Abstract
CD5 is constitutively expressed on T cells and a subset of mature normal and leukemic B cells in patients with chronic lymphocytic leukemia (CLL). Important functional properties are associated with CD5 expression in B cells, including signal transducer and activator of transcription 3 activation, IL-10 production and the promotion of B-lymphocyte survival and transformation. However, the pathway(s) by which CD5 influences the biology of B cells and its dependence on B-cell receptor (BCR) co-signaling remain unknown. In this study, we show that CD5 expression activates a number of important signaling pathways, including Erk1/2, leading to IL-10 production through a novel pathway independent of BCR engagement. This pathway is dependent on extracellular calcium (Ca2+) entry facilitated by upregulation of the transient receptor potential channel 1 (TRPC1) protein. We also show that Erk1/2 activation in a subgroup of CLL patients is associated with TRPC1 overexpression. In this subgroup of CLL patients, small inhibitory RNA (siRNA) for CD5 reduces TRPC1 expression. Furthermore, siRNAs for CD5 or for TRPC1 inhibit IL-10 production. These findings provide new insights into the role of CD5 in B-cell biology in health and disease and could pave the way for new treatment strategies for patients with B-CLL.
Keywords: B cells, calcium, CD5, MAPK/Erk, TRPC1
INTRODUCTION
CD5 is expressed on T cells and a subpopulation of B cells, specifically B1 cells.1 B1 cells comprise a large subset of B lineage cells during early life, but their frequency among total B cells declines with age.2 The B1 subset has important roles in the immune system, produces natural antibodies and contributes to innate immunity. However, these cells can also give rise to leukemic B cells in patients with chronic lymphocytic leukemia (CLL).3 The involvement of CD5 in the pathophysiology of B-CLL has yet to be conclusively established, but there is evidence that CD5 is involved in B-CLL development, at minimum through the production of IL-10.4 CD5+ B cells produce IL-10 and are the main B-cell source of this cytokine.5 This ability is relevant to B-CLL pathophysiology because IL-10 acts as a growth factor for B cells via its stimulatory6, 7 and anti-apoptotic properties.8 Furthermore, IL-10 production is associated with the outcome of CLL9 and with a malignant genotype.10
Recently, we revealed that CD5 induces IL-10 production by activating signal transducer and activator of transcription 3 (STAT3) and nuclear factor of activated T cells 2 (NFAT2) in a subset of B-CLL cells.11, 12 Interestingly, the activation of these transcription factors influences disease progression in patients with B-CLL.13, 14
CD5 is a member of the conserved scavenger receptor cysteine-rich superfamily.15 It has a cytoplasmic tail that exerts no enzymatic activity but contains a conserved motif with a threonine and four tyrosine residues. Two of these tyrosines (Y429 and Y441) serve as docking sites for phosphorylated Src homology 2 (SH2) domain-containing proteins.16 In T and B lymphocytes, CD5 associates with Src kinases such as Lyn, which phosphorylates the SH2 domain of CD5, creating docking sites for Lck, Zap70, PI3K, c-Cbl and the SH2/SH3 RasGap.17, 18 In contrast, the phosphatase SHP1 binds CD5 on Y378.19 In a yeast two-hybrid system, CD5 was found to associate with CAM kinase IIδ and casein kinase II (CK2), which phosphorylate CD5 serine 459 (Ser459) and serine 461 (Ser461), respectively.20, 21 CD5+ B lymphocytes exhibit delayed JNK activation and lack the ability to induce p38 MAPK and NF-κB activation upon B-cell receptor (BCR) crosslinking, although Erk1/2 and NFAT2 are constitutively active.22 Furthermore, CD5 reduces intracellular Ca2+ mobilization upon BCR engagement.23 On the basis of these findings, CD5 has been implicated in B-lymphocyte tolerance and leukemic transformation.24
In this study, we report changes in multiple intracellular signaling pathways resulting from CD5 expression. CD5 promotes constitutive MAPK activation through a Ca2+-dependent pathway, leading to Erk1/2 phosphorylation (pErk1/2) and IL-10 production. This IL-10 production is independent of BCR engagement but is associated with the expression of a non-selective channel permeable to Ca2+, transient receptor potential channel 1 (TRPC1). In addition, CD5 promotes the activation of the PI3K/Akt/mTOR pathway, which has important roles in B-cell survival and proliferation. These effects occur through the ability of CD5 to activate a range of key kinases.25 Furthermore, we show that in pErk1/2-positive CLL B cells, small interfering RNA (siRNA) against CD5 suppresses TRPC1 expression, while siRNAs against CD5 or TRPC1 inhibit IL-10 production.
MATERIALS AND METHODS
Patients
Twenty-six patients who fulfilled the criteria for CLL26 were recruited at the Centre of Ressources Biologiques-santé in Brest (Table 1). Disease assessment included Binet stage determination, progression-free survival, CD38 expression, cytogenetic abnormalities and lymphocyte counts. Informed consent was obtained from all patients, and the Ethical Committee at Brest University Medical School Hospital approved the study. B cells were enriched to >96% using an enrichment kit (StemCell Technologies, Cambridge, UK).
Table 1. Demographic, clinical and immunological information on patients with CLL included in the study.
| CLL | Age years | Sex | Binet | Follow-up (years) | PFS (months) | Ly (× 109/l) | CD19+ CD5+ CD38+ (%) | Cytogenetic | pErk1/2 statusa |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | F | B | 4 | 36 | 38,2 | 1 | del13q14 | neg |
| 2 | 55 | M | A | 10 | >120 | 41,7 | 0 | del13q14 | neg |
| 3 | 65 | M | C | 2 | 26 | 42,1 | 88 | del13q14 | neg |
| 4 | 86 | F | A | 7 | >84 | 23,9 | 2 | del13q14 | neg |
| 5 | 73 | M | A | 8 | 102 | 38,2 | 7 | del13q14 | neg |
| 6 | 74 | M | B | 6 | 57 | 25,8 | 2 | del13q14 | neg |
| 7 | 67 | F | A | 5 | >60 | 14,3 | 4 | normal | neg |
| 8 | 63 | M | B | 1 | 10 | 56 | 30 | del11q/ATM, del 13q14 | neg |
| 9 | 65 | F | B | 3 | 18 | 20.6 | 0 | del13q14 | neg |
| 10 | 85 | M | A | 9 | >108 | 35,6 | 8 | normal | neg |
| 11 | 53 | F | A | 8 | >96 | 26,8 | 0 | del13q14 | neg |
| 12 | 67 | F | A | 4 | >54 | 9,5 | 64 | trisomy 12 | neg |
| 13 | 77 | F | A | 6 | >77 | 20,4 | 0 | del13q14 | neg |
| 14 | 88 | F | A | 20 | >240 | 19,8 | 7 | ND | pos |
| 15 | 77 | M | B | 16 | 126 | 96 | 50 | del13q14 | pos |
| 16 | 71 | M | B | 7 | 72 | 69,2 | 20 | trisomy 12, del13q14 | pos |
| 17 | 83 | M | A | 5 | 65 | 57,8 | 11 | del13q14 | pos |
| 18 | 76 | M | A | 10 | >120 | 31,3 | 7 | del13q14 | pos |
| 19 | 79 | M | A | 2 | >24 | 14,9 | 4 | ND | pos |
| 20 | 74 | F | B | 10 | 123 | 45,7 | 7 | del13q14 | pos |
| 21 | 74 | F | A | 5 | >57 | 41.8 | 48 | del13q14 | neg |
| 22 | 77 | M | A | 3 | >36 | 16.7 | 26 | normal | pos |
| 23 | 66 | M | A | 12 | >130 | 62.5 | 0 | del13q14 | neg |
| 24 | 63 | F | B | 9 | 90 | 60.4 | 8 | del13q14 | neg |
| 25 | 84 | F | B | 5 | 46 | 61.9 | 23 | del17p/TP53, del13q14 | pos |
| 26 | 56 | F | B | 7 | 36 | 35.3 | 1 | del13q14 | pos |
Abbreviation: CLL, chronic lymphocytic leukemia; Ly, lymphocytes numbers; ND, not determined; neg, negative; PFS: progression-free survival; pos, positive.
Indicates CLL patient divided on the basis of the phosphorylation status of MAPK Erk1/2 in their B cells into neg (pErk1/2−); pos (pErk1/2+).
Cell culture
The CD5-negative hairy B-cell leukemia cell line Jok-1,27 which possesses the phenotypic characteristics of B-CLL cells,28 was transfected with cDNA for either the membrane isoform of CD5, E1A (Jok-E1A) or the cytoplasmic E1B isoform (Jok-E1B).11 Cells were maintained in RPMI-1640 containing 10% fetal calf serum, antibiotics and 0.5 mg/ml G418 (Sigma-Aldrich, Dorset, UK). For activation, 106 cells/ml were stimulated with 10 μg/ml goat F(ab’)2 anti-human IgM coated onto Sepharose beads (Bio-Rad, Hemel Hempstead, UK). For inhibition experiments, 106 cells/ml were incubated for 48 h with 50–100 μM PD98059 (inhibits Mek1; Calbiochem, Watford, UK), 100 μM lanthanum (La3+; blocks extracellular Ca2+ entry; Sigma-Aldrich) or 50 μM LY294002 (inhibits PI3K; Sigma-Aldrich) and 10 ng/ml rapamycin (inhibits mTOR; Pfizer, New York, NY, USA). IL-10 levels in culture supernatants were quantified using ELISA (BD OptiEIA; BD Biosciences, Oxford, UK).
