Abstract
Objective(s):
Production of effective tuberculosis (TB) vaccine is necessity. However, the development of new subunit vaccines is faced with concerns about their weak immunogenicity. To overcome such problems, polymers-based vaccine delivery systems have been proposed to be used via various routes. The purpose of this study was to determine the potential of polymeric particles as future vaccine delivery systems/adjuvants for parenteral and non-parenteral immunization against TB.
Materials and Methods:
PubMed, Scopus, Science-Direct, and the ISI web of knowledge databases were searched for related keywords. A total of 420 articles, written up to June 25, 2016, were collected on the potential of polymeric particles as TB vaccine delivery systems after parenteral and non-parenteral immunization. Thirty-one relevant articles were selected by applying inclusion and exclusion criteria.
Results:
It was shown that the immunogenicity of TB vaccines had been improved by using biodegradable and non-biodegradable synthetic polymers as well as natural polymers and they are better able to enhance the humoral and cellular immune responses, compared to TB vaccines alone. The present study revealed that various polymeric particles, after M. tuberculosis challenge in animal models, provide long-lasting protection against TB. PLGA (poly (lactide-co-glycolide)) and chitosan polymers were widely used as TB vaccine delivery systems/adjuvants.
Conclusion:
It seems that PLGA and chitosan polymers are well-suited particles for the parenteral and non-parenteral administration of TB vaccines, respectively. Non-biodegradable synthetic polymers in comparison with biodegradable synthetic and natural polymers have been used less frequently. Therefore, further study on this category of polymers is required.
Keywords: Mycobacterium tuberculosis, Non-parenteral immunization, Parenteral immunization, Polymeric particles, Vaccine
Introduction
Among main intracellular pathogens, Mycobacterium tuberculosis (M. tuberculosis) is known as the cause of a terrible infectious disease with a high mortality rate all over the world (1, 2). Tuberculosis (TB) vaccination is the most important approach to long-term control of the disease (3). The BCG vaccine, which is a live attenuated strain of Mycobacterium bovis, bacillus Calmette-Guérin, is the only licensed and cost-effective vaccine but with limited and variable effects on specific individuals in prevention of active disease (0-85%) (3, 4). Therefore, the development of new vaccines is an urgent need. Currently, at least 15 TB vaccine candidates are located in various phases of clinical trials, which are classified into three main groups: 1) prime group, includes vaccines that are designed as a replacement for the current BCG vaccine, 2) prime-boost group, includes vaccines that act as boosters for the BCG vaccine and 3) immunotherapy group, includes therapeutic vaccines for effective treatment of various forms of TB disease as well as drug-resistant strains of M. tuberculosis (5). However, most of these vaccines are weak immunogens, and polymer-based particles as vaccine delivery systems/adjuvants could help to attain the following goals: 1) for optimal stimulation of innate and adaptive immune systems, 2) protection of antigens from in vivo enzymatic degradation, 3) effective antigen targeting to antigen-presenting cells (APCs) followed by its processing by both MHC-I and II routes and then induction of CD4+ and CD8+ T cells, 4) control of inducing cell-mediated responses (Th1 or Th2) by controlling the particle size, and 5) usage as an adjuvant (6-8). Among various biomaterials used in the development of protein, peptide, and DNA vaccines, polymers are of great importance due to some advantages including biocompatibility with synthetic polymers, lower toxicity, biocompatibility, easy availability, and biodegrade-ability of natural polymers (8-12). Polymers could be cate-gorized as 1) biodegradable synthetic polymers like PLGA (poly (lactide-co-glycolide)), PLA (poly (lactide)) (both are FDA approved) (8-11), poly (alkyl cyanoacrylates), poly (ɛ-caprolactone), and polyphos-phates, 2) non-biodegradable synthetic polymers like polyvinylpyrrolidone, poloxamers, and poly (methyl methacrylates), and 3) natural polymers like proteins (albumin, collagen, and gelatin) and polysaccharides (chitosan/chitin and alginate) (13). In spite of the advantages of polymeric nanoparticles (NPs), they also have some disadvantages including toxicity, non-degradability, complexity, and costly synthesis process for synthetic polymers, and more complicated structure, complexity, and expensive extraction process for natural polymers (12).
There is two main delivery routes for vaccine administration, non-parenteral (mucosal) vaccination via intranasal, oral, vaginal, and rectal routes, and parenteral via subcutaneous and intramuscular (14). To date, parenteral routes are the common delivery routes for TB vaccine candidates, and there is much less research on mucosal vaccination (14). Since the respiratory mucosa is the site of TB entry, mucosal vaccination, especially the nasal route could provide good protection against TB (14). This could be performed by the production of neutralizing anti-bodies like secretory IgA (sIgA), systemic IgG, and also activation of various CD4+ T cells such as Th1, Th2, and Th17, and also CD8+ T cells (CTLs). These could strongly stimulate both mucosal and systemic immune responses (15, 16). Th1, Th17, and CTLs mediated immunity are essential for the host defense against intracellular pathogens that enter through various mucosal surfaces, whereas CD4+ Th2 cells are effective against extracellular organisms (16-18). Antibody responses via organism opsonization and conse-quently the more efficient processing by dendritic cells (DCs) have synergistic effects on the immune system (19). Additionally, the nasal cavity possesses other advantages such as patient compliance, better epithelium permeability, and needle-free and self-administration (14, 20). In the present review, the potential of various polymeric particles as future vaccine delivery systems/adjuvants for parenteral and mucosal immunization against TB has been investigated.
