Abstract
Poly-gamma-glutamic acid (γ-PGA) is a natural, edible and non-toxic polymer synthesized by Bacillus subtilis and is suggested as a safe biomaterial for the use in hydrogels and vaccine adjuvants. However, the effect of γ-PGA on inflammasome activation has not yet been studied in macrophages. Inflammasomes, which are intracellular multi-protein complexes, promote acute and chronic inflammation via interleukin-1β or interleukin-18 maturation, and they are known targets for metabolic syndromes and cancer. In this study, we observed that γ-PGA attenuated NLRP3, NLRC4 and AIM2 inflammasome activation, whereas it upregulated pro-inflammatory cytokine expression in human and murine macrophages. Although γ-PGA had conflicting effects on cytokine production and maturation, it clearly alleviated the severity of lipopolysaccharide-induced endotoxin shock in an animal model. Thus, we suggest γ-PGA as a candidate to control inflammasome-mediated disorders.
Keywords: Cheonggukjang, cytokines, inflammasome, macrophages, poly-gamma-glutamate
INTRODUCTION
Poly-gamma-glutamic acid (γ-PGA) is an anionic polypeptide in which the α-amino and γ-carboxyl groups of glutamic acid are polymerized by a γ-amid linkage.1, 2 γ-PGA is a water-soluble, mucilaginous and biodegradable compound that is edible and non-toxic to both humans and the environment.2, 3 γ-PGA has been widely used as a supplement in foods, cosmetics and drug delivery systems, and its peptides exhibit many bioactive functions. γ-PGA is a mucilaginous compound that is naturally synthesized by Bacillus subtilis in fermented soybeans and is present in traditional soy products, including cheonggukjang in Korea and natto in Japan. These foods are generally considered to be healthy, as they are rich in vitamins and other nutrients. The γ-PGA concentration in cheonggukjang is generally in the range of 13–17%, which is slightly higher than that of natto by 0.1–0.8%.4 There have been several reports describing the various physiological properties of γ-PGA, which include fibrinolytic, anti-cancer, anti-hypertensive, hypocholestrolemic, anti-oxidative and anti-inflammatory activities.5
Inflammasomes are multi-molecular complexes that promote inflammation in response to pathogenic, environmental and endogenous danger signals, such as microbes, asbestos and uric acid.6, 7 Inflammasomes, whose principal function is the activation of caspase-1, also mediate interleukin (IL)-1β maturation.8, 9, 10 Following activation, caspase-1 proteolytically cleaves pro-IL-1β or IL-18 into their biologically active forms. Inflammasomes have been found to regulate other important aspects of inflammation and tissue repair, such as pyroptosis, which is a form of cell death.11 Mutation of just one inflammasome-associated gene has been shown to induce auto-inflammatory disease.12 To date, there are several inflammasomes known to contain NOD-like receptors (NLRs), pyrin and HIN domain-containing proteins, including NLRP1, NLRP3, NLRC4 and AIM2.13 Collectively, inflammasomes have received increased attention as new therapeutic targets of tumors and metabolic disorders.14, 15 In this study, we investigated the effects of γ-PGA on inflammasome activation. γ-PGA attenuated IL-1β and IL-18 secretion, which are readouts of inflammasome activation, in the presence of NLRP3, NLRC4 and AIM2 triggers.
MATERIALS AND METHODS
Cell culture
For bone marrow-derived macrophages (BMDMs), bone marrow cells were obtained by flushing C57BL/6 mouse (6–12 weeks old; Narabio Co., Seoul, Republic of Korea) tibia and femur bones. The cells were then cultured in Dulbecco's Modified Eagle’s Medium (WELGENE Inc. Gyeongsan-si, Gyeongsanbuk-do, Republic of Korea) supplemented with 10% fetal bovine serum (FBS; Corning cellgro, Manassas, VA, USA), a penicillin and streptomycin solution (Corning cellgro), and L929 cell-conditioned medium containing granulocyte/macrophage colony-stimulating factor.16 Next, bone marrow cells were cultured in Petri dishes (SPL Life Science Co., Phcheon-si, Gyeonggi-do, Republic of Korea) at 37 °C in a 5% CO2 atmosphere for 7 days. THP-1 cells were obtained from the Korean Cell Line Bank (KCLB No. 40202; Seoul, Republic of Korea) and maintained in RPMI 1640 medium (WELGENE Inc.) containing 10% FBS, and penicillin and streptomycin solution at 37 °C in a 5% CO2 atmosphere. THP-1 cells were differentiated into macrophage-like cells using phorbol 12-myristate acetate (PMA, 100 nm; Cat. #tlrl-pma, InvivoGen, San Diego, CA, USA) for 72 h.