Antibodies
Antibodies (Abs) to Erk1/2, phosphorylated-Erk1/2 (pErk1/2), Syk/pSyk, Btk/pBtk, PLCγ2/pPLCγ2, SHP1/pSHP1, SHIP/pSHIP were obtained from Insight Biotechnology (Middlesex, UK). Abs to Lyn, c-Cbl, Vav1, CD79a, S6K/pS6K T389, STAT3/pSTAT3 S727, STAT1/pSTAT1 S727, Akt/pAkt S473 were obtained from Abcam (Cambridge, UK). The anti-CD5 clone UCHT2, the rabbit anti-extracellular TRPC1, and the mouse anti-β-actin Abs were obtained from BD Biosciences and Sigma-Aldrich, respectively.
Western blotting and immunoprecipitation
Cell lysates in 1% NP-40 buffer (1% NP-40, 150 mM NaCl, 2 mM EDTA, 10 mM Tris-HCl at pH 7.4, 5 mM sodium fluoride) containing protease/phosphatase inhibitors were separated using 10% SDS–polyacrylamide gel electrophoresis, blotted onto polyvinylidene fluoride membranes, probed with Abs and visualized with horseradish peroxidase-conjugated secondary Abs and enhanced chemiluminescence (Amersham-Pharmacia, Little Chalfont, UK). For immunoprecipitation, lysates were cleared using centrifugation, incubated with Abs coupled to protein G-Sepharose, washed and analyzed using western blotting (WB).
Flow cytometry
Expression of TRPC1 was detected with specific rabbit Abs followed by incubation with fluorescein isothiocyanate-conjugated goat (Fab’)2 anti-rabbit IgG (ImmunoResearch, Newmarket, UK). Data were acquired and analyzed using an FC500 flow cytometer (Beckman-Coulter, High Wycombe, UK) relative to staining with the isotype control. The results were expressed as the mean fluorescence intensity (MFI).
Measurement of intracellular calcium (iCa2+) levels
Imaging was performed to monitor iCa2+ mobilization in B cells loaded for 30 min at 37 °C with 2 μM Fura-2/AM. B cells in six independent experiments were washed and attached onto cell-Taq pre-coated coverslips. Fura-2-fluorescence was excited sequentially at 340 and 380 nm, emission recorded at 520 nm and excitation/emission ratios calculated. Extracellular Ca2+ depletion was monitored to measure iCa2+ release. Repletion with 1.8 mM Ca2+ was used to evaluate Ca2+ entry, and subsequent addition of 100 μM La3+ was used to block entry. In selected experiments, ratios were normalized to basal values (F0) at the beginning of each experiment and are provided as (ΔF/F0).
Transfection with small interfering RNA (siRNA)
A total of 106 cells were transfected with siRNA at 3 pM using a B-cell Nucleofector Transfection Kit (Lonza, Amboise, France). siRNAs complementary to CD5 RNA plus control siRNA were purchased from Ambion (Life Technologies, Paisley, UK). siRNAs complementary to TRPC1 (3′-GCAUCGUAUUUCACAUU CU-3′ 5′-UGAGCCUCUUGACAAACGA-3′) were obtained from Eurogentec (Seraing, Belgium).
Kinome array analysis
A kinome array (Pepscan Systems, Lelystad, The Netherlands) was performed as previously described.25 Briefly, 106 Jok-1, Jok-E1A or Jok-E1B cells were lysed in 50 μl lysis buffer and analyzed. The array comprises 1024 peptides representing phosphorylation sites in protein substrates of all known kinases spotted onto glass slides. Ten microliters of the peptide array incubation mix (50% glycerol, 50 μM ATP, 0.05% v/v Brij-35, 0.25 mg/ml bovine serum albumin, [33P] ATP (1 MBq)) was added to each of the lysates, and the samples were loaded onto the chips and permitted to phosphorylate for 90 min at 37 °C. Washed and dried slides were exposed using a phosphorimager for 72 h, and data were acquired (Storm, Amersham-Biosciences). The levels of incorporated radioactivity, which correspond to phosphorylation levels, were quantified using the array software Scanalyze (Eisen Software, Toronto, ON, Canada). Differential kinase activation in Jok-E1A and Jok-E1B cells was quantified and represented as significant fold changes in the ratio of phosphorylated peptides compared with untransfected Jok-1 cells. All analyses were carried out in triplicate and repeated on two separate occasions.
Construction of CD5 mutants
Two deletion mutants, S398Mstart and S415Mstart, and three proteins with amino acid replacements were generated. The extracellular domains and transmembrane (Tm) regions in both deletion mutants were deleted. The SHP-1 and the CaM-binding motifs were removed from the S398Mstart mutant in addition to the first CK2 motif from the S415Mstart mutant. These mutants were generated using PCR using ATG-containing sense/antisense primers, cloned into the pDNR-dual plasmid and subcloned into the pLPcmv vector using the Cre-recombinase system (BD Biosciences). The E1A-CD5 cDNA was mutated at three amino acid positions using the Quick-Change Site-Directed Mutagenesis Kit (Agilent Technologies, Les Ulis, France). Point mutations were introduced into the serine phosphorylation sites (422AS423→422VD423; 428EYS430→428AAA430; 459SDS461→459VDG461). All constructs were validated using sequencing.
cDNA microarray
cDNA microarray analysis was performed according to Agilent Technologies’ instructions as described.29 Thirteen micrograms of mRNA were reverse-transcribed and fluorescence-labeled using the cyanine 3-CTP-RNA Quick Amplification Kit. Labeled cDNAs were hybridized to the Agilent Whole Human Genome Oligo Microarray (4 × 44 k). Each sample was hybridized with three arrays as biological replicates, the slides were washed and dried and the fluorescence was quantified with a scanner (Agilent-G2565AA). The signals were analyzed after subtracting background outliers using the Feature Extraction Software (Les Ulis, France). Signal values were calculated as the ratios between the signal intensities from the Jok-E1A or Jok-E1B cells to the Jok-1 cells. The data can be viewed in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (accession number GSE50714). Data normalization, quality control and probe list processing were all carried out with GeneSpring GX using the Agilent Feature Extractor plug-in.29
RT-PCR and quantitative RT-PCR
RNA was extracted using the RNeasy kit (Qiagen, Les Ulis, France) and reverse-transcribed using oligo-dT. RT-PCR was used to amplify CD5 (sense: 5′-TCGGACGGCTCAGCTGGTATGAC-3′ antisense: 5′-TGCCATCCGTCCTTGAGGTAGAC-3′); TRPV2 (sense: 5′-TCACCGCTGTTGCCTACCATCA 3′ antisense: 5′-AGGGCTACAGCGAAGCCGAAAA-3′); TRPC1 (sense: 5′-ACCTTCCATTCGTTCATTGG-3′ antisense: 5′-TGGTGAGGGAATGATGTTGA-3′ and GAPDH (sense: 5′-TGCACCACCAACTGCTTAGC-3′, antisense: 5′-GGCATGGACTGTGGTCATGAG-3′). Amplification was performed with 150 ng of cDNA, 20 ng of genomic DNA, 200 nM primers and 2.5 units of Taq polymerase (Thermo-Fisher Scientific, Villebon-sur-Yvette, France). The protocol consisted of denaturation at 94 °C for 5 min; 40 cycles of 94 °C for 40 s, 60 °C for 40 s and extension at 72 °C for 1 min; and a final cycle at 72 °C for 10 min. For quantitative real-time PCR (qRT-PCR), TaqMan gene expression assay FAM/MGB probes (Hs 00901640_m1-human TRPV2, Hs 00608195_m1 human TRPC1, and Hs 99999905_m1 human GAPDH) were obtained from Applied Biosystems (Foster City, CA, USA). For CD5, specific primers (sense: 5′-TCGGACGGCTCAGCTGGTATGAC-3′ antisense: 5′-TGCCATCCGTCCTTGAGGTAGAC-3′) were used at 500 nM plus 1 × SYBR Green PCR Master Mix (Applied Biosystems). mRNA levels were normalized to GAPDH, and cycle thresholds were compared using the 2−ΔΔct method.
Gene ontology and the analysis of biological pathways
The FatiGO web-interface was employed to carry out data mining using the Gene Ontology database (www.geneontology.org). The signaling pathways were grouped according to functional classes and pathways.
