then every 12 weeks thereafter. Patients were followed up for a total of 48 weeks. Vital signs, side effects of treatment, and laboratory data, including spot urine protein to creatinine ratio, complete blood cell count, serum albumin, and serum creatinine, were prospectively collected at each visit. Lipid profile and 24-hour urinary protein were monitored at baseline, and at 12, 24, and 48 weeks.
Outcome measurements and safety assessment
The primary outcome was the rate of complete or partial remission of proteinuria at 48 weeks in the MMF and CsA groups. To assess remission of proteinuria at 48 weeks, level of 24-hour urinary protein was used. Complete remission was defined as a decrease in proteinuria to ≤ 200 mg/day and a sustained serum albumin level ≥ 3.5 g/dL. Partial remission was defined as a decrease in proteinuria to > 200 and < 3,500 mg/day or a decrease greater than 50% compared to baseline.19 As secondary outcomes, eGFR, relapse rate, and improvement of hypoalbuminemia and hypercholesterolemia at 48 weeks were compared between the 2 groups. A relapse was defined as proteinuria ≥ 3,500 mg/day after achievement of partial or complete remission or an increase in proteinuria greater than 50% in patients in whom proteinuria had improved initially by more than 50%.
To assess safety, any adverse events, such as leukopenia, infectious episodes, malignancy, and gastrointestinal symptoms, including diarrhea and epigastric discomfort, were recorded. To compare gastrointestinal symptoms in the 2 groups, patients were asked to fill in a questionnaire including the Gastrointestinal Symptom Rating Scale (GSRS)20,21 and Gastrointestinal Quality of Life Index (GIQLI)22 at baseline and at 48 weeks.
Measurement of anti-PLA2R Ab level by using the enzyme-linked immunosorbent assay method
Serum samples from the patients at baseline and at 48 weeks were obtained and stored at −80°C. Levels of anti-phospholipase A2 receptor (anti-PLA2R) antibodies (Ab) were measured using a commercial ELISA kit (Euroimmun AG, Lubeck, Germany) in accordance with the manufacturer's protocol, as described in previous studies.23,24 A value higher than 20 RU/mL was regarded as a positive result.
Statistical analysis
It was estimated that at least 28 patients in each group would be needed for 80% power assuming a 5% significance level. As we estimated a 10% screening failure and dropout rate, 31 patients would finally need to be included in each group.
The intention-to-treat (ITT) analyses included all randomly assigned patients. We compared complete or partial remission rates of proteinuria in the 2 treatment groups using a non-inferiority test: the lower limit of the confidence interval (CI) of the difference in proportions of patients who achieved complete or partial remission of proteinuria in the MMF and CsA groups could not exceed the threshold of −20%, which is 50% of the difference in remission rate between spontaneous remission (−30%)3,4 and CsA treatment (−70%).25,26 Mean values and frequencies of the parameters were compared by independent sample t-tests or χ2 tests, as appropriate. For non-normally distributed variables (body mass index, serum creatinine, and high-sensitivity C-reactive protein [hsCRP]), the Mann-Whitney U test was performed after a test for normality. If patients discontinued treatment due to a lack of efficacy, their last value was carried forward for the analysis. Cumulative probabilities of complete or partial remission were estimated using the Kaplan-Meier method. SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. A P value of < 0.05 was considered statistically significant.
Ethics statement
Approval was obtained from the Institutional Review Board of Kyungpook National University Hospital (KNUH_10-1096). Written informed consent was obtained from all patients prior to randomization. The trial was registered at ClinicalTrials.gov site (www.ClinicalTrials.gov NCT01282073).
RESULTS
Baseline characteristics of the patients
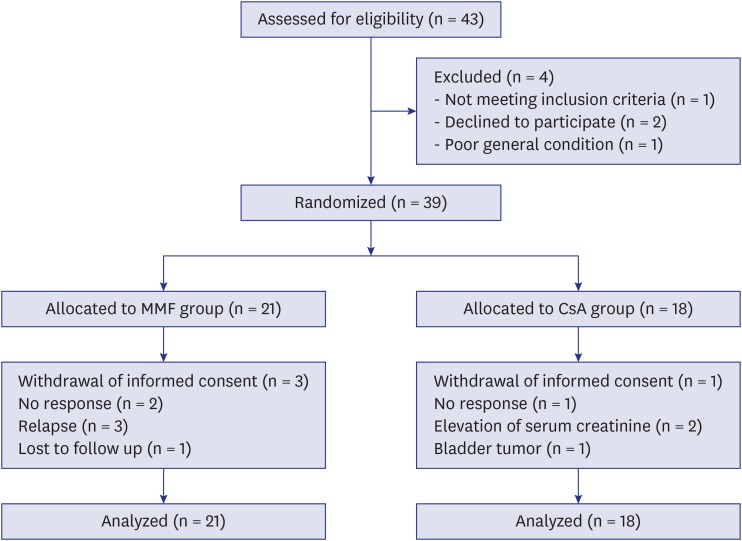
Of 43 MN patients with severe proteinuria screened for this study, 1 was not eligible, 2 refused to participate, and 1 was in poor general condition and had a rapidly rising serum creatinine level. Thus, 39 patients were included in the study. Of these, 21 and 18 were allocated to the MMF and CsA groups, respectively (Fig. 1). The baseline characteristics of patients were comparable between the 2 groups (Table 1).
