Abstract
Objective(s):
Melatonin, an important hormone secreted by the epiphysis, is a powerful anti-oxidant with a high potential to neutralize medical toxins. The goal of this study was to demonstrate the beneficial effect of melatonin on epididymal sperm and reproductive parameters in mice treated with acetylsalicylic acid (ASA).
Materials and Methods:
Male adult mice were divided into four treatment groups: control, ASA, melatonin, and ASA+melatonin. Mice were administered ASA (50 mg/kg, orally) and/or melatonin (10 mg/kg, intraperitoneally), or vehicle control, for 14 days. Sperm count, sperm motility, and sperm morphology were evaluated to assess fertility. A colorimetric assay was used to measure serum total antioxidant capacity (TAC). A sperm chromatin dispersion (SCD) test was used to assess sperm chromatin integrity. Sex hormone levels were measured by ELISA.
Results:
Compared to the control group, ASA treatment resulted in a significant decrease in sperm parameters (P<0.05), as well as a decrease in the integrity of sperm chromatin (P<0.01). ASA treatment also reduced serum testosterone and TAC levels (P<0.05). Co-administration of melatonin with ASA significantly improved epididymal sperm parameters and increased serum testosterone and TAC levels compared to the ASA-treated group. LH level was not different in the combined treatment group compared to control or ASA treatment.
Conclusion:
Short-term administration of ASA (50 mg/kg) has adverse effects on male reproductive function in mice. Co-administration of melatonin protects against ASA-induced impairment of male reproductive function by preventing the reduction in serum TAC and testosterone levels seen with ASA treatment alone.
Keywords: Acetylsalicylic acid, Antioxidants, Epididymis, Melatonin, Sperm, Testosterone
Approximately 10 to 20% of couples in the world are infertile, and nearly 50% of infertility cases are a result of male factors (1). Acetylsalicylic acid (ASA), or aspirin, is a non-steroidal anti-inflammatory drug (NSAID) with anti-prostaglandin effects (2). Prostaglandins regulate sexual maturity, steroidogenic activity of Leydig cells, production of testosterone, development of male sexual behaviors (3, 4), and are important for sperm motility, capacitation, and the acrosomal reaction (5, 6). Prostaglandins also stimulate contraction of seminiferous tubules (7). ASA is used as a prophylactic to prevent cardiovascular disease, prostate cancer, and gastrointestinal cancers including colorectal cancer (8, 9), and is also used for its anti-coagulant and analgesic properties. ASA is safe to use at low doses, but doses above 80 mg/kg/day cause drug intoxication symptoms in humans (9). Previous studies have shown that ASA impacts male fertility, resulting in reduced serum testosterone, FSH, and LH, as well as decreased spermatid numbers in rats (10, 11) and sheep (12).
Melatonin regulates several physiological functions and is secreted primarily by the epiphysis, but is also produced by other organs including the gastrointestinal tract and ovaries (13). Melatonin has neuroendocrine actions and in addition to its anti-cancer and anti-aging effects, it regulates reproduction, cell multiplication, proliferation, and differentiation (14, 15). Melatonin is a powerful antioxidant (16), and studies suggest that it could decrease drug intoxication and side effects (17). A previous study has shown that melatonin prevents testicular pathology induced by a single high dose of ASA (200 mg/kg) (18). However, no previous studies have directly evaluated the ability of melatonin to prevent ASA-induced changes in epididymal sperm parameters, specifically by measure of sperm morphology, sperm motility, sperm count, sperm chromatin dispersion test, sex hormone levels, and quality or quantity of spermatogenesis. Epididymal sperm analyses such as count, motility, morphology, and chromatin integrity are essential criteria for the assessment of male fertility. Therefore, the aim of this study was to evaluate the potential benefits of melatonin on epididymal sperm parameters, sex hormone levels, total serum antioxidant levels, and quality of spermatogenesis in adult mice following treatment with ASA for two weeks.
