Abstract
Haemoglobin Video Imaging (HVI) demonstrates conjunctival and episcleral blood flow in man with the resolution of a single erythrocyte. A new method for establishing vessel hierarchy in micro-circulations is described, which recognises either delivery or drainage vessels and references vessel order to the capillary. These tools have been used to characterise blood flow.
Anterior ciliary arteries show pulsatile variation in diameter. The episcleral arterial circle that they supply has functional apices with pulsatile flow reversal. Perfusion fields overlap: a single delivery vessel may project to many drainage vessels and vice-versa. Some vascular pathways remained inactive throughout a 1 min angiogram.
Small conjunctival delivery vessels have laminar flow, but advancing luminal constrictions are often observed within the blood column. Laminar flow is lost in low-order drainage vessels where erythrocytes aggregate, but quickly recovers, new striae being added to the blood column at each confluence. Aqueous forms a discrete column, which centralises in episcleral drainage vessels.
There is strong evidence that the luminal constrictions in small delivery vessels propel blood by peristalsis: they form spontaneously, remote from bifurcations; a single vessel may have multiple constrictions; they truly narrow the lumen, rarely contributing volume to post-capillary venules; they can proceed faster than the vessel contents; they never enter the drainage system; the trailing edges of erythrocyte boluses usually taper. They are rhythmically aligned with cardiac systole.
While blood is transported to the periphery by the heart, it is actively transferred through tissues by peristalsis in small delivery vessels.
Introduction
The micro-circulations of conjunctiva and episclera are visible and can be recorded, by clinical video-microscopy, with the resolution of a single erythrocyte. They are accessible samples of the myriad vessels, hidden from view in every tissue of the human body.
When the conjunctiva is illuminated by a waveband that corresponds with the long-wavelength absorption peak of haemoglobin, light reflected by sclera shows erythrocytes as dark objects against a bright background.1, 2 These images (Figure 1) have been used to explore the distribution and drainage of blood and aqueous in the normal human conjunctiva and episclera.
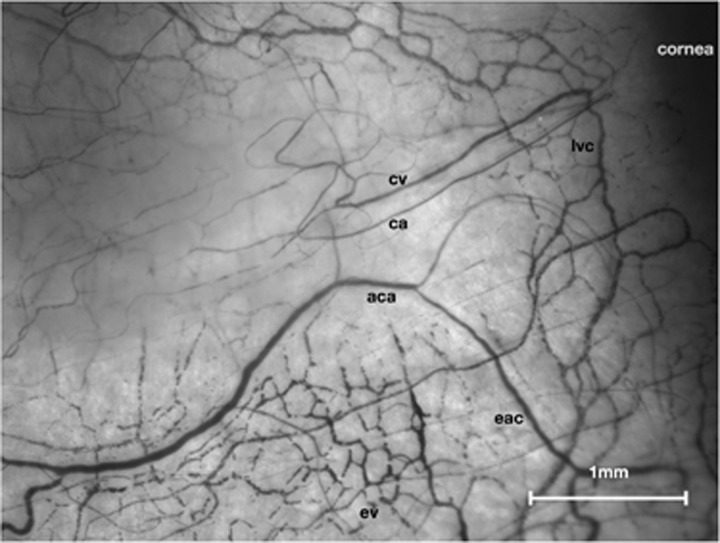
Figure 1.
Normal conjunctival and episcleral microcirculations. Left nasal field (low-magnification Haemoglobin Video Imaging study). aca, anterior ciliary artery; eac, episcleral arterial circle; ca, conjunctival arteriole; cv, conjunctival venule; lvc, limbal venous circle; ev, mantle of episcleral venules.
This technology has now been developed into a clinical imaging method (Haemoglobin Video Imaging, HVI).1, 2 A sensitive, high-resolution video camera is mounted on a clinical slit-lamp and real-time image stabilisation is undertaken to eradicate motion artefact due to fixation micro-saccades.
This paper presents initial findings from a continuing study of the normal conjunctival and episcleral circulations. It describes their organisation, the behaviour of flowing blood and certain interactions between vessels and the blood they transmit, offering new insights into microvascular physiology.
