Type 1 diabetes (T1D), also referred to as insulin-dependent diabetes mellitus, is a debilitating disease that follows the destruction of pancreatic insulin-producing β cells by autologous T-cells. T1D primarily affects children and has a strong genetic component, with >50 susceptibility loci identified, including HLA-DQβ chains.1 However, the striking differences between European regions with similar genetic backgrounds and a sharp rise in T1D incidence in developed countries over the last several decades suggest that environmental factor(s) also play a relevant etiopathogenic role.2 Given the accumulating evidence linking the gut microbiota to protection against metabolic diseases,3 Mariño et al.4 recently tested the hypothesis that short-chain fatty acids (SCFAs), which are the end products of fermentation of dietary fibers by anaerobic intestinal microbiota, can protect genetically susceptible mice from developing T1D. Interestingly, the authors found that diets enriched in acetate or butyrate (two of the main SCFAs) can protect animals from developing diabetes through different and complementary cellular mechanisms. Such findings significantly enhance our understanding of the role of diet and gut microbiota in the development of autoimmune diseases and indicate that the use of medicinal foods may be a cost-effective treatment against T1D and other autoimmune diseases with a cellular component. Given that current anti-T1D approaches (which focus on prevention or modulation of the adaptive specific immune response against autoantigens) have been generally disappointing,5 such a new and refreshing strategy has attracted a great deal of attention (reviewed in Ref. 2).
Over the last decade, an ever-growing body of evidence has established that the gut microbiota is one of the most important epigenetic determinants of prevalent metabolic disorders such as type 2 diabetes and metabolic syndrome.6 Similarly, accumulating experimental observations indicate that T1D incidence in non-obese diabetic (NOD) mice is influenced by the microbial environment, thus indicating that the gut microbiota is involved in T1D development.7 This concept has been well-illustrated by a recent report demonstrating that the interaction between the gut microbiota and the host immune system was essential for the prevention and treatment of T1D.8 In their study, Wen et al. generated myeloid differentiation primary response 88 (MyD88)-deficient mice in a NOD background (NOD.Myd88−/−). MyD88 is a master regulator of immune responses and is capable of detecting bacteria and other infectious agents by binding to Toll-like receptors and initiating a pro-inflammatory cascade dependent on nuclear factor kappa beta (NF-κB) activation. NOD.Myd88−/− mice kept under specific-pathogen free conditions were completely protected against T1D development, but this protection was dependent on commensal microbiota, as NOD.Myd88−/− mice housed in germ-free (GF) conditions developed robust diabetes. Importantly, when these animals were colonized with altered Schaedler’s flora, which is a consortium of six bacteria that are normally found in the human gut, protection against T1D was restored. Molecular analyses of cecal microbiota revealed that MyD88 ablation correlates with changes in the microbiota composition, with a significant increase in butyrate-producing Firmicutes, as well as Rikenellaceae and Porphoromadaceae.
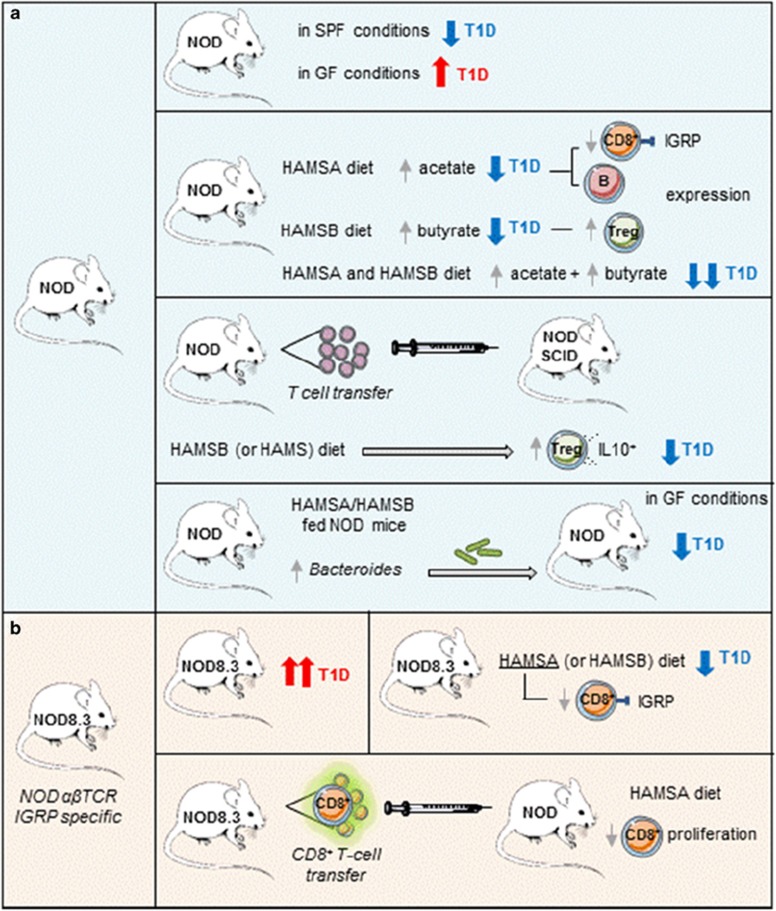
SCFA (namely, acetate, butyrate and propionate) are the main metabolites of the bacterial fermentation of dietary fiber and have been associated with anti-inflammatory effects via the up-regulation of regulatory T (Treg) cells and the inhibition of histone deacetylase (HDAC) activity.9 Given the instrumental role of SCFA in intestinal homeostasis, Mariño et al. compared the SCFA concentration in diabetes-prone NOD mice and their diabetes-resistant NOD.Myd88−/− counterparts. Although the propionate levels were similar between both mouse lines, the acetate and butyrate levels were much higher in the NOD.MyD88−/− animals, which suggested that T1D protection was mediated by SCFAs. In addition, T1D-prone NOD mice under GF conditions developed a more aggressive form of the disease, thus supporting the role of commensal bacteria-produced metabolites as a defense mechanism against diabetes (Figure 1).
