Abstract
Heat-shock protein (HSP)-based immunotherapy is established on its adjuvant effects when applied via an extracellular approach to pulse and activate dendritic cells (DCs). Our previous studies indicate that DCs pulsed with recombinant fusion proteins of antigenic fragment and HSP70-like protein 1 (HSP70L1) are potent in stimulating antigen-specific Th1 responses. We herein evaluated the cytotoxic T cell (CTL) response by an intracellular approach of priming DCs with transfection of recombinant adenovirus-expressing the fusion gene of the 576–699 fragment of carcinoembryonic antigen (CEA) and HSP70L1. As compared with DCs pulsed with extracellular fusion protein, the DCs transfected with recombinant adenovirus expressing the fusion gene displayed equivalent mature phenotypes but less inflammatory appearance. However, the transfected DCs were superior to the pulsed DCs in inducing CEA-specific CTLs. Consistently, immunization of HLA-A2.1/H-2Kb transgene mice with the transfected DCs could induce more quantities of HLA-A2.1-restricted CEA-specific CTLs, protecting nude mice more significantly from human CEA-expressing colon tumor challenge when adoptively transferred. Mechanistic investigation indicated that intracellular expression of the fusion protein empowered the transfected DCs by activation of STAT1 possibly via inducing IFN-β and ERK pathways. Therefore, the more potent ability to induce anti-CEA CTL responses enables the DCs, which transfected with recombinant adenovirus expressing the fusion gene of antigenic CEA fragment and Th1 adjuvant, as an alternative promising approach for the immunotherapy of CEA-positive tumors.
Keywords: CTL, dendritic cell, HSP70L1, immunotherapy
INTRODUCTION
Heat-shock proteins (HSPs) are highly conserved throughout the evolution from prokaryotes to eukaryotes, serving as a protective system at the time of environmental or pathologic insults such as hyperthemia, oxidative stress, glucose deprivation, heavy metal and viral intrusion, as well as cancerous transformation.1, 2 In human, HSPs are grouped into five main families, including HSP60, HSP70, HSP90, HSP100 and the small HSPs, according to their approximate molecular weight. Under steady physiological status, HSPs within cells function as 'chaperones' or 'cochaperones' participating in the proper conformation, assembly and transport of nascent peptides and degradation of misfolded proteins.3, 4 Under stress or pathological conditions, some HSP complexes including their chaperoned peptides are released from cells. Some of them such as glucose-regulated protein 96, HSP90 and HSP70 have been identified to possess the activities of adjuvant when outside cells, being helpful for induction of Th1 and cytotoxic T cell (CTL) responses specific for their chaperoned peptides.5, 6, 7 What are the mechanisms for the adjuvant activities of extracellular HSPs and how to modify HSPs to take the antigenic information for the activation of specific T-cell response remain to be further understood.
Induction of chaperoned peptide-specific immune responses by these HSP complexes is dependent on the presence of Antigen-presenting cells (APCs), particularly dendritic cells (DCs).8, 9 Extracellular HSPs induce the secretion of inflammatory cytokines and antigen-presenting functional maturation of APCs via activating the pathways of nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs).10, 11, 12, 13 On the other hand, HSPs promote the uptake and internalization of HSP/chaperoned peptide complexes by APCs via interaction with CD91, lectin-like oxidized low-density lipoprotein receptor-1 or other unknown receptors,14, 15, 16, 17 and enhance the cross-presentation of chaperoned peptides by APCs, subsequently eliciting a robust antigen (Ag)-specific T-cell response.18, 19, 20 Therefore, most HSP-based immunotherapeutic strategies are established via an extracellular priming approach. Numerous experimental data and several clinical trials have demonstrated that HSP complexes or fusion proteins containing tumor or viral antigens and tumor cell-derived or tumor-DC fusion cell-derived HSP complexes or HSP-containing exosomes display promising efficacy in immunotherapy of tumors and infectious diseases.21, 22, 23, 24, 25, 26, 27 However, the adjuvant activities of extracellular HSPs once transfected into APCs such as DCs remain to be discovered.
HSP70-like protein 1 (HSP70L1, also HSPA14) belongs to the HSP70 family, which contains at least 11 members with distinct intracellular locations and functions. HSP70L1 within cells is one component of mammalian ribosome-associated complex (mRAC) binding to the ribosome.28 Intracellular mRAC functions as a J-domain partner of HSP70 by M-phase phosphoprotein 11 (MPP11), another component of mRAC, to enhance the interaction of HSP70 with nascent peptide chains.29 The exact role of HSP70L1 within cell remains unclear. In Saccharomyces cerevisiae, RAC consists of Ssz1 (ortholog of HSP70L1), Z-DNA-binding protein 1 (ortholog of MPP11) serving as the J-domain protein and ribosome-associated Ssb1/2 (ortholog of classic HSP70s). Ssz1 may function as a scaffold in organizing Z-DNA-binding protein 1 and Ssbs into ribosomal protein-folding complexes.30 Similar to HSP70, extracellular HSP70L1 displays the characteristics of adjuvant by its induction of inflammatory responses and the maturation of DCs.31, 32, 33
Our previous studies have demonstrated that fusion proteins of the 576–669 fragment of carcinoembryonic antigen (CEA576–669) or the 341–456 fragment of human epidermal growth factor receptor-2 that fused to the N terminus of HSP70L1 efficiently induce CEA-specific or human epidermal growth factor receptor-2-specific CTLs by priming DCs via an extracellular approach.34, 35 We herein transfected human monocyte-derived DCs (MoDCs) with a recombinant adenovirus (Ad) expressing the fusion gene of CEA576–669 and HSP70L1 (AdCEA576–669HSP70L1-DCs), and interestingly found that priming of DCs via this intracellular approach could more efficiently elicit CTL responses for CEA-positive tumor. Our study outlines a new approach to induce antigen-specific antitumor immune response by more efficiently empowering DC vaccination via HSP70L1-mediated intracellular priming.
MATERIALS AND METHODS
Animals, cell lines and reagents
HLA-A2.1/H-2Kb transgene mice were obtained from Jax Lab (Bar Harbor, ME, USA), and nude mice (C57BL/6 J background) were from Joint Venture Sipper BK Experimental Animal (Shanghai, China). They were bred and housed in appropriate animal care facilities. All experimental manipulations were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University (Shanghai, China).
HEK293 cells, LS-174T, LoVo, SW480 and SW620 cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640 complete medium supplemented with 10% FCS (both from Gibco, Grand Island, NY, USA).