The anterior ocular blood supply
Delivery
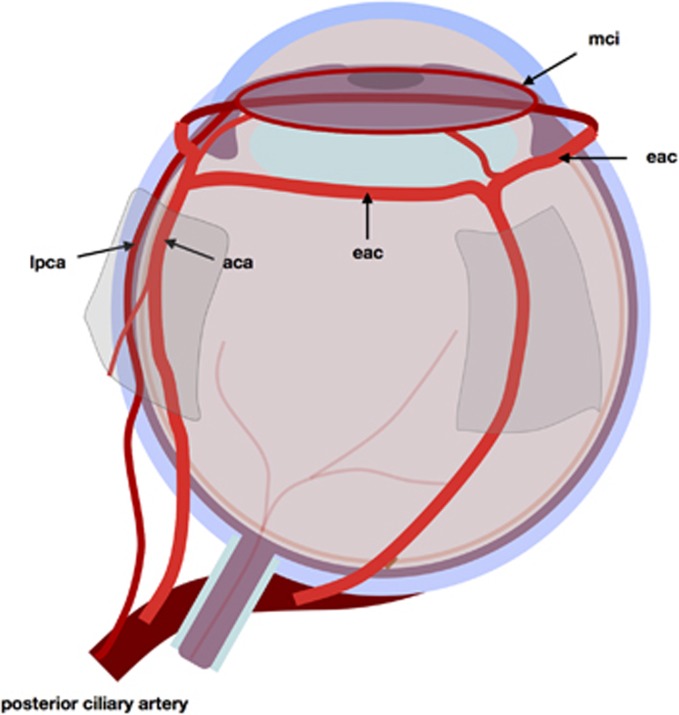
Blood reaches the eye through tiers of arcades (Figure 2). Branches of the ophthalmic artery perforate the rectus muscles and continue forward on their surfaces as anterior ciliary arteries.3 Beyond the muscle insertions, these run over and through episclera, joining to form a ring of arcades behind the limbus: the anterior episcleral arterial circle.4 Branches from this episcleral arterial circle perforate sclera to reach the major circle of the iris, which receives an additional blood supply from the two long posterior ciliary arteries.5 They, too, derive from the ophthalmic artery and enter the globe behind the equator.
Figure 2.
The anterior segment circulations: delivery of blood. aca, anterior ciliary artery; lpca, long posterior ciliary artery; eac, episcleral arterial circle; mci, major circle of iris.
Therefore, trans-scleral arcades supply and join two coronal circular arcades: one on the surface of the globe, the other at the iris root.6
Distribution
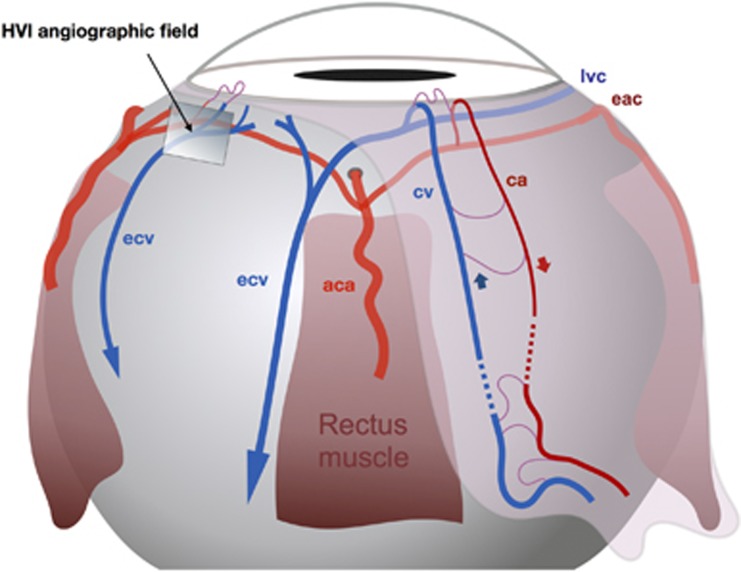
Illustrated in Figures 3, 4a and 4b. From the episcleral arterial circle, anterior roots run forward to the limbal conjunction of conjunctiva and episclera, at which they give rise to the limbal arcades, before reflecting posteriorly to form radial anterior conjunctival arterioles.2, 4
Figure 3.
The anterior segment circulations: distribution and drainage of blood. aca, anterior ciliary artery; eac, episcleral arterial circle; lvc, limbal venous circle; ecv, episcleral collecting vein; ca, conjunctival arteriole; cv, conjunctival venule.