Fig. 1.
Flow diagram of the multi-center, randomized trial evaluating the effect of MMF vs. CsA combined with low-dose corticosteroids in patients with idiopathic membranous nephropathy.
MMF = mycophenolate mofetil, CsA = cyclosporine.
Table 1. Baseline characteristics.
| Parameters | MMF group (n = 21) | CsA group (n = 18) | Total (n = 39) | P |
|---|---|---|---|---|
| Sex (male) | 16 (76.2) | 9 (50.0) | 25 (64.1) | 0.172 |
| Age, yr | 57.7 ± 10.0 | 52.7 ± 10.9 | 55.4 ± 10.6 | 0.141 |
| Body mass index, kg/m2 | 25.2 ± 3.8 | 26.4 ± 4.7 | 25.7 ± 4.2 | 0.385 |
| Systolic BP, mmHg | 125.0 ± 17.2 | 122.3 ± 17.5 | 123.7 ± 17.2 | 0.639 |
| Diastolic BP, mmHg | 77.0 ± 11.9 | 75.9 ± 11.1 | 76.5 ± 11.4 | 0.758 |
| Serum creatinine, mg/dL | 1.1 ± 0.5 | 0.9 ± 0.4 | 1.1 ± 0.4 | 0.154 |
| Albumin, g/dL | 2.3 ± 0.6 | 2.5 ± 0.6 | 2.4 ± 0.6 | 0.366 |
| Total cholesterol, mg/dL | 268.5 ± 73.3 | 243.9 ± 64.1 | 257.2 ± 69.4 | 0.277 |
| hsCRP, mg/dL | 0.52 ± 1.10 | 0.38 ± 0.63 | 0.45 ± 0.91 | 0.656 |
| Proteinuria, g/day | 8.9 ± 5.9 | 8.4 ± 3.5 | 8.7 ± 4.9 | 0.710 |
| Microscopic hematuria | 19 (90.5) | 15 (83.3) | 34 (87.2) | 0.853 |
| eGFR, mL/min/1.73 m2 | 73.9 ± 31.0 | 84.9 ± 25.2 | 78.9 ± 28.7 | 0.236 |
| Hypertension | 14 (66.7) | 9 (50.0) | 23 (59.0) | 0.466 |
| Diabetes | 4 (19.0) | 4 (22.2) | 8 (20.5) | 1.000 |
| ACEi and/or ARB | 16 (76.2) | 17 (94.4) | 33 (84.6) | 0.258 |
| Statin | 19 (90.5) | 18 (100.0) | 37 (94.9) | 0.538 |
| Proton pump inhibitor | 8 (38.1) | 11 (61.1) | 19 (48.7) | 0.266 |
| Trimethoprim/sulfamethoxazole | 3 (14.3) | 3 (16.7) | 6 (15.4) | 1.000 |
| Vitamin D/calcium | 1 (4.8) | 3 (16.7) | 4 (10.3) | 0.489 |
Values expressed as the mean ± SD, or number (%). Mean values were compared by independent sample t-tests and frequencies were compared by χ2 tests.
MMF = mycophenolate mofetil, CsA = cyclosporine, BP = blood pressure, hsCRP = high-sensitivity C-reactive protein, eGFR = estimated glomerular filtration rate, ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, SD = standard deviation.
Comparison of response to treatment
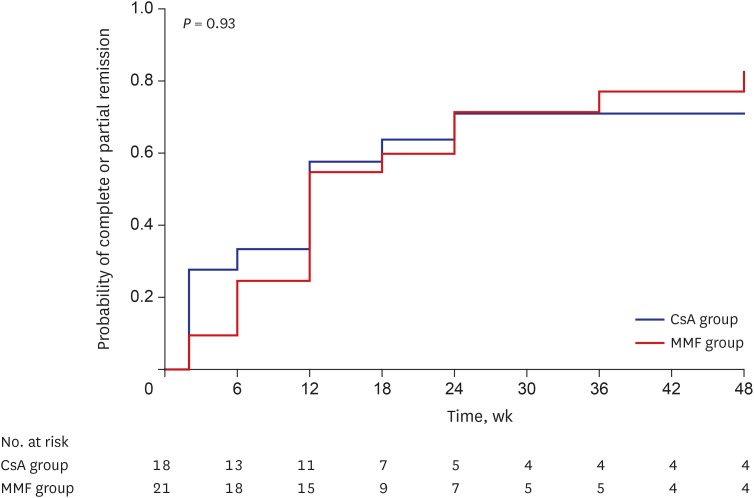
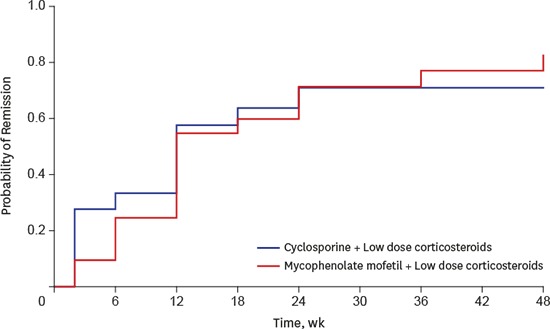
A total of 16 patients (76.1%) in the MMF group and 12 (66.7%) in the CsA group achieved complete or partial remission of proteinuria at 48 weeks (Table 2; P = 0.805). The absolute difference in complete or partial remission of proteinuria was 9.4% (95% CI, −0.18 to 0.38), which did not exceed the margin. The cumulative incidence of complete or partial remission of proteinuria at 48 weeks was 82.8% in the MMF group and 70.9% in the CsA group, which was not significantly different between the groups (Fig. 2; P = 0.929).