Statistical analyses
Differences between the cell lines were analyzed using Student’s t-test and/or the Mann–Whitney U-test when appropriate. P-values were determined using the GraphPad Prism Version 6.0 (San Diego, CA, USA) statistical software package, and values less than 0.05 were considered significant.
RESULTS
CD5 promotes constitutive activation of pErk1/2
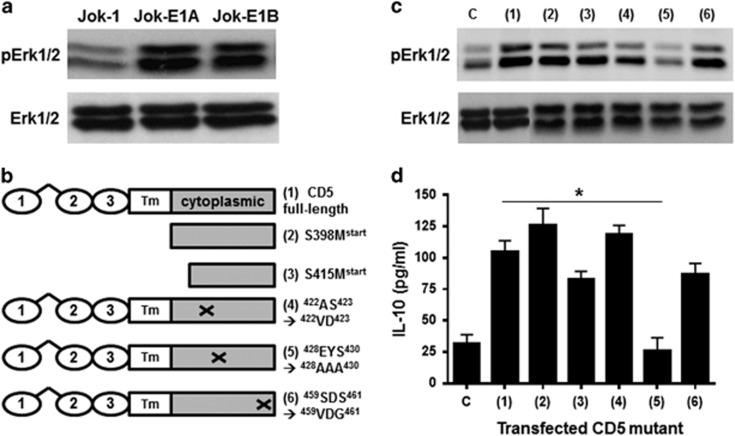
Erk1/2 is a key signaling molecule in normal and B-CLL cell survival and IL-10 production.13 An association between CD5 expression, constitutive Erk1/2 phosphorylation (pErk1/2) and IL-10 production has been suggested.22 To assess this association directly, we compared pErk1/2 levels in untransfected and CD5-transfected Jok-1 cells. The results revealed that Jok-1 transfection with either the membrane isoform of CD5 (Jok-E1A) or the cytoplasmic isoform (Jok-E1B) markedly enhanced constitutive pErk1/2 (Figure 1a). To explore the molecular mechanism(s) underpinning the increase in pErk1/2 induced by CD5, we transfected Jok-1 cells with CD5 mutants (Figure 1b). Transfection with CD5 lacking the extracellular-transmembrane domains S398Mstart or S415Mstart or with mutations in the intracellular domain (422AS423→422VD423 or 459SDS461→459VDG461) did not affect constitutive pErk1/2 and IL-10 production (Figures 1c and d). However, transfection with CD5 mutated in the intracellular domain (428EYS430→428AAA430) reduced pErk1/2 and IL-10 production to levels similar to those observed in untransfected Jok-1 cells (IL-10: 99.7±5.5 pg/ml with native CD5 versus 19.3±5.5 pg/ml in 428AAA430 CD5, P<0.05). This indicates that the 428EYS430 motif, which encompasses the Src kinase-docking site Y429, is critical for constitutive pErk1/2 and IL-10 production, irrespective of the subcellular location of CD5.
Figure 1.
Constitutive Erk1/2 activation and IL-10 production in CD5-expressing B cells is dependent on the phosphorylation of Y429 in the CD5 molecule. (a) The upper panel depicts WB analysis of phosphorylated Erk1/2 (pErk1/2) in untransfected Jok-1 cells and Jok-1 cells transfected with membrane (E1A-CD5) or cytoplasmic (E1B-CD5) CD5. The lower panel shows the total levels of Erk1/2. (b) Cartoons representing full-length CD5 and the mutants generated in this study to identify sites in CD5 involved in Erk1/2 activation. CD5 has three extracellular domains (1–3), a transmembrane (Tm) region and a cytoplasmic domain. Truncated CD5 molecules (S398Mstart and S415Mstart) are named according to their start codons. (c) WB of constitutive Erk1/2 phosphorylation in untransfected Jok-1 cells (labeled c) and cells transfected with native CD5 (1) or with the mutants generated as shown in the cartoons. (d) ELISA results for IL-10 levels produced by the corresponding cells in c. The cells were cultured for 48 h. The data in c and d represent three independent experiments. Statistical analyses were performed by calculating the Mann–Whitney U-test for IL-10 production. * indicates P<0.05 for significant differences in IL-10 production between Jok-1 cells transfected with 428AAA430 compared with the full-length CD5 molecule. Erk1/2, extracellular signal-regulated kinases 1/2; ELISA, enzyme-linked immunosorbent assay; pErk1/2; phosphorylated Erk1/2; WB, western blotting.
Constitutive Erk1/2 phosphorylation is independent of BCR engagement
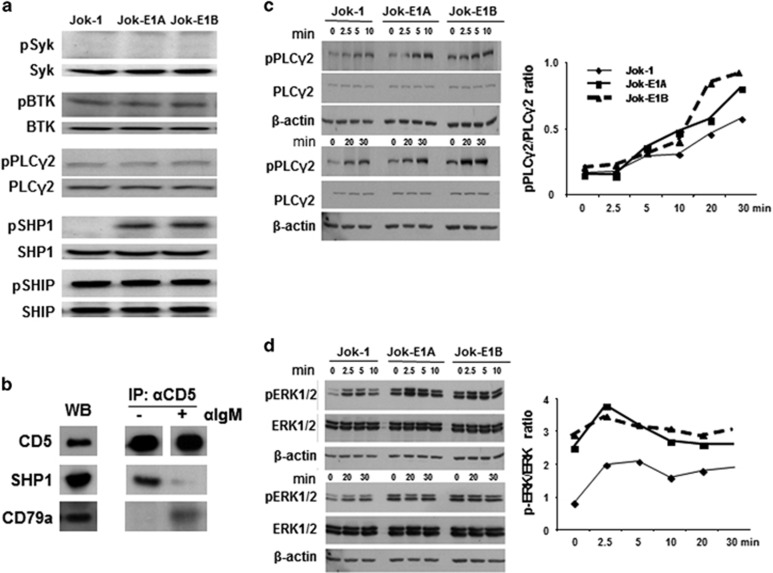
Canonical BCR-dependent Erk1/2 phosphorylation involves the activation of Syk/Btk/PLCγ2 pathway, which is regulated by two phosphatases, SHP1 and SHIP.30 To determine if constitutive Erk1/2 phosphorylation in Jok-E1A/E1B cells occurs as the result of an association between CD5 and the BCR, we determined the phosphorylation status of Syk, Btk, PLCγ2, SHP1 and SHIP in non-activated Jok-1 and Jok-E1A/E1B cells. The results showed that pSyk, pBtk, pPLCγ2 and pSHIP phosphorylation status not different in Jok-1 cells compared with Jok-E1A/E1B cells (Figure 2a). However, the level of pSHP1 phosphorylation was higher in Jok-E1A/E1B cells compared with Jok-1 cells, as previously reported in CD5+ CLL B cells.18
Figure 2.
Constitutive Erk1/2 phosphorylation in CD5-expressing B cells is BCR-independent. (a) WB analysis of Syk, BTK, PLCγ2, SHP1 and SHIP phosphorylation in Jok-1, Jok-E1A and Jok-E1B cells. (b) Anti-CD5 immunoprecipitation (IP: αCD5) followed by WB of Jok-E1A cells to assess the association between CD5 and the BCR complex in resting and F(ab’)2 anti-human IgM (α-IgM)-stimulated Jok-E1A cells. The left panel shows CD5, CD79a and SHP1 in Jok-E1A cell lysate (WB), tested as controls. The panel on the right depicts the association between CD5 and CD79a after α-IgM stimulation following IP with anti-CD5 mAb (c) WB to reveal the kinetics of PLCγ2 phosphorylation in unstimulated Jok-1, Jok-E1A and Jok-E1B cells or cells stimulated with anti-IgM. The upper three panels depict the kinetics of PLCγ2 activation from 0 to 10 min after BCR engagement with the anti-IgM. The blots indicate pPLCγ2; the middle band is total PLCγ2 protein (PLCγ2), and the lower band is β-actin protein. The bottom three panels depict the levels of pPLCγ2 at time points 0, 20 and 30 min after BCR engagement with anti-IgM. (d) Analysis of the kinetics of pErk1/2 following BCR engagement arranged as in c. The two graph panels to the right of the western blots represent semi-quantification data for the levels of pPLCγ2 as in c and pErk1/2 in d for the signaling molecules, represented as the ratio of band intensity for the phosphorylated proteins to the band intensity of the total protein. BCR, B-cell receptor; BTK, Bruton’s tyrosine kinase; Erk1/2, extracellular signal-regulated kinases 1/2; IP, immunoprecipitation; pPLCγ2, phosphorylated PLCγ2; SHIP, SH2-containing inositol phosphatase; SHP1; SH2 domain-containing protein tyrosine phosphatase-1; WB, western blotting.