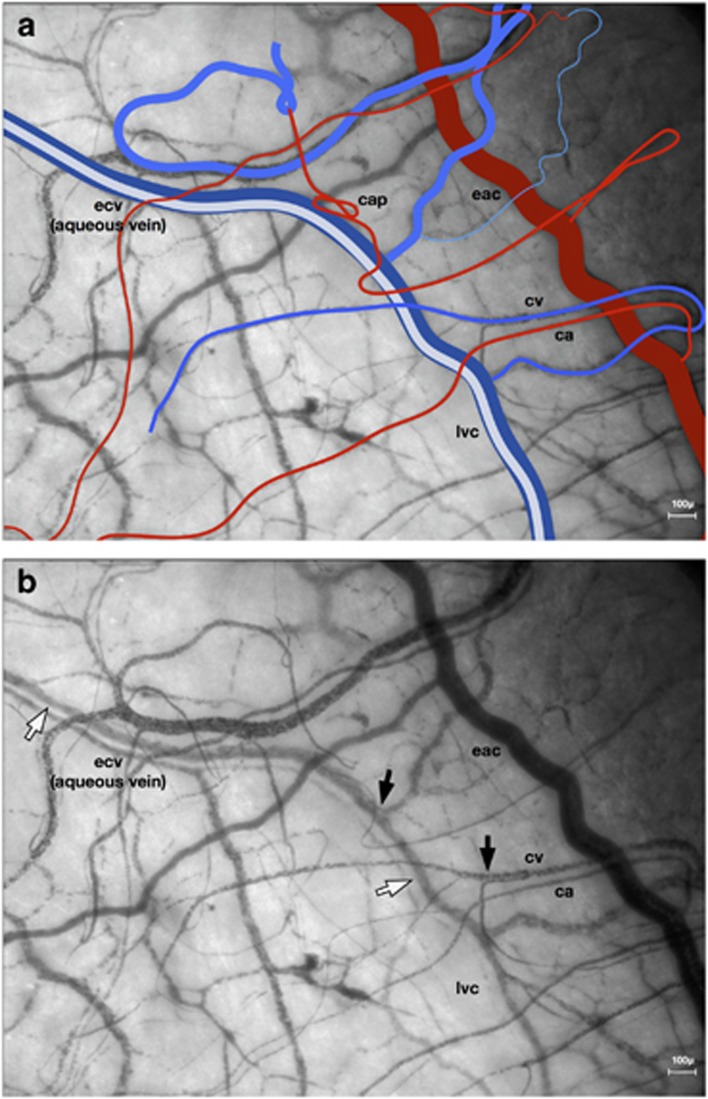
Figure 4.
(a, b) Distribution and drainage of anterior conjunctival blood; aqueous drainage. Left nasal field (NB, the limbal venous circle more commonly lies anterior to the episcleral arterial circle). eac, episcleral arterial circle; lvc, limbal venous circle (aqueous vein); ecv, episcleral collecting vein (aqueous vein); ca, conjunctival arteriole; cv, conjunctival venule; cap, capillary. (b) (black arrows show striation at venous confluences) (white arrows show aqueous column).
Anterior conjunctival venules return blood to the limbus, accept blood from the limbal arcades, and drain into a fragmentary limbal venous circle, which feeds radial episcleral collecting veins.1 These traverse the muscle cone to reach the superior and inferior ophthalmic veins, which flow into the cavernous sinus.
The anterior conjunctival arterioles and venules also communicate with the posterior conjunctival vessels which arise from, and drain into, the eyelid circulation.2, 4
Methods
The work presented here derives from HVI studies of normal conjunctiva in 20 subjects. They were performed as controls for another study (to be published separately) and the participants gave consent for their angiograms to be used to demonstrate basic physiology or pharmacology.
Haemoglobin Video Imaging exploits the haemoglobin absorption spectrum to demonstrate red cells as dark objects against a bright background of light reflected by sclera. The optics are calculated to resolve single erythrocytes (melanin can occasionally cause an artefactual signal on account of the overlap of its absorption spectrum with haemoglobin).
Angiographic technique
Image capture
The Haemoglobin Video Imaging station employs a Zeiss SL130 slit-lamp, mounted on a well-stabilised trolley. The slit-lamp illumination system is fitted with a band-pass interference filter (steep long and short wavelength cut-off; >50% transmission between 505 and 575 nm) and a hot mirror that stops light with wavelengths beyond 730 nm from reaching the camera.
The video camera is mounted on a 50% beam-splitter with a 220 mm focal length C-mount.
Images are captured at 30 frames per second, without compression, using a Prosilica monochrome CCD camera with GIG-E output.
In the present study, each patient received a single, 1 min angiographic examination of a nasal or temporal field in one eye. At the chosen nominal magnification of ‘ × 20’, each pixel covered 4 μm2.
Image processing
During the recording and initial interpretation of HVI angiograms, the images were displayed and analysed using bespoke software, incorporating real-time stabilisation in x and y axes. For more detailed analyses, correction of rotation was also undertaken using Image-J and the ‘StackReg’ plugin.