Table 2. Status of proteinuria at 48 weeks.
| Outcomes | MMF group (n = 21) | CsA group (n = 18) | Total (n = 39) | P | |
|---|---|---|---|---|---|
| Remission | 16 (76.1) | 12 (66.7) | 28 (71.7) | 0.805 | |
| Complete remission | 4 (19.0) | 3 (16.7) | 7 (17.9) | 1.000 | |
| Partial remission | 12 (57.1) | 9 (50.0) | 21 (53.8) | 0.901 | |
Values expressed as number (%). P, MMF vs. CsA group compared by χ2 tests.
MMF = mycophenolate mofetil, CsA = cyclosporine.
Fig. 2.
Probability of complete or partial remission of proteinuria in MMF and CsA groups. The cumulative incidence of complete or partial remission of proteinuria at 48 weeks was 82.8% in the MMF group and 70.9% in the CsA group, which did not significantly differ between the groups (P = 0.93).
MMF = mycophenolate mofetil, CsA = cyclosporine.
Rate of complete or partial remission was compared based on the categories of proteinuria at baseline. The results were presented in Table 3. Remission rate of proteinuria was higher in patients with proteinuria less than 8 g/day compared to patients with proteinuria > 8 g/day. However, there was no significant difference between the MMF and CsA group in patients with each categories of proteinuria (Table 3).
Table 3. Complete or partial remission of proteinuria based on the categories of proteinuria at baseline.
| Proteinuria category | Complete or partial remission | P | ||
|---|---|---|---|---|
| MMF group | CsA group | Total | ||
| > 8 g/day (n = 21) | 7/12 (58.3) | 5/9 (55.6) | 12/21 (57.1) | 1.000 |
| 3–8 g/day (n = 16) | 7/7 (100) | 7/9 (77.8) | 14/16 (87.5) | 0.568 |
| < 3 g/day (n = 2) | 2/2 (100) | - | 2/2 (100) | - |
Values expressed as number (%). P, MMF versus CsA group compared by χ2 tests.
MMF = mycophenolate mofetil, CsA = cyclosporine.
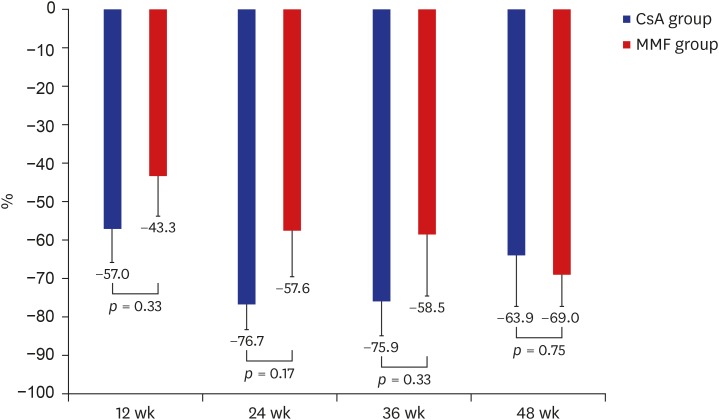
Proteinuria at baseline and 48 weeks measured 8.9 ± 5.9 and 2.1 ± 3.1 g/day, respectively, in the MMF group compared to 8.4 ± 3.5 and 3.2 ± 5.7 g/day, respectively, in the CsA group. Changes in proteinuria from baseline were comparable between the 2 groups at 12 (−57.0% CsA vs. −43.3% MMF group; P = 0.330), 24 (−76.7% CsA vs. −57.6% MMF group; P = 0.174), 36 (−75.9% CsA vs. −58.5% MMF group; P = 0.326), and 48 weeks (−63.9% CsA vs. −69.0% MMF group; P = 0.745) (Fig. 3). Proteinuria was significantly decreased at each time point compared to baseline in both groups. In the CsA group, there was no significant difference in reduction of proteinuria in each time point (−57.0% at 12 weeks vs. −76.7% at 24 weeks, P = 0.095; −57.0% at 12 weeks vs. −75.9% at 36 weeks, P = 0.145; −57.0% at 12 weeks vs. −63.9% at 48 weeks, P = 0.665; −76.7% at 24 weeks vs. −75.9% at 36 weeks, P = 0.947; −76.7% at 24 weeks vs. −63.9% at 48 weeks, P = 0.398; −75.9% at 36 weeks vs. −63.9% at 48 weeks, P = 0.472). In the MMF group, there was also no significant difference in reduction of proteinuria in each time point (−43.3% at 12 weeks vs. −57.6% at 24 weeks, P = 0.376; −43.3% at 12 weeks vs. −58.5% at 36 weeks, P = 0.414; −43.3% at 12 weeks vs. −69.0% at 48 weeks, P = 0.070; −57.6% at 24 weeks vs. −58.5% at 36 weeks, P = 0.963; −57.6% at 24 weeks vs. −69.0% at 48 weeks, P = 0.427; −58.5% at 36 weeks vs. −69.0% at 48 weeks, P = 0.531).
