Granulocyte macrophage colony stimulating factor (GM-CSF) is generally considered to be a proinflammatory cytokine; GM-CSF-based vaccines have been shown to elicit potent antitumor and antiviral immune responses in preclinical experiments. In clinical trials, however, these effects were not as robust and sometimes even contradicted the findings from animal models. In this commentary, we aim to discuss the latest advances in this field, hoping that an upto-date and comprehensive understanding of this molecule will help others design effective GM-CSF-based strategies for antiviral and anticancer vaccines.
It is recognized that sufficient immunogenicity capable of breaking host immune tolerance is the key factor contributing to the efficacy of therapeutic vaccines against established tumors or persistent infections. To augment vaccines with lower immunogenicities, adjuvant incorporation has become an indispensable and widely adopted practice. To date, Alum, the first adjuvant licensed for human vaccines, remains in extended use. Newer alternatives, such as MPL, CpG and nano-emulsions with bacterial components/derivatives, are also effective in improving the immunogenicities of a multitude of vaccines.1 However, some of these regimens are not yet free of safety concerns; extensive clinical use of these adjuvants is therefore still some distance away. On the positive side, several cytokines, such as GM-CSF and IL-12, can evoke equally effective immune responses to vaccines.2 These originally lab-based findings are being actively tested in vivo to develop a new class of clinical immune enhancers. However, despite their repeated success in the laboratory, GM-CSF-adjuvant vaccines do not usually fare well in clinical tests. In fact, some GM-CSF-based preparations have been found to have effects in humans that contradict animal findings.
Through upregulation of co-stimulatory (CD80 and CD86) and MHC class II molecules, GM-CSF regulates the development and functions of DC subsets as well as T-cell activation.3 In a mouse model of a vaccine-targeting bladder cancer stem cells, the experimental group using GM-CSF as an adjuvant treatment displayed increased IgG levels and increased dendritic, CD4+ T, and CD8+ T-cell counts.4 Chen et al. also demonstrated that mice vaccinated with GM-CSF-secreting breast tumor cells elicited antigen-specific CD8+ T-cell immunity and delayed type hypersensitivity (DTH). Moreover, patients receiving this tumor vaccine had prolonged survival.5 Consistent with these observations, a peptide vaccine adjuvant with GM-CSF achieved a 25% reduction in tumor progression in patients with Wilms’ tumor 1 (WT1), suggesting the potential for its use in human therapies.6 However, GM-CSF-boosted vaccines were less promising in other trials. Mitchell et al.7 showed that autologous DC vaccines combined with a GM-CSF adjuvant failed to elicit an adequate immune response in glioblastoma patients. Similarly, GM-CSF immune boosters did not produce any benefits in some clinical CTL peptide vaccines for HIV and tumor antigens.8, 9
While it has been noted that clinical responses to GM-CSF-based vaccines have not been as vigorously tested as those recorded in animal experiments, the reasons for these divergent outcomes are not fully defined. GM-CSF is a double-edged sword. It could, at least under some settings, induce production of immunosuppressive cells including myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs). These effects were mostly unintended outcomes from the effort to induce monocyte to DC conversion. For instance, in a tumor vaccine study using modified tumor cells expressing GM-CSF, serum GM-CSF concentrations above a threshold level induced the development of CD11b+Gr-1+ myeloid suppressor cells, aiding tumor development.10 In parallel, Parmiani et al.11 demonstrated that compared with the use of GM-CSF at lower doses (that is, 40–80 μg/day), the use of GM-CSF at doses above 100 μg/day had negative effects in patients with melanoma or colon cancers. Mechanistically, GM-CSF may achieve T-cell tolerance via induction of tolerogenic DCs.12 Antigens captured by tolerogenic DCs can directly cause T-cell anergy and/or hypo-responsiveness. Moreover, these tolerogenic DCs can expand CD4+CD25+ Tregs that suppress antigen-specific responses through production of TGF-β and/or IL-10.13 Collectively, while GM-CSF is in theory an ‘alternative adjuvant’ with a potential place in clinical interventions, in practice, the desired outcomes or the lack thereof are influenced by factors including dosing, timing, disease model, immune response characteristics, and the sparsity of standardized protocols.