Materials and Methods
Literature search
PubMed, Scopus, Science-Direct, and the ISI web of knowledge databases were searched to identify relevant studies up to June 25, 2016. Data from published English language articles were obtained by using the medical terms including “M. tuberculosis vaccine’’ or “TB vaccine” and “polymeric NPs” or “polymeric microparticles” and “PLGA” and “PLA” and “poly (alkyl cyanoacrylates)” and “poly (ɛ-caprolactone)” and “polyphosphates” particles and “polyvinylpyrrolidone” and “poloxamers” and “poly (methyl methacrylates)” particles and “protein (albumin, collagen and gelatin)” particles and “polysaccharide (chitosan/chitin and alginate)” particles and “immunization” or “vaccine delivery”. Finally, a supplementary search in the Google Scholar search engine and hand searching of reference lists to find the excluded articles was conducted.
Evaluation criteria
In this systematic review, the main inclusion criteria for selection of relevant articles included: 1) published original articles written in English, 2) evaluation of various TB vaccines only based on different polymeric particles, 3) parenteral and mucosal administration of TB vaccines, and 4) assessment of different polymeric particles as adjuvants. Exclusion criteria included: 1) abstracts of articles, 2) review articles and books, 3) use of other particles as vaccine delivery systems/adjuvants, 4) assessment of vaccine delivery in other organisms, 5) duplicate studies, and 6) assessment of drug delivery in M. tuberculosis.
Results
A total of 420 original articles were collected from different studies using systematic search in databases (Figure 1). Searching was limited to polymeric particles and their roles as vaccine delivery systems/adjuvants for parenteral and mucosal immunization against TB written up to June 25, 2016. All obtained studies in the present paper were reviewed to extract the following data: 1) year of study, 2) type of polymer and adjuvant, 3) antigen(s), 4) size, zeta-potential, and preparation method of particles, 5) route of administration, 6) animal, and 7) profile of immune responses. Thirty-one selected articles were classified into three groups based on the inclusion and exclusion criteria.
Figure 1.
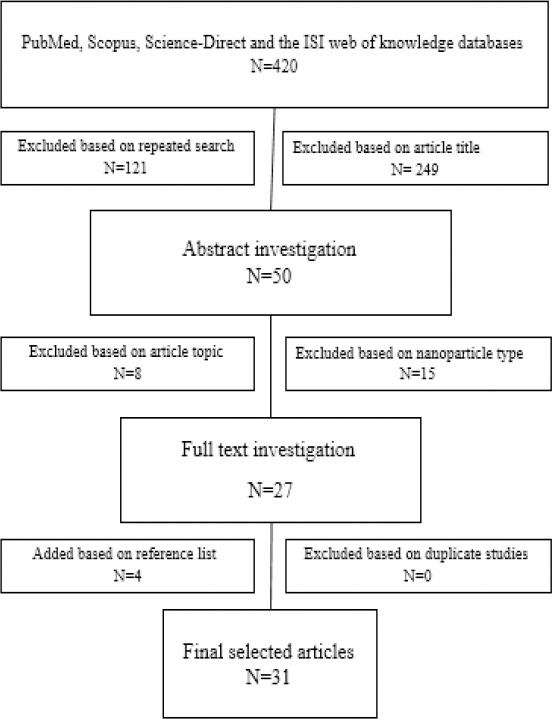
Flowchart of literature search and inclusion and exclusion criteria
Biodegradable synthetic polymers
Among biodegradable synthetic polymers (PLGA, PLA, poly (alkyl cyanoacrylates), poly (ɛ-capro-lactone), and polyphosphates), PLGA polymers are the most widely used as TB vaccine delivery systems/adjuvants. This systematic review includes 14 studies on PLGA polymers. It is evident that PLGA (50:50) is the most frequently used polymer. All studies have used the double emulsion/solvent evaporation method (W/O/W) to prepare PLGA polymers. After administration through various routes, PLGA polymers induced high levels of antibody and Th1, Th2, and Th17 immune responses. The impact of different adjuvants such as cationic lipid, DDA (dimethyldioctadecylammonium bromide), TDB (trehalose 6, 6’-dibehenate), PEI (polyethyleneimine), TDM (trehalose dimycolate), and lipid adjuvants, such as MPL (monophosphoryl lipid A), either separately or in combination, on the particle size, zeta-potential, and profile of immune responses were reviewed (Table 1).
Table 1.