To further test the hypothesis that constitutive pErk1/2 is BCR-independent in CD5+ B cells, we carried out immunoprecipitation experiments with anti-CD5 mAb (clone UCHT2) and WB (Figure 2b). These experiments confirmed that CD5 was not associated with the BCR complex (CD79a) when the BCR was not engaged, while engagement with F(ab’)2 anti-IgM resulted in the co-precipitation of CD79a with CD5. In contrast, SHP1 co-precipitated with CD5 in Jok-E1A cells only when the BCR was not engaged. These data are consistent with our previous findings showing that CD79a associates with CD5 in B-CLL only after BCR engagement.31
To rule out the possibility that our findings were attributable to defective BCR-mediated signaling in Jok-1 cells, the kinetics of PLCγ2 and Erk1/2 phosphorylation were studied before and after BCR engagement with the F(ab’)2 anti-IgM. As shown in Figure 2c, the level of pPLCγ2 phosphorylation was similar in all three cells lines before BCR engagement. PLCγ2 phosphorylation increased after 5 min and continued until 30 min post BCR engagement, confirming that BCR-mediated signaling is functional in Jok-1 cells (Figure 2c). Phosphorylation of Erk1/2 in the three cell lines with BCR engagement was highest at 2.5 min and declined thereafter (Figure 2d). Notably, increased pErk1/2 levels were merely additive and proportional to the baseline in the three cell lines. The data, therefore, indicate that enhanced Erk1/2 phosphorylation by CD5 occurs independent of the BCR and through different pathways.
CD5 expression induces multiple signaling pathways
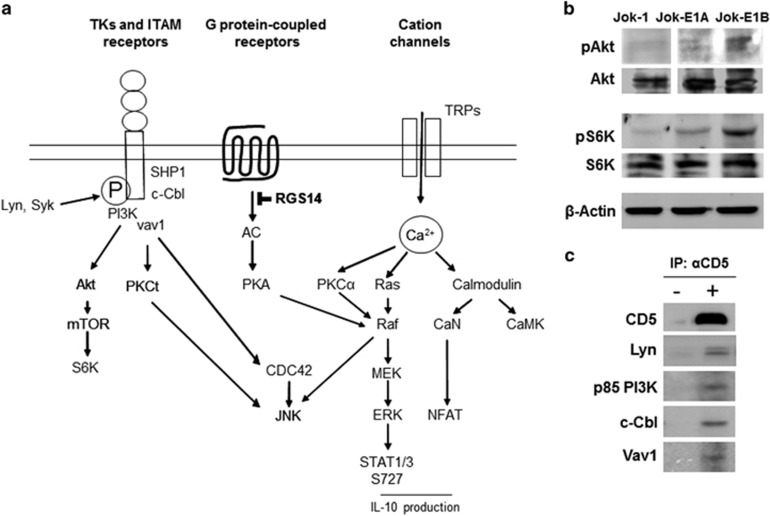
To identify the signaling pathway(s) through which CD5 enhances constitutive pErk1/2, the effects of plasma membrane and intracellular CD5 isoform expression on intracellular signaling were assessed through kinome array analyses. Significant changes (P<0.05) in the phosphorylation of 1024 substrates of all known kinases were reported when the increase was ⩾2-fold higher, while decreases were reported when levels were 0.5-fold or less. The phosphorylation of 154 substrates was increased, and the phosphorylation of 29 substrates was decreased in Jok-E1A/E1B cells compared with Jok-1 cells (key substrates are presented in Table 2). Analyses of kinases and the biological pathways reflected in these changes performed using FatiGO revealed that at least two pathways, one driving Ras/Erk, calmodulin and PKC through Ca2+ activation and another driving the PI3K/Akt/mTOR pathway, were either activated or activation was enhanced in the presence of CD5 (summarized in Figure 3a). Activation of PI3K/Akt/mTOR was further studied using WB (Figure 3b), which confirmed an increase in the constitutive phosphorylation of Akt and S6K in Jok-E1A/E1B cells compared with Jok-1 cells. Activation of the PI3K/Akt/mTOR pathway was related to the association between CD5 and the p85 unit of PI3K, as established by immunoprecipitation with a mAb for CD5 (Figure 3c). Moreover, we established a link between CD5 and Lyn, as well as with the U3-ubiquitin ligase c-Cbl and the kinase Vav1, as previously described in thymocytes.32
Table 2. Kinase activation profiles and change in their activity in Jok-E1A and Jok-E1B relative to untransfected Jok-1 cells.
| Peptide with phosphorylation site | Protein substrate | Kinase | Function | E1A/Ctrl | E1B/Ctrl | Change |
|---|---|---|---|---|---|---|
| FIGEHYVHVNA | HGFR/MET (Y1349) | Abl, autoP | TK receptor | 4.7 | 3.5 | Up |
| PESIHSFIGDG | MTOR (S2481) | Akt, autoP | Akt/mTOR | 2.7 | 2.2 | Up |
| EVPRRSGLSAG | MBD3 (S24 S26) | Aurora A | 4.4 | 3.4 | Up | |
| PGMKIYIDPFT | EPHB1 (Y594) | autoP | TK receptor | 4 | 3.9 | Up |
| DIKSDSILLTS | CDC42/PAK5 (S573) | autoP, Ca2+ | Prot Kinase | 6.1 | 5 | Up |
| CEEEFSDSEEE | HDAC1 (S421 S423) | autoP, CK2 | Cell cycle, DNA repair | 2 | 2.4 | Up |
| ETPAISPSKRA | dUTPase/DUT (S99) | CDC2 | Enzyme | 5.6 | 5.2 | Up |
| GDAAETPPRPR | MEK1/MAP2K1 (T286) | CDK1 | MAPK/Erk | 5.3 | 4.3 | Up |
| DPWGGSPAKPS | EPN1 (S357) | CDK1 | Vesicle formation | 7.5 | 9 | Up |
| SASPYTPEHAA | TP73 (T86) | CDK1/2, CDC2, autoP | Cell cycle | 5.6 | 6.1 | Up |
| LSRMGSLRAPV | E2F1 (S364) | CHK2 | TF | 3.3 | 3.2 | Up |
| PELARYLNRNY | HRS/HGS (Y329) | EGFR/MET | Vesicle formation | 4 | 4.9 | Up |
| DYDDMSPRRGP | HNRNPK (S284) | Erk | RNA binding | 3.5 | 2.7 | Up |
| AEVLPSPRGQR | TOP2A (S1213) | Erk1, CDK1 | Cell cycle | 3.7 | 3.5 | Up |
| GPHRSTPESRA | PSEN1 (S353 S357) | GSK3B | MAPK/Erk | 4.1 | 4.2 | Up |
| RSGLCSPSYVA | MYC (S71) | MAPK/JNK | TF | 2.3 | 2.3 | Up |
| EKPRLSFADRA | PKC theta (S676) | nd | PKC | 4.9 | 4.1 | Up |
| ALRRESQGSLN | RGS14 (S260) | PKA | GTPase | 9.8 | 11 | Up |
| SAWPGTLRSGM | HSPB8 (T63) | PKC | Small HSP | 3.5 | 3.2 | Up |
| TTCVDTRWRYM | HIR/KCNJ4 (T53) | PKC | K receptor | 3.1 | 2.8 | Up |
| KSFTRSTVDTM | CD88/C5AR1 (S334 S338) | PKC | G protein | 3.8 | 3.6 | Up |
| AGIQTSFRTGN | DDX5 (S557) | PKC, autoP | RNA binding | 2.7 | 2.9 | Up |
| LLREASARDRQ | TRPV1 (S801) | PKCalpha, Ca2+ | Ca receptor | 3 | 2.8 | Up |
| EHRKSSKPIME | HES1 (S37-38) | PKCalpha, Ca2+ | TR | 6.3 | 5.2 | Up |
| ESLESTRRILG | SNAP23 (S23 T24) | PKCalpha, Ca2+ | Vesicle | 2.5 | 2.8 | Up |
| EGKHLYTLDGG | Rack1/GNB2L1 (Y228) | Src kinase | G protein | 3.4 | 2.2 | Up |
| SRLSAYPALEG | CD5 (Y465) | Src kinase | PI3K | 2.8 | 2.9 | Up |
| EVERTYLKTKS | GRIN2A (Y1105) | Src kinase (Fyn) | Ca receptor | 4.1 | 3.1 | Up |
| PCTTIYVAATE | CD150/SLAM (Y307) | Src kinase (Fyn) | ITAM receptor | 2.7 | 2 | Up |
| EEGEGYEEPDS | CD19 (Y409) | Src kinase (lyn) | ITAM receptor | 2.3 | 2.5 | Up |
| GTDLEYLKKVR | OGT (Y989) | Src kinase, INSR | Enzyme | 2.5 | 2.3 | Up |
| GSPSVRCSSMS | SMAD2 (S464 S465 S467) | TGFBR, BMPR1 | TF | 3.8 | 4.7 | Up |
| FMRRTSLGTEQ | PTGER4 (S222) | unknown | G protein | 5 | 5 | Up |
| LDRFLSLEPVK | CCND1 (S90) | unknown | Cell cycle | 2.2 | 4 | Up |
| HSLPFSLPSQM | CBL (S623) | unknown | TK regulation | 2.9 | 2.8 | Up |
| TDGNRSSHSRL | BID (S64 S65) | unknown | TF | 2.4 | 2.3 | Up |
| ASKMDTCSSNL | F2R/PAR1 (S406) | unknown | G protein | 0.3 | 0.5 | Down |
Abbreviations: autoP, autophosphorylated; CK2, casein kinase 2; Ctrl, control, refers to activity of the kinase in the untransfected Jok-1 cells; RTK, receptor tyrosine kinase; TF, transcription factor; TK, tyrosine kinase; TR, transcription repressor.
