Abstract
Urokinase-type plasminogen activator receptor (uPAR), is a multifunctional receptor on cell surface, widely present in endothelial cells, fibroblasts, and a variety of malignant cells. Current studies have suggested that uPAR overexpressed on synovial tissues or in synovial fluid or plasma in patients with rheumatoid arthritis (RA). However, there are limited researches regarding the role of uPAR on fibroblast-like synoviocytes of rheumatoid arthritis (RA-FLSs) and its underlying mechanisms. Here, our studies show that the expression of uPAR protein was significantly higher in fibroblast-like synoviocytes (FLSs) from RA than those from osteoarthritis or traumatic injury patients. uPAR gene silencing significantly inhibited RA-FLSs cell proliferation, restrained cell transformation from the G0/G1 phase to S phase, aggravated cell apoptosis, interfered with RA-FLSs cell migration and invasion, and reduced activation of the PI3K/Akt signaling pathway, which may be associated with β1-integrin. Cell supernatants from uPAR gene-silenced RA-FLSs markedly inhibited the migration and tubule formation ability of the HUVECs (a human endothelial cell line). Therefore, we demonstrate that uPAR changes the biological characteristics of RA-FLSs, and affects neoangiogenesis of synovial tissues in patients with RA. All of these may be associated with the β1-integrin/PI3K/Akt signaling pathway. These results imply that targeting uPAR and its downstream signal pathway may provide therapeutic effects in RA.
Keywords: angiogenesis, cell viability, fibroblast-like synoviocytes, rheumatoid arthritis, uPAR
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by synovitis and progressive joint destruction. Several cell types, including macrophages, T cells, B cells, fibroblast-like synoviocytes (FLSs) and the complex interaction of many pro-inflammatory cytokines are involved in this process.1 Among the inflammatory cell populations that might participate in RA pathogenesis, FLSs are likely responsible for initiating and driving the inflammatory process, and are also the main effector cells responsible for the invasive nature of rheumatoid synovium.2 Recent studies have found that the RA-FLSs acquire unique morphology and have some characteristics of tumor-like cells when activated in the chronic inflammatory environment. Eventually these cells escape the growth limits of contact inhibition, enhancing migration and acquiring invasive ability, prompting angiogenesis. This process contributes to RA disease progression and ultimately to joint destruction.3
Urokinase-type plasminogen activator receptor (uPAR), a multifunctional cell surface receptor, is widely present on endothelial cells,4 fibroblasts,5, 6 and a variety of malignant cells.7, 8, 9 The combination of uPAR and its ligand urokinase-type plasminogen activator (uPA) not only activates the fibrinolytic system, degrading various matrix proteins and accelerating invasive metastasis, but also affects multiple signaling pathways through binding with other transmembrane proteins, regulating the transcription and expression of downstream signaling proteins and altering the biological properties of the cells.10, 11
Current studies have suggested that uPAR expression on synovial tissues or in synovial fluid or plasma in patients with RA, osteoarthritis (OA) and other inflammatory joint diseases is higher than in healthy subjects, and the elevated expression of uPAR is more obvious in patients with RA compared with OA and other inflammatory diseases.12 It has been confirmed using an in vitro system that multiple cells in inflammatory joint disease, such as RA and OA, could synthesize and overexpress uPAR; this is most pronounced in synovial lining layer cells of RA.13 However, the role uPAR has played on RA-FLSs and its underlying mechanisms remain unclear.
In this investigation, we report that elevated expression of uPAR markedly affects the proliferation, migration, and invasiveness of RA-FLSs, as wells as the process of angiogenesis in the synovial tissues of RA patients. We further show that the PI3K/Akt signaling pathway may be responsible for mediating effects of uPAR on FLSs and the process of neoangiogenesis.
Materials and methods
Patients
All synovial specimens were taken from patients who underwent knee replacement surgery, knee synovial debridement or meniscal repair surgery in the Third Affiliated Hospital at Sun Yat-sen University, from March 2011 to October 2013. Among them were eight patients with RA (consistent with the criteria of the American College of Rheumatology),14 four patients with OA (in line with 1995 American College of Rheumatology classification criteria of knee OA)15 and three patients with severe trauma who had, no other joint abnormalities or systemic diseases. All patients were age and sex matched and all samples were obtained from tissue otherwise discarded. The study was approved by an ethics board and got the patients’ informed consent.
Synovial cell culture
Synovial tissues were dissected free of fat, blood vessels and fibrous tissue, rinsed 2–3 times with phosphate-buffered solution (PBS), repeatedly shredded into ~1 mm3 pieces, moved to flasks and tiled uniformly with spacing of about 5 mm. The flasks containing an appropriate amount of DMEM (Hyclone, Thermo Fisher scientific, Waltham, MA, USA) culture medium supplemented with 10% fetal bovine serum (FBS; Hyclone, Thermo Fisher scientific) and were placed upright for tissue adherence in 37 °C, 5% CO2 thermostatic incubator. They were then laid flat slowly after 4 h, to continue culture. Cell culture medium was changed every 2–3 days. The monolayer (coverage >80%) was removed by a trypsin–EDTA solution and passaged 3–5 times for use in the experiments. The cells were considered as type B fibroblast-like synovial cells if the typical spindle-shaped, fibroblast-like appearance was present and anti-CD55 staining was positive. Such features were enriched by >90% of cells in passage 3. All FLS populations were used within the fifth passage in culture.
Transfection, uPAR interference and uPAR overexpression
The chemically synthesized small interference RNA (siRNA) used in this study was purchased from GenePharma (Shanghai, China) and siRNA reagents consisted of a pool of five siRNA oligonucleotides (Table 1). RA-FLSs were seeded in 6-well plates (1 × 105 cells per well for RNA extraction, western blot, ELISA, cell cycle, apoptosis, migration and invasion assays) or in 96-well plates (2.5 × 103 cells per well for proliferation) 24 h before transfection. When RA-FLSs reached 60–70% confluence, siRNAs were transfected using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) at a final concentration of 40 nM according to the manufacturer’s instructions. The interference efficiency for each RA-FLSs sample should be evaluated by real-time quantitative PCR at 24 h and western blot analysis at 48, 72 and 96 h to ensure accuracy of the follow-up tests. The supernatants of transfected RA-FLSs were replaced after 24 h with serum-free DMEM, and incubated for another 48 h. Then, the conditioned media of transfected RA-FLSs for total 72 h were collected, centrifuged, filtered through a 0.22-mm filter (Millipore, Billerica, MA, USA) and stored in fresh tubes at −20 °C, until used for Cytokine ELISA tests, the human vein endothelial cells (HUVECs) migration and tubule formation assays.