Vessel hierarchy and nomenclature
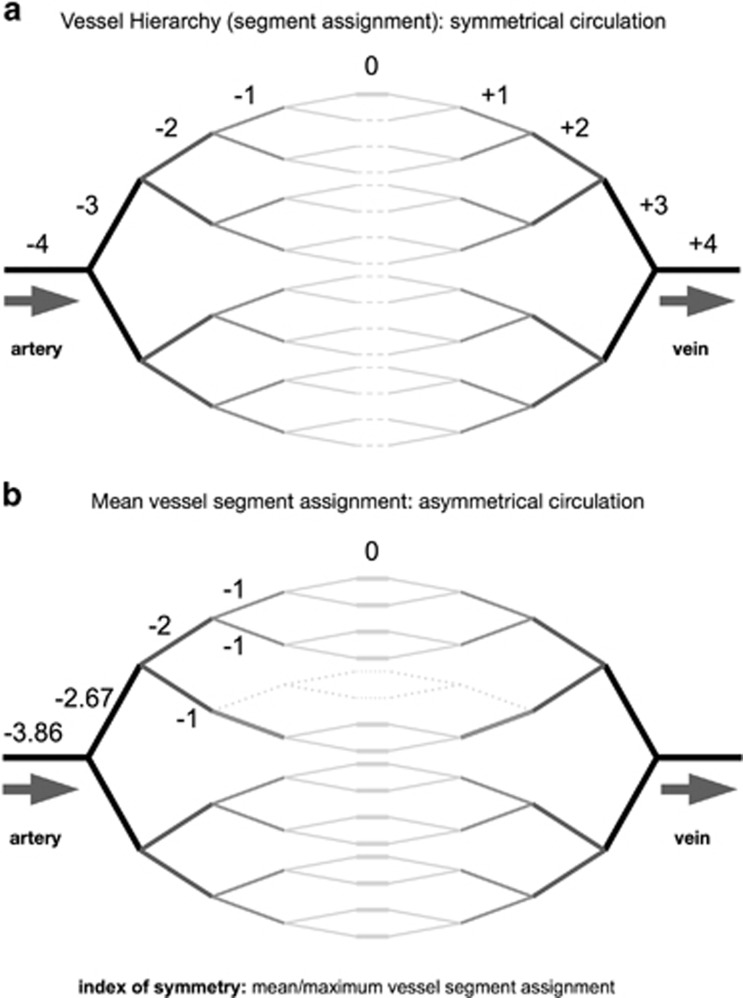
The comparison of micro-circulations over time and between individuals, and the collection of data from cohorts, requires an objective and repeatable vessel nomenclature (Figures 5a, b and 6). Following the Harverian model of the cardiovascular system,7 the heart has conventionally been used as the reference point for such classifications. However, most micro-circulations are too peripheral for vessel hierarchy to be derived in this way and a new nomenclature has been developed, referencing the capillary (Figures 5a and b).
Figure 5.
(a) Vessel hierarchy and nomenclature: symmetrical circulation. (b) Vessel hierarchy and nomenclature: mean vessel segment assignment and index of symmetry.
Figure 6.
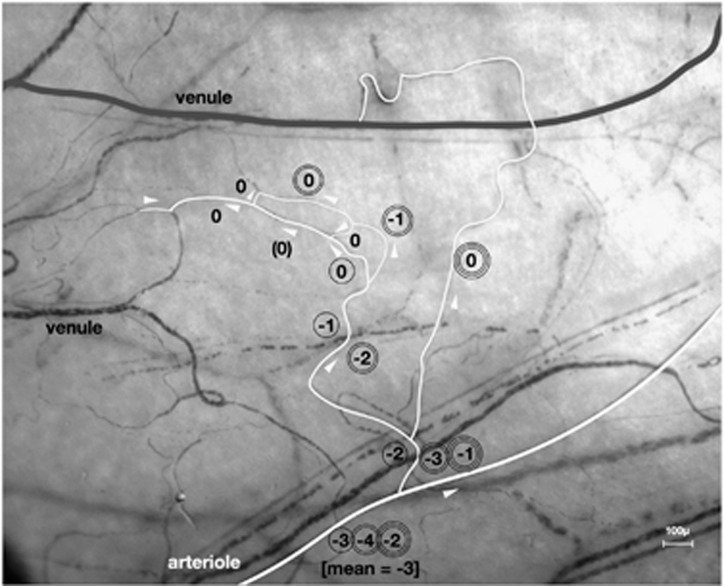
Vessel hierarchy and nomenclature: calculation of mean vessel segment assignment. 3 pathways through a microcirculation (brackets show a capillary receiving from a vessel confluence; delivering to a divergence). White arrows show direction of blood flow. At the arteriole, mean vessel segment assignment=−3. Index of symmetry (mean/maximum vessel segment assignment)=0.75.