Fluorescence-conjugated antibodies (Abs) were from BD Bioscience (Mountain View, CA, USA) or Biolegend (San Diego, CA, USA), mouse anti-CEA576–669HSP70L1 monoclonal antibody (mAb; clone 4E8) and enzyme-linked immunosorbent assay (ELISA) kit for CEA576–669HSP70L1 from AbMax (Beijing, China), and purified Abs for intracellular signal molecules from Abcam (Cambridge, UK). Human or mouse recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4, IL-2 and IL-7 were from R&D (Minneapolis, MN, USA), immunomagnetic microbeads from Miltenyi Biotec (Bergisch Gladbach, Germany), human IL-6, TNFα and IL-12/IL-23p40 CBA beads and interferon (IFN)-γ ELISPOT kits from BD Bioscience, human IFN-β ELISA kit from CUSABIO (Wuhan, China) and carboxyfluorescein diacetate succinimidyl ester (CFSE) from Molecular Probe (Eugene, OR, USA). Nonapeptides of CEA peptide-1 (CAP1), Her2435–443, EBV416–424, CEA636–644, CEA652–660 and Her2263–271 were synthesized and purified by Apeptide (Shanghai, China), and the pentamers of HLA-A2/CAP1 and HLA-A24/CEA652–660 were synthesized and purified by Proimmune (Oxford, UK) and HLA-A11/CEA636–644 pentamer by Immundex (Copenhagen, Denmark). CEA576–669HSP70L1 fusion protein was prepared as previously described.35 SiRNAs were synthesized by GenePharma (Shanghai, China) and primers by Sangon (Shanghai, China). Sequences of short interfering RNA (siRNA) and primers are listed in Supplementary Table1. Recombinant replication-defective Ad5 without exogenous inserted gene (AdCtrl) was constructed by Hanbio (Shanghai, China).
Preparation of human and mouse DCs
Human MoDCs from peripheral blood of healthy donors, obtained with informed consent and the approval by our institutional review board, and mouse bone marrow-derived DCs were induced in the presence of GM-CSF (50 ng/ml for human, 10ng/ml for mouse) plus IL-4 (10 ng/ml for human, 1 ng/ml for mouse) for 7 or 9 days. Immature human DCs (on d5) or mouse DCs (on d9) were stimulated with CEA576–669HSP70L1 (1 μg/ml) or were transfected with Ad vectors at indicated multiplicity of infection (MOI) after treatment with or without siRNAs at 20 nM or inhibitor of ERK signal pathway (UO126, 10 μM). After another 2 days of culture, DCs were collected for the next studies.
Construction and purification of recombinant Ad vectors
Ad expressing the fusion gene of CEA576–669HSP70L1 with or without a sequence of signal peptide from human Chemokine CCL17 (Supplementary Table1) at its 5’ end, which amplified from the recombinant pQE30 expression vector,35 was constructed using the pAdEasy1 System (Strategen, La Jolla, CA, USA), and all Ad vectors were packaged in HEK293 cells and purified using the Adeasy virus purification kits (Agilent Technologies, Santa Clara, CA, USA).
MLR
DCs were incubated with allogeneic CD3+ T cells purified by anti-CD3 microbeads at a ratio of 1:20 for 5 days. The proliferation of CD3+CD4+ and CD3+CD8+ T cells by CFSE dilution, the production of IFN-γ and IL-17 from Th cells gated as CD3+CD4+ cells and of granzyme B and perforin from CTLs gated as CD3+CD8+ cells were detected using FACS.
CTL generation
Purified autogeneic CD3+ T cells and DCs from healthy HLA-A2+, HLA-A24+ or HLA-A11+ donors were cocultured at an initial ratio of 20:1, and every other 7 days frozen autologous DCs were revived, and similar initial quantities of DCs were added into the DC/T coculture system. Beginning on day 10, 300 U/ml IL-2 and 30 ng/ml IL-7 were added every 2–3 days. After three cycles of culture, cells were collected for FACS analysis or further isolation of CD8+ T cells in CTL or ELISPOT assays.
CTL assay
CFSE-labeled tumor cells were cocultured with CD8+ T cells at a ratio of 1:30 overnight, and then the death of CFSE+ cells was evaluated using propidium iodide staining and FACS analysis.
IFN-γ-ELISPOT assay
CD8+ T cells (1 × 105/well) from the autologous DC/T coculture system were seeded onto 96-well polyvinylidene difluoride-backed microplates coated with anti-human IFN-γ mAb in the presence of autogeneic DCs at a ratio of 10:1 and indicated nonapeptide (5 μg/ml) for 24 h, and then cells were removed, and the plates processed following the manufacturer's protocol of the ELISPOT kit. Resulting spots were counted using ImmunoSpot Analyzer (Cellular Technology Ltd., Cleveland, OH, USA). All groups were performed in triplicate.
In vivo assay
HLA-A2.1/H-2Kb transgene mice (6–8 weeks) were immunized intravenously with 106 DCs/mouse (eight mice/group) and boosted every other week for a total of three times. After 7 days of the last immunization, lymphocytes from the spleen and axillary and inguinal lymph nodes were adoptively transferred into SW480 or LS-174 T-loaded nude mice (5 × 106 cells/mouse) or for the HLA-A2.1/CAP1 pentamer analysis. Tumor-loaded models were established by subcutaneous injection of tumor cells (1 × 106/mouse) into the nude mice (6–8 weeks, 6 or 8 mice/group). After 1 week, mice were injected with splenic and lymph node cells from immunized mice for three times at a 1-week interval, and the tumor growth and the survival of tumor-loaded mice were monitored.
Statistical analysis
Statistical analysis was performed using Prism (GraphPad software, La Jolla, CA, USA), and Students’ t-test, analysis of variance and log-rank (Mantel–Cox) test and FACS analysis were carried out using software including DIVA, SUITE (BD Bioscience) and FlowJo software (Ashland, OR, USA); significance was established at P<0.05.