Table 1. Sequence of siRNA sequences and primer sets used in this study.
| Oligonucleotides | Sequences |
|---|---|
| uPAR-siRNA-559 | F:5′-GCCGUUACCUCGAAUGCAUTT-3′ |
| R:5′-AUGCAUUCGAGGUAACGGCTT-3′ | |
| uPAR-siRNA-1048 | F:5′-GUGACGCCUUCAGCAUGAATT-3′ |
| R:5′-UUCAUGCUGAAGGCGUCACTT-3′ | |
| uPAR-siRNA-1392 | F:5′-GUGUGAAGCAGAAGAGAAATT-3′ |
| R:5′-UUUCUCUUCUGCUUCACACTT-3′ | |
| NC-siRNA | F:5-UUCUCCGAACGUGUCACGUTT-3′ |
| R:5′-ACGUGACACGUUCGGAGAATT-3′ | |
| GAPDH-siRNA | F:5′-GUAUGACAACAGCCUCAAGTT-3′ |
| R:5′-CUUGAGGCUGUUGUCAUACTT-3′ | |
| uPAR-primer | F: 5′-TGTAAGACCAACGGGGATTGC-3′ |
| R: 5′-AGCCAGTCCGATAGCTCAGG-3′ | |
| GAPDH-primer | F: 5′-AAGGTGAAGGTCGGAGTCAAC-3′ |
| R: 5′-GGGGTCATTGATGGCAACAATA-3′ |
Lentiviral transfection
The lentiviral vector was obtained from Addgene (Cambridge, MA, USA), and lentiviral expression vectors and production were constructed according to ViraPower Lentiviral Expression Systems. RA-FLSs cells were plated in each well of a 6-well plate as above. After reaching 50–70% confluence, media were changed to complete growth medium without antibiotics, containing lentiviral stock, and 10 μg/ml polybrene (Sigma-Aldrich, St Louis, MO, USA). Polybrene was used to improve infection efficiency. After incubation for 24 h, supernatants were replaced by complete DMEM and cultured for another 24 h, and observed under fluorescence microscope. Cells with GFP expression were harvested for further experiments. Each experiment was performed in triplicate.
Quantitative real-time reverse transcription PCR
Total cellular RNA was extracted using the RNAiso Plus reagent (Takara Bio, Shiga, Japan). Equal amounts of RNA from different samples were reverse-transcribed using the PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio) and underwent PCR reaction operation with SYBR Premix Ex TaqTMII kit (Takara Bio) on the ABI 7000 real-time PCR amplification equipment. The specific primers for quantitative PCR (qPCR) are listed in Table 1. Ct values of each sample were analyzed and 2−ΔΔCt was calculated to indicate relative quantification of target genes to control gene GAPDH.
Western blot analysis
Western blot analysis was performed as follows: total proteins were extracted using Total Protein Extraction Kit (Keygen, Nanjing, China) following the manufacturer’s instructions and deactivated at 100 °C for 5 min. Whole-cell lysates (30 μg) were fractionated on Tris-glycine-buffered 10% SDS-PAGE by electrophoresis and transferred to polyvinylidene fluoride membrane (Millipore). Membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline contained 0.1% Tween-20 at room temperature for 1 h, then incubated overnight at 4 °C with primary antibodies (anti-uPAR antibody, 1:1000 dilution, Cell Signaling, Danvers, MA, USA; anti-p-PI3K antibody, 1:1000 dilution, Millipore Merck, Darmstadt,Germany; anti-PI3K antibody, 1:1000 dilution, Cell Signaling; anti-p-Akt (Ser473) antibody, 1:800 dilution, Cell Signaling; anti-Akt antibody, 1:1000 dilution, Cell Signaling; anti-p-GSK3β antibody, 1:1000 dilution, Cell Signaling; anti-GSK3β antibody, 1:1000 dilution, Cell Signaling; anti-GAPDH, 1:4000 dilution, Bioss, Beijing, China). Subsequently, incubations with horseradish-peroxidase-conjugated secondary antibody were performed for 1 h at room temperature. Enhanced chemiluminescence (ECL, Keygen) was used to detect protein signals and AlphaImager HP was used to perform densitometry, which was analyzed with Alpha View SA software (San Jose, CA, USA). The protein expression was normalized to GAPDH expression.
Cytokines secretion by ELISA
The secretion of uPA and suPAR were analyzed in conditioned media using ELISA kits from R&D Systems (Minneapolis, MN, USA) as recommended by the manufacturer. The conditioned media of transfected RA-FLSs for 72 h was tested in triplicate and cytokine concentrations were determined according to a standard curve from recombinant mice cytokine (R&D System), and results were given in ng/ml.
Proliferation assay by CCK-8
Cell proliferation was measured using a cell counting kit-8 (CCK-8 kit, Yeasen, Guangzhou, China) assay according to the manufacturer’s protocol. Briefly, ~2.5 × 103 RA-FLSs in logarithmic growth phase in a volume of 100 μl DMEM with 10% FBS were planted in each well of 96-well plate. Cells were transfected with uPAR-siRNA following the above method and incubated for 24, 48, 72 and 96 h, or transfected with lentivirus-mediated uPAR overexpression and uPAR-siRNA for 72 h. Then 10 μl of the CCK-8 solution was added to each well and incubated at 37 °C for 3 h. The absorbance (A) at 450 nm was measured to evaluate cell viability.
Cell-cycle analysis by flow cytometry
RA-FLSs transfected for 72 h were digested with 0.25% trypsin, washed twice with cold PBS and fixed with 70% ethanol at 4 °C overnight. Fixed cells were then washed with PBS twice and treated with staining buffer (500 μl PBS containing 0.05 mg/ml propidium iodide (PI) and 0.2 mg/ml RNase A, Sigma-Aldrich) at 37 °C in the dark for 30 min. Samples were analyzed with a flow cytometer (FACS Calibur, BD Bioscience Influx, Franklin Lakes, NJ, USA). Fluorescence emitted from the PI–DNA complex was estimated at 488 nm using ~20 000 cells per sample and analyzed using Modfit software (Verity Software House, Topsham, ME, USA).
Flow cytometric analysis of apoptosis
The Annexin-V-FITC apoptosis detection kit (Keygen) was used to measure apoptosis. In brief, the RA-FLSs were treated for 24 h with SNP (Sodium nitroprusside, Sigma-Aldrich, at a final concentration of 1 mM), which served as apoptosis-inducing agent, and then transfected with uPAR-siRNA. 72 h later, cells were digested with EDTA-free trypsin (Invitrogen) and washed twice with pre-cooled PBS. Approximately 5 × 105 cells were collected and resuspended in a mixture comprising 500 μl of binding buffer, 5 μl of Annexin-V-FITC and 1 μl of PI, according to the protocol of the kit manufacturer, then incubated in the dark at room temperature for 15 min. Finally, the samples were analyzed by flow cytometer (FACS Calibur, BD Bioscience Influx). Annexin-V-FITC and propidium iodide were used to determine the phosphatidylserine exposure on the outer plasma membrane.