Figure 1.
Main experimental approach revealing the specific mechanisms of acetate and butyrate protection in T1D development. (a) NOD mice undergo exacerbated T1D in germ-free (GF) conditions. Oral administration of acetylated high-amylose maize starch (HAMSA) or butyrylated high-amylose maize starch (HAMSB) increased acetate or butyrate concentrations, respectively, which protected NOD mice from T1D development via independent mechanisms. Whereas HAMSA administration reduced specific IGRP (islet-specific antigen glucose-6-phospatase catalytic subunit-related protein) reactive CD8+ T-cells and induced a reduction in MHC-I and CD86 expression, HAMSB increased the Treg cell population. Splenic T-cells from NOD mice that were orally treated with HAMSB, when transferred into immunodeficient NOD-SCID mice, reduced T1D severity in receptor NOD-SCID mice. Fecal transplant of gut microbiota from NOD mice fed the HAMSA/HAMSB diet into GF NOD mice transferred diabetes protection. (b) NOD mice that express a transgene encoding the αβ TCR derived from a CD8+ T-cell clone specific against IGRP (NOD8.3) undergo acute T1D. The HAMSA diet inhibits the specific proliferation of IGRP reactive CD8+ T-cells NOD8.3 and, ultimately, T1D progression.
In an effort to provide both a mechanistic explanation and to increase the potential clinical relevance of their findings, Mariño et al. fed NOD mice with special diets designed to release large amounts of specific SCFAs after bacterial fermentation. As expected, mice fed acetylated high-amylose maize starch (HAMSA) showed higher concentrations of acetate, whereas mice fed butyrylated high-amylose maize starch (HAMSB) presented higher concentrations of butyrate. Notably, both diets induced local and systemic increases in the corresponding SCFA levels, but had no effect on body weight or food or energy intake. Confirming their hypothesis that bacterial metabolites can protect genetically prone animals from developing T1D, animals fed either HAMSA or HAMSB presented a significantly reduced incidence of diabetes. Mice fed a combination of these diets demonstrated an even higher protection against diabetes, suggesting different mechanisms of action for acetate and butyrate. This finding was further confirmed in experiments using NOD8.3 mice, which express a transgene encoding the αβ T-cell antigen receptor derived from a CD8+ T-cell clone specific against islet-specific antigen glucose-6-phospatase catalytic subunit-related protein (IGRP), which is a major target of autoreactive T-cells in pancreatic β-cells.10 Even in this model of aggressive and rapidly progressing disease, a diet designed to release large levels of acetate showed a protective effect, as evidenced by both a delay in diabetes progression and a diminished percentage of IGRP-specific CD8+ T-cells (Figure 1).
The authors also found a remarkable reduction in the number of B cells from the spleen and Peyer’s patches from NOD mice that were fed the HAMSA diet. Moreover, B-cells from spleen from animals fed the HAMSA diet also expressed lower levels of major histocompatibility complex class-I (MHC-I) and costimulatory CD86 molecules. These results strongly indicate that impaired antigen presentation likely causes a reduction in autoreactive CD8+ T-cells and concomitant protection against diabetes. These results fully agree with the previous report from the same group showing that cross-presentation by antigen presenting B-cells of islet-derived autoantigens drives the expansion and differentiation of self-reactive CD8+ T-cells in the pancreatic lymph node into effector cells, a critical process for the transition from clinically silent insulinitis to overt diabetes.11
Butyrate has been linked to an increase in the number and activation status of Treg cells.12 Because Tregs cells are known to play a critical role in controlling T1D,13 the authors next tested the hypothesis that HAMSB-fed animals would have increased numbers of Treg cells and would thus prevent autoreactive T-cells from inducing T1D. In an elegant reverse protection approach, spleen T-cells from NOD mice fed with the different diets were transferred into severe combined immunodeficiency mice (NOD-SCID). Because SCID mice are depleted of B- and T-cells, the experiment allowed the authors to study the direct effects of the individual diets on the donor T-cells. In this rapidly progressing T1D model, the authors found that the adoptive transfer of spleen T-cells derived from animals fed the HAMSB (but not HAMSA) diet almost completely protected host animals from diabetes development. Surprisingly, spleen T-cells obtained from animals fed with the original high-amylose resistant starch (HAMS) were also protective, albeit to a lesser extent. Further analysis showed that HAMSB-fed animals promoted the conversion of CD4+ T-cells into Foxp3+ IL-10-producing (Treg) cells. Although this result supports a putative role of Treg cells in butyrate-mediated protection against diabetes, a formal demonstration that such Treg cells are indeed responsible for ablating diabetes by inhibiting autoreactive T-cell proliferation was not provided (Figure 1).