Polymeric particles as tuberculosis vaccine delivery systems
| First author (Ref) | Year | Polymer | Antigen (s) | Adjuvant (s) | Preparation method | Size | Zeta-Potential (mV) | Route of administration | Animal | Profile of immune responses | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibody | Cytokine | Challenge | ||||||||||
| Rose et al (21) | 2015 | PLGA (75:25) | MOMP | DDA:TDB (CAF01) | O/W | <250 nm | >5 | Subcutaneous | Female B6C3F1 mice | IgG1 IgG2a IgA | IFN-γ IL-17a IL-5 | NA |
| Carletti et al (22) | 2013 | PLGA (50:50) | Apa | TDM | W/O/W | NA | NA | Subcutaneous Intramuscular | Female BALB/c mice | NA | NA | M. tuberculosis H37Rv |
| Shi et al (23) | 2010 | PLGA (75:25) | TB10.4- Ag85B | MDP-BSA | Emulsion /spray-drying | 3.0µm | −25 | Pulmonary | NA | NA | IL-2 | NA |
| Bivas-Benitaa et al (24) | 2009 | PLGA (53:47) | Rv1733c | PEI | W/O/W | 235 - 275 nm | +38.8 - +64.3 | Intramuscular Intranasal | Female BALB/c mice | NA | IFN-γ IL-12 TNF-α | NA |
| Kirby et al (25) | 2008 | PLGA (75:25) | Ag85B-ESAT-6 | DDA:TDB (CAF01) DDA TDB | W/O/W | 1.50± 0.13 µm | 0-15 | Subcutaneous | Female C57BL/6j mice | IgG1 IgG2b | IFN-γ | NA |
| de Paula et al (26) | 2007 | PLGA (50:50) | HSP65 | TDM | W/O/W | <10 µm | NA | Intratracheal | Female Hartley guinea pigs and Female BALB/c mice | IgG2a | IFN-γ | M. tuberculosis H37Rv |
| Lu et al (27) | 2007 | PLGA (75:25) | rAg85B | MDP TDB | Emulsion /spray-drying | 3.4-4.3 µm | NA | NA | NA | NA | IL-2 | NA |
| Ha et al (28) | 2006 | PLGA (NA) | rAg85A ESAT-6 | IL-12EM AS01B alum | W/O/W | NA | NA | Subcutaneous | Female C57BL/6 mice | Total IgG IgG1 IgG2a | IFN-γ | M. tuberculosis H37Rv |
| Cai et al (29) | 2005 | PLGA (50:50) | Ag85B MPT-64 MPT-83 | DDA | W/O/W | <5 µm | NA | Intramuscular | Female C57BL/6 mice | IgG | IFN-γ | M. tuberculosis H37Rv |
| Evans et al (30) | 2004 | PLGA (50:50) | Mtb8.4 | MPL RC-529 | Hydrophobic ion-pairing technique | 2 µm | NA | Intramuscular Subcutaneous | Female C57Bl/6 mice | IgG IgG2a | IFN-γ | NA |
| Lima et al (31) | 2003 | PLGA (50:50) | HSP65 | TDM | W/O/W | <5 µm | NA | Intramuscular | Female BALB/c mice | IgG1 IgG2a | IFN-γ IL-10 IL-4 | M. tuberculosis H37Rv |
| Lima et al (32) | 2001 | PLGA (50:50) | NA | TDM | W/O/W | NA | NA | Intraperitoneal Intratracheal | Female BALB/c mice | NA | IL-6 TNF-α IL-10 IFN-γ IL-12 IL-4 | M. tuberculosis H37Rv |
| Dhiman et al (33) | 1998 | PLGA (50:50) | 71-kDa cell wall protein | FIA | W/O/W | <0.65 µm | NA | Intramuscular Subcutaneous | NA | NA | NA | M. tuberculosis H37Rv |
| Carpenter et al (34) | 2005 | PLA | ESAT-6 | Alum | W/O/W | 1.179 μm±0.07 | NA | Pulmonary Intranasal Intramuscular | Female BALB/c mice | IgG | IFN-γ IL-4 | NA |
| Venkataprasad et al (35) | 1999 | PLA PLGA (75:25, 50:50) | 38 kDa protein | NA | W/O/W | PLA (3-5 µm) and PLG (0.3-0.8 µm) | NA | Subcutaneous | Female C57BL/10 mice | NA | IFN-γ IL-4 | NA |
| Todoroff et al (36) | 2013 | Poloxamer 407 | Ag85A | CpG oligonucleotide | NA | 27 nm | NA | Pulmonary (Intratracheal) | Female BALB/c mice | IgG, IgG1, and IgG2a | IFN-γ TNF-α IL-2 IL-17a | NA |
| Orr et al (37) | 2014 | NA | ID93 | GLA-SE | NA | < 120 nm | -13 | Intramuscular | Female C57Bl/6 mice | IgG1 IgG2c | IFN-γ TNF-α IL-2 | M. tuberculosis H37Rv |
| Yeboah et al (38) | 2009 | Albumin | Dead whole cells and whole cell lysate | NA | Spray-drying method | 3.52±0.13 µm 6.61±0.70 µm | -13.05 ±19.18 -40.28 ±7.86 | Oral | Rat | IgG IgA | NA | NA |
| Meerak et al (39) | 2013 | Chitosan | Ag85B | NA | Coacervation method | 100–200 nm | NA | Subcutaneous Intranasal | Female BALB/c inbred | total IgG IgG2a | IFN-γ IL-2 IL-4 | NA |
| Feng et al (40) | 2013 | Chitosan | Esat-6/3e-FL | NA | Ionic crosslink and coacervation | 311.2±34 nm | 30.6 mV | Intramuscular Intranasal | Female C57Bl/6 mice | NA | IFN-γ IL-12 IL-10 IL-4 | M. tuberculosis H37Rv |
| Ai et al (41) | 2013 | Chitosan | pHSP65pep | NA | Coacervation method | 350 -400 nm | NA | Intranasal Intradermal | Female BALB/c mice | IgA IgG IgG1 IgG2a | IFN-γ | M. bovis |
| Verma et al (42) | 2013 | Chitosan-TPP | CFP-10 CFP-21 | Ionotropic gelation | 250 -300 nm | 41 ±5.29 | NA | NA | NA | IFN-γ IL-4 | NA | |