Materials and Methods
Animals
In this experimental study, 32 adult NMRI male mice aged 8–10 weeks (weighing 35–40 g) were used. Animals were allowed to acclimate in their cages with free access to water and food, with a 12 hr dark-light cycle at a temperature of 25°C. All procedures were done according to the guidelines of the Animal and Human Ethical Committee of Guilan University of Medical Sciences. After acclimation, animals were divided into 4 groups, with 8 mice per group. The control group received 0.5 ml normal saline through gavage daily for 14 days. The ASA group received 50 mg/kg ASA (Sigma-USA) through gavage daily for 14 days. The melatonin group received 10 mg/kg melatonin (Sigma-USA) intraperitoneally (IP) daily for 14 days. The combined treatment group received both 10 mg/kg melatonin (IP) and 50 mg/kg ASA (oral gavage), daily for 14 days. All animals were sacrificed by cervical dislocation 15 days after the beginning of treatment. Testes and epididymides were removed from the abdominal cavity and carefully separated from one another. For tissue fixation, testicles were fixed in Bouin’s solution for 72 hr at room temperature.
Evaluation of sperm parameters
For evaluation of sperm parameters, the caudal parts of both epididymides were used. The tissue was placed in a Petri dish containing 2 ml pre-warmed (37°C) Ham’s F10 (Sigma) solution, cut into small pieces using a scalpel and was then incubated at 37°C with 5% CO2 for 30 min. Sperm suspension was pipetted several times to mix, and then two drops were placed on a microscope slide and covered with a coverslip to evaluate motility. For each slide, at least five microscopic fields with 400×magnification were observed, and the percent of motile sperm was recorded. For sperm counts, 1 ml of sperm suspension was diluted with 4 ml formaldehyde solution (1:5 dilutions). Then, 10 µl of diluted sperm suspension was placed on a Neubauer, and the number of sperm heads was counted in five fields and expressed as 106 sperm per milliliter. For analysis of sperm morphology, 20 µl of sperm suspension was smeared on a slide and allowed to air dry, fixed in 96% ethanol, and stained with Papanicolaou stain. Stained sperm were evaluated under a light microscope with a 1000× magnification. Sperm meeting one or more of the following criteria were classified as abnormal: small or big head, abnormal form of the head or double head, head-tail separation, short, long, coiled, bent, or double tail. Abnormal sperm were expressed as a percentage of total sperm counted (19, 20).
Histologic study
After 3 days of fixation and ascertaining tissue fixation of testicles, tissue passaging was done on samples. Ethanol with ascending degree, xylene, and melted paraffin were used for passaging. To maximize the number of ideal cuts in seminiferous tubule in cross-section, testicles were molded in paraffin along length axis. Sections with 5-micron thickness were cut using rotatory microtome (Leitz, Germany). For each animal, five slides were stained with Hematoxylin and Eosin and studied using an optical microscope with 400× magnification.
Thickness of germinal epithelium
Linear scaled grades inserted on the eyepiece of microscope were used to evaluate the thickness of germinal epithelium. In each animal 20 seminiferous tubules in stages of VII and VIII in round or almost round cross sections were randomly chosen to be studied. Tubules that were oval or cut angled were not studied. Tubules were measured with a 400× (22).
Study of quality and maturity of seminiferous tubule with Johnsen score
The Johnsen score is used to study maturity and quality of seminiferous tubule (22). For this purpose cross sections of 100 seminiferous tubules in each animal were used and each tubule was assigned a mark ranging from 1 to 10. Percentages of tubules with high maturity were also calculated (23).
Hormone assay
Blood samples were taken from inferior vena cava and their sera were separated and then were stored at -80°C until assay. The serum LH and testosterone were measured using RIA as kit’s instructions (Monobind, USA). Measurements were repeated twice.
Serum total antioxidant capacity (TAC)
Serum TAC was measured using a commercial kit on the basis of kit’s instructions (ZellBio GmbH, Germany) using a colorimetric assay at a wavelength of 490 nm. TAC level was considered as the amount of antioxidant in the sample that was compared with ascorbic acid action as a standard. This method can determine TAC with 0.1 mM sensitivity (100 µmol/L). The intra- and inter-assay variation coefficient is claimed to be less than 3.4% and 4.2%, respectively. Measurement was repeated twice.