Cell migration assay
The 24-well microchemotaxis chamber with a pore size of 8 μm (Corning, Cambridge, MA, USA) was used for cell migration assay. A total of 1 × 105 cells transfected for 48 h were resuspended in serum-free medium and seeded in the upper chamber. Overall, 600 μl culture medium containing 10% FBS was added into the lower chamber as chemoattractant. After 24 h incubation at 37 °C, cells remaining on the upper filter were removed with cotton swabs and the ones adhering to the lower surface of the filter were fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS for 20 min, stained with crystal violet for 15 min and counted under a microscope. Data were presented as mean number of migrated cells in five randomly selected fields at × 100 magnification in every membrane. Every well was studied at least three times.
Cell invasion assay
Cell invasion was determined using the same modified two-chamber plates as the migration assay described above. To evaluate the cell invasion, the 8 μm pore filter separating the well was coated with 300 μl/ml Matrigel (BD Biosciences, San Jose, CA, USA) in serum-free DMEM. After air-dry and sterilization, 600 μl culture medium containing 10% FBS into the lower chamber and 1 × 105 cells, which transfected for 48 h resuspended in serum-free DMEM into the upper were seeded, respectively. After 24 h of culture at 37 °C, the subsequent operations are similarly conducted as the migration assays.
Immunofluorescence staining and confocal imaging
RA-FLSs were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 10 min, permeabilized using 0.1% Triton X-100. After washing cells with PBS, samples were incubated with 1% BSA for 1 h to block non-specific binding. Then, cells were incubated with primary antibody (β1-integrin from Abcam (Cambridge, MA, USA); uPAR from Cell Signaling) overnight at 4 °C, washed, and incubated with the respective secondary antibody (Alexa Fluor conjugates 488, 546, Invitrogen) for 1 h at room temperature. Dilutions ratios were 1:200 and 1:1000 for the primary and secondary antibodies, respectively. Lastly, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Invitrogen) was used to stain cell nuclei at a working concentration of 100 ng/ml. After washing, the sections were viewed and captured using a Nikon Eclipse Ti A1R-A1 confocal microscope with 40 × oil-immersion objective. Images were acquired and analyzed using ImageJ software (NIH, Bethesda, MD, USA), finally processed with Photoshop program (Adobe, San Jose, CA, USA).
Culture of HUVECs
HUVECs were obtained as a kind gift from Dr Xiaoxian Qian (Division of Cardiology, The Third Affiliated Hospital at Sun Yat-Sen University). Passage and count operation of HUVECs were similarly achieved as FLSs. Cells were incubated in complete medium containing 10% FBS in DMEM, under 37 °C, 5% CO2 environment. The culture medium was replaced every other day and HUVECs were passaged once every 2–3 days, depending on the growth state of the cells.
HUVECs CCK-8 assay
This assay was carried out as the RA-FLSs CCD8 test. Briefly, ~2.5 × 103 HUVECs in logarithmic growth phase in a volume of 100 μl RA-FLSs conditioned medium were planted in each well of 96-well plate. Cells were incubated for 24 and 48 h. Then 10 μl of the CCK-8 solution was added to each well and incubated at 37 °C for 3 h. The absorbance (A) at 450 nm was measured to evaluate cell viability.
HUVECs migration assay
This assay was performed by chemotaxis chamber with a pore size of 8 μm (Corning). Overall, 5 × 104 HUVECs were suspended in 100 μl conditioned medium from transfected RA-FLSs and planted in the upper chamber. HUVECs suspended in serum-free DMEM was used as blank control. The lower chamber was filled with 600 μl complete medium containing 10% FBS. The migration of HUVECs was analyzed at 37 °C at 24 h. The follow-up processes were similar to those for the RA-FLSs migration assay.
HUVECs tubule formation assay
The matrix gel tubule formation assay was used to investigate tube-formation ability. Overall, 70 μl of Matrigel Basement Membrane Matrix (BD Biosciences) were pipetted into each well of a 96-well plate and polymerized for 30 min at 37 °C. HUVECs were harvested after trypsin treatment and resuspended in conditioned medium from RA-FLSs transfected with reagents. Then 2 × 104 HUVECs with these conditioned medium were added into each well and incubated at 37 °C, 5% CO2, for 24 h. Imaging was performed using a Nikon Eclipse microscope with six images taken from triplicate wells and analysis carried out using ImageJ (NIH). Figures were generated using Graphpad Prism displaying the average total tubule length per field of view.
Statistics
Statistical analysis was performed by SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). Data were expressed as mean±standard error of the mean (s.e.m.). Multiple groups of samples were analyzed by one-way ANOVA, pairwise comparisons were adjusted by Bonferroni method. Differences were considered statistically significant when P<0.05.
Results
Overexpression of uPAR in RA-FLSs
RA-FLSs was successfully cultured and identified by morphology under an optical microscope and cell surface marker of CD55-positive, which was the characteristic of RA-FLSs (Supplementary Figure 1).
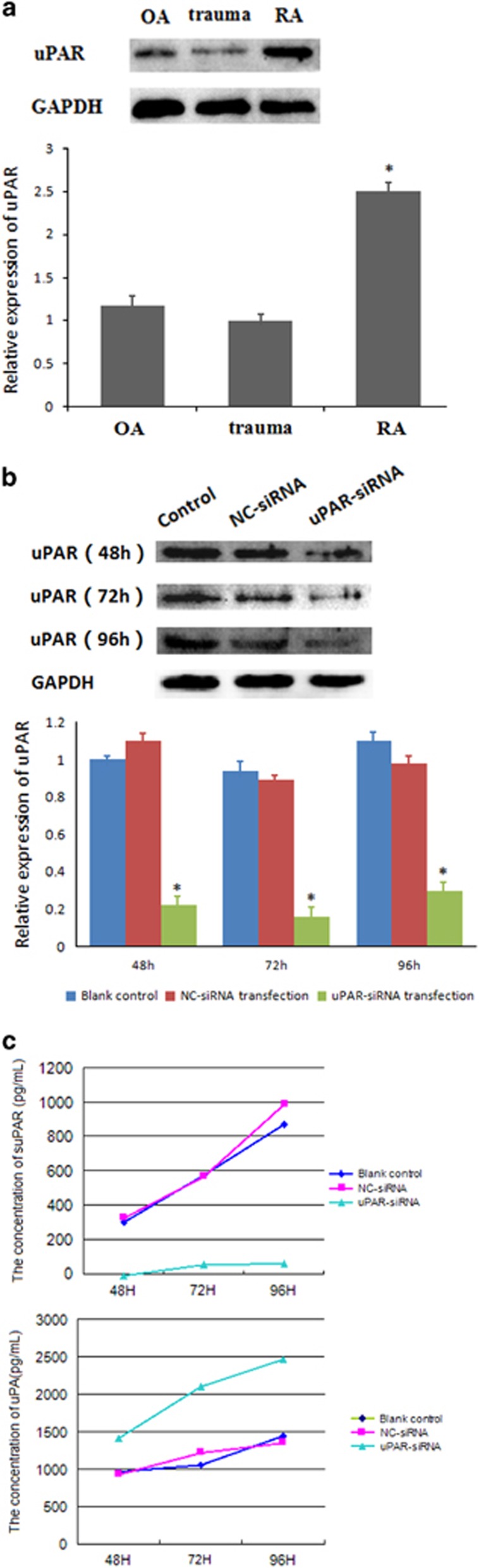
The expression of uPAR in RA-FLSs determined by western blot showed that uPAR protein level in FLSs was significantly higher in each of eight RA patients than in OA or trauma patients. uPAR expression in OA patients was slightly higher than the trauma group, but this difference was not statistically significant (Figure 1a). We next sought to determine whether the elevated expression of uPAR can be regulated using uPAR-siRNA to decrease the uPAR levels in RA-FLSs. The qPCR and western blot assays were used to detect inhibition efficiency on the mRNA and protein expression, respectively. As shown in Table 2, uPAR knockdown markedly decreased uPAR mRNA to (16±0.04)%, compared with the blank and NC-siRNA controls following siRNA transfer for 24 h. Quantifications of the blots showed that uPAR silencing for 48, 72 and 96 h leads to significant decreases of the uPAR protein expression, by (75.16±9.63)%, (89.35±11.12)% and (57.08±10.49)%, respectively (Figure 1b). Interference efficiency was significant compared with the blank control and NC-siRNA group.