| Caetano et al (43) | 2013 | Chitosan/Alginate/TPP | BCG | NA | Ionotropic gelation | 33.865 µm ±5.347 | +10.91 ± 3.55 | Intranasal Subcutaneous | Female BALB/c mice | IgG IgG 1 IgG2a IgA | NA | NA |
| Zhu et al (44) | 2007 | Chitosan | AMM | IFA | Precipitation/coacervation method | 5.78 ± 0.65 µm | 32.77 ± 1.51 | Subcutaneous | Female C57Bl/6 mice | IgG1 IgG2a | IFN-γ IL-4 | NA |
| Bivas-Benita et al (45) | 2004 | Chitosan | DNA vaccine encoding eight HLA-A*0201-restricted T-cell epitopes | NA | Complexation-coacervation method | 376±59 nm | 21±4 | Pulmonary Intramuscular | Female HLA-A2 transgenic mice | NA | IFN-γ | NA |
| Dobakhti et al (46) | 2009 | NA | BCG | Sodium alginate | NA | NA | NA | Subcutaneous | Female BALB/c mice | Total IgG IgG2a IgG1 | IFN-γ | M. bovis |
| Ajdary et al (47) | 2007 | Sodium alginate | BCG | NA | NA | 11.5 μm | NA | Subcutaneous Oral | Female BALB/c mice | Total IgG IgG2a IgG1 | IFN-γ IL-4 | M. bovis |
| Dobakhti et al (48) | 2006 | Calcium alginate | BCG | NA | Internal emulsification method | 11 μm | NA | Subcutaneous Oral | Female BALB/c mice | Total IgG IgG2a IgG1 | NA | NA |
| Wilkinson et al (49) | 2000 | Polystyrene | Ag85A, B and C | NA | NA | 2 μm | NA | NA | NA | NA | IFN-γ | NA |
| Yu et al (50) | 2012 | Fe3O4-Glu-polyethyleneimine | Ag85A-ESAT-6-IL-21 | NA | NA | NA | +36 mV | Intramuscular Subcutaneous | Male C57BL/6 mice | NA | IFN-γ | M. tuberculosis H37Rv |
| Ballester et al (51) | 2011 | Pluronic-stabilized polypropylene sulfide | Ag85B | CpG | Emulsion polymerization and surface functionalized | 30 nm | NA | Intradermal Pulmonary | Female C57BL/6 mice | NA | IFN-γ TNF-α IL-6 IL-1β IL-17a IL-2 | M. tuberculosis Erdman strain |
Abbreviations: O/W: Oil-in-water single emulsion, W/O/W: Double emulsion/solvent evaporation, MOMP: Major outer-membrane protein, Apa: Alanine-proline antigen, MDP: Muramyl dipeptide, BSA: Bovine serum albumin, AS01B: Composed of MPL and QS21 (saponin molecule), RC-529 adjuvant: A synthetic ω-amin-oalkyl-2-amino-2-deoxy-4-phosphono-²-d-glucopyranoside (AGP) which is structurally related to the major hexaacyl component of MPL adjuvant, FIA: Freund’s incomplete adjuvant, ID93: Fusion protein containing M. tuberculosis genes Rv3619, Rv1813, Rv3620, and Rv2608, GLA-SE: A mixture of squalene, DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine), poloxamer 188, glycerol, and ammonium phosphate buffer, Esat-6/3e-FL: Esat-6 three T cell epitopes (Esat-6/3e) and fms-like tyrosine kinase 3 ligand (FL) genes, pHSP65pep: pECANS plasmid with four epitopes cast in the gene backbone of HSP65, namely ESAT-664-76, Ag85A124–135, CFP-1055–69, and Ag85B141–153, TPP: Tripolyphosphate, AMM: Ag85B–MPT64190–198–Mtb8.4, Fe3O4-Glu-PEI: Fe3O4-Glutamic acid-Polyethyleneimine, NA: Not available
So far, just two studies have investigated the application of PLA polymers as vaccine delivery systems for TB subunit vaccines. Data shows that the microencapsulated antigen was highly immunogenic and nasal delivery of the microencapsulated TB antigen generated specific cellular immune responses (Table 1).
However, no study on the application of other biodegradable synthetic polymers, poly (alkyl cyano-acrylates), poly (ɛ-caprolactone), or polyphosphates, as TB vaccine delivery systems/adjuvants was found for the present systematic review.
Non-biodegradable synthetic polymers
Our study shows the higher capacity of poloxamer polymers amongst other non-biodegradable synthetic polymers, like polyvinylpyrrolidone and poly (methyl methacrylates), in the induction of protective immunity against M. tuberculosis. It has been shown that after pulmonary delivery of the TB antigen with poloxamer polymers, as single adjuvants or combined with other adjuvants; these polymers have good potentials in stimulation of humoral and cell-mediated immune responses. However, no study was found on polyvinylpyrrolidone or poly (methyl methacrylates) to better compare the non-biodegradable synthetic polymers as adjuvants, nano-, or micro-carriers for TB vaccines.