Cell treatments
One million cells plated in 12-well plates (SPL Life Science Co.) were primed with lipopolysaccharide (LPS, 1 μg/ml; Sigma-Aldrich Co., St. Louis, MO, USA) in RPMI 1640 medium containing 10% FBS and antibiotics for 3 h.17 LPS-primed cells were subjected to the following activation steps. For NLRP3 inflammasome activation, medium was replaced with RPMI 1640 medium supplemented with ATP (2 mm; InvivoGen) or nigericin (NG, 40 μm; Tocris Bioscience, Bristol, UK) for 1 h. To activate the AIM2 or NLRC4 inflammasomes, media were replaced with 1 μg/ml of double-stranded DNA (dsDNA) with 2 μl/ml of jetPRIME (Polyplus-transfection, New York, NY, USA) or flagellin (0.5 μg/ml; #tlrl-stfla, InvivoGen) with Lipofectamine 2000 (10 μl/ml, Invitrogen, Grand Island, NY, USA) for 1 h. To determine the inhibitory effects of Cheonggukjang extracts on inflammasome activation, l-glutamate (Daejeung Chemicals & Materials Co., Shiheung-city, Gyeonggi-do, Republic of Korea) and γ-PGA (20 mg/ml, 2850 kDa, <0.5 EU/ml; PHOENYX, Dongbang Co., Seoul, Republic of Korea) were co-treated with the above activators. In addition, BMDMs were treated with a Toll-like receptor 4 inhibitor (TAK-242, CLI-095, tlrl-cli95, InvivoGen) to elucidate whether γ-PGA interacts with Toll-like receptor 4. Cheonggukjang (1 kg) extract was prepared with 1000 ml of ethanol (99.99% (v/v), Daejeung Chemicals & Materials Co.) at room temperature overnight. The ethanol extract was filtered through filter paper (no. 1, Advantec MFS, Inc., Dublin, CA, USA) and evaporated in vacuo at 55–65 °C. The ethanol-free extract was further filtered through filter paper (no. 2, Advantec MFS, Inc.) and adjusted to pH 7.4. The solution was then dried at 105 °C and dissolved in distilled water (100 mg/ml).18 Cellular supernatant (Sup), lysate (Lys) and cross-linked pellets (Pellet) with suberic acid bis (Sigma-Aldrich Co.) were collected for further analyses.
Western blot analysis
Proteins were separated on SDS-polyacrylamide gel electrophoresis (10 or 16%) under denaturating and reducing conditions, followed by transfer onto a polyvinylidene difluoride membrane (Pall Co., Port Washington, NY, USA).19 Proteins of interest were detected with anti-mouse IL-1β (AF-401-NA, R&D Systems, Minneapolis, MN, USA), anti-human IL-1β (AF-201-NA, R&D Systems), anti-caspase-1 p20 (06-503, EMD Millipore Co., Darmstadt, Germany, or #AG-20B-0042, AdipoGen Co., San Diego, CA, USA), anti-ASC (sc-22514, Santa Cruz Biotechnology, Dallas, TX, USA), anti-caspase-1 (sc-622, Santa Cruz Biotechnology), anti-TNFα (sc-1351, Santa Cruz Biotechnology), anti-actin antibodies (sc-1615, Santa Cruz Biotechnology) or horseradish peroxidase-conjugated second anti-sera (sc-2020 or sc-2004, Santa Cruz Biotechnology). The membranes were visualized using Power-Opti ECL solution (BioNote Co., Hwansung-si, Gyeonggi-do, Republic of Korea) and a cooled charge-coupled device camera System (AE-9150 EZ-Capture II, ATTO Technology, Tokyo, Japan).
LPS lethality assay
For LPS-induced endotoxin shock, 40 mice (male, C57BL/6, 10 mice per group) obtained from Narabio Co. were divided into four groups: LPS, LPS+γ-PGA (4 mg), LPS+γ-PGA (10 mg) and γ-PGA (10 mg). Three groups were intraperitoneally (i.p.) injected with LPS (25 mg/kg). After 1 h, four groups were further i.p. injected with vehicle saline or γ-PGA (4 mg per mouse or 10 mg per mouse). The animals were observed every 8 h for 4 days. All mice were housed at 24±1 °C under 50% humidity and a 12- h light/dark cycle. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Kangwon National University (IACUC; approval no. KW-150313-1).
Cytotoxicity assay
BMDMs (10 000 cells per well) were plated in a 96-well plate (SPL Life Science Co.) and allowed to attach overnight. The BMDMs were treated with the indicated agents for 1 h, followed by replacement with fresh media (RPMI 1640 medium containing 10% FBS and antibiotics) and further incubation for 3 h. Triton x-100 (0.01%, Triton) was used to obtain complete cell death (0% survival rate), whereas the non-treated group was set as 100%. Cytotoxicity was measured using a Cell Counting Kit-8 assay (Dojindo Molecular Technologies, Inc., Rockville, MD, USA) according to the manufacturer’s protocol.
Caspase-1 activity assay
For the caspase-1 activity assay, human recombinant caspase-1 (1 unit/rx, #1081, BioVison Inc., Milpitas, CA, USA) was incubated with YVAD-pNA, a substrate of caspase-1, in the presence of γ-PGA or Z-VAD-FMK (#FMK001, R&D Systems). Caspase-1 activity was measured using a caspase-1/ICE colorimetric assay kit (#K111, BioVison Inc.) according to the manufacturer’s protocol.
RNA extraction and real-time qPCR
For RNA extraction, BMDMs (2.0 × 106 cells per well) were plated in six-well plates (SPL Life Science Co.) and primed with LPS (10 ng/ml) in RPMI 1640 medium containing 10% FBS and antibiotics for 1 h. Total RNA was extracted using Trizol (Invitrogen) and reverse-transcribed to first-stand complementary DNA using an M-MLV cDNA synthesis kit (Enzynomics, Daejeon, Republic of Korea). Transcription was quantitated using SYBR Green (TOPreal qPCR 2X PreMIX, Enzynomics) and an Eco Real-Time PCR system (Illumina, San Diego, CA, USA). Quantitation was normalized with β-actin (Actb). The following primers were utilized: IL-1β (Il1b; Genebank ID: NM_008361) 5′-CCC AAG CAA TAC CCA AAG AA-3′ and 5′-GCT TGT GCT CTG CTT GTG AG-3′ IL-1α (Il1a; NM_010554) 5′-CCG ACC TCA TTT TCT TCT GG-3′ and 5′-GTG CAC CCG ACT TTG TTC TT-3′ IL-6 (Il6; NM_031168) 5′-CCG GAG AGG AGA CTT CAC AG-3′ and 5′-TCC ACG ATT TCC CAG AGA AC-3′ Tumor necrosis factor (TNF)-α (Tnfa; NM_013693) 5′-ACG GCA TGG ATC TCA AAG AC-3′ and 5′-GTG GGT GAG GAG CAC GTA GT-3′ NLRP3 (Nlrp3; NM_145827) 5′-CAG GCG AGA CCT CTG GGA AA-3′ and 5′-CCC AGC AAA CCC ATC CAC TC-3′ IL-10 (Il10; NM_010548) 5′-TGC TAT GCT GCC TGC TCT TA-3′ and 5′-TCA TTT CCG ATA AGG CTT GG-3′ IL-1RN (Il1rn, NM_001039701) 5′- GCC TGA TCA CTC TGG CCA TC-3′ and 5′- AGG CCA GCC AAC AGA CTG AG-3′ and β-actin (Actb; NM_007393) 5′-AGC CAT GTA CGT AGC CAT CC-3′ and 5′-CTC TCA GCT GTG GTG GTG AA-3′.