Figure 1.
(a) RA-FLSs expressed higher levels of uPAR than OA and trauma groups. Western blot assays were used to detect uPAR expression in RA, OA, and traumatic FLSs. Densitometry analysis of protein expression (means±s.e.m.) were representative of three independent experiments. (b) uPAR-siRNA efficiently interferes with uPAR expression in RA-FLSs. Cells were transfected with uPAR-siRNA (40 nM) or a negative control (NC, 40 nM) for 48, 72, 96 h and western blot analysis was used to detect the effect of uPAR-siRNA on uPAR expression. Upper panel, representative images are shown; lower panel, quantification (means±s.e.m.) of three independent experiments normalized to 1 in the untreated sample. (c) uPAR knockdown affects suPAR/uPA secretion. Secretion curves of suPAR/uPA in the supernatants of transfected RA-FLSs for 48 , 72 , 96 h was made by ELISA assays of three independent experiments. *P<0.05, compared with the controls and n=4. FLSs, fibroblast-like synoviocytes; OA, osteoarthritis; uPAR, Urokinase-type plasminogen activator receptor.
Table 2. mRNA expression of uPAR knockdown RA-FLSs (2−ΔΔCt)  (n=4).
(n=4).
| Group | Gene | Relative mRNA expression |
|---|---|---|
| Blank control | uPAR | 1±0.00 |
| NC-siRNA transfection | uPAR | 1.16±0.24 |
| uPAR-siRNA transfection | uPAR | 0.16±0.04* |
*P<0.01 vs NC-siRNA group.
uPAR knockdown decreases suPAR secreted but increases uPA extracellular
In addition to uPAR expression cut down after transfection, soluble uPAR (suPAR) secreted by RA-FLSs was also decreased. Results showed that suPAR in transfected culture medium reduced to (7.49±0.86)% for 48 h, (11.54±1.14)% for 72 h and (7.52±1.09)% for 96 h, improving as the extension of time. In contrast, secretion levels of uPA, a important ligand to uPAR, increased significantly to (158.21±8.34)% for 48 h, (190.82±9.18)% for 72 h and (137.74±7.93)% for 96 h, compared with the controls (Figure 1c).
uPAR knockdown inhibits RA-FLSs proliferation by regulating cell cycles and apoptosis
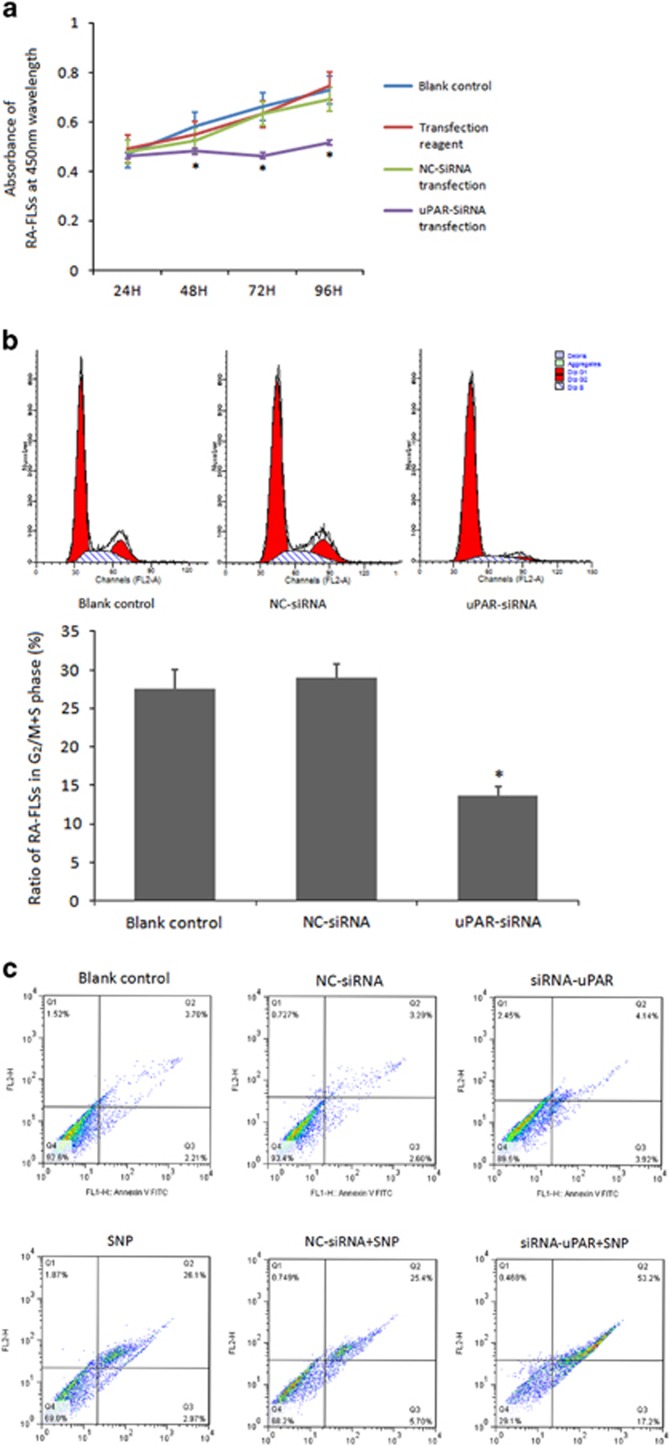
To determine whether the elevated uPAR expression on RA-FLSs is functional, we conducted uPAR-siRNA silencing as above to explore whether this knockdown alters the proliferation of RA-FLSs using the CCK-8 assay. Growth curves of RA-FLSs (Figure 2a) showed proliferation levels of these cells with different treatments at different time points. In the uPAR-siRNA group, a significant inhibition rate in growth was first observed (17.51±2.27%) at 48 h after transfection, more apparent (28.62±4.82%) at 72 h and largely maintained (22.91±5.78%) at 96 h after transfection, all significantly different to the controls (Figure 2a).