Sperm chromatin dispersion (SCD) test
Sperm chromatin integrity was evaluated by SCD test. For this purpose, the processed sperm solution was diluted with PBS (1:3). Then 30 µl sperm solution was well mixed with 70 µl low melted agarose at 37°C then 10 µl of the above-mixed solution was put on a slide which was already covered with 0.65% agarose. Then a lamella was put on the slide and transferred to the refrigerator and left for 4 min. Then the lamella was removed slowly in a horizontal position. Each slide was then put in Hcl 0.12 N for 7 min and left in the dark. Slides were immersed in the acid denaturation solution (acidic Tris 0.4 m, 2-mercaptoethanol 0.8 m, sodium dodecyl sulfate (SDS) 1%, ethylene-diamine-tetra-acetic acid (EDTA) 50 mM, sodium chloride (NaCl) 2 m at room temperature for 25 min. In order to wash out the lysate solution, the slides were transferred into a container of distilled water twice. Slides were placed into a tray containing 70%, 90%, 100% ethanol for 2 min in each step and were dried in the air. The slides were stained with PBS-Wright (1:1) for 10 min. Then they were observed with a light microscope (Olympus, Japan) with 400x magnification. For each animal, 200 cells were studied. The better cells had a larger pink hallo and the cells with small hallo or no hallo were considered as fragmented DNA. The percent of sperm DNA fragmentation (SDF) and chromatin integrity as intact sperm DNA was calculated.
Detection of apoptosis in germ cells
For assay of apoptosis, we used TUNEL staining using kit’s instructions (Roche, Germany). After staining positive cells containing fragmented nuclear chromatin characteristic of apoptosis would exhibit a bright yellow color. The apoptotic cell number was calculated and averaged by counting cells in 15 microscopic fields in each section. In each animal 3 sections were evaluated with 400× magnification. A master histologist blinded to the source of testis tissue performed all measurements. The amounts of apoptosis were expressed as a percentage.
Statistical analysis
Data were analyzed using SPSS statistical program and one-way analysis of variance (ANOVA) test. Tukey’s Post hoc test was used to evaluate the difference between groups. Findings were expressed in terms of mean and standard deviation and P<0.05 was considered significant.
Results
Epididymis sperm parameters
Following administration of 50 mg/kg ASA for two weeks in adult mice, all measured parameters of sperm quality were decreased compared to the control group (P<0.001), including sperm number, percentage of motile sperm, and percentage of abnormal sperm. Sperm count and percent motility were decreased with ASA treatment compared to the control group (P<0.001; Table 1). ASA treatment increased the percentage of sperm with abnormal morphology in the head and tail regions compared to the control group (P<0.001), but treatment with melatonin alone did not significantly alter sperm count, percent motility, or head morphology compared to controls (Table 1). Combined treatment of ASA and melatonin resulted in an increase in sperm count and percent motility compared to the ASA-treated group (P<0.05; Table 1). The percentage of sperm with abnormal head morphology in the combined treatment group was decreased compared to the ASA group, but this decrease was not statistically significant (Table 1). However, the percentage of sperm with abnormal tail morphology was significantly reduced in the combined treatment group compared to the ASA treatment group (P<0.05; Table 1).
Table 1.
Effect of acetylsalicylic acid (ASA) and melatonin on sperm parameters in adult mice
| Groups | Motility (%) | Number (×106/ml) | Abnormal head morphology (%) | Abnormal tail morphology (%) | Sperm chromatin integrity (%) | SDF (%) |
|---|---|---|---|---|---|---|
| Control | 63.6 ±5.6 b | 29.7 ± 3.4 b | 4.9±1.7 b | 5.5 ±0.6 b | 82.18±6.17 b | 18.2±2.4 |
| ASA | 51.50 ± 40 adc | 12.6±3.1 acd | 9.21 ± 1.1acd | 14.0 ±1.4 acd | 65.18±3.08 acd | 35.4±4.15 acd |
| Melatonin | 67.4 ± 5.2 b | 32.2 ±3.6 b | 5.2±1.3 b | 5.1 ±0.8 b | 84.18±6.1 b | 16.8±3.3 b |
| ASA+ Melatonin | 60.1 ±3.0 b | 26.8±2.6 b | 6.65±.3 | 7.4±0.3 ab | 78.46±7.2 b | 23.6±3.6 b |
Data are shown as mean±SD. SDF: Sperm DNA fragmentation. a: P<0.001, compared with the control group within the same column; b: P<0.01, compared with the ASA treated group within the same column; c: P<0.01, compared with the melatonin-treated group within the same column; d: P<0.05, compared with the ASA+ melatonin-treated group within the same column
The percentage of sperm chromatin integrity in the control and melatonin-treated groups was 82.18 ± 6.17 and 84.18±6.1, respectively. Treatment with ASA resulted in a significant reduction in chromatin integrity compared to the control group (P<0.01), and melatonin alone did not have a significant effect on chromatin integrity compared to the controls (Table 1). Combined treatment of ASA with melatonin resulted in a significant increase in sperm chromatin integrity compared to the ASA-treated group (P<0.05; Table 1). ASA treatment significantly increased the percentage of sperm DNA fragmentation (SDF) compared to the control group, but treatment with melatonin alone did not significantly change SDF compared to controls. Co-administration of ASA and melatonin reduced the SDF value significantly compared to the ASA-treated group (P<0.05).