RESULTS
The expression and intracellular distribution of CEA576–669HSP70L1 fusion protein in AdCEA576–669HSP70L1-transfected DCs
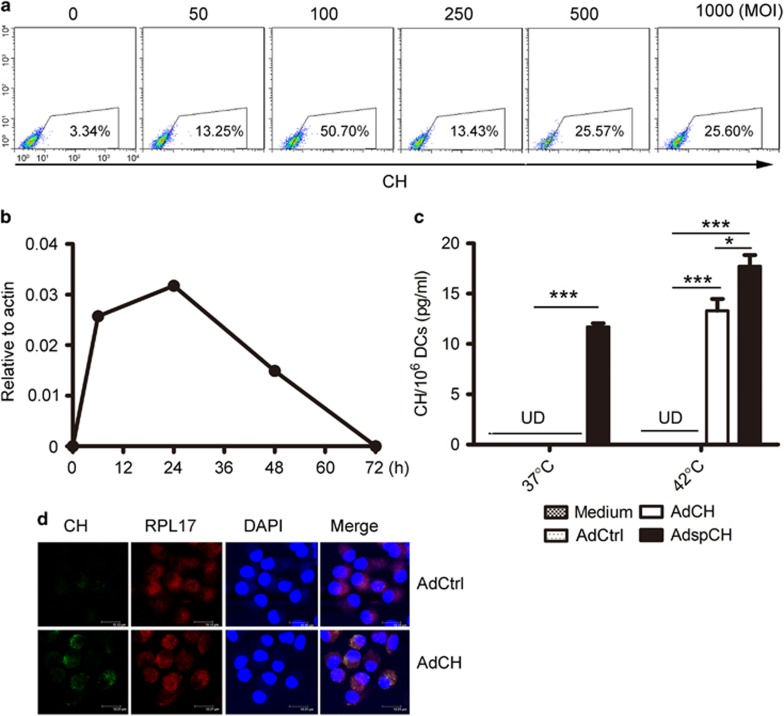
To detect the effect of intracellular CEA576–669HSP70L1 on the maturation and T-cell stimulatory activity of DCs, we transfected human monocyte-derived immature DCs with AdCEA576–669HSP70L1 (AdCEA576–669HSP70L1-DCs) at indicated MOI for 48 h, and found that AdCEA576–669HSP70L1-DCs expressed the highest level of CEA576–669HSP70L1 once transfected with the recombinant Ad at a MOI of 100 (Figure 1a). Therefore, we selected MOI 100 throughout the subsequent studies. The expression of CEA576–669HSP70L1 in AdCEA576–669HSP70L1-DCs could be obviously detectable at 6 h, up to peak at 24 h, and significantly downregulated to an undetectable level at 72 h after transfection (Figure 1b). Therefore, we selected Ad-transfected DCs after 48 h of transfection for the subsequent functional experiments. The amount of CEA576–669HSP70L1 in the culture supernatant of AdCEA576–669HSP70L1-DCs was undetectable under a steady status, excluding the possibility that the secreted, extracellular CEA576–669HSP70L1 could exert the DCs via an autocrine/paracrine way. While under hyperthermia condition CEA576–669HSP70L1 could be markedly released from AdCEA576–669HSP70L1-DCs into extracellular environments, despite its amount being lower than the level of secreted CEA576–669HSP70L1 from DCs transfected with Ad expressing a sequence of signal peptide at the 5’ end of the CEA576–669HSP70L1 fusion gene (Figure 1c). Intracellular CEA576–669HSP70L1 expressed in the transfected DCs mainly distributed in the cytoplasm but little in the nucleus, and could colocalize with the 60S ribosomal protein L17 (RPL17) subunit of the ribosome (Figure 1d). Taken together, intracellular CEA576–669HSP70L1 by Ad-mediated transfection may exert its function in the cytoplasm of the transfected DCs under steady status.
Figure 1.
The intracellular expression and localization of CEA576–669HSP70L1 in DCs transfected with AdCEA576–669HSP70L1. (a, b) Immature DCs were transfected with AdCEA576–669HSP70L1 at indicated MOI for 48 h (a) or at a MOI of 100 for indicated time (b), and then the expression of intracellular CEA576–669HSP70L1 was measured, respectively, using FACS (a) or real-time PCR (b). Representative results from three independent experiments were shown. (c) Immature DCs were transfected with or without control Ad (AdCtrl), AdCEA576–669HSP70L1 (AdCH) or Ad expressing gene of secreted CEA576–669HSP70L1 (AdspCH) at a MOI of 100 for 24 h, followed by treated with or without hyperthermia of 40°C for 2 h and continuously cultured for another 24 h, and then the amount of CEA576–669HSP70L1 in the culture supernatants was measured with ELISA. Values are mean±s.d. of three determinants, and one representative result of three experiments was shown. ***P<0.0001; *P<0.05 (one-way ANOVA). (d) Immature DCs were transfected with AdCtrl or AdCH for 48 h, and then sequentially stained with mouse-derived anti-CEA576–669HSP70L1 followed by anti-mouse FITC-Ab, rabbit-derived anti-RPL17, anti-rabbit TsRed-Ab and DAPI, and then visualized with confocal microscopy (TCS-SP2, Leica, Wetzlar, Germany). Representative results were presented from three experiments. Indicated bars are 15 μm (AdCtrl) and 12 μm (AdCEA576–669HSP70L1) representatively. ANOVA, analysis of variance; CEA, carcinoembryonic antigen; DAPI, 4,6-diamidino-2-phenylindole; DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; MOI, multiplicity of infection; PCR, polymerase chain reaction; UD, undetectable.
AdCEA576–669HSP70L1-DCs display the characteristics of mature DCs
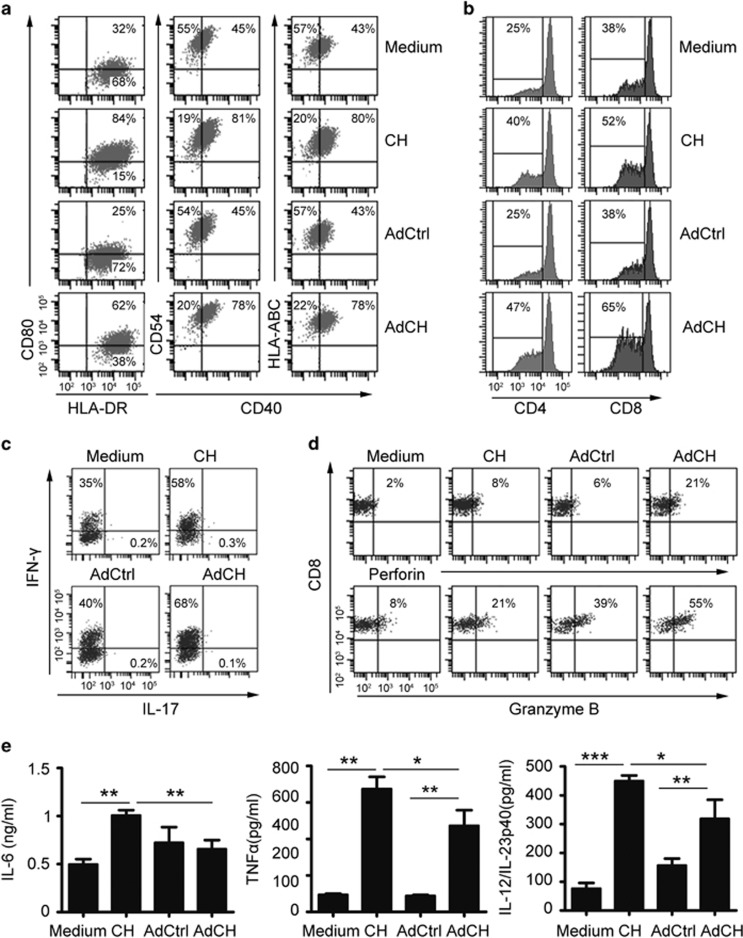
Next, we evaluated the phenotypes and allogeneic T-stimulatory activities of AdCEA576–669HSP70L1-DCs. AdCEA576–669HSP70L1-DCs expressed higher levels of CD40, CD80, HLA-ABC, HLA-DR, CD83 and CD54 (Figure 2a and Supplementary Figure S1) and could induce allogeneic T-cell responses more efficiently, including the proliferation of CD4+ and CD8+ T cells (Figure 2b) and the differentiation of IFN-γ-producing Th1 (Figure 2c) and of perforin/granzyme B-producing CD8+ CTLs (Figure 2d) than control Ad-transfected DCs (AdCtrl-DCs). AdCEA576–669HSP70L1-DCs displayed a similar mature phenotype and activities in inducing the proliferation of T cells and Th1 differentiation as extracellular CEA576–669HSP70L1 fusion protein-pulsed DCs (CEA576–669HSP70L1-DCs). However, AdCEA576–669HSP70L1-DCs displayed a more efficient ability in inducing the generation of CD8+ CTLs but lesser inflammatory appearance because of the less secretion of inflammatory cytokine IL-6, TNFα and IL-12/IL-23p40 as compared with CEA576–669HSP70L1-DCs (Figure 2e). Therefore, AdCEA576–669HSP70L1-DCs are fully matured but less inflammatory, with potent APC function, as compared with CEA576–669HSP70L1-DCs.