Figure 2.
(a) Knocking-down uPAR in RA-FLSs decreases cell proliferation. RA-FLSs were transfected with uPAR-siRNA (40 nM) or a negative control (NC, 40 nM) for 24 h, 48 h, 72 h, 96 h, respectively, and cell proliferation was assessed by the CCK-8 assay. (b) uPAR knockdown in RA-FLSs influences cell cycle. Flow cytometry analysis was performed to analyze the effects of uPAR on cell-cycle distribution. The upper panel was a representative histogram, the lower panel is the cumulative data of the effects. (c) uPAR knockdown in RA-FLSs aggravates cell apoptosis. After treatment of apoptosis inducer SNP and transfection, flow cytometry assay was also used to detect the RA-FLSs cell apoptotic rates. Annexin-V-FITC(+)PI(+) and Annexin-V-FITC(+)PI(−) population represented apoptotic cells. *P<0.05, compared with the controls and n=4. RA-FLSs, fibroblast-like synoviocytes of rheumatoid arthritis; uPAR, Urokinase-type plasminogen activator receptor.
Cell-cycle analysis was immediately conducted to determine if uPAR reductions also affect the cell-cycle changes in RA-FLSs. As expected, uPAR silencing produced cell-cycle arrest at G0/G1 phase. As shown in Figure 2b, the percentages of cells that stayed in G2/M+S phase were markedly decreased in uPAR-siRNA compared with the NC-siRNA group (13.60±4.31% vs 28.99±4.45%, *P<0.05).
To exclude the possibility that uPAR silencing inhibits the cell-cycle changes and proliferation through a non-specific toxic effect, we examined the apoptosis levels of these cells in uPAR-siRNA and controls. As shown in Figure 2c, RA-FLSs had so low rate of apoptosis that uPAR-siRNA group had no significant difference with the controls. After apoptosis-inducing by SNP, RA-FLS apoptosis rate increased, and the cell populations of Annexin-V+/PI− and Annexin-V+/PI+FLSs in uPAR-siRNA group were obviously less than the controls. This result indicates that uPAR in RA-FLSs may affect cell apoptosis.
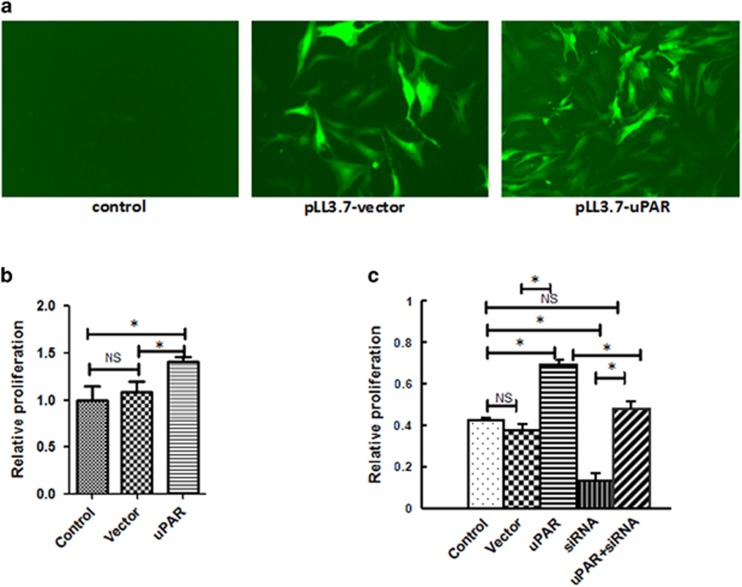
uPAR overexpression reverses RA-FLSs proliferation inhibition
To be sure that the effects above are due specifically to uPAR levels, we performed rescue experiments by transfecting the uPAR overexpressing plasmid. As shown in Figure 3a, transfection efficiency of lentivirus-mediated overexpression in FLS cells was evaluated by GFP signal under fluorescence microscope. CCK-8 analysis showed that lentivirus-mediated uPAR overexpression not only significantly promotes RA-FLS cells proliferation (Figure 3b), but also reverses inhibition effects by uPAR-siRNA (Figure 3c), which suggests the specific role uPAR played on RA-FLSs proliferation.
Figure 3.
Lentivirus-mediated uPAR overexpression promotes RA-FLS cells proliferation. Structures of pLentilox 3.7 contains GFP reporter gene. (a) Transfection efficiency of lentivirus-mediated overexpression in FLS cells was evaluated by GFP signal under fluorescence microscope (magnification × 100). (b) CCK-8 analysis showed that lentivirus-mediated uPAR overexpression significantly promotes RA-FLS cells proliferation. (c) Rescued experiment revealed that lentivirus-mediated uPAR overexpression reverses inhibition effects by uPAR-siRNA (c). *P<0.05, compared with the controls and n=4. RA-FLSs, fibroblast-like synoviocytes of rheumatoid arthritis; uPAR, Urokinase-type plasminogen activator receptor.
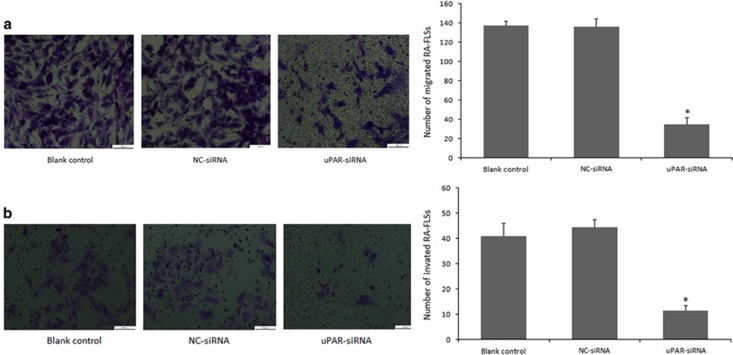
uPAR knockdown decreases RA-FLSs migration and invasion in vitro
Given that migration and invasion are other biological features of RA-FLSs, we next asked whether uPAR expression also affects these properties. An in vitro transwell migration system was developed to test the cell migration ability regulated by uPAR. As illustrated in Figure 4a, the transmembrane cell numbers decreased significantly in uPAR knockdown RA-FLSs (34.67±10.69) compared with the numbers in NC-siRNA (136±19) and blank control groups (137.67±21.36) at 72 h following siRNA transfection. These results suggest that uPAR may enhance RA-FLSs migration ability.
Figure 4.
Cell migration and invasion ability is impaired after uPAR knockdown in RA-FLSs. In both (a) and (b), RA-FLSs were transfected with uPAR-siRNA (40 nM) or a negative control (NC, 40 nM) for 72 h. (a) The RA-FLSs migration was measured in a transwell chamber assay. (b) The RA-FLSs invasive ability was assessed by a Matrigel invasion assay. In both (a) and (b), left panels show representative images; right panels present quantification (means±s.e.m.) of three independent experiments. *P<0.05, compared with blank control and the NC-siRNA control and n=4. RA-FLSs, fibroblast-like synoviocytes of rheumatoid arthritis; uPAR, Urokinase-type plasminogen activator receptor.