Histological findings and quality of spermatogenesis
Following histological analysis of seminiferous tubules from the control group, active spermatogenesis in different stages was observed, with the presence of mature sperm and sperm undergoing maturation. Inside the tubules, Sertoli cells and spermatogenic cells in various stages of division were observed (Figure 1). The quality and maturity of seminiferous tubules were high, and almost all tubules had a high level of sperm maturation, as measured by the Johnsen score (Table 2). Although various types of germ cells and Sertoli cells were observed in tubules of ASA-treated mice, the total number was decreased. Furthermore, the thickness of the germinal epithelium was decreased in ASA-treated mice compared to the control group (P<0.05; Table 2). In addition, vacuoles were observed in the epithelial cells of some tubules from ASA-treated mice, but no vacuoles were found in the control group (Figure 1). In interstitial tissue, Leydig cell numbers were not considerably different in the ASA-treated group compared to the control group. Treatment of mice with 50 mg/kg ASA for two weeks significantly decreased the Johnsen score and the maturity of seminiferous tubules, and significantly increased the percent of apoptotic cells compared to the control group (Table 2). Administration of 10 mg/kg melatonin alone did not alter the morphology of seminiferous tubules or interstitial tissue (Figure 1). Active spermatogenesis was observed in all tubules from melatonin-treated mice, and most tubules contained mature sperm, similar to observations from the control group (Table 2). Following combined treatment of melatonin and ASA, seminiferous tubules
Figure 1.
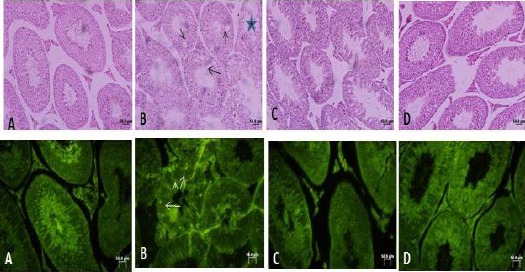
Optical photomicrograph of mouse seminiferous tubules, A: Control, B: under treatment with ASA, C: under treatment with melatonin, D: under treatment with ASA+ melatonin. In the first row in control pay attention to active spermatogenesis in seminiferous tubules. ASA has caused a reduced quality of spermatogenesis. Black arrows in B show vacuoles inside the germinal epithelium. Large star shows a damaged tubule with thin epithelium in ASA treated mouse. In D note a better quality of seminiferous tubules. The first row is stained with Hematoxylin-Eosin, 200×. In the second row, white arrows show apoptotic bright yellow cells and a reduced level of bright yellow apoptotic cells in ASA+ melatonin-treated group compared with the ASA treated group. The second row is showing TUNEL staining, 400× Bar: 50 µm displayed improved morphology compared to the ASA-treated group. Sertoli cells and all types of germ cells were observed in the combined treatment group and vacuoles were not observed in the epithelial cells of seminiferous tubules from the combined treatment group (Figure 1). Combined administration of melatonin with ASA increased the quality and percentage of mature sperm and reduced the percentage of apoptotic cells compared to ASA treatment alone (P<0.001; Table 2).
Table 2.