IL-1β and IL-18 ELISA detection
To quantitate secreted IL-1β or IL-18, BMDM cell culture supernatants were measured using a mouse IL-1β/IL-1F2 Quantikine ELISA kit (R&D Systems) or a mouse IL-18 platinum ELISA kit (eBioscience, San Diego, CA, USA). The enzyme-linked immunosorbent assay (ELISA) plates were read using a microplate reader (Bio-Rad, Hercules, CA, USA).
Statistical analyses
Statistical analyses were performed using one-way analysis of variance followed by the Tukey’s test for multiple groups, the Mann–Whitney t-test for two-group comparisons or the Kaplan–Meier method and log-rank test for survival data using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). The P-values are indicated in the figures.
Results
Cheonggukjang inhibits inflammasome activation
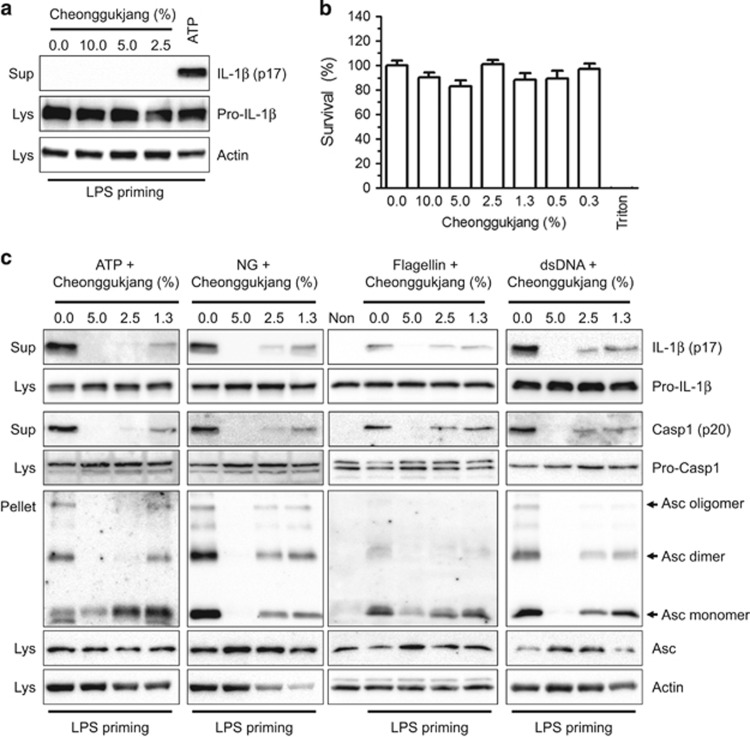
To elucidate the effect of cheonggukjang (fermented soybean paste) on inflammasome activation, we treated LPS-primed BMDMs with cheonggukjang extracts and observed IL-1β secretion as an inflammasome activation readout. As shown in Figure 1a, the cheonggukjang extracts induced inflammasome activation, whereas ATP, as an NLRP3 inflammasome activator, induced IL-1β secretion. The cheonggukjang extract did not exhibit any cytotoxicity (Figure 1b). We sequentially confirmed the inhibitory effects of cheonggukjang on inflammasome activation (Figure 1c). LPS-primed macrophages were treated with inflammasome activators (ATP and NG for NLRP3, flagellin for NLRC4 and dsDNA for AIM2) in the presence of cheonggukjang extract as indicated. We observed IL-1β and caspase-1 (Casp1) secretion along with Asc pyroptosome formation, as indicators of inflammasome activation. As a result, cheonggukjang attenuated IL-1β and Casp1 secretion, as well as Asc pyroptosome formation caused by NLRP3, NLRC4 or AIM2 inflammasome activation. These results imply that cheonggukjang is a potent inflammasome activation inhibitor.
Figure 1.
Effect of cheonggukjang on inflammasome activation. BMDMs were primed with LPS (1 μg/ml) in RPMI medium containing 10% FBS and antibiotics for 3 h. (a) Cells were treated with medium containing the indicated percentages of cheonggukjang extracts or ATP (2 mm) and incubated for 1 h. The immunoblot assays were analyzed as indicated. (b) Cytotoxicity was measured after applying the indicated cheonggukjang dosage to BMDMs for 1 h, which was similar to the inflammasome activation step. Triton (0.01% of Triton x-100) was used to obtain complete cell death (0% survival rate), whereas the non-treated group was set as 100%. The data represent the mean±s.d. of three independent experiments, each performed in triplicate. (c) LPS-primed BMDMs were treated with ATP (2 mm), NG (40 μm), flagellin (0.5 mg/ml) or dsDNA (1 μg/ml) for 1 h in the presence of the indicated cheonggukjang concentration. Cellular supernatant (Sup), lysate (Lys) and cross-linked pellets (Pellet) from whole-cell lysates were analyzed with the indicated anti-sera in an immunoblot assay. All immunoblot data shown are representative of at least two independent experiments. BMDMs, bone marrow-derived macrophages; FBS, fetal bovine serum; LPS, lipopolysaccharide; NG, nigericin.