In HVI angiograms, capillaries (or thoroughfare vessels) can be defined as those vessels that receive blood from a bifurcation and deliver into a confluence, and each is ascribed zero order.
Moving away from the zero order vessel in both directions, increasing orders are assigned after every branch-point (negative prefix for vessels delivering blood and positive prefix for those draining blood).
In the event of capillary arcades, certain vessels receive blood from a convergence and deliver to a divergence (Figure 6). These are also allocated a zero order.
When using this nomenclature on an asymmetrical circulation, the assignments of higher order vessel segments vary according to the reference capillary that is chosen. This can be used to advantage, since a mean of all possible assignments that can be allocated to a vessel segment reflects the symmetry of the more distal micro-circulation. Mean/maximum vessel segment assignment becomes an index of symmetry (Figures 5b and 6).
Haemoglobin Video Imaging records vessel contents and cannot demonstrate vessel wall structure. Therefore, in this study of flow characteristics, the microcirculation was divided into delivery vessels (arteries, arterioles, metarterioles, thoroughfare vessels and capillaries) and drainage vessels (post-capillary venules, collecting venules and collecting veins), defined using the new nomenclature.
(In this paper, conventional nomenclature will also be used wherever anatomical features provided sufficient cues).
Results
HVI characteristics of flowing blood
The behaviour of blood elements can be observed in exquisite detail in small delivery and drainage vessels (Figures 4a, b; 7 and 8). The texture of the blood column is usually homogeneous in fast-flowing delivery vessels and more granular in the slower-flowing drainage vessels. Individual erythrocytes can be resolved in the capillary lumina of at least one well-focused sequence, in most angiograms.
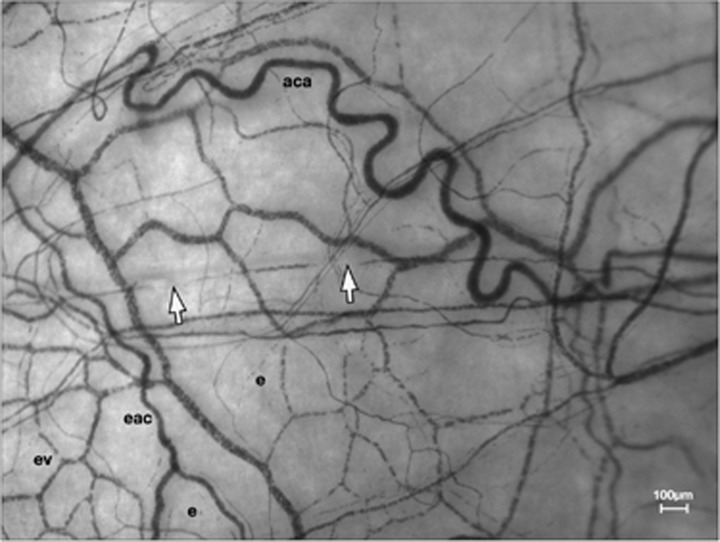
Figure 7.
Erythrocyte bolus in thoroughfare vessel. Left temporal field. White arrows indicate two constrictions in the blood column of a conjunctival thoroughfare vessel, demarcating an erythrocyte bolus. e, static erythrocytes in inactive circulations; ev, mantle of episcleral venules; aca, anterior ciliary artery; eac, episcleral arterial circle.
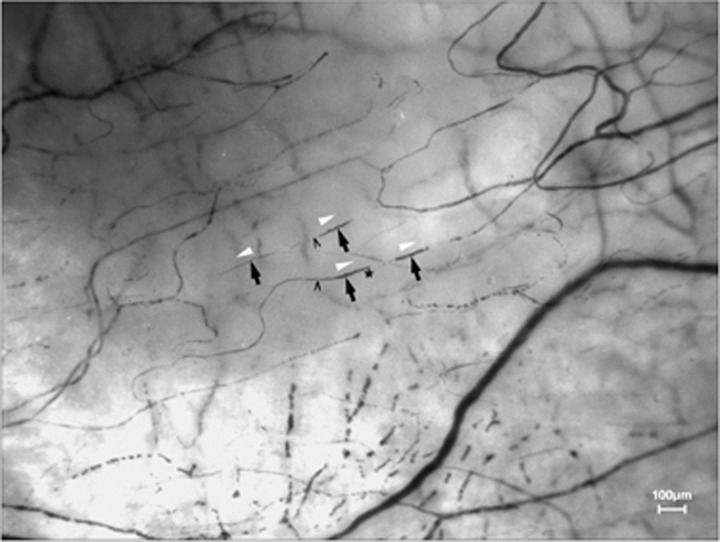
Figure 8.