Natural polymers
Studies on albumin, collagen, and gelatin as natural polymers were not found except one on the albumin polymer. It has been reported that albumin polymers can induce antigen-specific mucosal and systemic immune responses. To date, there are no studies on collagen and gelatin as TB vaccine carriers. Ten studies on chitosan/chitin and alginate (natural polysaccharide polymers) were found. Chitosan polymers are widely used as TB vaccine delivery systems/adjuvants. It was shown that chitosan can induce increased levels of antibody and IFN-γ secretion against TB antigens.
Discussion
Main prevention strategies against TB are prophylactic and post-exposure vaccination. Prophylactic vaccines may be used either for replacing BCG, like live mycobacterial vaccines, or as a booster for BCG, like subunit vaccines. Post-exposure vaccines are used for elimination of the disease in latently-infected individuals and to prevent disease reactivation (52). Ag85A, Ag85B, Ag85C, TB10.4, MPT-64, MPT-83, ESAT-6, CFP-10, Mtb8.4, and hsp65 are among main TB antigens that are used as DNA or subunit vaccines, either alone or as a fusion protein. These antigens could induce specific antibodies and cell-mediated immune responses (Table 1). These are mostly expressed in the early phase of infection by the replicating bacteria, however, to induce a broad spectrum of immune responses, antigens expressed in latency phase of non-replicating bacteria are also important, because they are often hidden from the immune system (53). This review suggests that ideal vaccination approach against both acute and latent TB infections is using both early and late stage antigens of M. tuberculosis (multistage booster vaccines).
TB DNA and subunit vaccines have high safety profiles and have shown promising results in animal models, however, in human clinical trials, they were poor immunogens (8, 24). To overcome this problem, polymer-based particles have been used as adjuvant/delivery systems to potentiate the immune responses. Among them, PLGA particles were mostly used for delivery of prophylactic TB vaccines (Table 1). The PLGA (50:50) copolymer as compared with the various polymer compositions (like 75:25 and 53:47), was the most frequently used PLGA polymer. The PLGA (75:25) as an intermediate copolymer has more efficient protein release (due to faster degradation) than the PLA homopolymer and more prolonged release profile in comparison with the PLGA (50:50) copolymer (25). As presented in Table 1, using cationic lipids as adjuvants in combination with PLGA polymers could affect the size, zeta-potential, polydispersity index (PDI), entrapment efficiency, in vitro release profile, and also elicit simultaneously humoral and cell-mediated immune responses (8, 25). The cationic lipids skew the immune response towards the Th1 branch of immunity and IgG2a production, which is important for immunization against TB. In the presence of cationic lipids, reduction in particle size and PDI, change of zeta potential from negative to positive, and the decrease in antigen entrapment were observed. Similar results have been shown by Wedlock et al and Jensen et al (54, 55). As shown in Table 1, encouraging results have been observed after administration of PLGA-based TB vaccines via various routes. Several studies have shown that the PLGA polymer is well-tolerated in the most common injection sites, i.e., subcutaneous and intramuscular spaces (56). Due to biocompatibility, adjuvanticity, and prolonged release profile of PLGA particles, mucosal (nasal, oral, and pulmonary) administration of PLGA-based vaccines have shown promising results (25).
PLA particles can be built with the same method as PLGA particles and induced robust Th1-type responses (IFN-γ) against TB (Table 1). However, the main disadvantages of PLA polymers are the possibility of denaturation of encapsulated protein and also change in immunogenicity of loaded antigens during particle formulation and in the release period (35). To preserve the antigens’ integrity, antigens could be adsorbed onto the PLA or PLGA particles.
Other biodegradable synthetic polymers, such as poly (ɛ-caprolactone), are also suitable for preparation of vaccine delivery systems (56). However, these polymers have not been tried for delivery of TB vaccines.
Non-biodegradable synthetic polymers compared to biodegradable synthetic and natural polymers have been less frequently used as carrier/adjuvant for TB vaccines. However, as discussed in the Todoroff et al study, Poloxamer 407 (a non-biodegradable synthetic polymer) combined with a CpG oligonucleotide, induced immune responses against the M. tuberculosis antigen (Ag85A) (Table 1) (36).
It is suggested that natural polymers due to milder preparation process, lower prices, and their adjuvant potential are attractive materials for replacement with biodegradable synthetic polymers (56). The protective and immunogenic efficacy of the vaccines based on the natural polymers has been shown in many studies. Yeboah et al and Dobakhti et al have shown that oral delivery of albumin and alginate microspheres loaded with TB vaccines could induce mucosal and systemic immune responses (38, 46). As Table 1 represents, alginate-based TB vaccines have been administered subcutaneously and orally. According to these studies, alginates may be appropriate for utilization in oral vaccination and will protect encapsulated BCG against degradation in the stomach and induce strong Th1 immune responses against TB (46-48, 56). Several studies have indicated that chitosan-based vaccines can significantly promote Th1 and CTL immune responses and prevent TB infection (39-45). According to the different studies shown in Table 1, due to antigen depot, efficient uptake by membrane epithelium, and mucoadhesive and adjuvant properties of these biodegradable particulate delivery systems, they are ideal intranasal vaccine delivery systems/adjuvants against TB. As mentioned in Table 1, polymer-based TB vaccines have shown promising results in pre-clinical studies and were able to protect animal models against challenge with the virulent M. tuberculosis H37Rv strain, however, none of these vaccines entered clinical trials. Due to the results obtained in this study, we are optimistic about introduction of polymer-based TB vaccines to the clinical trial pipeline as potential antigen delivery systems/adjuvants for parenteral and non-parenteral immunization.