Fig. 3.
Changes in proteinuria from baseline in MMF and CsA groups. Changes in proteinuria from baseline to 12, 24, 36, and 48 weeks were comparable between the two groups.
MMF = mycophenolate mofetil, CsA = cyclosporine.
Serum albumin was significantly increased at each time point in both groups compared to baseline except 12 weeks in CsA group (Table 4). Four patients (19.0%) in the MMF group and 4 (22.2%) in the CsA group had relapse of proteinuria during the study period (P = 1.000).
Table 4. Changes in laboratory parameters.
| Group | Parameters | |||||
|---|---|---|---|---|---|---|
| Proteinuria, g/day | Serum albumin, g/dL | Serum creatinine, mg/dL | eGFR, mL/min/1.73 m2 | Total cholesterol, mg/dL | ||
| MMF group | ||||||
| Baseline | 8.9 ± 5.9 | 2.3 ± 0.6 | 1.1 ± 0.5 | 73.9 ± 31.0 | 268.5 ± 73.3 | |
| 12 wk | 3.6 ± 2.9a | 3.0 ± 0.8a | 1.1 ± 0.3 | 72.6 ± 21.4 | 266.1 ± 87.5 | |
| 24 wk | 2.7 ± 3.8a | 3.2 ± 0.9a | 1.1 ± 0.3 | 69.8 ± 22.0 | 242.3 ± 97.4 | |
| 36 wk | 2.1 ± 2.2a | 3.6 ± 0.7a,b | 1.1 ± 0.3 | 71.5 ± 32.9 | - | |
| 48 wk | 2.1 ± 3.1a | 3.6 ± 0.9a | 1.1 ± 0.3 | 70.1 ± 19.2 | 206.2 ± 57.3 | |
| CsA group | ||||||
| Baseline | 8.4 ± 3.5 | 2.5 ± 0.6 | 0.9 ± 0.4 | 84.9 ± 25.2 | 243.9 ± 64.1 | |
| 12 wk | 3.8 ± 3.8a | 2.9 ± 0.7 | 0.9 ± 0.3 | 83.2 ± 24.8 | 267.9 ± 72.8 | |
| 24 wk | 1.7 ± 1.7a | 3.4 ± 0.7a | 1.0 ± 0.4 | 81.3 ± 25.3 | 213.1 ± 63.2 | |
| 36 wk | 2.0 ± 3.2a | 3.5 ± 0.6a,b | 1.0 ± 0.4 | 75.3 ± 26.8 | - | |
| 48 wk | 3.2 ± 5.7a | 3.6 ± 0.7a,b | 1.0 ± 0.4 | 84.0 ± 32.6 | 204.2 ± 85.0 | |
MMF = mycophenolate mofetil, CsA = cyclosporine, eGFR = estimated glomerular filtration rate.
aP < 0.05 vs. baseline; bP < 0.05 vs. 12 weeks in each group compared by independent sample t-tests.
eGFRs at baseline and at 48 weeks were 73.9 ± 31.0 and 70.1 ± 19.2 mL/min/1.73 m2, respectively, in the MMF group compared to 84.9 ± 25.2 and 84.0 ± 32.6 mL/min/1.73 m2, respectively, in the CsA group. There was no significant difference in eGFR between the 2 groups at each visit. Hypercholesterolemia were improved at 48 weeks compared to baseline in both groups, but did not show significant differences between the groups at each visit (Table 4).
The trough CsA level in the CsA group was 134.6 ± 55.8 and 131.7 ± 36.4 ng/mL at 18 and 36 weeks, respectively. The dose of MMF and CsA was not significantly different at each time point in both groups (Supplementary Table 1).