The table lists peptide substrates whose phosphorylation status is different in Jok-1 cells transfected with the E1A or E1B isoforms of CD5, corresponding protein substrates, kinases whose activity is altered and the ratio of activity of the kinase in the transfected cells compared with untransfected Jok-1 cells (according to phosphositeplus database at http://www.phosphosite.org). Ratio: refers to the ratio of activity of the kinase in Jok-E1A/E1B cells compared with Jok-1 cells. The analyses were carried out in triplicates for each cell line and the analysis repeated on two separate occasions. Differences were analyzed using student’s t-test. P<0.05 are considered significant and shown in the table. Change: indicates whether activity of the kinase in question was upregulated (Up), or downregulated (Down).
Figure 3.
Key signaling pathways affected by CD5 expression. (a) A cartoon representing the major kinases and signaling pathways whose activities are affected by CD5 expression in Jok-1 B cells. Only the major kinases and signaling pathways are shown based on the kinome array analysis and WB in the current study and data from the literature. (b) WB showing phosphorylation (top) and total protein levels (bottom) of Akt and S6K in Jok-1, Jok-E1A and Jok-E1B cells. (c) Immunoprecipitation with anti-CD5 mAb in Jok-E1A cells reveals that CD5 associates with Lyn, the p85 regulatory unit of PI3K, c-Cbl and Vav1. Representative of three independent experiments. ITAM, immune receptor tyrosine-based activation motifs; TK, tyrosine kinase; WB, western blotting.
Comparing the effects of membrane versus cytoplasmic CD5 revealed not only the overlaps between the effects of the two isoforms on kinase activation but also the differences in how they impact signaling (Table 3). Both isoforms activated the Ca2+-dependent Ras/Erk, PKC and PI3K/Akt/mTOR pathways.
Table 3. Altered phosphorylation of kinase substrates by CD5 expression in Jok-1 B cells.
| Kinase |
CD5-E1A |
CD5-E1B |
||
|---|---|---|---|---|
| Up | Down | Up | Down | |
| PI-3K/Akt/mTOR | 6 | 0 | 3 | 0 |
| CaMkII | 4 | 0 | 1 | 0 |
| Cell cycle (CDK, CDC) | 12 | 0 | 15 | 0 |
| CK1/CK2 | 2 | 4 | 4 | 1 |
| GSK3B | 2 | 0 | 5 | 0 |
| NF-κB | 1 | 0 | 1 | 0 |
| Jak/STAT | 1 | 0 | 0 | 0 |
| Ras-Erk | 6 | 0 | 9 | 1 |
| PKA | 9 | 0 | 11 | 0 |
| PKC | 11 | 0 | 11 | 3 |
| Src kinases | 9 | 0 | 12 | 0 |
This table lists the number of target peptide substrates whose phosphorylation status is upregulated (Up) or down-regulated (Down) in Jok-E1A and Jok-E1B cells when compared with untransfected Jok-1 cells. The analysis was carried out as described in Table 2 legend and is drawn on data summarized in the same Table.
CD5 expression impacts the Ca2+ pathway
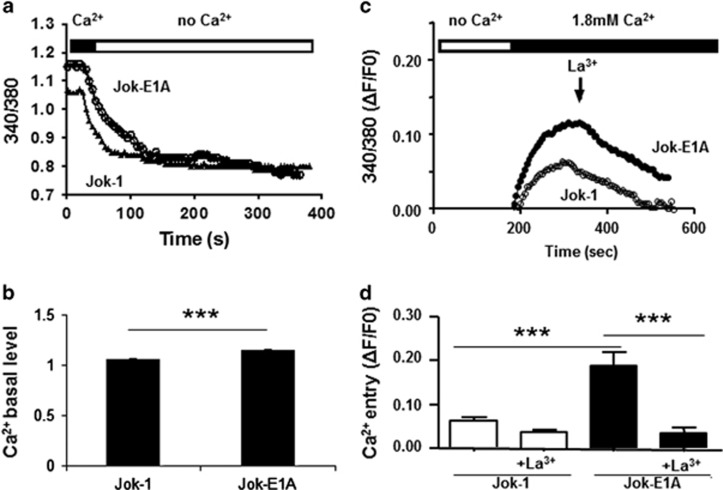
Kinome analysis indicated that constitutive Erk1/2 phosphorylation in CD5+ B cells is dependent on the Ca2+ pathway. To verify this observation, we carried out single-cell video microscopy and observed an elevated resting initial fluorescence ratio, suggesting an increase in the basal level of iCa2+ in Jok-E1A cells compared with Jok-1 cells (340/360: 1.155±0.009, n=1723, in Jok-E1A cells versus 1.067±0.007, n=1623, in Jok-1 cells; P<0.001, Figures 4a and b). Jok-E1B cells were excluded from this analysis because these cells constitutively express the fluorescent marker GFP. On the basis of the observation that such effects could be reversed when Ca2+ was depleted from the media in the absence of stimulation (Figure 4b), we next assessed whether this increase could be the result of elevated constitutive extracellular Ca2+ entry. To test this, we carried out Ca2+ repletion experiments followed by the addition of the non-selective plasma membrane Ca2+ channel blocker La3+. The results revealed that with Ca2+ repletion, iCa2+ increased in resting Jok-E1A cells (ΔF/F0: 0.16±0.01 Jok-E1A cells versus 0.08±0.01 Jok-1 cells, P<0.001) (Figures 4c and d). In addition, the experiments indicated that this effect can be reversed with La3+. These findings confirm the hypothesis that the effect of CD5 is dependent on membrane Ca2+ channels.
Figure 4.
CD5 expression modulates the Ca2+ pathway in B cells. (a) CD5 expression in Jok-E1A cells increases the basal levels of intracellular Ca2+ (iCa2+) compared with Jok-1 cells. (b) Histograms representing basal levels of iCa2+ in Jok-1 and Jok-E1A cells. The increase in basal iCa2+ in Jok-E1A cells is sensitive to extracellular Ca2+ depletion (no Ca2+), as can be noted in a and confirmed in c. Re-addition of extracellular Ca2+ to resting Jok-1 and Jok-E1A cells as shown in c reveals a high extracellular and constitutive Ca2+ influx in Jok-E1A cells. This influx can be reversed in the presence of lanthanum (La3+); ratios are normalized to basal values (F0), indicated as (ΔF/F0). The mean and s.e.m. of the ΔF/F0 values in c are from six independent experiments presented in histograms in d. *** indicates P<0.001 values for the difference between the two cell lines, as determined using Student’s t-test.
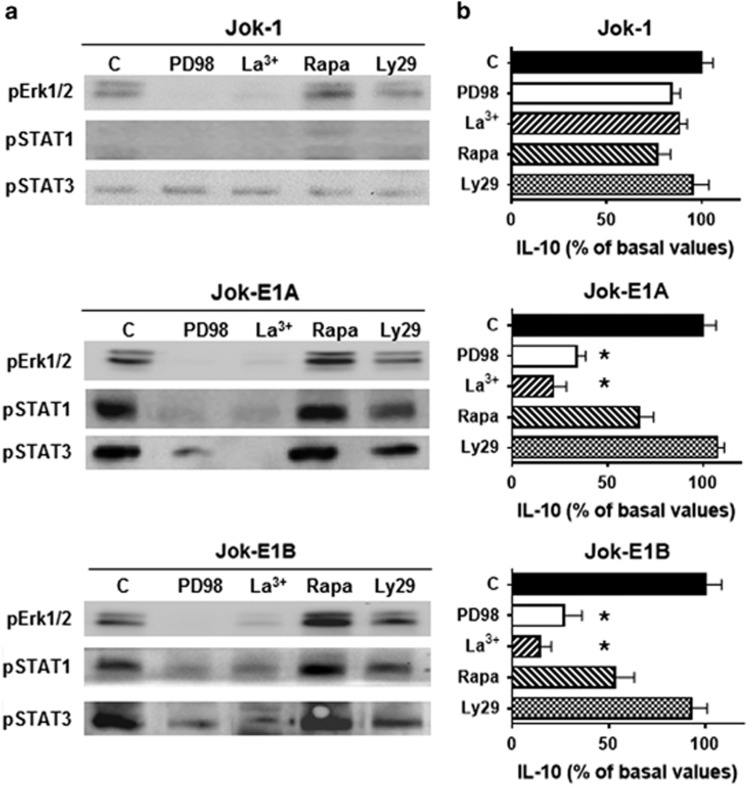
We next carried out inhibition experiments to confirm the dependence of Erk1/2 phosphorylation by CD5 on Ca2+ using PD98059 and La3+. PD98059 inhibits MEK/Erk activation, while La3+ inhibits extracellular Ca2+ entry. In addition, as increases in iCa2+ and Ca2+ influx in lymphocytes may involve the PI3K/Akt/mTOR pathway, which is activated in resting CD5+ B cells as shown in this study, pErk1/2 activation was evaluated in the presence of LY294002, which inhibits PI3K, and rapamycin, which inhibits mTor.33, 34 Interestingly, LY294002 and rapamycin had no effects on pErk1/2 in contrast to PD98059 and La3+ (Figure 5a). To confirm this observation, we assessed whether the phosphorylation of STAT1/3 S727 also occurred independent of the PI3K/mTor pathway.35 Again, PD98059 and La3+, but not LY294002 or rapamycin, inhibited pSTAT1/3 S727 in Jok-E1A/E1B cells. Furthermore, PD98059 and La3+ also inhibited IL-10 production (Figure 5b). These data, therefore, indicate that iCa2+ and constitutive Erk1/2-STAT1/3 phosphorylation increase when CD5 is expressed in B cells, bypassing the PI3K/Akt/mTOR pathway, and this may result from the transient upregulation of Ca2+ membrane channel(s).