Figure 2.
The phenotype, allogenetic T-stimulatory activities and inflammatory cytokine secretion by AdCEA576–669HSP70L1 DCs. (a–e) Immature DCs were pulsed with CEA576–669HSP70L1 (CH) or transfected with indicated Ad for 48 h, and then the phenotypes (a), allogeneic T-cell-stimulatory activities including the proliferation by CFSE dilution of CD3+CD4+ and CD3+CD8+ T cells (b) and the differentiation of IFN-γ or IL-17-producing CD3+CD4+Th cells (c) and granzyme B/perforin-producing CD3+CD8+ CTL (d) and the secretion of IL-6, TNF-α and IL-12/IL-23p40 (e) were detected using FACS. Representative results of three independent experiments were shown. Values are % in FACS graphs (a–d) or mean±s.e.m of three independent experiments (e). *P<0.05; **P<0.01; ***P<0.001 (one-way ANOVA). Ad, adenovirus; ANOVA, analysis of variance; CEA, carcinoembryonic antigen; FACS, fluorescence-activated cell sorting; IFN, interferon.
AdCEA576–669HSP70L1-DCs induce more potent CEA-specific CD8+ CTL responses
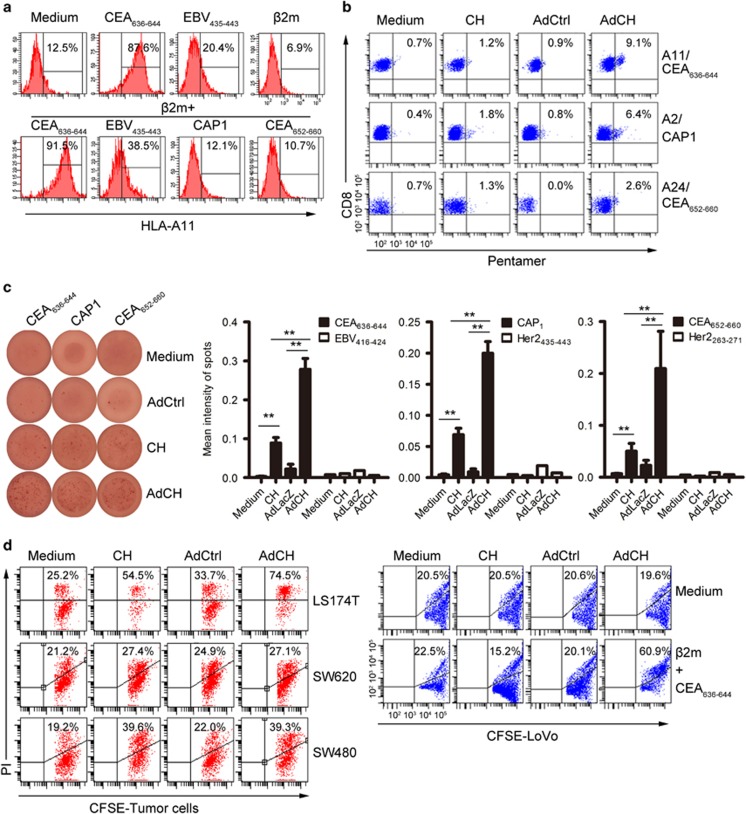
As AdCEA576–669HSP70L1-DCs display the mature phenotypes of DCs, we evaluated their ability to induce CEA-specific CD8+ CTLs. The CEA576–669 fragment contains two known major histocompatibility complex (MHC)-I-restricted epitopes, respectively, HLA-A2.1-restricted CAP1 and HLA-A24.2-restricted CEA652–660, and one predicted HLA-A11.1-restricted CEA636–644 using http://www.cbs.dtu.dk/services/NetMHC/. The peptide of CEA636–644 whether together with or without β2 microglobulin (β2 m) significantly upregulated the expression of HLA-A11 on LoVo cells, a HLA-A11.1+donor-derived colon tumor cell line but expressing undetectable level of HLA-A11 in the absence of HLA-A11-restricted peptides, and a known HLA-A11.1-restricted EBV416–424 also upregulated the expression of HLA-I on LoVo cells but at a less extend, whereas peptides of HLA-A2.1-restricted CAP1 and HLA-A24.2-restricted CEA652–660 had no such effect, indicating that the CEA636–644 peptide was a potential HLA-A11.1-restricted epitope (Figure 3a). We stained CD3+T cells, which had been restimulated by autogeneic DCs with indicated treatments, with pentamers of HLA-A2/CAP1, HLA-A11/CEA636–644 or HLA-A24/CEA652–660 and found that T cell stimulated by autogeneic AdCEA576–669HSP70L1-DCs contained more frequencies of the CD8+T cells, which could bind these pentamers of HLA-A2/CAP1, HLA-A24/CEA652–660 or HLA-A11/CEA636–644 than those by DCs, AdCtrl-DCs or CEA576–669HSP70L1-DCs (Figure 3b). Next, ELISPOT assays demonstrated that autogenous CD8+ T cells stimulated by AdCEA576–669HSP70L1-DCs produced more amounts of IFN-γ in response to CAP1, CEA652–660 or CEA636–644 peptides as compared with those by DCs, CEA576–669HSP70L1-DCs or AdCtrl-DCs (Figure 3c).
Figure 3.