Anti-PLA2R Ab levels
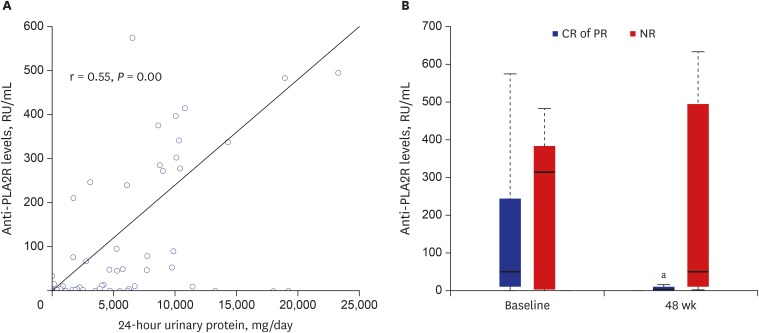
Serum samples could be obtained from 33 of the 39 patients (18 patients in the MMF group and 15 in the CsA group) for measurement of anti-PLA2R Ab levels at baseline and 48 weeks. Anti-PLA2R Ab levels from the samples in baseline and 48 weeks were strongly correlated with 24-hour urinary protein (r = 0.546, P = 0.000, Fig. 4A). Twenty-one patients (63.6%) showed anti-PLA2R Ab positivity. No significant difference in anti-PLA2R Ab level was found between the MMF and CsA groups at baseline and 48 weeks. Anti-PLA2R Ab levels at baseline significantly decreased at 48 weeks in the complete or partial remission group (P = 0.001), but showed no significant difference between baseline and 48 weeks in the no-response group (P = 0.679, Fig. 4B). In 10 patients (5 patients in the MMF group and 5 in the CsA group), positive anti-PLA2R Ab at baseline turned negative at 48 weeks. Of the patients, 9 had complete or partial remission.
Fig. 4.
Anti-PLA2R Ab levels. (A) Correlation between anti-PLA2R Ab levels and 24-hour urinary protein. Anti-PLA2R Ab levels from the samples in baseline and 48 weeks were strongly correlated with 24-hour urinary protein (r = 0.546, P = 0.000). (B) Anti-PLA2R Ab levels in CR or PR groups and the NR group at baseline and at 48 weeks. Horizontal lines at the bottom, middle, and top of the boxes show the 25th, 50th, and 75th percentiles, respectively, in the box plot. Anti-PLA2R Ab levels at baseline were significantly decreased at 48 weeks in the CR or PR groups (P = 0.001), but were not significantly changed in the NR group (P = 0.679).
anti-PLA2R = anti-phospholipase A2 receptor, Ab = antibodies, CR = complete remission, PR = partial remission, NR = no-response.
aP < 0.05 vs. baseline.
Safety
Adverse events occurred in 12 (57.1%) and 9 (50.0%) patients in the MMF and CsA groups, respectively (Table 5; P = 0.901). Adverse events, such as leukopenia and thrombocytopenia, did not occur in either group during treatment. One patient in each group developed anaemia. Two patients in each group had diarrhea, which was mild and transitory. Three patients in the MMF group and 4 in the CsA group developed epigastric discomfort (P = 0.742). Infectious episodes occurred with a similar frequency in both groups. One patient in each group developed malignancy. The patient in the MMF group was diagnosed with early gastric cancer, and the patient in the CsA group was diagnosed with a bladder tumour. There were no significant differences between the two groups in changes in the GSRS and GIQLI scores from baseline to 48 weeks (Table 6; P = 0.660 for GSRS and 0.221 for GIQLI).
Table 5. Safety profiles.
| Outcomes | MMF group (n = 21) | CsA group (n = 18) | Total (n = 39) | P | |
|---|---|---|---|---|---|
| Bone marrow suppression | |||||
| Leukopenia | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Anaemia | 1 (5.0) | 1 (6.2) | 2 (5.6) | 1.000 | |
| Gastrointestinal symptoms | |||||
| Diarrhea | 2 (10.0) | 2 (12.5) | 4 (11.1) | 1.000 | |
| Epigastric discomfort | 3 (15.0) | 4 (25.0) | 7 (19.4) | 0.742 | |
| Infections | |||||
| Upper respiratory | 6 (30.0) | 3 (18.8) | 9 (25.0) | 0.699 | |
| Urinary tract | 1 (5.0) | 0 (0.0) | 1 (2.8) | 1.000 | |
| Skin | 2 (10.0) | 1 (6.2) | 3 (8.3) | 1.000 | |
| New onset diabetes mellitus | 1 (5.0) | 1 (6.2) | 2 (5.6) | 1.000 | |
| Malignancy | 1 (5.0) | 1 (6.2) | 2 (5.6) | 1.000 | |
Values expressed as number (%).
MMF = mycophenolate mofetil, CsA = cyclosporine.