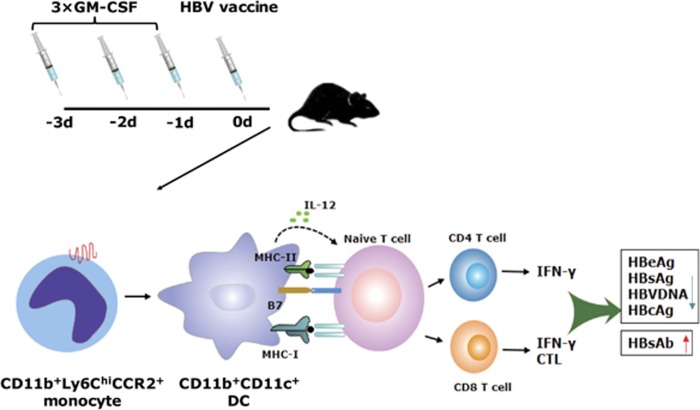
As a therapeutic vaccine adjuvant promote antigen-specific cellular responses, the GM-CSF mode of action should be rigorously tested in animal models prior to clinical testing. As discussed above, there are a number of variables that need to be tested, and each variable should be quantified in a systematic manner. With this in mind, we recently hypothesized that the in vivo conversion from monocytes to DCs by GM-CSF would require a treatment duration longer than those previously tested in the clinic. We tested this hypothesis by pre-treating animals one, two, and three days (one injection per day) prior to antigen delivery. We observed that one or two days of GM-CSF pretreatment in vivo did not potently convert monocytes to DCs. However, extending the treatment to 3 days induced sufficient conversion (Figure 1). This outcome inspired us to design a novel protocol utilizing a 3-day GM-CSF pretreatment prior to HBV antigen vaccination. Using this protocol, we observed that CD11b+Ly6Chi monocyte-derived DCs were effectively induced, leading to robust antigen-specific immune responses in wild type C57BL/6 mice. Strikingly, a similar enhanced response was also observed in two mouse models of HBV immunotolerance. Treatment with vaccines utilizing extended GM-CSF pretreatment resulted in significant serum HBeAg and HBsAg clearance and a 95% reduction of HBV-positive hepatocytes. Large numbers of infiltrating CD8+ T cells were found in the livers of animals treated with this new protocol. The anti-HBV-specific T-cell responses were induced via the conversion of CCR2-dependent CD11b+ Ly6Chi monocytes to CD11b+CD11c+ DCs, since a depletion of Ly6Chi monocytes nullified the immune enhancement.14, 15 As a follow-up to this animal work, this protocol is being adapted to a clinical trial attempting to treat chronic HBV-infected patients (Registration No.: ChiCTR-TRC-13003254). Promising results against persistent HBV infections would not only rejuvenate GM-CSF as an effective adjuvant against this particular virus but also open the door to its extended use in other infection controls and cancer therapies.
Figure 1.
The 3 × GM-CSF+VACCINE regimen elicits potent HBV-specific immune responses in HBV mouse models. Three-day pretreatment with GM-CSF prior to HBV vaccination promotes the proliferation and maturation of CD11b+Ly6Chi monocyte-derived CD11b+CD11c+ DCs, which mediate HBV-specific T-cell immune responses and lead to the elimination of the HBV antigen. HBV, hepatitis B virus; DCs, dendritic cells; CTL, cytotoxic T lymphocytes.
Acknowledgments
This work is supported by grants from the National Science and Technology Major Program of Infectious Diseases (2012ZX10002002004-001, 2012ZX10004701 and 2013ZX10002001) and the Natural Science Foundation of China (31430027 and 81672016) to BW. We thank Dr Yan Shi for his critical review and suggestions and Dr Douglas Lowrie for his proofreading.
Footnotes
The authors declare no conflict of interest.
References
- O'Hagan DT, Fox CB. New generation adjuvants—from empiricism to rational design. Vaccine 2015; 33 (Suppl 2): B14–B20. [DOI] [PubMed] [Google Scholar]
- Decker WK, Safdar A. Cytokine adjuvants for vaccine therapy of neoplastic and infectious disease. Cytokine Growth Factor Rev 2011; 22: 177–187. [DOI] [PubMed] [Google Scholar]
- van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood 2012; 119: 3383–3393. [DOI] [PubMed] [Google Scholar]
- Zhu YT, Zhao Z, Fu XY, Luo Y, Lei CY, Chen W et al. The granulocyte macrophage-colony stimulating factor surface modified MB49 bladder cancer stem cells vaccine against metastatic bladder cancer. Stem Cell Res 2014; 13: 111–122. [DOI] [PubMed] [Google Scholar]
- Chen G, Gupta R, Petrik S, Laiko M, Leatherman JM, Asquith JM et al. A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol Res 2014; 2: 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Okuyama R, Aruga A, Sugiyama H, Yamamoto M. Phase I trial of Wilms' Tumor 1 (WT1) peptide vaccine with GM-CSF or CpG in patients with solid malignancy. Anticancer Res 2012; 32: 2263–2269. [PubMed] [Google Scholar]
- Mitchell DA, Sayour EJ, Reap E, Schmittling R, DeLeon G, Norberg P et al. Severe adverse immunologic reaction in a patient with glioblastoma receiving autologous dendritic cell vaccines combined with GM-CSF and dose-intensified temozolomide. Cancer Immunol Res 2015; 3: 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman P, Kalams S, Elizaga M, Metch B, Chiu YL, Allen M et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine 2009; 27: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi K, Dawson NA. Update on castrate-resistant prostate cancer: 2010. Curr Opin Oncol 2010; 22: 263–267. [DOI] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 2004; 64: 6337–6343. [DOI] [PubMed] [Google Scholar]
- Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 2007; 18: 226–232. [DOI] [PubMed] [Google Scholar]
- Gaudreau S, Guindi C, Menard M, Benabdallah A, Dupuis G, Amrani A. GM-CSF induces bone marrow precursors of NOD mice to skew into tolerogenic dendritic cells that protect against diabetes. Cell Immunol 2010; 265: 31–36. [DOI] [PubMed] [Google Scholar]
- Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol 2010; 184: 1765–1775. [DOI] [PubMed] [Google Scholar]
- Wang X, Dong A, Xiao J, Zhou X, Mi H, Xu H et al. Overcoming HBV immune tolerance to eliminate HBsAg-positive hepatocytes via pre-administration of GM-CSF as a novel adjuvant for a hepatitis B vaccine in HBV transgenic mice. Cell Mol Immunol 2016; 13: 850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhou X, Zhao G, Lin Q, Wang X, Yu X et al. Enrichment of Ly6Chi monocytes by multiple GM-CSF injections with HBV vaccine contributes to viral clearance in a HBV mouse model. Hum Vaccin Immunother 2017. e-pub ahead of print 12 July 2017 doi:10.1080/21645515.2017.1344797. [DOI] [PMC free article] [PubMed]