Figure 5.
CD5 promotes the phosphorylation of Erk1/2 and STAT1/STAT3 S727 and IL-10 production, which are dependent on extracellular Ca2+ entry. (a) Analysis of constitutive Erk1/2 phosphorylation, STAT1 S727 phosphorylation, STAT3 S727 phosphorylation and (b) IL-10 production in Jok-1, Jok-E1A and Jok-E1B cells after 48 h of culture in the presence of PD98059 (at 50 μM for WB and at 100 μM for IL-10 production), lanthanum (La3+), rapamycin (Rapa) or Ly294002 (Ly29). PD98059 inhibits MEK1 and 2; La3+ blocks extracellular Ca2+ entry; rapamycin inhibits PI3-K/mTOR; and Ly294002 inhibits PI3K/Akt. Cells cultured without inhibitors are used as controls and marked ‘c’. IL-10 levels produced by cells cultured either alone or with the indicated inhibitors were determined using ELISA, and IL-10 levels are expressed as the percentage of basal values; % reduction is presented as the mean and s.e.m. for three independent experiments. The basal value of IL-10 was 32±6.9 pg/ml in Jok-1 cells and 105±8.7 pg/ml for CD5-transfected cells. * indicates P<0.05 for IL-10 production levels in the presence of a given inhibitor compared with cultured cells without inhibitors as determined by the Mann–Whitney U-test. ELISA, enzyme-linked immunosorbent assay. WB, western blotting.
CD5 expression alters the transcriptome of B lymphocytes
To provide further insight into the impact of CD5 on B cell biology, we analyzed the transcriptome of the Jok-E1A and Jok-E1B cell lines compared with the Jok-1 cell line using a whole-human genome oligonucleotide microarray. The analyses revealed that the expression levels of 621 unique genes were altered in Jok-E1A cells compared with Jok-1 cells. Specifically, the expression of 502 (80.8%) genes increased by >1.5-fold in CD5-E1A cells compared with Jok-1 cells, while the expression of 119 (19.1%) genes decreased by >1.5-fold in CD5-E1A cells compared with Jok-1 cells. These results reveal the similarities between some of the altered genes and those identified in a previous study in Daudi B cells transfected with CD5 (Supplementary Data). Thus, among those genes whose expression was altered >2-fold, the expression of 45 genes was found to be upregulated and seven were downregulated in both cell lines. Some of the genes demonstrating altered expression were genes encoding cytokines and chemokines (IL-10, IL2RG and CCL3), signaling molecules (MKNK2 and RGS1), apoptosis inhibitors (Bcl-2), transcription factors (NF-KB2, Spi-C) and cell surface receptors (CD83, CD74, CD54/ICAM1 and CD69).10 With the exception of 4 genes (TRIM68, FRDM6, DYNLRB1 and FLJ11710), no differences were observed between Jok-E1A and Jok-E1B cells.
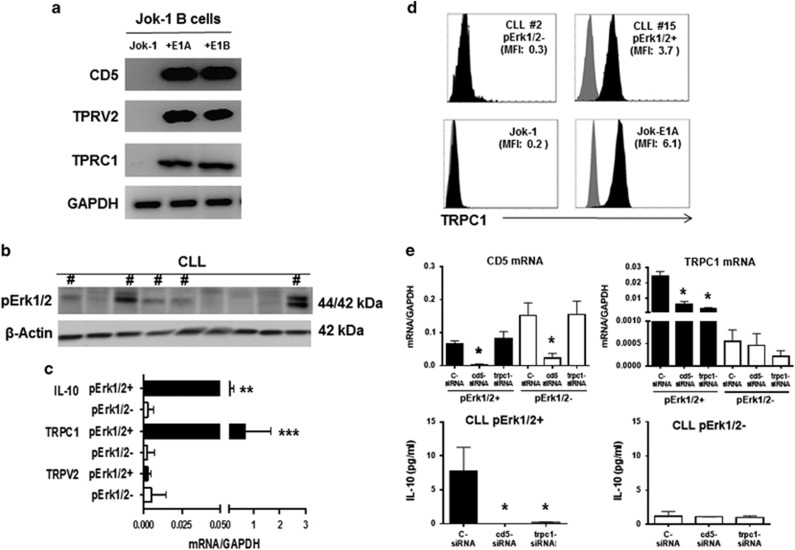
Analysis of alterations in Ca2+-permeable channel expression revealed upregulation of genes encoding the cationic channel TRPV2 and TRPC1 in both Jok-E1A and Jok-E1B cells compared with Jok-1 cells. Upregulation of both the TRPV2 and TRPC1 genes was confirmed using RT-PCR in Jok-E1A/E1B cells (Figure 6a).
Figure 6.
TRPC1 regulates extracellular Ca2+ entry by CD5 in Jok-1 B cells and B cells from Erk1/2+ B-CLL patients. (a) Transcripts of CD5, TRPV2, TRPC1 and GAPDH in Jok-1, Jok-E1A and Jok-E1B B cells as determined using RT-PCR. (b) B-CLL patients were divided into two groups based on the phosphorylation status of the Erk1/2 protein as assessed using WB. # indicates B cells from CLL patients positive for constitutively phosphorylated Erk1/2. (c) Levels of IL-10 (n=26 patients), TRPC1 (n=26) and TRPV2 (n=12) transcripts relative to GAPDH mRNA as determined using real-time PCR in B cells from pErk1/2+ and pErk1/2− B-CLL patients. ** indicates P<0.01 and *** indicates P<0.001 for the relative levels of IL-10 and TRPC1 transcripts between pErk1/2+ and pErk1/2− B-CLL patients, respectively, as determined using Student’s t-test. (d) Representative FACS plot of extracellular TRPC1 protein expression (black histograms) in CLL#2 (pErk1/2−), CLL#15 (pErk1/2+), Jok-1, and Jok-E1A cells. MFI, indicated for each cell; isotype controls are presented as gray histograms. (e) Histograms depicting levels of TRPC1, CD5 and IL-10 transcripts in B cells from pErk1/2+ (black histograms) and pErk1/2− (white histograms) B-CLL patients following transfection with c-siRNA, CD5-siRNA and TRPC1-siRNA. The top two histograms depict relative levels of CD5 (left) and TRPC1 (right) transcripts relative to GAPDH mRNA. The lower two histograms represent relative levels of IL-10 transcripts relative to GAPDH in pErk1/2+ (left) and pErk1/2− patients (right). B cells from three pErk1/2+ and three pErk1/2− B-CLL patients were studied in these experiments. * indicates P<0.05 for the levels of CD5, TRPC1 and IL-10 transcripts observed when using siRNA targeting CD5 or TRPC1 compared with c-siRNA. Statistical analyses were carried out using Student’s t-test. CLL, chronic lymphocytic leukemia; c-siRNA, control siRNA; MFI; mean fluorescence intensity; RT-PCR, PCR with reverse transcription; WB, western blotting.