Table 6. Changes in GSRS and GIQLI scores from baseline to 48 weeks.
| Scales | MMF group | CsA group | P | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 48 wk | Changes | Baseline | 48 wk | Changes | ||
| GSRS | 3.9 ± 3.1 | 4.8 ± 4.0 | 0.6 ± 3.4 | 3.9 ± 5.4 | 3.9 ± 5.0 | −0.1 ± 5.2 | 0.660 |
| GIQLI | 107.2 ± 19.9 | 106.4 ± 19.9 | −2.5 ± 22.4 | 105.5 ± 24.0 | 113.6 ± 21.0 | 6.3 ± 17.2 | 0.221 |
P value: changes in scores from baseline to 48 weeks in the 2 groups by paired t-test.
GSRS = Gastrointestinal Symptom Rating Scale, GIQLI = Gastrointestinal Quality of Life Index, MMF = mycophenolate mofetil, CsA = cyclosporine.
DISCUSSION
In this study, we demonstrated similar efficacy of MMF compared to CsA in combination with low dose corticosteroids for high-risk patients with idiopathic MN. To the best of our knowledge, this is the first randomized controlled trial to compare the effect of MMF versus CsA combined with low dose corticosteroids in patients with idiopathic MN since Cattran et al.6 had conducted a randomized trial comparing CsA plus low-dose prednisone to placebo plus prednisone.
The therapeutic regimen may depend on the natural course of idiopathic MN because of the side effects of therapeutic agents or complications of idiopathic MN.5,7 In our study, we included patients with idiopathic MN at high risk for progression to end-stage renal disease, including those with severe proteinuria over 8 g/day or decreased renal function. These patients were to receive immunosuppressive agents without an observation period. CsA with corticosteroids has been used in patients with idiopathic MN who do not tolerate alkylating agents; however, this requires careful monitoring of serum creatinine and cyclosporine level in the blood due to the nephrotoxicity of the agents.27 Therefore, the use of cyclosporine is limited by nephrotoxicity, and the regimen should be modified or avoided if patients show deterioration of renal function.28
MMF has been used for idiopathic MN as an alternative to cytotoxic agents or calcineurin inhibitors.29,30 Dussol et al.31 showed that MMF monotherapy was not effective at decreasing proteinuria. Previous randomized trials had shown the efficacy of MMF with corticosteroids to be similar to that with alternating months of corticosteroids and a cytotoxic drug.17,18 In this study, MMF treatment was shown to be efficacious in reducing proteinuria in comparison to CsA-based regimen, when each was combined with low dose corticosteroids. In our study, 76.1% of patients in the MMF group showed complete or partial remission of proteinuria at 48 weeks; this remission rate was higher than that reported in previous studies (63.0%–66.0%)17,18,19 and recently published results (70.0%)32 for an MMF-based regimen. In addition, the rate of complete or partial remission of proteinuria at 48 weeks in the CsA group was 66.7% in this study, which is comparable to previous studies.25 Moreover, serum albumin increased and total cholesterol level in patients of both groups decreased at 48 weeks, compared to baseline. The eGFR in patients of both groups was also stably maintained during the study period. The higher rate of remission and improvement in laboratory parameters in our study might be attributed to the following: early commencement of immunosuppressive agents without a period of observation, and inclusion of patients with relatively preserved renal function at diagnosis, even though they had severe proteinuria.
Relapse after achievement of complete or partial remission occurred in 19% of the MMF group and 22.2% of the CsA group. Few trials have reported rates of relapse after using an MMF-based regimen in patients with idiopathic MN; however, 38% of an MMF group in a study by Branten et al.19 and 23.1% of patients who were treated with MMF or chlorambucil combined with corticosteroids in a study by Chan et al.17 experienced a relapse. Our patients showed similar relapse rates.
Anti-PLA2R Ab levels were also measured at baseline and at 48 weeks in our study. The prevalence of anti-PLA2R Ab positivity was 63.6% in our study population, similar to the results of previous studies that reported anti-PLA2R Ab positivity in approximately 50%–80% of patients.33 While anti-PLA2R Ab levels at baseline were not significantly changed at 48 weeks in the no-response group, they significantly decreased at 48 weeks in the complete or partial remission group. Our results support previous evidence that a decline in anti-PLA2R Ab levels could be a strong predictor of proteinuria remission and that serial follow-up of anti-PLA2R Ab levels provide better prognostic information.34,35,36
Leukopenia associated with MMF treatment was reported in patients with lupus nephritis and in kidney transplant recipients, as well as in those with MN.14,29,37 However, there was no occurrence of leukopenia in our patients, and the frequency of anaemia was similar in the MMF and CsA groups. Infectious complications and adverse events of malignancy were also not significantly different between the groups. Gastrointestinal symptoms, such as diarrhea, dyspepsia, and epigastric discomfort, are well known adverse effects of MMF treatment.37 To compare the gastrointestinal complications in the 2 groups quantitatively, we obtained GSRS and GIQLI scores at baseline and at 48 weeks. The changes in GSRS and GIQLI scores from baseline to 48 weeks were similar in the 2 groups; therefore, the gastrointestinal symptom burden from MMF seemed acceptable. There have been no data for quantitative assessment of gastrointestinal symptoms in idiopathic MN patients treated with MMF. Indeed, the results from 2 questionnaires support the preference for MMF treatment without serious gastrointestinal complications in patients with idiopathic MN.