In addition, the transwell chamber with Matrigel assay was employed to detect the invasive ability of RA-FLSs at 72 h following transfection. The Matrigel planted in the membrane was used to simulate the extracellular matrix in the human body environment. Results revealed that siRNA-mediated knockdown of uPAR decreases the numbers of membrane-invading RA-FLSs, which were reduced by 72.37% compared with blank control and 74.44% with NC-siRNA, respectively (Figure 4b). These results suggest that uPAR stimulates RA-FLSs invasiveness speculatively.
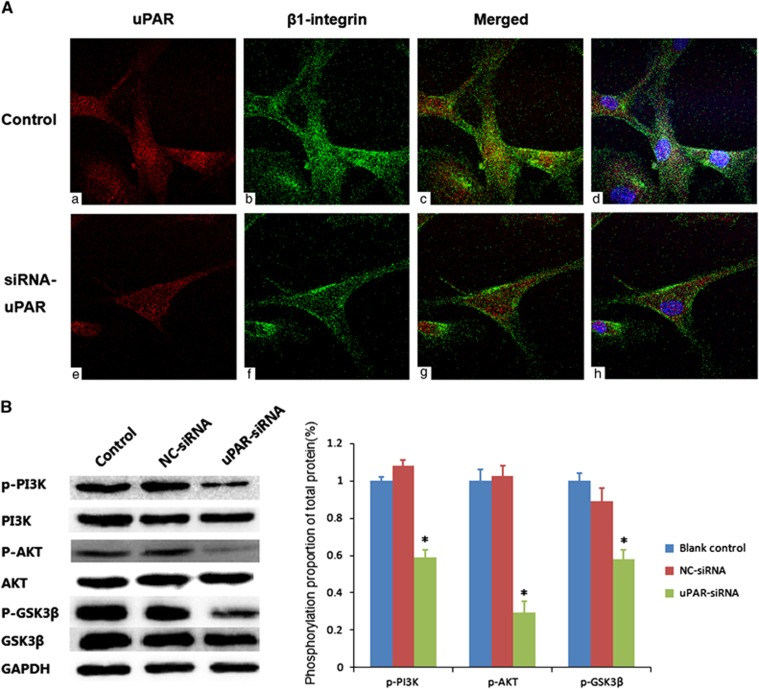
uPAR knockdown interfered β1-integrin internalization
For integrins had affection on many cell biological activities, to explore whether uPAR played a role through them, receptor co-clustering experiments were performed (Figure 5A). As we supposed, uPAR and β1-integrin co-location in the RA-FLSs membrane and cytoplasm visualized by confocal microscopy. Colocalization of uPAR with β1-integrin was significantly reduced in knockdown-uPAR-expressing RA-FLSs and β1-integrin expressed less in intracellular, concentrated mainly on cell surface. This indicated uPAR in RA-FLSs might affect β1-integrin transmembrane movement somehow, such as complex.
Figure 5.
(A) uPAR co-locates with β1-integrin and interferes its internalization. To facilitate visualization, images were converted to pseudo color using ImageJ software: uPAR (red, a, e), β1-integrin (green, b, f), and sites of colocalization (yellow, c, g). The nuclear staining was added in the merged images for easy location and shown on the right (blue, d, h). The above experiments were performed three times with similar results. (B) uPAR silencing reduces PI3K/Akt downstream signaling activation in RA-FLSs. Western blot showed the levels of phosphorylated PI3K, Akt and GSK3β in RA-FLSs after uPAR-siRNA (40 nM) or negative control (NC, 40 nM) following transfection for 72 h. Left panels show representative protein strips; right panels present densitometry quantification of protein expression (means±s.e.m.) of three independent experiments. *P<0.05, compared with the controls and n=4. RA-FLSs, fibroblast-like synoviocytes of rheumatoid arthritis; uPAR, Urokinase-type plasminogen activator receptor.
uPAR inhibition reduces the phosphorylation of PI3K/Akt pathway in RA-FLSs
Previous results have demonstrated that uPAR promotes cancer cell growth through a PI3K/Akt signal.16 Given that RA-FLSs share some features of tumor cells, we then investigated whether PI3K/Akt signaling is responsible for the role of uPAR in promoting the proliferation, migration and invasion of RA-FLSs. RA-FLSs transfected were subjected to western blotting for PI3K/Akt-related proteins. As shown in Figure 4b, uPAR knockdown for 72 h significantly reduced the phosphorylation levels of PI3K, Akt, GSK3β in RA-FLSs (Figure 5B). Thus, it is likely that uPAR modulates the activation of PI3K/Akt signaling in RA-FLSs.
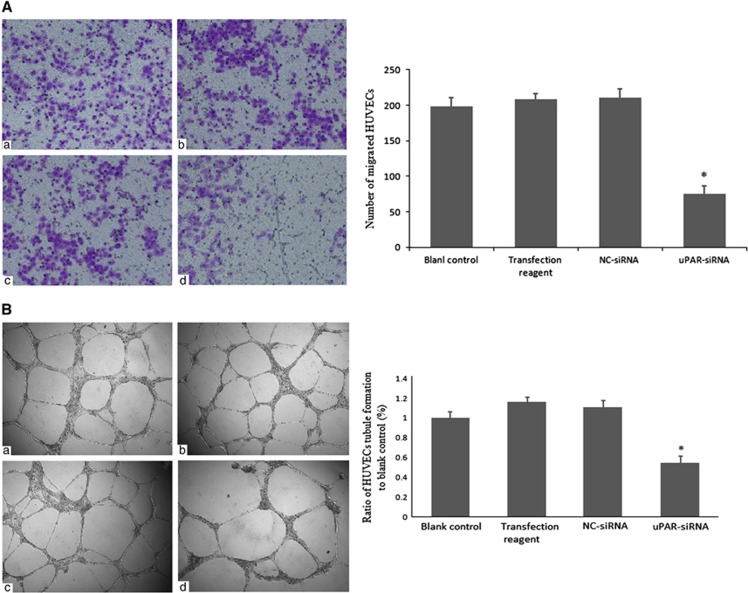
uPAR knockdown in RA-FLSs suppresses endothelial cell angiopoiesis
Pannus is a characteristic structure representing synovial hyperplasia and its formation is associated with angiopoiesis.17 To test the hypothesis that uPAR expression contributes to angiopoiesis and pannus formation, a transwell migration assay using HUVECs was employed. After incubation with different media for 24 h, we observed that HUVEC cells treated with conditioned medium of uPAR-siRNA RA-FLSs had only about 36% transmembrane cells of the controls (Figure 6A). The migration ability of HUVECs cultured in blank control, conditioned medium of transfection reagent and NC-siRNA conditioned medium had no statistically significant differences. These results suggest that uPAR in RA-FLSs could accelerate endotheliocyte migration, thereby contributing to angiopoiesis.
Figure 6.