CD5 drives TRPC1 expression and IL-10 production in pErk1/2-positive B-CLL cells
To verify that CD5 drives IL-10 production through the upregulation of TRPC1 and/or TRPV2, B cells from 26 patients with CLL were segregated into two groups based on the phosphorylation status of pErk1/2 as determined using WB (Figure 6, and data not shown). As expected, pErk1/2 activation was associated with IL-10 production (P<0.01) in B-CLL cells (Figure 6c). TRPV2 was detectable at low levels in B cells from some CLL patients, but no differences were observed between pErk1/2+ and pErk1/2− B-CLL in terms of TRPV2 transcripts as assessed using qRT-PCR (Figure 6c). In contrast, TRPC1 transcripts were detectable at significantly higher levels in pErk1/2+ B-CLL patients compared with pErk1/2− B-CLL patients (P<0.001). Notably TRPV2 and TRPC1 were not detectable in B or T cells from healthy controls (data not shown). Flow cytometry confirmed that the TRPC1 protein was expressed on B cells from pErk1/2+ CLL patients (MFI TRPC1: 1.9±1.3 in pErk1/2+ CLL patients versus 0.4±0.1 in pErk1/2− CLL patients, P<0.05) (Figure 6d). These data are consistent with the expression of TRPC1 in the Jok-E1A cell line (MFI: 5.9±2.4 versus 0.4±0.3 in Jok-1 cells, P<0.01). The levels of Erk1/2 phosphorylation and TRPC1 expression were independent of age, sex, CLL stage, disease progression, CD38 expression or the cytogenetic status of the patients.
Finally, to confirm that CD5 induces TRPC1 expression and promotes IL-10 production, we employed siRNA targeting CD5 and TRPC1 to transfect B-CLL cells from 3 pErk1/2+ and 3 pErk1/2− patients. After 2 days of culture, reductions in CD5 were evaluated at the mRNA level in CD5 and TRPC1 siRNA transfected B-CLL cells from both groups. The expression of TRPC1 was reduced with cd5-siRNA and TRPC1-siRNA in pErk1/2+ B-CLL cells (Figure 6e). Both siRNAs resulted in the inhibition of IL-10 production in pErk1/2+ B-CLL cells. Collectively, these results indicate that in pErk1/2+ B-CLL cells, CD5 promotes IL-10 production through a BCR-independent Ca2+-dependent pathway that involves the non-selective Ca2+ channel protein TRPC1.
DISCUSSION
This study reveals that CD5 directly alters the biology of B cells and induces IL-10 production. The molecular pathways through which CD5 modulates B-cell biology appear to be mediated through Erk1/2 activation in a Ca2+-dependent pathway and involve the non-selective Ca2+ channel TRPC1. Interestingly, the changes induced by CD5 are distinct from the negative modulating effects that it exerts on BCR signaling. Furthermore, the data reveal that pathways induced by CD5 in B cells are similar to those activated in B-CLL, as induced CD5 expression replicates several characteristics of neoplastic B cells, including constitutive basal Erk1/2 phosphorylation. This observation is consistent with previous studies13, as is the ability of CD5 to activate STAT1/335 and IL-10 production;8 these are all features of neoplastic B-CLL cells. In addition, CD5 expression resulted in the perturbation of Ca2+ homeostasis, leading to increased basal iCa2+.36
Consistent with our observation that the expression of CD5 induces biological changes in a manner that is distinct from its role in modulating effects on BCR-mediated signaling, we observed that CD5 exerts a distinct effect on Ca2+ mobilization in both settings.31 The characteristics noted in CD5+ B cells are similar to those observed in anergic37, 38 B cells and CD5+ transitional B cells.39, 40, 41 This ‘anergic signature’ was previously shown to be a characteristic feature of B-CLL cells.42 Interestingly, the anergic phenotype of B cells was shown to be reversed in hen egg lysozyme (HEL) transgenic mice when the mice were CD5-deficient.24
The current study also provides in-depth analysis of the pathways leading to the constitutive activation of Erk1/2 and IL-10 production in B-CLL cells. Thus, the study shows that a Ca2+ influx-dependent pathway is involved in constitutive Erk1/2 phosphorylation and IL-10 production. Unlike conventional CD5− B2 cells in which Erk1/2 phosphorylation is mediated through Syk/BTK/PLCγ2 and PI3K activation following BCR engagement,43 constitutive Erk1/2 phosphorylation by CD5 occurs independent of this pathway. This was demonstrated through the finding that Syk, BTK and PLCγ2 were not activated in unstimulated CD5+ B cells and that the inhibition of PI3K by LY294002 was ineffective at suppressing constitutive Erk1/2 phosphorylation, which stands in contrast to the effectiveness of the non-selective Ca2+ channel blocker La3+. The newly identified pathway is compatible with the observations that inhibition of Erk1/2 phosphorylation in B-CLL cells does not occur immediately after BTK inhibition44 and that Erk1/2 phosphorylation in leukemic B cells in patients with CLL failed to mobilize Ca2+ upon BCR crosslinking.13, 42
Given that CD5+ B cells in healthy individuals, patients with autoimmune diseases such as systemic lupus erythematosus, and also patients with CLL cells express both isoforms of CD5,45 albeit at different levels, we studied whether these two isoforms differentially impact intracellular signaling. The results indicated that there were no major differences in terms of the effects that the two isoforms exert on intracellular signaling in B cells with the exception that E1B-CD5 cells downregulated the level of CD5 expression on the membrane.12, 45 These results indicate that the 428EYS430 motif is functional in both isoforms, which is in agreement with previous studies showing that the CD5 Y429 is constitutively phosphorylated in B-CLL cells,10 most likely by Lyn,18 and that this phosphorylation has a positive effect on transcription but a negative effect on BCR-mediated signaling.
The mechanisms through which CD5 exerts a dual role in modulating B-cell signaling and biology, however, remain unclear. This is in part due to the capacity of CD5 to activate a large array of kinases and phosphatases, as shown in our current study. Consistent with the inhibitory effects of CD5 on BCR-mediated signaling, we observed that CD5 associates with SHP1 and c-Cbl in resting cells but with CD79a following BCR engagement. The positive effect of CD5 on gene transcription, however, appears to be attributable to the recruitment of key kinases, including Lyn, the p85 unit of PI3K and Vav1. The molecular mechanism(s) through which CD5 modulates Ca2+ homeostasis and the role of TRPC1 in the process have yet to be defined.
The expression of TRP channels has been associated with cancer, and, in particular, TRPC1 overexpression was described in a transformed CD5+ chicken DT-40 cell line and in human B-lymphoblast cell lines.46, 47 In DT-40 cells, TRPC1 was linked to increases in intracellular Ca2+ and the activation of NFAT2,46 a signaling cascade that leads to cytokine/chemokine production in B-CLL cells. Interestingly, mice deficient in TRPC1 have defective B cell functions, similar to those observed in NFAT2-deficient mice.48 Consequently, TRPC1 upregulation in CD5+ B cells may be an important mechanism that promotes B-CLL cell survival.
CONCLUSIONS
This study provides molecular evidence that CD5 expression alters B cell biology, including the constitutive activation of key signaling pathways leading to IL-10 production. Pathways and transcription factors activated by CD5 include those involved in regulating B-cell survival, proliferation, cytokine/chemokine production and transformation. The findings reported in this study facilitate a better understanding of the biology and regulatory properties of CD5+ B cells in health and in diseases, including in patients with B-CLL, and how CD5 may potentially contribute to B-cell abnormalities. These findings may aid in the design of new treatment strategies, particularly for CLL patients identified as refractory to currently available treatments. Such treatment strategies could involve the use of monoclonal antibodies targeting membrane proteins relevant to B-CLL cell transformation, such as TRPC1 or CD5, in the form of mono or combination therapies.28 Alternatively, signaling pathways mediated by CD5 and involved in B-CLL cell transformation may be modulated. For example, high basal Ca2+ levels36 or upstream kinases could be targeted, a strategy that has successfully been employed to treat patients with autoimmune diseases.49
Acknowledgments
This study was supported by a grant from Arthritis Research-UK to RAM and by grants from the “Cancéropole Grand Ouest”, the “Région Bretagne” and the “Ligue contre le cancer” to YR. We thank Catherine Riou and Professor Valérie Ugo (Brest) for the clinical material and Ms Simone Forest and Geneviève Michel for helping with typing the manuscript.