Conclusion
In summary, the present systematic review reveals that to develop a new TB vaccine, more attention to antigens expressed in early and latency phase of M. tuberculosis infection is needed. On the other hand, for improving the weak immunogenicity of these antigens, co-delivery of TB vaccines with polymeric particles could be beneficial in reduction of obstacles against development of new and more efficient TB vaccines. The present study revealed that various polymeric particles could be used as carriers for parenteral and mucosal administration of TB vaccine candidates. Among the polymers reviewed, PLGA and chitosan polymers are the most outstanding ones that show promising results, after subcutaneous and mucosal administration, respectively. Non-biodegradable synthetic polymers in comparison with biodegradable synthetic and natural polymers have been used less frequently. Therefore, further study on this category of polymers is required.
Acknowledgment
The authors would like to thank Dr Ramin Sadeghi for his technical support in the design of this systematic review. This plan does not have any financial source, and there is no conflict of interest.
References
- 1.Khademi F, Yousefi-Avarvand A, Derakhshan M, Meshkat Z, Tafaghodi M, Ghazvini K, et al. Mycobacterium tuberculosis HspX/EsxS Fusion Protein: Gene Cloning, Protein Expression, and Purification in Escherichia coli. Reports of Biochemistry and Molecular Biology. 2017;6:15–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Khademi F, Yousefi-Avarvand A, Derakhshan M, Vaez H, Sadeghi R. Middle East Mycobacterium tuberculosis Antibiotic Resistance: A Systematic Review and Meta-Analysis. Infection, Epidemiology and Medicine. 2017;3:25–35. [Google Scholar]
- 3.Karimi SM, Sankian M, Khademi F, Tafaghodi M. Chitosan (CHT) and trimethylchitosan (TMC) nanoparticles as adjuvant/delivery system for parenteral and nasal immunization against Mycobacterium tuberculosis (MTb) ESAT-6 antigen. Nanomed J. 2016;3:223–229. [Google Scholar]
- 4.Khademi F, Derakhshan M, Sadeghi R. The role of Toll-Like Receptor Gene Polymorphisms in Tuberculosis Susceptibility: A Systematic Review and Meta-Analysis. Rev Clin Med. 2016;3:133–140. [Google Scholar]
- 5.Da Costa C, Walker B, Bonavia A. Tuberculosis Vaccines–state of the art, and novel approaches to vaccine development. Int J Infect Dis. 2015;32:5–12. doi: 10.1016/j.ijid.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Islam MA, Firdous J, Choi Y-J, Yun C-H, Cho C-S. Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomedicine. 2012;7:6077–6093. doi: 10.2147/IJN.S38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27:573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Garg NK, Dwivedi P, Jain A, Tyagi S, Sahu T, Tyagi RK. Development of novel carrier (s) mediated tuberculosis vaccine: More than a tour de force. Eur J Pharm Sci. 2014;62:227–242. doi: 10.1016/j.ejps.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Tafaghodi M, Sajadi Tabassi S, Jaafari MR. Nasal Immunization by (PLGA) Nanospheres Encapsulated with Tetanus Toxoid and (CpG-ODN) IJPR. 2010:151–158. [Google Scholar]
- 10.Tafaghodi M, Khamesipour A, Jaafari MR. Immunization against leishmaniasis by PLGA nanospheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODN. Parasitol Res. 2011;108:1265–1273. doi: 10.1007/s00436-010-2176-4. [DOI] [PubMed] [Google Scholar]
- 11.Mohaghegh M, Tafaghodi M. Dextran microspheres could enhance immune responses against PLGA nanospheres encapsulated with tetanus toxoid and Quillaja saponins after nasal immunization in rabbit? Pharm Dev Technol. 2011;16:36–43. doi: 10.3109/10837450903479962. [DOI] [PubMed] [Google Scholar]
- 12.Muhamad12 II, Selvakumaran S, Lazim NAM. Designing Polymeric Nanoparticles for Targeted Drug Delivery System. Nanomed. 2014:287–312. [Google Scholar]
- 13.Tafaghodi M, Rastegar S. Preparation and in vivo study of dry powder microspheres for nasal immunization. J Drug Target. 2010;18:235–242. doi: 10.3109/10611860903434035. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Liu H, Zhang X, Qian F. Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein & cell. 2015:1–24. doi: 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renegar KB, Small PA, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 16.Yuk J-M, Jo E-K. Host immune responses to mycobacterial antigens and their implications for the development of a vaccine to control tuberculosis. Clin Exp Vaccine Res. 2014;3:155–167. doi: 10.7774/cevr.2014.3.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2009;157:235–243. doi: 10.1111/j.1365-2249.2009.03967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Valliere S, Abate G, Blazevic A, Heuertz R, Hoft D. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73:6711–6720. doi: 10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tafaghodi M, Tabassi SAS, Jaafari M-R, Zakavi SR, Momennejad M. Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. Int J Pharm. 