Effects of acetylsalicylic acid (ASA) and melatonin on the quality of seminiferous tubules of adult mice
| Groups | Johnsen score | Maturity of seminiferous tubules (%) | Germinal epithelium height (µ) | Apoptotic cells (%) |
|---|---|---|---|---|
| Control | 9.1 ±0.2 b | 96.6 ± 1.4 bd | 68.01 ± 4.2 b | 2.04±0.06 bd |
| ASA | 7.08 ± 0.1acd | 79.3 ± 4.3 acd | 52.3 ± 3.6 acd | 9.1±0.2 acd |
| Melatonin | 8.9 ± 0.6 | 95.8 ± 3.9 bd | 63.01 ± 6.63 b | 2.1±0.05 b |
| ASA+ Melatonin | 8.6 ± 0.1 ab | 88.1 ± 1.4 abc | 60.34 ±3.1 | 4.01±0.1 b |
Data are shown as mean±SD. a: P<0.05, compared with the control group within the same column; b: P<0.001, compared with the ASA treated group within the same column; c: P<0.01, compared with the melatonin-treated group within the same column; d: P<0.01, compared with the ASA+ melatonin-treated group within the same column
Table 3 shows the mean amount of serum total antioxidant capacity (TAC), testosterone, and LH. Serum testosterone was reduced with ASA treatment compared to the controls (P<0.05), whereas co-administration of ASA and melatonin increased testosterone levels compared to ASA treatment alone (P<0.05; Table 3). There were no significant differences in LH levels between groups (Table 3). A significant decrease in serum TAC was observed in the ASA-treated group compared to the control group (P<0.05). However, co-administration of ASA and melatonin significantly increased TAC levels compared to ASA treatment alone (P<0.05; Table 3).
Table 3.
Effect of acetylsalicylic acid (ASA) and melatonin on serum TAC, testosterone, and LH
| Groups | Serum TAC (mM) | Testosterone (ng/ml) | Serum LH (Iu/l) |
|---|---|---|---|
| Control | 1.32±0.3 b | 3.13±0.68 b | 0.38±0.14 |
| ASA | 0.76±0.3 acd | 1.52±0.48 acd | 0.21±0.09 |
| Melatonin | 1.42±0.4 b | 3.0 8±0.24 b | 0.28±0.06 |
| ASA+ Melatonin | 1.12±0.2 b | 2.78±0.17 b | 0.26±0.07 |
Data are shown as mean±SD. a: P<0.05, compared with the control group within the same column; b: P<0.05, compared with the ASA treated group within the same column; c: P<0.05, compared with the melatonin-treated group within the same column; d: P<0.05, compared with the ASA+ melatonin-treated group within the same column
Discussion
In the present study, we demonstrate the beneficial effects of melatonin on male fertility in mice after treatment with ASA. Co-administration of melatonin increased sperm count, motility, and sperm chromatin integrity, and reduced the percentage of abnormal sperm in ASA-treated mice. Melatonin also improved maturation of seminiferous tubules in ASA-treated mice by increasing serum levels of testosterone and TAC. In this study, administration of 50 mg/kg ASA for 14 days decreased all parameters of sperm analysis (number, motility, and morphology). Administration of 50 mg/kg ASA for 14 days or 30 days has also been shown to decrease sperm motility in rats (10). Similar to our study, usage of ASA in rats (15, 24-26), humans (16), and sheep (27) decreases some sperm parameters in the epididymis. These effects are likely a result of reduced serum testosterone, which we have shown is a side effect of ASA treatment in the current study. Testosterone is produced by Leydig cells under the influence of LH and is essential for spermatogenesis (27). It has been shown that use of ASA is detrimental to metabolism in the testis, epididymis, and seminal vesicles, and promotes anti-androgen and anti-anabolic effects in rats (28). Sperm motility, sperm count, and sperm morphology are all androgen-regulated and therefore are most likely altered following ASA treatment as a result of reduced serum testosterone. Decreased sperm number with ASA treatment may also be explained by a decrease in the contractile power of the epididymis and vas deferens (29, 30).
Our finding of decreased sperm numbers with ASA treatment is in accordance with the observed decrease in the maturity level of sperm and the maturity of seminiferous tubules with ASA treatment. The presence of vacuoles in the germinal epithelium of seminiferous tubules could indicate a loss of cellular connectivity or a decrease of adhesion molecules such as cadherin and could be indicative of pre-apoptosis (23). Indeed, we observed increased DNA fragmentation in sperm with ASA treatment, which suggests that cells are pre-apoptotic. Apoptosis is a physiological mechanism of cell death and occurs in cells throughout embryonic, postnatal, and adult time periods. The amount of apoptosis is increased in the testis following exposure to toxic chemical drugs, chemotherapy, radiotherapy, or X-ray, and also in cases of cancer and cryptorchidism (23).