γ-PGA inhibits inflammasome activation
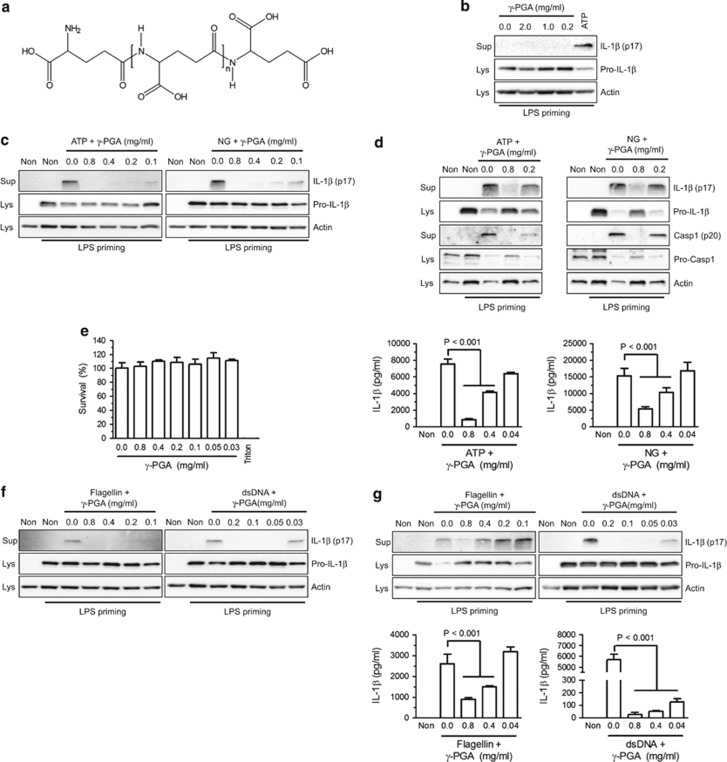
To identify which cheonggukjang extract components regulate inflammasome activation, we focused on γ-PGA (Figure 2a), which has been reported as an immune modulator in cheonggukjang.20 To elucidate the effect of γ-PGA on inflammasome activation, increasing γ-PGA dosages were applied to LPS-primed human macrophages (PMA-stimulated THP-1 cells) without any inflammasome activators (Figure 2b). IL-1β was not secreted from the cells treated with γ-PGA, whereas ATP-induced IL-1β secretion was observed. We further confirmed the inhibitory effect of γ-PGA on inflammasome activation by measuring IL-1β secretion following co-treatment of LPS-primed macrophages with γ-PGA and NLRP3 inflammasome triggers (ATP or NG; Figure 2c). ATP- or NG-mediated IL-1β secretion was attenuated by γ-PGA co-treatment. This result suggests that γ-PGA was the component responsible for the anti-inflammasome properties of cheonggukjang. We further confirmed the inhibitory effect of γ-PGA on NLRP3 inflammasome activation in LPS-primed BMDMs (Figure 2d). γ-PGA attenuated IL-1β maturation, as well as IL-1β and Casp1 secretion in murine macrophages. In addition, γ-PGA showed no cytotoxic effects on BMDMs (Figure 2e). Next, we examined the effects of γ-PGA on other inflammasome activators, such as NLRC4 and AIM2. To this end, we introduced purified flagellin for NLRC4 or dsDNA for AIM2 into LPS-primed THP-1 cells (Figure 2f) or BMDMs (Figure 2g) in the presence of increasing dosages of γ-PGA and then monitored IL-1β secretion. Similar to cheonggukjang, γ-PGA inhibited flagellin- or dsDNA-induced IL-1β secretion. Furthermore, γ-PGA attenuated IL-18 maturation similar to IL-1β (Supplementary Figure 1a) and ASC pyroptosis (Supplementary Figure 1b). Thus, γ-PGA from cheonggukjang can be considered as an NLRP3, NLRC4 and AIM2 inflammasome activation inhibitor.
Figure 2.
Effect of γ-PGA on inflammasome activation. (a) Chemical structures of γ-PGA were drawn using Chemdraw ultra 8.0 (http://www.cambridgesoft.com). (b) BMDMs were primed with LPS (1 μg/ml) and then treated with the indicated percentages of γ-PGA or ATP. IL-1β secretion was analyzed by immunoblotting. (c) PMA-treated THP-1 cells were primed with LPS for 3 h and then treated with ATP or NG with increasing concentrations of γ-PGA. (d) LPS-primed BMDMs were treated with ATP and NG for 1 h in the presence of the indicated γ-PGA concentrations. Secreted IL-1β was quantitated by an ELISA-based assay kit and presented with bar graphs. (e) Cytotoxicity was measured after applying the indicated γ-PGA dosage to BMDMs. (f) PMA-treated THP-1 cells were primed with LPS and then treated with flagellin or dsDNA. (g) LPS-primed BMDMs were treated with flagellin or dsDNA. Secretion of IL-1β was analyzed with immunoblots and ELISAs as indicated. All immunoblot and ELISA data shown are representative of at least two independent experiments. The bar graphs represent the mean±s.d. BMDMs, bone marrow-derived macrophages; dsDNA, double-stranded DNA; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; γ-PGA, poly-gamma-glutamic acid; LPS, lipopolysaccharide; NG, nigericin; PMS, phorbol 12-myristate acetate.