Erythrocyte boluses in low order delivery vessels. Left nasal field. White arrows show direction of blood flow. Black arrows indicate erythrocyte boluses. * convex leading edge ^ concave, tapering trailing edge.
Erythrocyte aggregates can rarely be distinguished in larger arteries and veins: the thick blood column may absorb so much light that insufficient is returned to activate the CCD. Discrimination of single or aggregated cells is further confounded by motion artefact.
When venules converge, their blood columns remain separated, for a short distance, by erythrocyte-poor laminae (Figure 4b). The low erythrocyte concentration at the periphery of a venous blood column indicates that cells centre in flowing blood; also, that their flow is laminar even when they are aggregated.
Where aqueous enters episcleral veins, it flows to the centre of the blood column, displacing red cells (Figures 4a and b).
The micro-circulation
Arterio-venous projection
HVI angiograms routinely demonstrated complete arterio-venous maps of the conjunctival micro-circulations (Figure 6). Although examples of one-to-one communication between arteries and veins were found, projection of a single artery to multiple veins and multiple arteries to a single vein were much more common. This gave rise to elaborate overlap of delivery and drainage fields.
The episcleral circulation was rarely recorded in its entirety; therefore, its arterio-venous projection could not be characterised.
Active and inactive circulations
Almost every angiogram demonstrated vascular pathways in which blood flow was arrested, often throughout the study, leaving aggregated or individual erythrocytes static for long periods (Figure 7). Examples were also found in which collections of blood cells, which had been static, suddenly resumed their transit and were expelled from the microcirculation.
Delivery vessels
Arteries
The more proximal delivery vessels (arteries) are usually tortuous, with a continuous blood column (Figure 7). They sometimes pulsate, with subtle rippling of their walls and periodic variation in the density of their contents.
Arterial arcades (inter-arterial communications)
In this study, it was possible to examine erythrocyte motion in the episcleral arterial circle and demonstrate functional apices: areas of almost static blood with erythrocyte aggregation, drifting to and fro as the demand for blood from different branch arterioles waxed and waned. Beat to beat oscillations were superimposed over this.
Conjunctival delivery vessels
Constrictions can commonly be seen to advance - singly or in groups - along conjunctival delivery vessels with orders from 0 to at least -4 (Figures 7 and 8). It is a limitation of HVI that it cannot establish the histology of the vessels in which these occur (whether they are arterioles or capillaries); however, constrictions are never seen in arteries or drainage vessels.
Their periodicity bears a relationship to the arrival of the cardiac pulse within the angiographic field and they may propagate in groups of 2 or 3, commensurate with pulse waves arriving by two or more separate routes through tiered arcades (Figures 2, 3 and 7).
Characteristics of the interruptions in blood columns of conjunctival delivery vessels are listed in Table 1.
Table 1. Observations on constrictions in delivery vessels.
| The constrictions that sweep along delivery vessel walls are triggered by the systolic pulse |
| •They are rhythmically aligned with cardiac systole. |
| They represent true constrictions of the vessel lumen (Figure 8) |
| •The gaps between boluses rarely contribute volume to post-capillary venules, indicating that they are not haemoglobin-free zones in otherwise open vessels. |
| •The constrictions define erythrocyte boluses with discrete leading and trailing boundaries. |
| •The leading edges of boluses are commonly convex; however, their trailing portions usually taper, suggesting that they may be driven by advancement of wall constriction. |
| Their formation is spontaneous: not a mere consequence of the systolic delivery of blood |
| •When it can be observed, their appearance, disappearance or re-modelling arises between branch-points and remote from the origin of a vessel segment. |
| Advancing vessel wall motion is active and the cause of blood flow, not its consequence |
| •Several discrete boluses can often be seen to advance simultaneously along a single vessel (Figure 8). |
| •The constrictions may sweep along vessels faster than their contents, overtaking microscopic features within the blood column (for example, erythrocyte aggregates, or haemoglobin-free lucencies). |
| Vessel wall constrictions are propagated locally |
| •During their course along a vessel, the lengths, separation and velocities of constrictions may vary. |
| •At vessel bifurcations, strictures often split to pass along both daughter vessels. |
| •They are always lost when they arrive at +1 vessels (post-capillary venules). |
This evidence suggests that blood is driven through these micro-circulations by peristalsis.
Drainage vessels
Conjunctival drainage vessels
Conjunctival post-capillary venules (+1) are characterised by widening of the blood column, reduction of flow velocity and disorganised erythrocyte distribution. Aggregation of erythrocytes, and striation of convergent blood columns indicating laminar flow, were an invariable characteristic of small venules (Figures 4a, b and 7).