2004;280:125–135. doi: 10.1016/j.ijpharm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Rose F, Wern JE, Ingvarsson PT, van de Weert M, Andersen P, Follmann F, et al. Engineering of a novel adjuvant based on lipid-polymer hybrid nanoparticles: A quality-by-design approach? J Control Rel. 2015;210:48–57. doi: 10.1016/j.jconrel.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Carlétti D, da Fonseca DM, Gembre AF, Masson AP, Campos LW, Leite LC, et al. A Single Dose of a DNA Vaccine Encoding Apa Coencapsulated with 6, 6′-Trehalose Dimycolate in Microspheres Confers Long-Term Protection against Tuberculosis in Mycobacterium bovis BCG-Primed Mice. Clin Vaccine Immunol. 2013;20:1162–1169. doi: 10.1128/CVI.00148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Hickey AJ. PLGA microparticles in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen TB10 4-Ag85B. Pharmaceut Res. 2010;27:350–360. doi: 10.1007/s11095-009-0028-7. [DOI] [PubMed] [Google Scholar]
- 24.Bivas-Benita M, Lin MY, Bal SM, van Meijgaarden KE, Franken KL, Friggen AH, et al. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA–PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine. 2009;27:4010–4017. doi: 10.1016/j.vaccine.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Kirby DJ, Rosenkrands I, Agger EM, Andersen P, Coombes AG, Perrie Y. PLGA microspheres for the delivery of a novel subunit TB vaccine? J Drug Target. 2008;16:282–293. doi: 10.1080/10611860801900462. [DOI] [PubMed] [Google Scholar]
- 26.de Paula L, Silva CL, Carlos D, Matias-Peres C, Sorgi CA, Soares EG, et al. Comparison of different delivery systems of DNA vaccination for the induction of protection against tuberculosis in mice and guinea pigs. Genet Vaccines Ther. 2007;5:1–7. doi: 10.1186/1479-0556-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu D, Garcia-Contreras L, Xu D, Kurtz SL, Liu J, Braunstein M, et al. Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen 85B. Pharmaceut Res. 2007;24:1834–1843. doi: 10.1007/s11095-007-9302-8. [DOI] [PubMed] [Google Scholar]
- 28.Ha S-J, Park S-H, Kim H-J, Kim S-C, Kang H-J, Lee E-G, et al. Enhanced immunogenicity and protective efficacy with the use of interleukin-12-encapsulated microspheres plus AS01B in tuberculosis subunit vaccination. Infect Immun. 2006;74:4954–4959. doi: 10.1128/IAI.01781-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai H, Hu X, Yu D, Li S, Tian X, Zhu Y. Combined DNA vaccine encapsulated in microspheres enhanced protection efficacy against Mycobacterium tuberculosis infection of mice. Vaccine. 2005;23:4167–4174. doi: 10.1016/j.vaccine.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Evans JT, Ward JR, Kern J, Johnson ME. A single vaccination with protein-microspheres elicits a strong CD8 T-cell-mediated immune response against Mycobacterium tuberculosis antigen Mtb8. 4. Vaccine. 2004;22:1964–1972. doi: 10.1016/j.vaccine.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Lima K, Santos S, Lima V, Coelho-Castelo A, Rodrigues J, Silva C. Single dose of a vaccine based on DNA encoding mycobacterial hsp65 protein plus TDM-loaded PLGA microspheres protects mice against a virulent strain of Mycobacterium tuberculosis. Gene Ther. 2003;10:678–685. doi: 10.1038/sj.gt.3301908. [DOI] [PubMed] [Google Scholar]
- 32.Lima VM, Bonato VL, Lima KM, Dos Santos SA, Dos Santos RR, Gonçalves ED, et al. Role of trehalose dimycolate in recruitment of cells and modulation of production of cytokines and NO in tuberculosis. Infect Immun. 2001;69:5305–5312. doi: 10.1128/IAI.69.9.5305-5312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhiman N, Khuller G. Protective efficacy of mycobacterial 71-kDa cell wall associated protein using poly (DL-lactide-co-glycolide) microparticles as carrier vehicles. FEMS Immunol Med Microbiol. 1998;21:19–28. doi: 10.1111/j.1574-695X.1998.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter ZK, Williamson ED, Eyles JE. Mucosal delivery of microparticle encapsulated ESAT-6 induces robust cell-mediated responses in the lung milieu? J Control Rel. 2005;104:67–77. doi: 10.1016/j.jconrel.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Venkataprasad N, Coombes A, Singh M, Rohde M, Wilkinson K, Hudecz F, et al. Induction of cellular immunity to a mycobacterial antigen adsorbed on lamellar particles of lactide polymers. Vaccine. 1999;17:1814–1819. doi: 10.1016/s0264-410x(98)00372-7. [DOI] [PubMed] [Google Scholar]
- 36.Todoroff J, Ucakar B, Inglese M, Vandermarliere S, Fillee C, Renauld J-C, et al. Targeting the deep lungs, Poloxamer 407 and a CpG oligonucleotide optimize immune responses to Mycobacterium tuberculosis antigen 85A following pulmonary delivery. Eur J Pharm Biopharm. 2013;84:40–48. doi: 10.1016/j.ejpb.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Orr MT, Kramer RM, Barnes L, Dowling QM, Desbien AL, Beebe EA, et al. Elimination of the cold-chain dependence of a Nano emulsion adjuvanted vaccine against tuberculosis by lyophilization. J Control Rel. 2014;177:20–26. doi: 10.1016/j.jconrel.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeboah KG, D'souza MJ. Evaluation of albumin microspheres as oral delivery system for Mycobacterium tuberculosis vaccines? J Microencaps. 2009;26:166–179. doi: 10.1080/02652040802211717. [DOI] [PubMed] [Google Scholar]