γ-PGA does not alter caspase-1 activity, whereas glutamate does not alter inflammasome activation
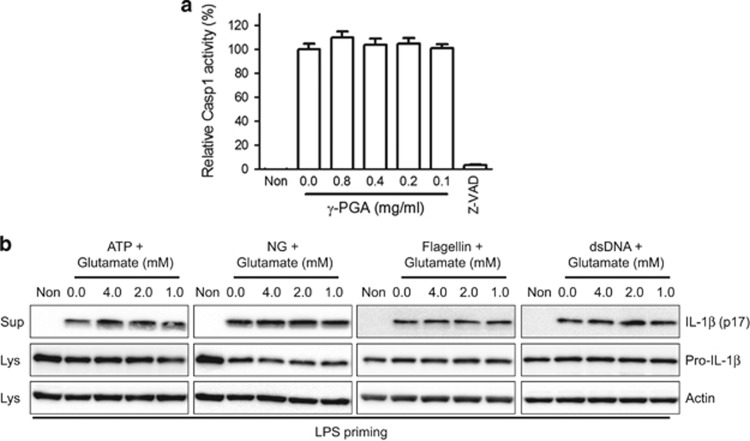
On the basis of the inhibitory effects of γ-PGA on all three inflammasomes, we next determined whether γ-PGA directly modulates Casp1 activity (Figure 3a). γ-PGA did not alter human recombinant Casp1 activity, suggesting that γ-PGA acts upstream of Casp1. We also investigated the effects of glutamic acid on inflammasome activation. For this, LPS-primed BMDMs were treated with l-glutamic acid and inflammasome triggers (ATP, NG and flagellin or dsDNA; Figure 3b). Treatment with glutamate did not alter IL-1β secretion due to inflammasome activation, suggesting that glutamic acid is not an anti-inflammasome agent.
Figure 3.
Effects of γ-PGA on caspase-1 activity and glutamate on inflammasome activation. (a) Human recombinant caspase-1 (Casp1) activity was measured in the presence of γ-PGA or Z-VAD-FMK (Z-VAD, a pan-caspase inhibitor). (b) LPS-primed BMDMs were treated with ATP, NG, flagellin or dsDNA in the presence of the indicated l-glutamic acid dosages. IL-1β secretion was measured by immunoblotting. All immunoblot data shown are representative of at least two independent experiments. BMDMs, bone marrow-derived macrophages; dsDNA, double-stranded DNA; IL, interleukin; γ-PGA, poly-gamma-glutamic acid; LPS, lipopolysaccharide; NG, nigericin.
γ-PGA induces expression of inflammatory cytokines via TLR4 signaling
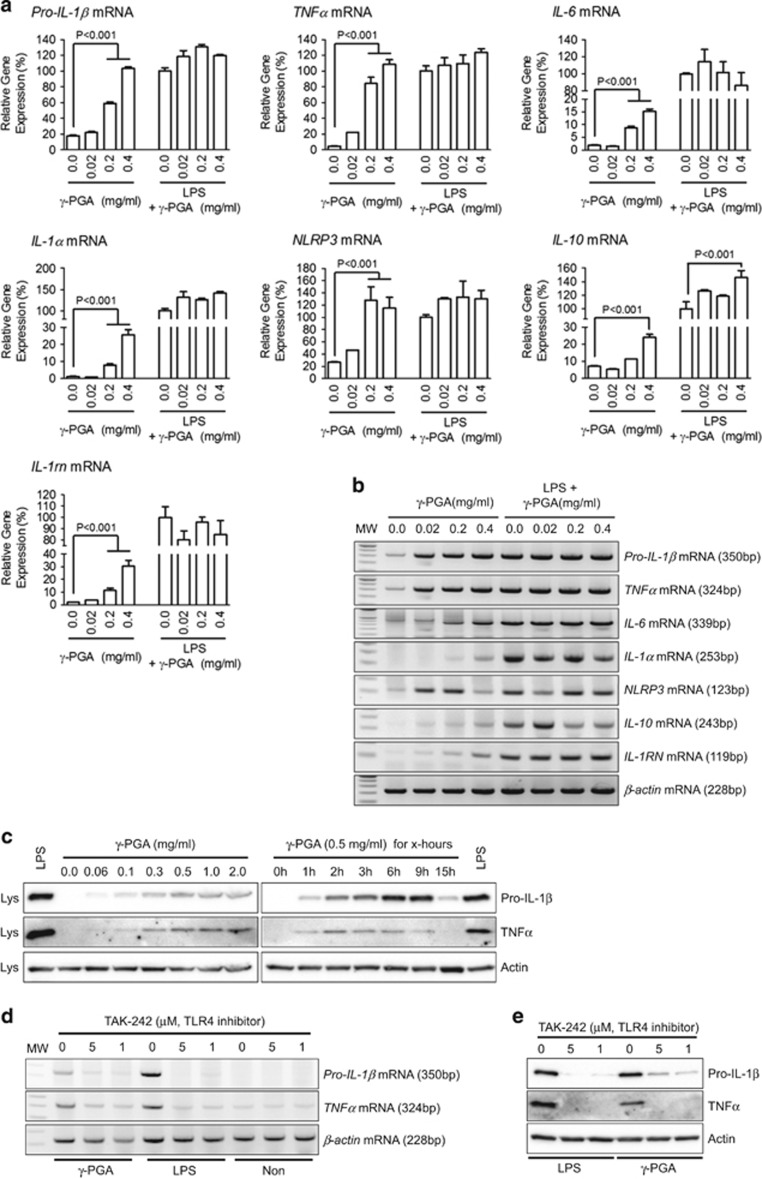
Emerging literature has suggested that γ-PGA derived from Bacillus subtilis directly interacts with Toll-like receptor 4 (TLR4) and induces myeloid differentiation factor 88 (MyD88)-mediated nuclear factor (NF)-κB signaling.21, 22, 23 In this study, we applied γ-PGA to BMDMs with/without LPS, and the pro-IL-1β, TNFα, IL-6, IL-1α, NLRP3, IL-10 and IL-1rn transcript levels were observed (Figures 4a and b). γ-PGA upregulated all cytokines and NLRP3 in a dose-dependent manner, although the synergy between γ-PGA and LPS could not be confirmed as the cytokine expression could have been induced by LPS alone. In addition, γ-PGA induced pro-IL-1β and TNFα translation (Figure 4c). The γ-PGA-induced upregulated cytokines were attenuated by the TLR4 inhibitor TAK-242 (Figures 4d and e). In summary, γ-PGA interacted with TLR4 to mediate production of inflammatory cytokines and NLRP3 while attenuating inflammasome activation.