Finally, the authors analyzed changes in the gut microbiota of animals fed with the specialized diets. As expected, NOD mice fed the HAMSA and, to a lesser extent, the HAMSB diets presented a higher percentage of Bacteroides—a genus that has been linked with diabetes protection.8 Importantly, fecal transplant of gut microbiota from NOD mice fed with the acetate-rich HAMSA diet into GF NOD mice was sufficient to elevate levels of acetate and transfer diabetes protection, thus further highlighting the relevance of gut microbiota in T1D pathogenesis (Figure 1). However, from a translational point of view, fecal transplants are associated with technical challenges and an almost overwhelming physiological stress and social stigma.14 Thus, future experiments should address whether individual bacterial species isolated from colonized GF could also transfer diabetes protection.
Collectively, the data presented by Mariño et al.4 highlight that acetate and butyrate, which are two of the main products of bacterial fermentation, can provide disease protection in a mouse model of autoimmune diabetes. Because each metabolite acts through different molecular mechanisms (acetate reduces the proliferation of autoreactive T-cells by minimizing B cell antigen presentation to T-cells, whereas butyrate increases the number and activity of Treg cells), their additive effects could be beneficial for controlling other immune-based disorders, particularly those of gastrointestinal origin (for example, chronic inflammatory bowel diseases such as Crohn’s disease or colitis ulcerosa). Investigations in this regard are warranted and are largely fueled by the increasingly accepted role of the gut microbiota in the development and control of several metabolic diseases, as well as the growing interest in the potential use of prebiotics and probiotics as therapeutic tools to improve gut integrity.15 The sheer complexity and inter-personal variation of the gut microbiota make any attempt to manipulate it extremely challenging. However, this study opens the door for the use of medicinal food (nutraceuticals) that is rich in bacterial metabolites as a promising and cost- effective treatment against T1D and other autoimmune diseases.
Acknowledgments
The work by FL is supported by grants from the Worldwide Cancer Research (14-1275), Fundació La Marató TV3 (201319-30), and Spanish Ministerio de Economía y Competitividad (Plan Nacional I+D+i, SAF2013-46151-R and SAF2016-80535-R) -co-financed by European Development Regional Fund ‘A way to achieve Europe’ ERDF. FA holds Sara Borrell (CD15/00016) and M-AES (MV16/00002) fellowships from Instituto de Salud Carlos III. TA is supported by the Cultural Mission of the Royal Embassy of Saudi Arabia (Qassim University).
Footnotes
The authors declare no conflict of interest.
References
- Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 1987; 329: 599–604. [DOI] [PubMed] [Google Scholar]
- Paun A, Yau C, Danska JS. The influence of the microbiome on Type 1 diabetes. J Immunol 2017; 198: 590–595. [DOI] [PubMed] [Google Scholar]
- Shi Y, Mu L. An expanding stage for commensal microbes in host immune regulation. Cell Mol Immunol 2017; 14: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 2017; 18: 552–562. [DOI] [PubMed] [Google Scholar]
- Kolb H, von Herrath M. Immunotherapy for Type 1 diabetes: why do current protocols not halt the underlying disease process? Cell Metab 2017; 25: 233–241. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009; 1: 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005; 23: 447–485. [DOI] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008; 455: 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 2000; 406: 739–742. [DOI] [PubMed] [Google Scholar]
- Marino E, Tan B, Binge L, Mackay CR, Grey ST. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes 2012; 61: 2893–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504: 446–450. [DOI] [PubMed] [Google Scholar]
- Takiishi T, Cook DP, Korf H, Sebastiani G, Mancarella F, Cunha JP et al. Reversal of diabetes in NOD mice by clinical-grade proinsulin and IL-10-secreting lactococcus lactis in combination with low- dose anti-CD3 depends on the induction of Foxp3-positive T cells. Diabetes 2017; 66: 448–459. [DOI] [PubMed] [Google Scholar]
- Jayasinghe TN, Chiavaroli V, Holland DJ, Cutfield WS, O'Sullivan JM. The new era of treatment for obesity and metabolic disorders: evidence and expectations for gut microbiome transplantation. Front Cell Infect Microbiol 2016; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord 2015; 16: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]