Episcleral micro-circulation
Small episcleral delivery vessels were rarely observed: the visible microcirculation mainly constituted an elaborate mantle of interconnecting channels with open lumina, which communicated with the episcleral collecting veins (Figures 1 and 7). They had the characteristics of low-order drainage vessels, in which erythrocyte aggregates, with intervening clear zones, flowed slowly and reversed freely. No constrictions were seen to propagate along vessel walls in this circulation.
Large episcleral collecting veins (limbal venous circle or episcleral collecting veins: orders of +4 to +6) received blood from both the conjunctival and the episcleral circulations. Where they received new venous contributions, their columns usually became striated.
Aqueous veins
Aqueous was seen to flow into the mantle of episcleral venules, usually displacing blood to form a discrete central column (Figures 4a and b). These vessels drained into the limbal venous circle and radial episcleral collecting veins, in which the segregation of aqueous from blood continued for many millimetres.
The drainage of aqueous is frequently pulsatile, evidenced by the transmission of pulsation to episcleral venules that contribute to the same collecting vein. This may occasionally cause pulsatile venous flow reversal.
Discussion
Haemoglobin video imaging and re-classification of the micro-circulation
Blood corpuscles contain their own contrast (haemoglobin), whilst sclera, which backs the conjunctival and episcleral circulations, exhibits polychromatic reflection. With suitable light filtration, clinical incident-light microscopy can demonstrate these living micro-circulations, revealing blood flow with exquisite resolution—sufficient to distinguish individual erythrocytes.
Such images show blood columns only and, without histology, vessel identification would be speculative. However, the problem can be overcome by establishing vessel hierarchy with reference to capillaries: the only vessels in a microcirculation that can be reliably recognised. This systematic nomenclature of every delivery and drainage vessel has made it possible to characterise blood flow and the re-modelling of micro-circulations, during normal life, disease and pharmacological interventions, and to make comparisons between subjects. It also provides a readily calculable mathematical index of symmetry for any microcirculation.
Blood flow in delivery and drainage vessels
The elaborate arterial arcades that deliver and distribute blood to the ocular anterior segment have been known about for decades;2 however, this imaging technique now illustrates the behaviour of blood within them. The streams of arterial blood, delivered to an arcade such as the episcleral arterial circle, meet at a functional apex. The location of this apex changes according to the volumes of blood supplied by its contributing arteries and demanded by its various branches. Since the contributions to arcades arrive asynchronously, pulsatile flow reversal takes place at the apex, disrupting laminar flow. Flow velocity and volume also diminish towards the apex.
Delivery and drainage fields overlap extensively. These communications underlie the enormous resilience of micro-circulations to damage.
The depth of the blood column and rapidity of flow in large arteries interfered with the ability of HVI to detect erythrocytes (single or aggregated) and estimate flow or shear rates. However, it was possible to demonstrate subtle wall motion, both in anterior ciliary arteries and at the origin of arterial arcades; also, to show turbulence at the apices of arcades.
The homogeneity of blood in small conjunctival delivery vessels (an indication of rapid laminar flow) was lost immediately on entering first order drainage vessels. Here, the blood column became disorganised, with tumbling, slow-flowing, aggregated erythrocytes, interrupted by clear zones.
Higher order drainage vessels had larger calibre and slower flow than their complementary arteries. Although the erythrocytes formed aggregates, these became uniformly distributed. The feature which best distinguished drainage vessels was the addition of new erythrocyte-poor striae to the blood column whenever vessels converged: confirmation, if required, that their flow is laminar.
Peristaltic delivery of blood
Vessel wall motion in vertebrate micro-circulations has been known to exist for decades, but this activity was presumed to regulate blood flow and not to drive it. The most remarkable observation to emerge from the studies presented here was that blood columns in low-order delivery vessels were often fragmented into multiple short boluses. The constrictions separating them represented true luminal occlusions and progressed along the vessels, apparently propelling blood towards the venous system.
The origin of such constrictions, remote from bifurcations, and their multiplicity in single vessels, indicated that they must result from active wall motion and refuted any notion that they could represent passive vasodilation, due purely to the systolic introduction of blood. The peristalsis of blood by vessel walls was supported by the tapering trailing edges of most boluses, and their various and independent flow velocities. Constrictions also often travelled faster than the vessel contents.
Micro-circulations vary enormously. In this study, conjunctiva contained delicate delivery vessels with visible peristaltic activity. However, the episcleral circulation was composed mostly of inter-connecting open drainage channels and delivery vessels were rarely seen. They were presumably short or beneath the scleral surface, rendering peristaltic activity difficult to demonstrate.