Figure 4.
Effects of γ-PGA on inflammatory cytokine and NLRP3 expression levels. BMDMs (2 × 106 cells per well for qPCR and 1 × 106 cells per well for immunoblotting) were treated with the indicated γ-PGA concentrations with/without LPS (10 ng/ml). Pro-IL-1β, TNFα, IL-6, IL-1α, NLRP3, IL-10 and IL-1rn transcript levels were analyzed using SYBR green-based quantitative real-time PCR (a) and general PCR analyzed by 2% agarose gel electrophoresis (b). The data represent the mean±s.d. of three independent experiments. (c) Effects of γ-PGA on pro-IL-1β and TNFα translational levels in dose- or time-dependent manners were detected by immunoblotting. The effect of a TLR4 signaling inhibitor (TAK-242) on γ-PGA-mediated cytokine induction was elucidated with BMDMs treated with γ-PGA (0.4 mg/ml) or LPS (10 ng/ml). Pro-IL-1β and TNFα were analyzed using SYBR green-based quantitative real-time PCR (d) and general PCR analyzed by 2% agarose gel electrophoresis (e). All data shown are representative of at least two independent experiments. BMDMs, bone marrow-derived macrophages; IL, interleukin; γ-PGA, poly-gamma-glutamic acid; LPS, lipopolysaccharide; TNFα, tumor necrosis factor-α TLR4, Toll-like receptor 4.
γ-PGA produces conflicting effects on inflammatory reactions
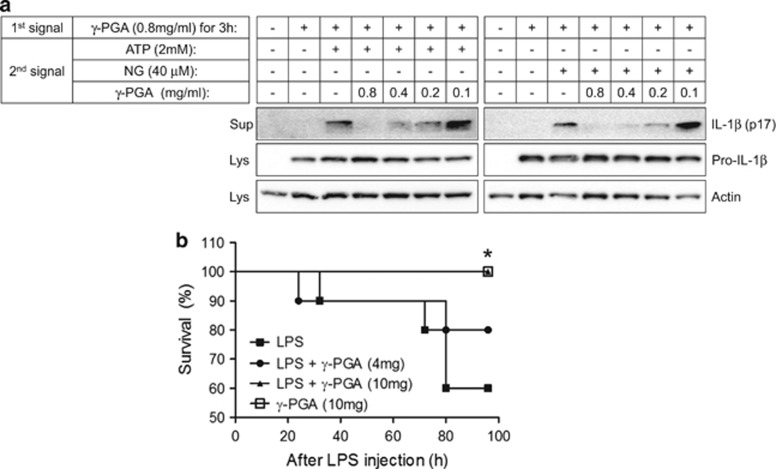
Inflammasome activation requires two steps: priming and activation.9, 11 During the priming step, TLR stimuli induce the production of pro-IL-1β and inflammasome components, such as NLRP3. In the activation step, inflammasome activators facilitate assembly of inflammasome complexes to induce self-cleavage of Casp1. With regards to these two steps, γ-PGA presented conflicting properties on inflammasome activation. Specifically, γ-PGA acted as an inhibitor of activation, whereas it induced pro-IL-1β production during priming. To determine the role of γ-PGA in inflammasome activation, we primed BMDMs with γ-PGA instead of LPS, followed by treatment with γ-PGA and inflammasome triggers (ATP or NG; Figure 5a). γ-PGA priming was sufficient for inflammasome activation, whereas γ-PGA treatment during the activation step blocked IL-1β maturation.
Figure 5.
Conflicting effects of γ-PGA on inflammasome activation. (a) BMDMs were primed with γ-PGA (0.8 mg/ml) for 3 h. γ-PGA-primed BMDMs were further treated with ATP or NG in the presence of γ-PGA as indicated. IL-1β secretion was detected with an immunoblot assay. All immunoblot data shown are representative of at least two independent experiments. (b) Mice (n=10, each group) were intraperitoneally injected with saline or γ-PGA 1 h after LPS (25 mg/kg) administration. The survival data were analyzed using the Kaplan–Meier method and log-rank test. *P<0.05 versus the LPS-treated group. BMDMs, bone marrow-derived macrophages; IL, interleukin; γ-PGA, poly-gamma-glutamic acid; LPS, lipopolysaccharide; NG, nigericin.
During inflammation, production and maturation of pro-inflammatory cytokines occur at the same time. However, our results show that γ-PGA displayed conflicting properties during inflammasome priming and activation. Therefore, we evaluated the role of γ-PGA in an animal model in which the priming and activation steps are indistinguishable. To this end, we adopted a LPS lethality model, as NLRP3 gene-depleted mice present lower susceptibility to LPS-induced endotoxin shock than wild-type mice.24 As shown in Figure 5b, mice treated with LPS alone presented a 60% survival rate at 96 h post injection, whereas 80 or 100% of γ-PGA (4 or 10 mg per mouse)-treated mice before LPS injection were alive at the same time point. Thus, γ-PGA ameliorated the severity of LPS-induced endotoxin shock through anti-inflammasome activity while simultaneously inducing pro-inflammatory cytokine production.