This study has several limitations. First, the number of enrolled patients did not reach the initial goal of the study. Thirty-one patients were needed to be included in each group, but only 43 were assessed for eligibility and 39 were included for final analyses. However, it is difficult to recruit patients with idiopathic MN as the global incidence of this type of kidney disease is low.38 Thus, this study is considered meaningful despite the small number of enrolled patients. Second, the duration of follow-up was not long; however, 48 weeks of treatment was adequate to assess the efficacy of the immunosuppressive agents. Third, the dropout rate in both groups exceeded the expected dropout rate. Fourth, the study results may not be generalisable to other populations because only Korean patients were included in this study. Nevertheless, it was sufficient to show that MMF was not inferior to CsA in combination with low-dose corticosteroids for treatment of high-risk patients with idiopathic MN. Future studies with long-term follow-up will be needed to evaluate the relapse rate after 48 weeks. Additionally, further studies using other regimens, including rituximab, might be used to treat idiopathic MN.
In conclusion, MMF with low dose corticosteroid may not be an inferior treatment option for high risk patients with idiopathic MN compared to treatment with CsA and low dose corticosteroids; adverse effects including gastrointestinal symptoms were similar. Further study with a larger sample size and longer period of follow-up can provide evidence for the efficacy of MMF versus CsA in combination with low-dose corticosteroids in patients with idiopathic MN.
Footnotes
Funding: This work was supported by a grant from the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1129; HI15C0001; HI13C1232). The drugs and placebo used for conducting the study were provided by Hanmi Pharmaceutical, Co., Ltd. (Seoul, Korea), which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Choi JY, Cho JH, Park SH.
- Data curation: Choi JY, Cho JH, Jung HY, Kim CD, Kim YL, Park SH.
- Formal analysis: Choi JY, Jung HY, Park SH.
- Investigation: Kim DK, Kim YW, Yoo TH, Lee JP, Chung HC, Cho KH, An WS, Lee DH, Park SH.
- Writing - original draft: Choi JY, Cho JH, Jung HY.
- Writing - review & editing: Choi JY, Kim CD, Kim YL, Park SH.
SUPPLEMENTARY MATERIAL
The dose of MMF and CsA in each time point
References
- 1.Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis. 1997;30(5):621–631. doi: 10.1016/s0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 2.Naumovic R, Pavlovic S, Stojkovic D, Basta-Jovanovic G, Nesic V. Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant. 2009;24(3):877–885. doi: 10.1093/ndt/gfn564. [DOI] [PubMed] [Google Scholar]
- 3.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329(2):85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 4.Honkanen E, Törnroth T, Grönhagen-Riska C. Natural history, clinical course and morphological evolution of membranous nephropathy. Nephrol Dial Transplant. 1992;7(Suppl 1):35–41. [PubMed] [Google Scholar]
- 5.Kidney Disease; Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 6.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59(4):1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines--application to the individual patient. Kidney Int. 2012;82(8):840–856. doi: 10.1038/ki.2012.280. [DOI] [PubMed] [Google Scholar]
- 8.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18(6):1899–1904. doi: 10.1681/ASN.2007020166. [DOI] [PubMed] [Google Scholar]
- 9.Eriguchi M, Oka H, Mizobuchi T, Kamimura T, Sugawara K, Harada A. Long-term outcomes of idiopathic membranous nephropathy in Japanese patients treated with low-dose cyclophosphamide and prednisolone. Nephrol Dial Transplant. 2009;24(10):3082–3088. doi: 10.1093/ndt/gfp251. [DOI] [PubMed] [Google Scholar]
- 10.McQuarrie EP, Stirling CM, Geddes CC. Idiopathic membranous nephropathy and nephrotic syndrome: outcome in the era of evidence-based therapy. Nephrol Dial Transplant. 2012;27(1):235–242. doi: 10.1093/ndt/gfr220. [DOI] [PubMed] [Google Scholar]
- 11.Allison AC, Eugui EM. Preferential suppression of lymphocyte proliferation by mycophenolic acid and predicted long-term effects of mycophenolate mofetil in transplantation. Transplant Proc. 1994;26(6):3205–3210. [PubMed] [Google Scholar]