Peristaltic propulsion of blood is likely to be ubiquitous, its embodiment varying from one tissue to another. The pulsatile arterial delivery of blood to micro-circulations carries a risk of reflux during diastole. In the absence of valves, this can only be prevented by constriction of vessel lumina, and it is a small evolutionary step for such constrictions to advance the blood.
We should not be surprised to discover active propulsion of blood through micro-circulations in man. Local control of perfusion pressure is required to overcome variations in systemic blood pressure and inconsistent tissue tension. Furthermore, it would be very remarkable if the heart alone could maintain the perfusion of an estimated 10 billion capillaries with a total length of >25 thousand miles.
There is also a phylogenetic precedent. In the primitive closed circulation of the earthworm, peristalsis is the main motive force: it drives blood along a dorsal vessel and into contractile circumferential vessels containing valves, which supply the more ventral longitudinal channels.8, 9
Much recent work has identified pericytes, which contain actin and are contractile, as a source of vessel wall motion.10, 11 They are plausible mediators of peristalsis, constricting vessel lumina when depolarised and (in the brain) relaxing in response to prostaglandin E2.12 The spread of capillary wall motion from cell to cell is slower after electrical stimulation in vitro than the flow rates observed in the present study, suggesting that peristalsis is more likely to be generated by a blood-borne or neuronal signal.
Clinical applications of HVI
As well as providing new insights into microvascular physiology, conjunctival HVI is a valuable clinical tool. Ocular surface blood vessels can easily be identified during clinical slit lamp microscopy, and their flow directions and flow characteristics can be recorded.
Patients with cANCA-positive vasculitis show abnormal erythrocyte aggregation in conjunctival veins, a phenomenon that can be used to assess disease activity.
In thyroid eye disease, elevated cone pressure causes reversal of blood flow in anterior conjunctival venules, as blood is diverted into posterior tarsal veins: the regional distribution of this sign identifies the most severely affected rectus muscles (paper in preparation).
Conjunctival venous flow reversal has also been observed in patients with carotico-cavernous fistula.
Recently, we have used conjunctival HVI to record the effects of systemically administered drugs on micro-circulations (paper in preparation).
Conjunctival HVI is transforming our knowledge about micro-circulations and the diseases that afflict them. This paper presents the first evidence that, while blood is transported to the periphery by the heart, it is actively transferred through tissues by small delivery vessels, themselves.
Dedication
This paper is dedicated to the memory of Peter G Watson (1930–2017). He had great generosity and the wonderful gift of facilitating achievement in others. He enabled me to undertake research, introduced me to the ocular anterior segment circulations and challenged me to image them.
Acknowledgments
The new data that is presented results from unfunded research, undertaken in the University of Cambridge Department of Ophthalmology.
Footnotes
The author declares no conflict of interest.
Supplementary Material
References
- Meyer PA. The circulation of the human limbus. Eye 1989; 3: 121–127. [DOI] [PubMed] [Google Scholar]
- Meyer PA. Anterior segment vascular imaging. In: Easty DL, Sparrow JM (eds) Oxford Textbook of Ophthalmology. Oxford University Press: Oxford, UK, 1999. pp 160–174. [Google Scholar]
- Henle J. Handbuch der Systematischen Anatomie des Menschen Vol III: Handbuch der Gefasslehre des Menschen. Friedrich Vieweg u Sohn: Braunschweig, 1868. [Google Scholar]
- Meyer PA, Watson PG. Low-dose fluorescein angiography of the conjunctiva and episclera. Br J Ophthalmol 1987; 71: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N, Smith R. Anatomical study of Schlemm’s canal and aqueous veins by means of Neoprene casts III. Arterial relations of Schlemm’s canal. Br J Ophthalmol 1953; 37: 577–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PA. Patterns of blood flow in episcleral vessels studied by low-dose fluorescein videoangiography. Eye 1988; 2: 533–546. [DOI] [PubMed] [Google Scholar]
- Harvey W. Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus 1628. [PubMed]
- Johnston JB. On the blood vessels, their valves and the course of the blood in Lumbricus Biol. Bull 1903; 5: 74–84. [Google Scholar]
- Monahan-Earley R, Dvorak AM, Aird WC. Evolutionary origins of the blood vascular system and endothelium. Thromb Haemost. 2013; 11(Suppl 1): 46–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Atobe Y, Goris RC, Yazama F, Ono M, Sawada H et al. Ultrastructure of the capillary pericytes and the expression of smooth muscle alpha-actin and desmin in the snake infrared sensory organs. Anat Rec 2000; 260: 299–307. [DOI] [PubMed] [Google Scholar]
- Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab 2016; 36: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508(7494): 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.