Discussion
In this study, we elucidated the effect of γ-PGA on inflammasome activation. In addition, the effect of cheonggukjang, a γ-PGA-enriched food, was further tested. In our results, γ-PGA and cheonggukjang attenuated NLRP3, NLRC4 and AIM2 inflammasome activation. However, γ-PGA did not alter Casp1 activity, nor did glutamate (monomer of γ-PGA) affect inflammasome activation. Interestingly, γ-PGA has been reported as a TLR4 agonist,25 and it upregulated pro-inflammatory cytokines and NLRP3 in the current study. Specifically, γ-PGA blocked inflammasome activation while simultaneously upregulating pro-IL-1β and NLRP3 production, which are required for inflammasome activation during the priming step. These conflicting effects of γ-PGA on inflammasome activation were further assessed both in vitro and in vivo. In cell culture, γ-PGA served as a TLR4 agonist during priming but as an inhibitor during activation. However, γ-PGA reduced lethality in an LPS-induced inflammation model. In addition, γ-PGA has been shown to ameliorate disease activity indexes in dextran sodium sulfate-induced mouse colitis models of inflammatory bowel disease, and NLRP3-deficient mice present less severe symptoms in a colitis model.25, 26 These results show that γ-PGA attenuates the severity of inflammasome-mediated inflammatory diseases while upregulating production of pro-inflammatory cytokines.
Until now, γ-PGA has been reported to have several immune-modulating properties. Specifically, γ-PGA is known to induce antigen uptake by antigen-presenting cells (APCs; dendritic cells and macrophages), cytokine production (IL-1β, IL-6, IL-12p40 and TNFα) and co-stimulatory molecules (CD40, CD86 and major histocompatibility complex class I and II) upregulation, and T-cell stimulatory capacity enhancement.22 In addition, γ-PGA has been shown to increase IFN-γ and antigen-specific antibody production, suggesting γ-PGA conjugation with antigens can induce cellular and humoral immune responses by acting as an effective vaccine adjuvant.22 γ-PGA also has been shown to have an effect on T-cell differentiation.27, 28 Kim et al. reported that γ-PGA upregulates the master genes (T-bet and ROR-γt) of Th1 and Th17 cells, whereas it decreases GATA-3-inducing Th2 cell levels.27 In addition, γ-PGA was found to induce IL-12p40, CD80 and CD86 expression in APCs, resulting in polarization of native CD4+ T cells into Th1 cells instead of Th2 cells.27 Lee et al. further reported that γ-PGA promotes selective differentiation of native CD4+ T cells into Treg cells and suppresses Th17 cell differentiation.28 It has also been reported that γ-PGA induces anti-tumor immunity.20, 21, 29 Specifically, oral administration of high-molecular-mass γ-PGA was found to be associated with smaller tumor sizes following challenge with tumor cell lines through induction of natural killer (NK) cell-mediated cytotoxicity and IFN-γ secretion.21, 29 γ-PGA was also shown to induce interferon-γ, TNFα and IL-12 production in NK dendritic cells, which are key factors in anti-cancer immunity.20 In the current study, γ-PGA showed anti-inflammasome activity, which increases its potential as a promising target for cancer therapy and metabolic diseases.13, 14 Indeed, treatments targeting IL-1β (IL-1 receptor antagonist or IL-1β monoclonal antibodies), caspase-1 inhibitors or inflammasome inhibitors have been shown to control inflammasome-mediated diseases.15, 30 However, these diseases require continuous treatment because their symptoms manifest slowly, which furthers the physical and economic burden for patients. Thus, a therapeutic agent targeting inflammasome diseases is required to be non-toxic, stable and economically feasible. In the current study, we provide evidence that γ-PGA is an inflammasome activation inhibitor or an alternative source for IL-1β-targeted treatments.
In the current study, we tested the effects of glutamate, a monomer of γ-PGA, on inflammasome activation. However, glutamate showed no inhibitory effect. In a similar study, intact γ-PGA (>2000 kDa) induced TNFα production via TLR4-MyD88 signaling, whereas low-molecular-mass γ-PGA (<50 kDa) digested with γ-PGA-degrading enzymes (γ-glutamyltransferase and glutamyl amidohydrolase) did not produce the same result.27 High-molecular-weight γ-PGA (>2000 kDa) induces strong anti-tumor immune responses mediated by NK cells in a TLR4- and dendritic cell-dependent manner.21, 29 By contrast, low-molecular-weight γ-PGA (<50 kDa) induces Th1 differentiation though an antigen-presenting cell-dependent manner.27 Taken together, the structural property of γ-PGA due to its high molecular mass may be responsible for its ability to induce cytokine production and maturation.
We also observed that γ-PGA induced pro-inflammatory cytokine (IL-1α, pro-IL-1β, IL-6 and TNFα) and anti-inflammatory cytokine (IL-10 and IL-1rn) expression via TLR4 signaling. It has been previously demonstrated that γ-PGA induces TNFα secretion from BMDMs via TLR4 and MyD88 signaling but not TLR2.21 In addition, IFN-γ-inducible protein 10 (IP-10) production, which normally occurs independent of MyD88, in response to γ-PGA treatment was shown to be almost completely abolished in TLR4-defective mice.21 Ligand-dependent activation of TLR4 induces two signaling pathways: MyD88-dependent and -independent signaling. The MyD88-dependent pathway involves signal transduction intermediates, such as IL-1R-associated protein kinases and transforming growth factor-activated kinase, resulting in NF-κB activation and pro-inflammatory cytokine production.31 The MyD88-independent pathway involves signal transduction intermediates, such as Toll-like receptor adapter molecule (TICAM)1 and TICAM2, resulting in transcription factor interferon regulatory factor 3 activation and IFN-β production.32 During inflammasome activation, cell priming through multiple signaling receptors, such as TLRs, results in an induction of NLRP3 expression in response to NF-κB, which is a critical checkpoint for inflammasome activation.33 Thus, γ-PGA mediates NF-κB signaling, which is sufficient for NLRP3 inflammasome activation.