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol 2002; 20: 253–300. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K, K. CD5B cells, a fetal B cell lineage. Adv Immunol 1994; 55: 297–339. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Formica AM, Colombo MJ, Ichikawa D, Shinton SA, Brill-Dashoff J et al. B cells generated by B-1 development can progress to chronic lymphocytic leukemia. Ann NY Acad Sci 2015; 1362: 250–255. [DOI] [PubMed] [Google Scholar]
- Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood 2002; 100: 4537–4543. [DOI] [PubMed] [Google Scholar]
- Burdin N, Rousset F, Banchereau J. B-cell-derived IL-10: production and function. Methods 1997; 11: 98–111. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19: 683–765. [DOI] [PubMed] [Google Scholar]
- Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med 1992; 175: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabayashi A, Hirokawa M, Miura AB. The role of interleukin-10 (IL-10) in chronic B-lymphocytic leukemia: IL-10 prevents leukemic cells from apoptotic cell death. Int J Hematol 1995; 62: 99–106. [DOI] [PubMed] [Google Scholar]
- Fayad L, Keating MJ, Reuben JM, O’Brien S, Lee BN, Lerner S et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood 2001; 97: 256–263. [DOI] [PubMed] [Google Scholar]
- Gary-Gouy H, Sainz-Perez A, Marteau JB, Marfaing-Koka A, Delic J, Merle-Beral H et al. Natural phosphorylation of CD5 in chronic lymphocytic leukemia B cells and analysis of CD5-regulated genes in a B cell line suggest a role for CD5 in malignant phenotype. J Immunol 2007; 179: 4335–4344. [DOI] [PubMed] [Google Scholar]
- Garaud S, Le Dantec C, de Mendoza AR, Mageed RA, Youinou P, Renaudineau Y. IL-10 production by B cells expressing CD5 with the alternative exon 1B. Ann NY Acad Sci 2009; 1173: 280–285. [DOI] [PubMed] [Google Scholar]
- Garaud S, Morva A, Lemoine S, Hillion S, Bordron A, Pers JO et al. CD5 promotes IL-10 production in chronic lymphocytic leukemia B cells through STAT3 and NFAT2 activation. J Immunol 2011; 186: 4835–4844. [DOI] [PubMed] [Google Scholar]
- Apollonio B, Scielzo C, Bertilaccio MT, Ten Hacken E, Scarfò L, Ranghetti P et al. Targeting B-cell anergy in chronic lymphocytic leukemia. Blood 2013; 121: 3879–3888. [DOI] [PubMed] [Google Scholar]
- Li P, Grgurevic S, Liu Z, Harris D, Rozovski U, Calin GA et al. Signal transducer and activator of transcription-3 induces MicroRNA-155 expression in chronic lymphocytic leukemia. PLoS One 2013; 8: e64678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrias MR, Grønlund J, Padilla O, Madsen J, Holmskov U, Lozano F. The scavenger receptor cysteine-rich (SRCR) domain: an ancient and highly conserved protein module of the innate immune system. Crit Rev Immunol 2004; 24: 1–37. [DOI] [PubMed] [Google Scholar]
- Gary-Gouy H, Bruhns P, Schmitt C, Dalloul A, Daëron M, Bismuth G. The pseudo-immunoreceptor tyrosine-based activation motif of CD5 mediates its inhibitory action on B-cell receptor signaling. J Biol Chem 2000; 275: 548–556. [DOI] [PubMed] [Google Scholar]
- Gary-Gouy H, Lang V, Sarun S, Boumsell L, Bismuth G. In vivo association of CD5 with tyrosine-phosphorylated ZAP-70 and p21 phospho-zeta molecules in human CD3+ thymocytes. J Immunol 1997; 159: 3739–3747. [PubMed] [Google Scholar]
- Tibaldi E, Brunati AM, Zonta F, Frezzato F, Gattazzo C, Zambello R et al. Lyn-mediated SHP-1 recruitment to CD5 contributes to resistance to apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia 2011; 25: 1768–1781. [DOI] [PubMed] [Google Scholar]
- Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol 1999; 19: 2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch A, Campbell KS, Reth M. Interaction of the CD5 cytoplasmic domain with the Ca2+/calmodulin-dependent kinase IIdelta. Eur J Immunol 1998; 28: 2167–2177. [DOI] [PubMed] [Google Scholar]
- Raman C, Kuo A, Deshane J, Litchfield DW, Kimberly RP. Regulation of casein kinase 2 by direct interaction with cell surface receptor CD5. J Biol Chem 1998; 273: 19183–19189. [DOI] [PubMed] [Google Scholar]
- Wong SC, Chew WK, Tan JE, Melendez AJ, Francis F, Lam KP. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells. J Biol Chem 2002; 277: 30707–30715. [DOI] [PubMed] [Google Scholar]
- Gary-Gouy H, Harriague J, Dalloul A, Donnadieu E, Bismuth G. CD5-negative regulation of B cell receptor signaling pathways originates from tyrosine residue Y429 outside an immunoreceptor tyrosine-based inhibitory motif. J Immunol 2002; 168: 232–239. [DOI] [PubMed] [Google Scholar]
- Hippen KL, Tze LE, Behrens TW. CD5 maintains tolerance in anergic B cells. J Exp Med 2000; 191: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh K, Poppema S, Peppelenbosch MP, Visser L. Extracellular ligation-dependent CD45RB enzymatic activity negatively regulates lipid raft signal transduction. Blood 2009; 113: 594–603. [DOI] [PubMed] [Google Scholar]
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011; 117: 5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross HJ, Merling A, Moldenhauer G, Schwartz-Albiez R. Ecto-sialyltransferase of human B lymphocytes reconstitutes differentiation markers in the presence of exogenous CMP-N-acetyl neuraminic acid. Blood 1996; 87: 5113–5126. [PubMed] [Google Scholar]
- Loisel S, André PA, Golay J, Buchegger F, Kadouche J, Cérutti M et al. Antitumour effects of single or combined monoclonal antibodies directed against membrane antigens expressed by human B cells leukaemia. Mol Cancer 2011; 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devauchelle-Pensec V, Cagnard N, Pers JO, Youinou P, Saraux A, Chiocchia G. Gene expression profile in the salivary glands of primary Sjogren’s syndrome patients before and after treatment with rituximab. Arthritis Rheum 2010; 62: 2262–2271. [DOI] [PubMed] [Google Scholar]
- Richards JD, Davé SH, Chou CH, Mamchak AA, DeFranco AL. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J Immunol 2001; 166: 3855–3864. [DOI] [PubMed] [Google Scholar]
- Nédellec S, Renaudineau Y, Bordron A, Berthou C, Porakishvili N, Lydyard PM et al. B cell response to surface IgM cross-linking identifies different prognostic groups of B-chronic lymphocytic leukemia patients. J Immunol 2005; 174: 3749–3756. [DOI] [PubMed] [Google Scholar]
- Roa NS, Ordoñez-Rueda D, Chávez-Rios JR, Raman C, García-Zepeda EA, Lozano F et al. The carboxy-terminal region of CD5 is required for c-CBL mediated TCR signaling downmodulation in thymocytes. Biochem Biophys Res Commun 2013; 432: 52–59. [DOI] [PubMed] [Google Scholar]
- Buhl AM, Pleiman CM, Rickert RC, Cambier JC. Qualitative regulation of B cell antigen receptor signaling by CD19: selective requirement for PI3-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J Exp Med 1997; 186: 1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fang Y. A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling. Biochem Pharmacol 2002; 64: 1071–1077. [DOI] [PubMed] [Google Scholar]
- Frank DA, Mahajan S, Ritz J. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Invest 1997; 100: 3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggen AF, Pillai SY, Kil LP, van Zelm MC, van Dongen JJ, Hendriks RW et al. Basal Ca(2+) signaling is particularly increased in mutated chronic lymphocytic leukemia. Leukemia 2015; 29: 321–328. [DOI] [PubMed] [Google Scholar]
- Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity 1997; 6: 419–428. [DOI] [PubMed] [Google Scholar]
- Chumley MJ, Dal Porto JM, Cambier JC. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J Immunol 2002; 169: 1735–1743. [DOI] [PubMed] [Google Scholar]
- Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood 2005; 105: 4390–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T et al. STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat Immunol 2011; 12: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limnander A, Zikherman J, Lau T, Leitges M, Weiss A, Roose JP. Protein kinase Cdelta promotes transitional B cell-negative selection and limits proximal B cell receptor signaling to enforce tolerance. Mol Cell Biol 2014; 34: 1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood 2008; 112: 188–195. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Ogasawara T, Yasuyama M. Activation of extracellular signal-regulated kinase through B-cell antigen receptor in B-cell chronic lymphocytic leukemia. Int J Hematol 2002; 75: 508–513. [DOI] [PubMed] [Google Scholar]
- Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia 2014; 28: 649–657. [DOI] [PubMed] [Google Scholar]
- Garaud S, Le Dantec C, Berthou C, Lydyard PM, Youinou P, Renaudineau Y. Selection of the alternative exon 1 from the cd5 gene down-regulates membrane level of the protein in B lymphocytes. J Immunol 2008; 181: 2010–2018. [DOI] [PubMed] [Google Scholar]
- Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M et al. Transient receptor potential 1 regulates capacitative Ca(2+) entry and Ca(2+) release from endoplasmic reticulum in B lymphocytes. J Exp Med 2002; 195: 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roedding AS, Li PP, Warsh JJ. Characterization of the transient receptor potential channels mediating lysophosphatidic acid-stimulated calcium mobilization in B lymphoblasts. Life Sci 2006; 80: 89–97. [DOI] [PubMed] [Google Scholar]
- Yildirim E, Carey MA, Card JW, Dietrich A, Flake GP, Zhang Y et al. Severely blunted allergen-induced pulmonary Th2 cell response and lung hyperresponsiveness in type 1 transient receptor potential channel-deficient mice. Am J Physiol Lung Cell Mol Physiol 2012; 303: L539–L549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Laurence A, McInnes IB. Back to the future: oral targeted therapy for RA and other autoimmune diseases. Nat Rev Rheumatol 2013; 9: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.