- 39.Meerak J, Wanichwecharungruang SP, Palaga T. Enhancement of immune response to a DNA vaccine against Mycobacterium tuberculosis Ag85B by incorporation of an autophagy inducing system. Vaccine. 2013;31:784–790. doi: 10.1016/j.vaccine.2012.11.075. [DOI] [PubMed] [Google Scholar]
- 40.Feng G, Jiang Q, Xia M, Lu Y, Qiu W, Zhao D, et al. Enhanced immune response and protective effects of nano-chitosan-based DNA vaccine encoding T cell epitopes of Esat-6 and FL against Mycobacterium tuberculosis infection. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0061135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Ai W, Yue Y, Xiong S, Xu W. Enhanced protection against pulmonary mycobacterial challenge by chitosan-formulated polyepitope gene vaccine is associated with increased pulmonary secretory IgA and gamma-interferon+T cell responses. Microbiol Immunol. 2013;57:224–235. doi: 10.1111/1348-0421.12027. [DOI] [PubMed] [Google Scholar]
- 42.Verma A, Pandey R, Chanchal A, Siddiqui I, Sharma P. Encapsulation of Antigenic Secretory Proteins of Mycobacterium tuberculosis in Biopolymeric Nanoparticles for Possible Aerosol Delivery System. Journal of Bionanoscience. 2011;5:88–95. [Google Scholar]
- 43.Caetano LA, Figueiredo L, Almeida AJ, Gonçalves L. Bioengineering (ENBENG), 2012 IEEE 2nd Portuguese Meeting in. IEEE; 2012. Alginate-chitosan particulate delivery systems for mucosal immunization against tuberculosis. [Google Scholar]
- 44.dong Zhu B, qing Qie Y, ling Wang J, Zhang Y, zhong Wang Q, Xu Y, et al. Chitosan microspheres enhance the immunogenicity of an Ag85B-based fusion protein containing multiple T-cell epitopes of Mycobacterium tuberculosis. Eur J Pharm Biopharm. 2007;66:318–326. doi: 10.1016/j.ejpb.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 45.Bivas-Benita M, van Meijgaarden KE, Franken KL, Junginger HE, Borchard G, Ottenhoff TH, et al. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A* 0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine. 2004;22:1609–1615. doi: 10.1016/j.vaccine.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 46.Dobakhti F, Naghibi T, Taghikhani M, Ajdary S, Rafinejad A, Bayati K, et al. Adjuvanticity effect of sodium alginate on subcutaneously injected BCG in BALB/c mice. Microb Infect. 2009;11:296–301. doi: 10.1016/j.micinf.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Ajdary S, Dobakhti F, Taghikhani M, Riazi-Rad F, Rafiei S, Rafiee-Tehrani M. Oral administration of BCG encapsulated in alginate microspheres induces strong Th1 response in BALB/c mice. Vaccine. 2007;25:4595–4601. doi: 10.1016/j.vaccine.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 48.Dobakhti F, Ajdary S, Taghikhani M, Rafiei S, Bayati K, Rafiee-Tehrani M. Immune response following oral immunization with BCG encapsulated in alginate microspheres. Iran J Immunol. 2006;3:114–120. [PubMed] [Google Scholar]
- 49.Wilkinson KA, Belisle JT, Mincek M, Wilkinson RJ, Toossi Z. Enhancement of the human T cell response to culture filtrate fractions of Mycobacterium tuberculosis by microspheres. J Immunol Methods. 2000;235:1–9. doi: 10.1016/s0022-1759(99)00200-8. [DOI] [PubMed] [Google Scholar]
- 50.Yu F, Wang J, Dou J, Yang H, He X, Xu W, et al. Nanoparticle-based adjuvant for enhanced protective efficacy of DNA vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis infection. Nanomedicine: Nanotechnology, Biology and Medicine. 2012;8:1337–1344. doi: 10.1016/j.nano.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Ballester M, Nembrini C, Dhar N, De Titta A, De Piano C, Pasquier M, et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis Vaccine. 2011;29:6959–6966. doi: 10.1016/j.vaccine.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 52.Andersen P. Vaccine strategies against latent tuberculosis infection. Trends Microbiol. 2007;15:7–13. doi: 10.1016/j.tim.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Principi N, Esposito S. The present and future of tuberculosis vaccinations. Tuberculosis. 2015;95:6–13. doi: 10.1016/j.tube.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Wedlock D, Keen D, McCarthy A, Andersen P, Buddle B. Effect of different adjuvants on the immune responses of cattle vaccinated with Mycobacterium tuberculosis culture filtrate proteins. Vet Immunol Immunopathol. 2002;86:79–88. doi: 10.1016/s0165-2427(02)00017-x. [DOI] [PubMed] [Google Scholar]
- 55.Jensen DK, Jensen LB, Koocheki S, Bengtson L, Cun D, Nielsen HM, et al. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA? J Control Rel. 2012;157:141–148. doi: 10.1016/j.jconrel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 56.McHugh KJ, Guarecuco R, Langer R, Jaklenec A. Single-injection vaccines: Progress, challenges, and opportunities. J Control Release. 2015;219:596–609. doi: 10.1016/j.jconrel.2015.07.029. [DOI] [PubMed] [Google Scholar]