γ-PGA has received attention as a good biological material due to its biodegradability, non-toxicity, compatibility and edibility.5 Thus far, the multi-functionalities of γ-PGA have made it a promising biopolymer for use as a health food, biodegradable thickener, humectant, sustained-release material, chemical food vehicle, cosmetic, medicine and pharmaceutical, cryoprotectant, curable biological adhesive, biodegradable fiber, and biopolymer flocculant.5 On the basis of the current results, we suggest that γ-PGA may induce pro-inflammatory cytokine expression and inhibit inflammasome activation.
Acknowledgments
We thank Young Jin Kim (Korea Food Research Institute) for providing the cheonggukjang extracts. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1A2A2A01004183).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Ashiuchi M, Kamei T, Baek DH, Shin SY, Sung MH, Soda K et al. Isolation of Bacillus subtilis (chungkookjang), a poly-gamma-glutamate producer with high genetic competence. Appl Microbiol Biotechnol 2001; 57: 764–769. [DOI] [PubMed] [Google Scholar]
- Ogunleye A, Bhat A, Irorere VU, Hill D, Williams C, Radecka I. Poly-gamma-glutamic acid: production, properties and applications. Microbiology 2015; 161: 1–17. [DOI] [PubMed] [Google Scholar]
- Shih IL, Van YT. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour Technol 2001; 79: 207–225. [DOI] [PubMed] [Google Scholar]
- Lee EH, Son WC, Lee SE, Kim BH. Anti-obesity effects of poly-gamma-glutamic acid with or without isoflavones on high-fat diet induced obese mice. Biosci Biotechnol Biochem 2013; 77: 1694–1702. [DOI] [PubMed] [Google Scholar]
- Moraes LP, Brito PN, Alegre RM. The Existing Studies on Biosynthesis of Poly(ɣ-glutamic acid) by Fermentation. Food Public Health 2013; 3: 28–36. [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820. [DOI] [PubMed] [Google Scholar]
- Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 2010; 327: 296–300. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140: 821–832. [DOI] [PubMed] [Google Scholar]
- Lee GS. Inflammasomes, multi-cellular protein complex in myeloid cells, induce several metabolic diseases via interleukin-1β maturation. J Biomed Res 2013; 14: 195–200. [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012; 492: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol 2012; 13: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell 2010; 140: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012; 481: 278–286. [DOI] [PubMed] [Google Scholar]
- Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell 2014; 5: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases—did Warburg miss inflammation? Nat Immunol 2012; 13: 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ahn H, Woo HM, Lee E, Lee GS. Characterization of porcine NLRP3 inflammasome activation and its upstream mechanism. Vet Res Commun 2014; 38: 193–200. [DOI] [PubMed] [Google Scholar]
- Ahn H, Kim J, Jeung EB, Lee GS. Dimethyl sulfoxide inhibits NLRP3 inflammasome activation. Immunobiology 2014; 219: 315–322. [DOI] [PubMed] [Google Scholar]
- Ahn H, Kim J, Lee MJ, Kim YJ, Cho YW, Lee GS. Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine 2014; 71: 223–231. [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn H, Han BC, Lee SH, Cho YW, Kim CH et al. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett 2014; 158: 143–150. [DOI] [PubMed] [Google Scholar]
- Lee SW, Park HJ, Park SH, Kim N, Hong S. Immunomodulatory effect of poly-gamma-glutamic acid derived from Bacillus subtilis on natural killer dendritic cells. Biochem Biophys Res Commun 2014; 443: 413–421. [DOI] [PubMed] [Google Scholar]
- Lee TY, Kim YH, Yoon SW, Choi JC, Yang JM, Kim CJ et al. Oral administration of poly-gamma-glutamate induces TLR4- and dendritic cell-dependent antitumor effect. Cancer Immunol Immunother 2009; 58: 1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M et al. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol 2007; 178: 2979–2986. [DOI] [PubMed] [Google Scholar]
- Lee W, Lee SH, Ahn DG, Cho H, Sung MH, Han SH et al. The antiviral activity of poly-gamma-glutamic acid, a polypeptide secreted by Bacillus sp., through induction of CD14-dependent type I interferon responses. Biomaterials 2013; 34: 9700–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006; 440: 228–232. [DOI] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010; 59: 1192–1199. [DOI] [PubMed] [Google Scholar]
- Davaatseren M, Hwang JT, Park JH, Kim MS, Wang S, Sung MJ. Poly-gamma-glutamic acid attenuates angiogenesis and inflammation in experimental colitis. Mediators Inflamm 2013; 2013: 982383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yang JY, Lee K, Oh KH, Gi M, Kim JM et al. Bacillus subtilis-specific poly-gamma-glutamic acid regulates development pathways of naive CD4(+) T cells through antigen-presenting cell-dependent and -independent mechanisms. Int Immunol 2009; 21: 977–990. [DOI] [PubMed] [Google Scholar]
- Lee K, Hwang S, Paik DJ, Kim WK, Kim JM, Youn J. Bacillus-derived poly-gamma-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis. Clin Exp Immunol 2012; 170: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Lee TY, Bae HC, Hahm JH, Kim YH, Park C et al. Oral administration of high molecular mass poly-gamma-glutamate induces NK cell-mediated antitumor immunity. J Immunol 2007; 179: 775–780. [DOI] [PubMed] [Google Scholar]
- Moll M, Kuemmerle-Deschner JB. Inflammasome and cytokine blocking strategies in autoinflammatory disorders. Clin Immunol 2013; 147: 242–275. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 2006; 125: 943–955. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 2003; 4: 1144–1150. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009; 183: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.