AdCEA576–669HSP70L1-DCs induce CEA-specific CD8+ CTLs. (a) The expression of HLA-A11 on LoVo cells with and without β2 m or/plus indicated that nanopeptide (1 μg/ml) was detected using FACS. (b, c) Immature DCs, respectively, from HLA-A11+, HLA-A2.1+ or HLA-A24+ healthy donors were pulsed with CEA576–669HSP70L1 (CH) or were transfected with indicated Ad for 48 h, then restimulated with autogenous CD3+T cells for a total of three cycles and then the frequencies of CD8+T cells specifically recognizing epitopes of HLA-A11-restricted CEA636–644, HLA-A2-restricted CAP1 or HLA-A24-restricted CEA652–660 were detected, respectively, using Pentamer staining and FACS analysis (b) or IFN-γ/ELISPOT (c), and the cytotoxicity by autogenous CD8+T cells to LS-174 T, SW480, SW620 or LoVo tumor cells labeled by CFSE was evaluated using cytotoxic assays and FACS analysis (d). Representative blots of IFN-γ/ELISPOT (c, left); HLA-24.2-restricted Her2263–271, HLA-A2.1-restricted Her2435–443 and HLA-A11.1-restricted EBV416–424 were used as negative control in c (right three panels). All results representative of the three independent experiments were shown. Values are % in FACS graphs (a, b, d) mean ±s.d. of three determinants (c, below). One-way ANOVA (c). **P<0.01. ANOVA, analysis of variance; CAP1, CEA peptide-1; CEA, carcinoembryonic antigen; FACS, fluorescence-activated cell sorting; IFN, interferon.
Finally, we evaluated the cytotoxicity of autogenous CD8+T cells induced by AdCEA576–669HSP70L1-DCs to colon tumor cell lines with distinct levels of CEA expression according to the information by ATCC and expression of HLA-A2, HLA-A11 and HLA-A24 (Supplementary Figure S2), including HLA-A2loA11-A24loCEAhiLS-174 T, HLA-A2-A11loA24loCEAhiLoVo, HLA-A2+A11-A24-CEAloSW480 and HLA-A2-A11-A24-CEA-SW620 cell lines. We found that autogenous CD8+ T cells induced by AdCEA576–669HSP70L1-DCs killed LS-174 T cells more efficiently than those induced by DCs, CEA576–669HSP70L1-DCs or AdCtrl-DCs. The cytotoxicities of autogenous CD8+T cells induced by AdCEA576–669HSP70L1-DCs and CEA576–669HSP70L1-DCs to SW480 cells were similar, but more efficient than those induced by DCs or AdCtrl-DCs, whereas the cyotoxicities of autogenous CTLs induced by AdCEA576–669HSP70L1-DCs, AdCtrl-DCs, DCs or CEA576–669HSP70L1-DCs to LoVo and SW620 cells were similar but poor (Figure 3d), possibly because of the weak expression of MHC-I, such as LoVo cells, or the poor expression of CEA, such as SW620. As the expression of HLA-A11 on LoVo cells could be significantly upregulated by the presence of CEA636–644, we examined the cytotoxicity of autogenous CD8+T cells induced by DCs to LoVo cells in the presence of CEA636–644 and β2 m. As expected, the cytotoxicity of AdCEA576–669HSP70L1-DC-stimulated autogenous CD8+T cells to LoVo cells was significantly enhanced and more powerful than those induced by AdCtrl-DC or CEA576–669HSP70L1-DC-stimulated auto-CD8+ T cells (Figure 3e). Collectively, AdCEA576–669HSP70L1-DCs could more efficiently induce CEA-specific MHC-I-restricted CTL responses.
AdCEA576–669HSP70L1-DCs are potential therapeutic vaccines for CEA-positive tumors
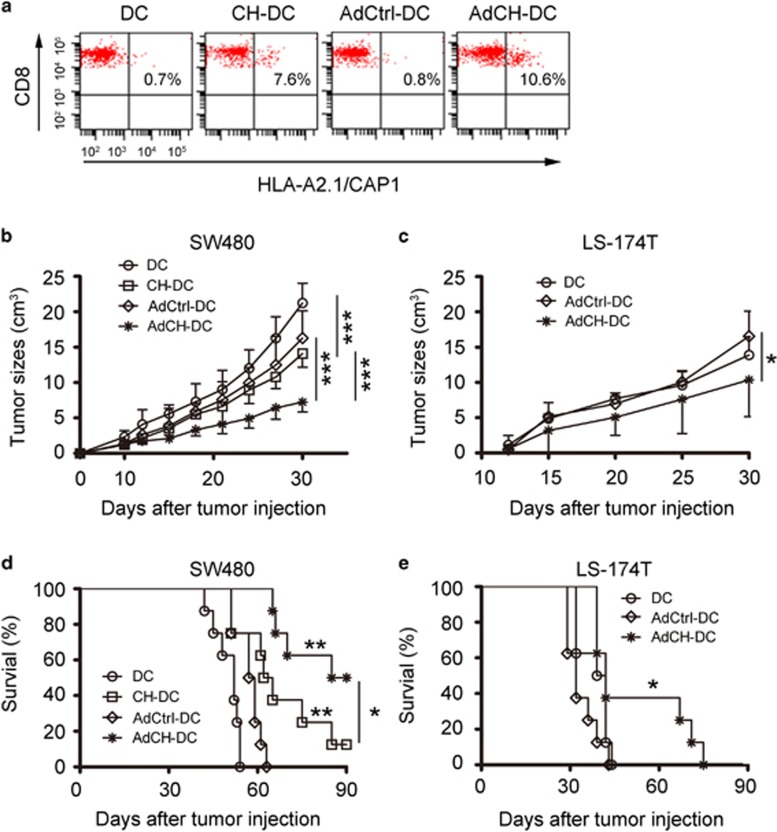
To testify whether AdCEA576–669HSP70L1-DCs can be as a potent therapeutic vaccine to treat CEA-positive tumors in vivo, we intraventrally immunized the HLA-A2.1/H-2Kb mice with AdCEA576–669HSP70L1-DCs derived from the bone marrow of the same strain of mice every other week for three times. We found that immunization with AdCEA576–669HSP70L1-DCs could more efficiently induce the generation of CD3+CD8+ splenocytes, recognizing HLA-A2.1-CAP1 pentamer compared with DCs with other treatments (Figure 4a).
Figure 4.
Immunization with AdCEA576–669HSP70L1-DCs protects mice from CEA-positive tumor challenge in vivo. (a–e) Lymphatic and splenic cells from HLA-A2.1/H-2 kb mice immunization with DCs, CEA576–669HSP70L1-DCs (CH-DC), AdCtrl-DCs (AdCtrl-DC) or AdCEA576–669HSP70L1-DCs (AdCH-DC) (106/mice) from the same strain of mice were evaluated for the frequencies of CEA-specific CTLs by HLA-A2/CAP1 pentamer staining and FACS analysis (a), or were adaptively transferred into SW480 or LS-174 T-loaded nude mice (5 × 106/mice) every other week for three times after 1 week of injection of tumor cells, and then the tumor growth (b, c, all n=6 mice/group) and survival (d, e, all n=8 mice/group) of mice were monitored at indicated time. Representative results of three (a) or two (b–e) independent experiments are shown. ***P<0.001; **P<0.01 compared with DC or AdCtrl-DC; *P<0.05. Two-way ANOVA (b, c) or log-rank (Mantel–Cox) test (d, e). ANOVA, analysis of variance; CAP1, CEA peptide-1; CEA, carcinoembryonic antigen; FACS, fluorescence-activated cell sorting.