- 12.Allison AC, Kowalski WJ, Muller CD, Eugui EM. Mechanisms of action of mycophenolic acid. Ann N Y Acad Sci. 1993;696(1):63–87. doi: 10.1111/j.1749-6632.1993.tb17143.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343(16):1156–1162. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 14.Mathew TH. A blinded, long-term, randomized multicenter study of mycophenolate mofetil in cadaveric renal transplantation: results at three years. Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1998;65(11):1450–1454. doi: 10.1097/00007890-199806150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation. 1997;63(1):39–47. doi: 10.1097/00007890-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Choi MJ, Eustace JA, Gimenez LF, Atta MG, Scheel PJ, Sothinathan R, et al. Mycophenolate mofetil treatment for primary glomerular diseases. Kidney Int. 2002;61(3):1098–1114. doi: 10.1046/j.1523-1755.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan TM, Lin AW, Tang SC, Qian JQ, Lam MF, Ho YW, et al. Prospective controlled study on mycophenolate mofetil and prednisolone in the treatment of membranous nephropathy with nephrotic syndrome. Nephrology (Carlton) 2007;12(6):576–581. doi: 10.1111/j.1440-1797.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 18.Senthil Nayagam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, Joshi K, et al. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrol Dial Transplant. 2008;23(6):1926–1930. doi: 10.1093/ndt/gfm538. [DOI] [PubMed] [Google Scholar]
- 19.Branten AJ, du Buf-Vereijken PW, Vervloet M, Wetzels JF. Mycophenolate mofetil in idiopathic membranous nephropathy: a clinical trial with comparison to a historic control group treated with cyclophosphamide. Am J Kidney Dis. 2007;50(2):248–256. doi: 10.1053/j.ajkd.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastroenterol. 1995;30(11):1046–1052. doi: 10.3109/00365529509101605. [DOI] [PubMed] [Google Scholar]
- 21.Dimenäs E, Carlsson G, Glise H, Israelsson B, Wiklund I. Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand J Gastroenterol Suppl. 1996;221:8–13. doi: 10.3109/00365529609095544. [DOI] [PubMed] [Google Scholar]
- 22.Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82(2):216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 23.Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, Ayalon R, Beck LH, Jr, Schlumberger W, et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol. 2014;142(1):29–34. doi: 10.1309/AJCP8QMOY5GLRSFP. [DOI] [PubMed] [Google Scholar]
- 24.Dou Y, Zhang L, Liu D, Wang C, Quan S, Ma S, et al. The accuracy of the anti-phospholipase A2 receptor antibody in the diagnosis of idiopathic membranous nephropathy: a comparison of different cutoff values as measured by the ELISA method. Int Urol Nephrol. 2016;48(6):845–849. doi: 10.1007/s11255-016-1263-6. [DOI] [PubMed] [Google Scholar]
- 25.Kosmadakis G, Filiopoulos V, Smirloglou D, Skarlas P, Georgoulias C, Michail S. Comparison of immunosuppressive therapeutic regimens in patients with nephrotic syndrome due to idiopathic membranous nephropathy. Ren Fail. 2010;32(5):566–571. doi: 10.3109/08860221003728754. [DOI] [PubMed] [Google Scholar]
- 26.Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, Clark WF, et al. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int. 1995;47(4):1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 27.Hogan J, Mohan P, Appel GB. Diagnostic tests and treatment options in glomerular disease: 2014 update. Am J Kidney Dis. 2014;63(4):656–666. doi: 10.1053/j.ajkd.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet. 2013;381(9868):744–751. doi: 10.1016/S0140-6736(12)61566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller G, Zimmerman R, 3rd, Radhakrishnan J, Appel G. Use of mycophenolate mofetil in resistant membranous nephropathy. Am J Kidney Dis. 2000;36(2):250–256. doi: 10.1053/ajkd.2000.8968. [DOI] [PubMed] [Google Scholar]
- 30.Ballarin J, Poveda R, Ara J, Pérez L, Calero F, Grinyó JM, et al. Treatment of idiopathic membranous nephropathy with the combination of steroids, tacrolimus and mycophenolate mofetil: results of a pilot study. Nephrol Dial Transplant. 2007;22(11):3196–3201. doi: 10.1093/ndt/gfm366. [DOI] [PubMed] [Google Scholar]
- 31.Dussol B, Morange S, Burtey S, Indreies M, Cassuto E, Mourad G, et al. Mycophenolate mofetil monotherapy in membranous nephropathy: a 1-year randomized controlled trial. Am J Kidney Dis. 2008;52(4):699–705. doi: 10.1053/j.ajkd.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Peng L, Wei SY, Li LT, He YX, Li B. Comparison of different therapies in high-risk patients with idiopathic membranous nephropathy. J Formos Med Assoc. 2016;115(1):11–18. doi: 10.1016/j.jfma.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Dai H, Zhang H, He Y. Diagnostic accuracy of PLA2R autoantibodies and glomerular staining for the differentiation of idiopathic and secondary membranous nephropathy: an updated meta-analysis. Sci Rep. 2015;5(1):8803. doi: 10.1038/srep08803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6(6):1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radice A, Trezzi B, Maggiore U, Pregnolato F, Stellato T, Napodano P, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN) Autoimmun Rev. 2016;15(2):146–154. doi: 10.1016/j.autrev.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25(6):1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dooley MA, Cosio FG, Nachman PH, Falkenhain ME, Hogan SL, Falk RJ, et al. Mycophenolate mofetil therapy in lupus nephritis: clinical observations. J Am Soc Nephrol. 1999;10(4):833–839. doi: 10.1681/ASN.V104833. [DOI] [PubMed] [Google Scholar]
- 38.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The dose of MMF and CsA in each time point