Interference of uPAR in RA-FLSs suppresses endotheliocyte angiopoiesis by reducing cell migration and tubule formation. (A) The HUVECs cell migration ability in different conditioned media were evaluated by a transwell chamber assay. (a) Blank control group, serum-free DMEM; (b) Transfection reagent group, conditioned medium of RA-FLSs transfected only with transfection reagent; (c) NC-siRNA group, conditioned medium of RA-FLSs transfected with a negative control (NC, 40 nM); (d) uPAR-siRNA group, conditioned medium of RA-FLSs transfected with uPAR-siRNA (40 nM). (B) The HUVECs cells with different conditioned media were added into Matrigel coated 96-well for 24 h to form tubule. Conditioned media treatment just reference as A. In both (A) and (B), left panels show representative images; right panels present quantification of tubule length ratio to controls (means±s.e.m.) of three independent experiments. *P<0.05, compared with blank control and n=4. HUVECs, human endothelial cell lines; RA-FLSs, fibroblast-like synoviocytes of rheumatoid arthritis; uPAR, Urokinase-type plasminogen activator receptor.
To exclude the influence of cell number and density on migration ability, CCK-8 test was employed to detect HUVECs proliferation in conditioned medium. The results showed that in the uPAR-siRNA transfected RA-FLSs cell supernatants, cell proliferation of HUVECs had no significant difference with the controls at 24 and 48 h (Table 3).
Table 3. Proliferation inhibition rate of HUVECs in conditioned medium of uPAR-siRNA (%,  (n=4).
(n=4).
|
Proliferation inhibition rate (100%) |
||
|---|---|---|
| 24 h | 48 h | |
| Blank control | 0±0 | 0±0 |
| Transfection reagent | 1.35±2.52 | 0.22±1.17 |
| NC-siRNA | 4.4±3.22 | 4.66±1.93 |
| uPAR-siRNA | 3.6±2.01# | 5.83±3.24# |
#P>0.05, compared with the controls.
A tubule formation assay was also performed to study effects of uPAR knockdown RA-FLSs on HUVECs. The extensive HUVECs tube formation can be observed in the corresponding controls (Figure 6B). HUVECs cultured in conditioned medium collected from the RA-FLSs for 24 h with transfection reagent and NC-siRNA had more tube structures compared with HUVECs with blank control. However, when the HUVEC were treated with conditioned media from the uPAR-siRNA RA-FLSs, the average number of complete tubular structures decreased to nearly 40%, relative to the transfection reagent control group. Thus, uPAR knockdown in FLSs markedly reduces the ability of RA-FLSs to promote the tube formation of endothelial cells.
Discussion
uPAR, also known as CD87, a single-chain glycoprotein, anchors in the cell membrane at Ser282 and Gly283 by glycosylation of phosphatidylinositol and has a serine proteolytic activity. After binding with uPA and prouPAR, uPAR not only converts non-active proPAR to active uPA, but also increases uPA activity.18 uPA/uPAR complexes concentrate uPA on the cell surface and stimulate plasminogen transforming into the fibrinolytic enzymes. They then induce premetalloproteinases, activate and release collagenase, cytokines and growth factors in the extracellular matrix, resulting in the degradation of extracellular matrix and basement membrane components, accelerating cell transfer and invasion, and promoting angiogenesis.19 Thus, intracellular signaling molecules associated with these transmembrane proteins are affected by uPA/uPAR complexes, such as tyrosine and serine protein kinases. This is followed by regulating a broad spectrum of fundamental physiological processes including cell proliferation, differentiation, apoptosis, migration and invasive characteristics.10, 11 For these reasons, the urokinase system has increasingly been thought to be important in cancer development and progression.20
As a multifunctional cell surface receptor, uPAR expression was significantly higher in papillary thyroid carcinoma,16 lung cancer,21 prostate cancer22 and other malignancies. The uPA/uPAR system in oncology has been demonstrated to involve signaling pathways. It has been observed that uPAR affects cell proliferation, differentiation, migration and invasion in glioma, papillary thyroid carcinoma, lung cancer, prostate cancer and other malignancies; all of these functions may be associated with the activation of signaling pathway intracelluar, such as Akt and ERK, as well as angiogenesis within the tumor.23 In addition, TLR2-mediated synthesis and release of TNF-α and IL-6 by neutrophils was closely related to uPAR.24 RA-FLSs, as a particular population in synovium, have tumor cell characteristics under chronic inflammatory stimulation, and in which, TNF-α and IL-6 are crucial inflammatory cytokines. In line with previous reports,13 we conformed the expression of uPAR was highly elevated in RA-FLSs and it is likely that uPAR contributes to the tumor-like features of RA-FLSs.
We now provide evidence that the elevated expression of uPAR on RA-FLSs is functionally relevant. Gene-specific interference technology can dramatically diminish the uPAR expression, which could be reversed by uPAR overexpression. Moreover, uPAR knockdown inhibites suPAR secretion by RA-FLSs, but increases soluble uPA extracellular, which may since uPA-uPAR composite reduction lead to uPA less transporting into the cell and more secreted to the condition. Besides this, knockdown markedly suppresses the proliferation of RA-FLSs time-dependingly. Cell proliferation is a dynamic process, including cell regeneration and cell death and the ratio between these two aspects determines the overall number of cells. We demonstrate that uPAR predominately affects cell generation and apoptosis, since uPAR gene silencing could significantly restrain RA-FLSs transformation from the G0/G1 phase to G2/M+S phase, what is more, after apoptosis-inducing by SNP, uPAR-siRNA could prevent cell death program. Thus, uPAR may influence the proliferation of RA-FLSs through modulation of cell cycle and death.
Bone erosion and joint damage is one of the major determinants of outcome in patients with rheumatoid arthritis. In the pathology of RA, the early inflammatory environment stimulates synovial tissue to migrate, invade intra-articular structures and damage or destroy cartilage and subchondral bone. Serretti et al.25 found that RA-FLSs could still migrate to distant joints and induce bone tissue erosion in SCID mice even without external stimulation. Moreover, this capacity cannot be suppressed by TNF-α antagonist therapy.26 This suggests that the RA-FLS has a potent ability to migrate and then destroy joint tissues. Our study has exactly revealed that the uPA/uPAR pathway has an important role in the migration of RA-FLSs.
Many studies have suggested that the PI3K/Akt signaling pathway is abnormally activated in RA-FLSs and contributes to excessive cell proliferation,27 migration and invasion.28 Our study findings indicate that uPAR silencing does decrease the expressions of p-PI3K, p-Akt and p-GSK3β, further validating that uPAR promotes the functional features of RA-FLSs through the PI3K/Akt signaling pathway. As we mentioned above, uPAR has no transmembrane structure, there must have some medium to contact uPAR with the signaling pathway. To conform this, the confocal microscope was used to detect if uPAR and β1-integrin had some location and expression relationship. The study suggested that not only uPAR and β1-integrin co-localized on cell membrane and cytoplasm, but also uPAR knockdown could inhibit internalization of β1-integrin towards the cytoplasm. This implies that targeting of the interaction site between uPAR and β1-integrin may impairs receptor internalization and thereby prevent changes of cell biological properties and signaling pathway.