Next, we detected the therapeutic effects of adoptively transferred lymphocytes derived from the immunized mice on the HLA-A2+CEA+ tumor cell-loaded nude mice. We established two CEA-positive tumor models, SW480 or LS-174 T tumor-bearing nude mice, because the two tumor cells in vitro could be killed by CEA-specific CTLs more efficiently than other tumor cells. However, the two tumors have some intrinsic defects, as SW480 cells express HLA-A2 but small amount of CEA, and LS-174 T cells express CEA abundantly but an undetectable level of HLA-A2. We transferred splenic and lymph node cells from the immunized mice into SW480 or LS-174 T-bearing nude mice, and observed that the tumor growth was inhibited (Figures 4b and c) and the survival was improved (Figures 4d and e), with more pronounced effects in the group of AdCEA576–669HSP70L1-DC immunization as compared with that in the group of AdCtrl-DC immunization. Thus, in vivo administration of AdCEA576–669HSP70L1-DCs could efficiently induce CEA-specific CTL to kill CEA-positive tumors.
The pathways of STAT1 and ERK are involved in the maturation of AdCEA576–669HSP70L1-DCs
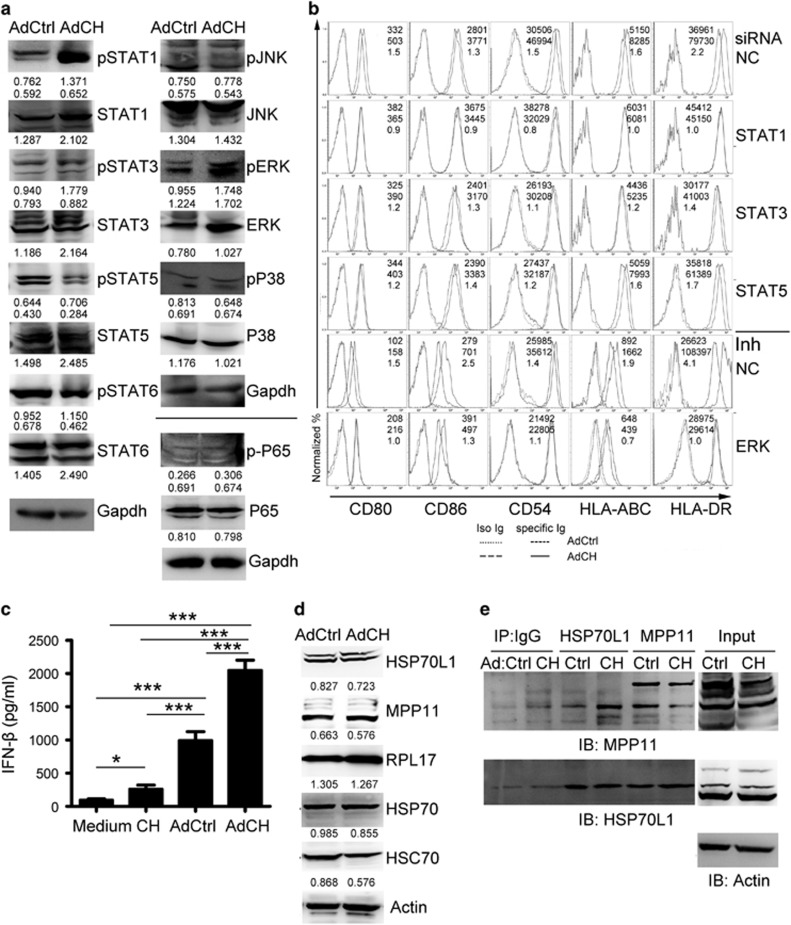
It has been demonstrated that DCs pulsed by extracellular CEA576–669HSP70L1 fusion protein are induced into maturation dependent on the activation of the pathways of MAPKs and NF-κB by TLR4;32, 33 therefore, we investigated which signal pathways were involved in the full maturation of AdCEA576–669HSP70L1-DCs. Interestingly, the activation of the STAT1, STAT3 and ERK pathways was significantly enhanced in AdCEA576–669HSP70L1-DCs as compared with AdCtrl-DCs, and the activation of the STAT5, STAT6, NF-κB, P38 and JNK pathways was similar in the two DCs (Figure 5a), suggesting that the pathways in DCs activated by intracellular CEA576–669HSP70L1 were different from those by extracellular CEA576–669HSP70L1. To investigate the roles of these pathways in the full maturation of AdCEA576–669HSP70L1-DCs, we blocked these pathways using siRNA or inhibitors, and found that blockade of the STAT1 or ERK pathway significantly inhibited the maturation of AdCEA576–669HSP70L1-DCs; however, blockade of STAT3 and STAT5 had less inhibitory effect (Figure 5b). Therefore, the intracellular CEA576–669HSP70L1 may be responsible for the Ag-presenting functional maturation of DCs via the activation of the STAT1 or ERK pathway. Furthermore, to investigate which mechanisms are involved in the activation of STAT1, we detected the expression of type I IFNs and found that the expression of IFN-α was undetectable in all indicated treated DCs (data not shown), but the secretion of IFN-β was strongly induced in AdCEA576–669HSP70L1-DCs (Figure 5c), suggesting that the activation of STAT1 might be a secondary response to the production of IFN-β.
Figure 5.
The pathways of STAT1 and ERK involved in the maturation of AdCEA576–669HSP70L1-DCs. (a, d, e). Immature DCs were transfected with indicated Ad for 24 h, and then the expression or/and phosphorylation of STAT1,3,5,6, ERK, JNK, P38 and P65 (a) and of HSP70L1, MPP11, HSP70, HSC70 and RPL17 (d) was detected using western blot, and the interaction between HSP70L1 and MPP11 was detected using CoIP/western blot (e). (b) Immature DCs were transfected with indicated siRNA (20 nM) or ERK inhibitor (UO126,10μM) 2 h before with indicated Ad for another 48 h, and then the phenotype of DCs was detected using FACS. (c) Immature DCs were pulsed with CEA576–669HSP70L1 (CH) or transfected with indicated Ad for 18 h, and then the production of IFN-β was detected using ELISA. Representative results of three (a–d) or two (e) independent experiments are shown. Values are relative gray-intensity to GAPDH (a, up), corresponding total protein (a, below) or to Actin (d) using the ImageJ software, mean fluorescence intensity (AdCtrl, up; AdCH, middle) and mean fluorescence intensity ratio (bottom) of AdCH/AdCtrl (b), or mean±s.e.m. (c). CEA, carcinoembryonic antigen; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; IFN, interferon; MPP11, M-phase phosphoprotein 11; siRNA; short interfering RNA.
Intrinsic HSP70L1 is a component of mRAC via interaction with MPP11. We then evaluated whether intracellular CEA576–669HSP70L1 could affect the interaction of intrinsic HSP70L1 and MPP11. We found that the expression levels of intrinsic HSP70L1, MPP11 and mRAC-related HSPs such as HSP70 and heat-shock cognate 71-kDa protein (HSC70) were similar in AdCEA576–669HSP70L1-DCs and AdCtrl-DCs (Figure 5d), and the interaction of intrinsic HSP70L1 with MPP11 was also not significantly affected (Figure 5e). Therefore, intracellular CEA576–669HSP70L1 might not be as a potent competitor of intrinsic HSP70L1 to exert significant interference on the roles by intrinsic HSP70L1.
DISCUSSION
Effective induction of tumor-specific CTLs is the crux for tumor immunotherapy. Numerous evidence over the past two decades has indicated that tumor-derived suppressive factors limit the efficacy of several tumor vaccines. HSP-based immunotherapy aims to overcome tumor-derived suppression, which has been confirmed to be excellently effective in a robust body of animal experiments because of its powerful adjuvant effect and Ag cross-presentation function when administered via an extracellular approach. In this study, we show that AdCEA576–669HSP70L1-DCs, as an alternative HSP-based vaccine via an intracellular approach, can also efficiently induce CEA-specific CTLs.
AdCEA576–669HSP70L1-DCs display the characterisitics of mature DCs dependent on the activation of STAT1 and ERK pathways, among which IFN-β might be responsible for the activation of STAT1, indicating that intracellular CEA576–669HSP70L1-triggered signal pathways were distinct from those triggered by extracellular CEA576–669HSP70L1, which cannot strongly induced the production of IFN-β but activates NF-κB and MAPKs via TLR4.32, 33 As the expression of IFN-β can be also induced from control Ad-transfected DCs compared with DCs alone or extracellular CEA576–669HSP70L1-pulsed DCs, Ad-mediated infection also contributes to the activation of the STAT1 pathway. Such an effect is consistent with other reports that the replication-defective recombinant Ad deleted in E1- and E3-coding domains activate secondary type I IFN-stimulated pathways of STAT1 and STAT2 via Cyclic GMP–AMP synthase/stimulator of IFN genes/TANK-binding kinase 1 DNA-sensing cascade.36 In addition to IFN-β-induced secondary response, the increased activation of STAT1 in AdCEA576–669HSP70L1-DCs might also result from the increase in total STAT1 by intracellular CEA576–669HSP70L1. Similar to STAT1, the expression of other STATs including STAT3, STAT5 and STAT6 was also increased in AdCEA576–669HSP70L1-DCs. As intracellular CEA576–669HSP70L1 mainly localizes in the cytoplasm, indicating it not as a transcription regulator, we therefore speculate that intracellular CEA576–669HSP70L1 enhances the expression of STATs via regulating ubiquitin/proteasome-dependent degradation or other mechanisms to be further elucidated. In addition, mechanisms underlying the activation of STAT1 by intracellular CEA576–669HSP70L1 beyond the induction of IFN-β and upregulation of the STAT1 expression also remain to be further explored. Interestingly, the inhibition on the STAT5 pathway seems to have no significant affection on the maturation of AdCEA576–669HSP70L1-DCs, possibly because of the compensated effects by the activation of the STAT1 and ERK pathways. Although the pathway of STAT5 by GM-CSF is critical for the differentiation of monocytes into DCs, its activation might be redundant for the maturation of MoDCs at the late stage of development because blockade of STAT5 only exerts less inhibitory effect on the expression of co-stimulatory and MHC molecules in AdCtrl-transfected DCs.
Intrinsic HSP70L1 inhibits the activation of the STAT1 pathway as well as the STAT3 pathway, and in fact also inhibits the maturation of DCs, as characterized by the expression of co-stimulatory and MHC molecules on DCs inhibited by overexpression of HSP70L1 but enhanced by blockade of intrinsic HSP70L1 (our unpublished data). The contrast roles in the STAT1 and STAT3 pathways, respectively, by intracellular CEA576–669HSP70L1 and intrinsic HSP70L1 imply that the presence of the CEA576–669 fragment fused to the N terminus of HSP70L1 endowed CEA576–669HSP70L1 with novel functions different from intrinsic HSP70L1. We speculated that intracellular CEA576–669HSP70L1 might not interact with MPP11 despite its ribosomal colocalization, as the interaction between MPP11 and HSP70L1 was not affected in AdCEA576–669HSP70L1-DCs significantly.
Our previous study indicated that DCs pulsed by HSP70L1-based fusion protein were also potent in induction of HLA-A2.1-restricted CTLs.34, 35 Such ability should be dependent on Ag cross-presentation by HSP70L1, the underlying mechanisms of which remain to be elucidated. In the present study, we showed that CEA576–669HSP70L1-pulsed DCs were not as powerful as AdCEA576–669HSP70L1-DCs in inducing CTLs. Such difference is possibly because of the reasons that intracellular CEA576–669HSP70L1 could follow the classical MHC-I pathway to present antigen, could stimulate the expression of MHC-I more efficiently than extracellular CEA576–669HSP70L1 fusion protein via STAT1 and ERK pathways or intracellular CEA576–669HSP70L1 might also interact with those yet unknown molecules that mediate Ag cross-presentation by extracellular CEA576–669HSP70L1 fusion protein. Another advantage of AdCEA576–669HSP70L1-DCs as DC vaccine compared with CEA576–669HSP70L1-pulsed DCs is that the amount of inflammatory cytokines including IL-6, TNF-α and IL-12/IL-23p40 produced by AdCEA576–669HSP70L1-DCs is less than that by CEA576–669HSP70L1-pulsed DCs, which may bring some suppressive effects on antitumor immune responses such as IL-6 or severely inflammatory problems. IL-6 has been identified as a component of the tumor microenvironment that promotes tumor cell growth, invasion, metastasis and drug resistance.37, 38 In addition, IL-6 as a Th17-type cytokine also promotes the differentiation of Th17, whose frequencies are increased in many human tumors, and derived IL-17 might drive the tumorigenesis, particularly colorectal cancer.39, 40 As we all know, most colorectal tumors are CEA-producing, therefore, as DC vaccine specific for CEA, CEA576–669HSP70L1-pulsed DCs might give rise to some negative effects in vivo via secreting large amounts of IL-6. In addition, excess production of IL-6 together with other inflammatory cytokines may also bring potential cytokine storm when large quantities of CEA576–669HSP70L1-pulsed DCs are administered individually. Given that DCs primed by the AdCEA576–669HSP70L1-mediated intracellular approach is better in induction of adaptive responses than by the CEA576–669HSP70L1-mediated extracellular approach, although the latter is better in eliciting innate responses than the former, HSP70L1-base DC combination vaccines, containing appropriate dosages of AdCEA576–669HSP70L1-DCs and CEA576–669HSP70L1-DCs, might be more effective than either one individually used.