Pannus is a characteristic structure representing high synovial hyperplasia in patients with RA. Neovascularization plays an important role in providing nutrition for synovium and in increasing the inflammatory response.29, 30 In our study, we also noted that uPAR expression is related to angiogenesis in RA-FLSs. These results suggest that abnormal expression of uPAR in RA-FLSs not only changes the biological characteristics of RA-FLSs, but also indirectly affects the angiogenesis capability of vascular endothelial cell and the formation of pannus, which are not associated with HUVECs proliferation. As this effect is achieved by cell supernatant, we speculate that uPAR overexpression has changed certain cytokines or other inflammatory mediators released by the RA-FLSs to promote pannus formation. The detailed mechanism is worth further exploration.
Collectively, our studies have strongly suggested that elevated uPAR expression and the uPA/uPAR signaling pathway have important roles in the tumor-like biologic features of RA-FLSs in RA joints. Targeting uPAR not only decreases cell proliferation, migration and invasiveness, but also angiogenesis. Thus, the development of pharmacological inhibitors may have a potential as a therapeutic approach for patients with RA or other relevant diseases.
Acknowledgments
This work was supported in part by the grants from Science and Technology Planning Project of Guangdong Province, China (2012B031800363); Science and Technology Program of Guangzhou, China (Special Project on the Integration of Industry, Education and Research); Developing Program of the Major Research Plan of the National Natural Science Foundation of Guangdong, China (2014A030308005); National Natural Science Foundation of China (81671611); and Major National developing program of the national level in Higher Education of Guangdong, China (Natural Science).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Henry J, Roulot E, Gaujoux-Viala C. The rheumatoid hand. Presse Med 2013; 42: 1607–1615. [DOI] [PubMed] [Google Scholar]
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 2010; 233: 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology 2006; 45: 669–675. [DOI] [PubMed] [Google Scholar]
- Rao JS, Gujrati M, Chetty C. Tumor-associated soluble uPAR-directed endothelial cell motility and tumor angiogenesis. Oncogenesis 2013; 2: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S, Kumar A, Johnson AR, Pueblitz S, Holiday D, Raghu G et al. Differential expression of the urokinase receptor in fibroblasts from normal and fibrotic human lungs. Am J Respir Cell Mol Biol 1996; 15: 78–87. [DOI] [PubMed] [Google Scholar]
- Postiglione L, Montuori N, Riccio A, Di Spigna G, Salzano S, Rossi G et al. The plasminogen activator system in fibroblasts from systemic sclerosis. Int J Immunopathol Pharmacol 2010; 23: 891–900. [DOI] [PubMed] [Google Scholar]
- Alpizar-Alpizar W, Nielsen BS, Sierra R, Illemann M, Ramirez JA, Arias A et al. Urokinase plasminogen activator receptor is expressed in invasive cells in gastric carcinomas from high- and low-risk countries. Int J Cancer J Int Cancer 2010; 126: 405–415. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Rank F, Illemann M, Lund LR, Dano K. Stromal cells associated with early invasive foci in human mammary ductal carcinoma in situ coexpress urokinase and urokinase receptor. Int J Cancer J Int Cancer 2007; 120: 2086–2095. [DOI] [PubMed] [Google Scholar]
- Ulisse S, Baldini E, Sorrenti S, D’Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets 2009; 9: 32–71. [DOI] [PubMed] [Google Scholar]
- Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 2010; 11: 23–36. [DOI] [PubMed] [Google Scholar]
- Alfano D, Franco P, Vocca I, Gambi N, Pisa V, Mancini A et al. The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thromb Haemost 2005; 93: 205–211. [DOI] [PubMed] [Google Scholar]
- Slot O, Brunner N, Locht H, Oxholm P, Stephens RW. Soluble urokinase plasminogen activator receptor in plasma of patients with inflammatory rheumatic disorders: increased concentrations in rheumatoid arthritis. Ann Rheum Dis 1999; 58: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci S, Del Rosso A, Cinelli M, Margheri F, D’Alessio S, Fibbi G et al. Rheumatoid synovial fibroblasts constitutively express the fibrinolytic pattern of invasive tumor-like cells. Clin Exp Rheumatol 2005; 23: 364–372. [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324. [DOI] [PubMed] [Google Scholar]
- Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum 1995; 38: 1541–1546. [DOI] [PubMed] [Google Scholar]
- Nowicki TS, Zhao H, Darzynkiewicz Z, Moscatello A, Shin E, Schantz S et al. Downregulation of uPAR inhibits migration, invasion, proliferation, FAK/PI3K/Akt signaling and induces senescence in papillary thyroid carcinoma cells. Cell Cycle 2011; 10: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z, Koch AE. Targeting angiogenesis in rheumatoid arthritis. Curr Rheumatolo Rev 2008; 4: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RW, Pollanen J, Tapiovaara H, Leung KC, Sim PS, Salonen EM et al. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol 1989; 108: 1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris GM, Sidenius N. Urokinase plasminogen activator receptor: a functional integrator of extracellular proteolysis, cell adhesion, and signal transduction. Sem Thromb Hemost 2013; 39: 347–355. [DOI] [PubMed] [Google Scholar]
- Noh H, Hong S, Huang S. Role of urokinase receptor in tumor progression and development. Theranostics 2013; 3: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CH, Hill ML, Brumwell AN, Chapman HA, Wei Y. Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins. J Cell Sci 2008; 121: 3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalla AK, Gorantla B, Gondi CS, Lakka SS, Rao JS. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther 2010; 17: 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla R, Gopinath S, Alapati K, Gondi CS, Gujrati M, Dinh DH et al. Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway in gliomas. PLoS One 2010; 5: e13731. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu G, Yang Y, Yang S, Banerjee S, De Freitas A, Friggeri A et al. The receptor for urokinase regulates TLR2 mediated inflammatory responses in neutrophils. PLoS One 2011; 6: e25843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrati S, Margheri F, Chilla A, Neumann E, Muller-Ladner U, Benucci M et al. Reduction of in vitro invasion and in vivo cartilage degradation in a SCID mouse model by loss of function of the fibrinolytic system of rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2011; 63: 2584–2594. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003; 423: 356–361. [DOI] [PubMed] [Google Scholar]
- Xu C, Sun G, Yuan G, Wang R, Sun X. Effects of platycodin D on proliferation, apoptosis and PI3K/Akt signal pathway of human glioma U251 cells. Molecules 2014; 19: 21411–21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang C, Jiang Y, Wu B. Effect of miR-92b on migration, adhesion and invasion of human gastric cancer cell line SGC7901. Nan Fang Yi Ke Da Xue Xue Bao 2014; 34: 1748–1752. [PubMed] [Google Scholar]
- Yoo SA, Kwok SK, Kim WU. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediators Inflamm 2008; 2008: 129873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleolog EM. The vasculature in rheumatoid arthritis: cause or consequence? Int J Exp Pathol 2009; 90: 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.