In summary, we here present an intracellular approach of priming DC for HSP70L1-based tumor immunotherapy. This approach is more outstanding in induction of CEA-specific CTLs but less powerful in stimulating innate responses than the classical extracellular priming approach. Given the pros and cons, combining priming DCs with AdCEA576–669HSP70L1 and extracellular CEA576–669HSP70L1 is expected to be a promising immunotherapeutic strategy for CEA-positive tumors.
Acknowledgments
We thank Drs Chaofeng Han, Meng Xia and Kun Chen for technical assistance. This work was supported by grants from the National High-Tech Projects (2012AA020808 and 2007AA021003).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Doyle SM, Genest O, Wickner S. Protein rescue from aggregates by powerful molecular chaperone machines. Nat Rev Mol Cell Biol 2013; 14: 617–629. [DOI] [PubMed] [Google Scholar]
- Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 2005; 67: 225–257. [DOI] [PubMed] [Google Scholar]
- Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 2013; 14: 630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 2010; 11: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Sarzotti M, Nicchitta CV. Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo. J Immunol 2004; 172: 4195–4203. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Okuya K, Kutomi G, Takaya A, Kajiwara T, Kanaseki T et al. Heat shock protein 90 targets a chaperoned peptide to the static early endosome for efficient cross-presentation by human dendritic cells. Cancer Sci 2015; 106: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S et al. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol 2010; 184: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ID, Shin SJ, Lee MG, Kang TH, Han HD, Lee SJ et al. Enhancement of tumor-specific T cell-mediated immunity in dendritic cell-based vaccines by Mycobacterium tuberculosis heat shock protein X. J Immunol 2014; 193: 1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinis B, Capobianco A, Esposito F, Bianchi A, Rovere-Querini P, Manfredi AA et al. Human recombinant heat shock protein 70 affects the maturation pathways of dendritic cells in vitro and has an in vivo adjuvant activity. J Leukoc Biol 2008; 84: 199–206. [DOI] [PubMed] [Google Scholar]
- Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol 2014; 193: 5765–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol chem 2002; 277: 20847–20853. [DOI] [PubMed] [Google Scholar]
- Saito K, Kukita K, Kutomi G, Okuya K, Asanuma H, Tabeya T et al. Heat shock protein 90 associates with Toll-like receptors 7/9 and mediates self-nucleic acid recognition in SLE. Eur J Immunol 2015; 45: 2028–2041. [DOI] [PubMed] [Google Scholar]
- Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A et al. T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J Immunol 2009; 183: 3092–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Jasuja H, Toes RE, Spee P, Münz C, Hilf N, Schoenberger SP et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med 2000; 191: 1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001; 14: 303–313. [DOI] [PubMed] [Google Scholar]
- Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L et al. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol 2010; 185: 2306–2313. [DOI] [PubMed] [Google Scholar]
- Jockheck-Clark AR, Bowers EV, Totonchy MB, Neubauer J, Pizzo SV, Nicchitta CV. Re-examination of CD91 function in GRP94 (glycoprotein 96) surface binding, uptake, and peptide cross-presentation. J Immunol 2010; 185: 6819–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun 2011; 2: 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Kato Y, Kajiwara C, Mizukami S, Ishige I, Ichiyanagi T et al. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc Natl Acad Sci USA 2011; 108: 16363–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyanagi T, Imai T, Kajiwara C, Mizukami S, Nakai A, Nakayama T et al. Essential role of endogenous heat shock protein 90 of dendritic cells in antigen cross-presentation. J Immunol 2010; 185: 2693–2700. [DOI] [PubMed] [Google Scholar]
- Ampie L, Choy W, Lamano JB, Fakurnejad S, Bloch O, Parsa AT. Heat shock protein vaccines against glioblastoma: from bench to bedside. J Neurooncol 2015; 123: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Chen J, Liu Y, Luo W. Dendritic-tumor fusion cells derived heat shock protein70-peptide complex has enhanced immunogenicity. PLoS One 2015; 10: e0126075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhu T, Ye X, Yang L, Wang B, Liang X et al. Long-term protection against human papillomavirus e7-positive tumor by a single vaccination of adeno-associated virus vectors encoding a fusion protein of inactivated e7 of human papillomavirus 16/18 and heat shock protein 70. Hum Gene Ther 2010; 21: 109–119. [DOI] [PubMed] [Google Scholar]
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol 2008; 26: 955–962. [DOI] [PubMed] [Google Scholar]
- Tischer S, Basila M, Maecker-Kolhoff B, Immenschuh S, Oelke M, Blasczyk R et al. Heat shock protein 70/peptide complexes: potent mediators for the generation of antiviral T cells particularly with regard to low precursor frequencies. J Transl Med 2011; 9: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RG, Dee MJ, Malek TR, Podack ER, Levy RB. Heat shock protein vaccination and directed IL-2 therapy amplify tumor immunity rapidly following bone marrow transplantation in mice. Blood 2014; 123: 3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Yoshimura K, Matsui H, Shindo Y, Tamesa T, Tokumitsu Y et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol Immunother 2015; 64: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H, Conz C, Maier P, Wölfle T, Suzuki CK, Jenö P et al. The chaperones MPP11 and Hsp70L1 form the mammalian ribosome-associated complex. Proc Natl Acad Sci USA 2005; 102: 10064–10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal H, Conz C, Otto H, Wölfle T, Fitzke E, Mayer MP et al. The chaperone network connected to human ribosome-associated complex. Mol Cell Biol 2011; 31: 1160–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidig C, Bange G, Kopp J, Amlacher S, Aravind A, Wickles S et al. Structural characterization of a eukaryotic chaperone—the ribosome-associated complex. Nat Struct Mol Biol 2013; 20: 23–28. [DOI] [PubMed] [Google Scholar]
- Wan T, Zhou X, chen G, An H, chen T, Zhang W et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood 2004; 103: 1747–1754. [DOI] [PubMed] [Google Scholar]
- Fang H, Wu Y, Huang X, Wang W, Ang B, Cao X et al. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J Biol chem 2011; 286: 30393–30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Wu Y, Yan F, Wang N, Wang W, Cao X et al. Efficient induction of a Her2-specific anti-tumor response by dendritic cells pulsed with a Hsp70L1-Her2(341-456) fusion protein. Cell Mol Immunol 2011; 8: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y et al. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol 2014; 11: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wan T, Zhou X, Wang B, Yang F, Li N et al. Hsp70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine. Cancer Res 2005; 65: 4947–4954. [DOI] [PubMed] [Google Scholar]
- Lam E, Stein S, Falck-Pedersen E. Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. J Virol 2014; 88: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015; 34: 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanga D, De Marco C, Guerriero I, Colelli F, Rinaldo N, Scrima M et al. The Akt1/IL-6/STAT3 pathway regulates growth of lung tumor initiating cells. Oncotarget 2015; 6: 42667–42686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Mu J, Tseng M, Wattenberg B, Zhuang X, Egilmez NK et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat Commun 2015; 6: 6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med 2015; 212: 1571–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.