Treatment with NSAID nimesulide leads to increased apoptosis of epithelial cells of the vas deferens (4). Most of the NSAID drugs induce apoptosis of inflammatory cells by inhibiting cyclooxygenase enzymes (4). ASA treatment resulted in DNA fragmentation and reduced chromatin integrity of sperm. Sperm DNA integrity is essential for the accurate transmission of genetic content (31). Some studies report that sperm DNA damage occurs in 5–8% of infertile men with normal spermatogram (32). An increased level of male germ cell apoptosis and reduced level of TAC in the ASA-treated group may account for more SDF or a reduction in the level of sperm chromatin integrity. In this regard, it has been shown that apoptosis, DNA fractures without restoration, oxidative stress, drug use, cigarette use, and illness are all known causes of DNA damage in sperm (33).
Melatonin, the most important secretion of the epiphysis, is a very effective antioxidant and scavenger of free radicals (34). Due to its small size and lipophilic characteristics, melatonin can easily cross cellular membranes. The concentration of melatonin is very high in the nucleus, where it functions to protect DNA against harmful factors (35). The present study demonstrated that co-administration of 10 mg/kg melatonin with ASA decreases the harmful effects of ASA on spermatogenesis and sperm parameters, which can be explained by several potential mechanisms. First, melatonin has strong anti-oxidant properties and stimulates antioxidant enzymes including superoxide dismutase, glutathione reductase, and glutathione peroxidase (17). Indeed, we found an increase in TAS in the serum of mice treated with melatonin and ASA compared to mice treated with ASA alone. The decrease in TAS observed in the ASA-treated group may be a result of ASA toxicity. Second, melatonin reduces ASA-induced apoptosis of germ cells, thus preventing impairment of spermatogenesis (36). The anti-apoptotic properties of melatonin have also been demonstrated in other tissues (23, 36-38). Third, melatonin may inhibit the differentiation of spermatogonia to spermatocytes by inhibiting proliferation of spermatogonia, which would also make any harmful effects of ASA on germ cells less noticeable (23). Fourth, melatonin may alter production of prostaglandins (39). Further analysis of prostaglandin levels after treatment with ASA and/or melatonin would provide further insight into this potential mechanism of melatonin action. Lastly, melatonin is known to affect the hypothalamic–pituitary–gonadal (HPG) axis, resulting in modification of sex hormone production, including estrogen, testosterone, FSH, and LH (11). Our results suggest that melatonin does alter testosterone, but no changes in LH were observed with melatonin treatment in this study. Further studies are required to elucidate the precise mechanism(s) by which melatonin ameliorates the effects of ASA on male reproduction and fertility.
Conclusion
Treatment of adult mice with 50 mg/kg ASA for two weeks decreases the percentage of mature seminiferous tubules, increased fragmentation of sperm DNA, reduces serum TAC and testosterone levels, and increases apoptosis in germ cells. The use of melatonin in combination with ASA increases the level of maturity of sperm and increases the percentage of mature seminiferous tubules via its anti-oxidant and anti-apoptotic properties. Future studies are needed to complete molecular, genetic, and ultrastructural analysis of Leydig cells, Sertoli cells, and spermatogonia in mice after treatment with ASA and melatonin.
Acknowledgment
This work was supported financially by Research & Technology Vice-chancellor, Guilan University of Medical Sciences, Rasht, Iran.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Wiweko B, Utami P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl. 2017;27:1–7. doi: 10.1186/s12610-016-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortan P, Hawkey C. Drug-induced gastrointestinal disorders. Medicine. 2007;35:210–215. [Google Scholar]
- 3.Gupta C, Gentlejewski C. Role of prostaglandins in the testosterone dependent wolffian duct differentiation of the fetal mouse. Biol Reprod. 1992;47:1151–1160. doi: 10.1095/biolreprod47.6.1151. [DOI] [PubMed] [Google Scholar]
- 4.Balaji T, Ramanathan M, Menon V. Localization of cyclooxygenase-2 in mice vas deferens and its effects on fertility upon suppression using nimesulide: a preferential cyclooxygenase-2 inhibitor. Toxicol. 2007;234:135–144. doi: 10.1016/j.tox.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb C, Svanborg K, Eneroth P, Bygdeman M. Effect of prostaglandins on human sperm function in vitro and seminal adenosine triphosphate content. Fertil Steril. 1988;49:322–327. doi: 10.1016/s0015-0282(16)59723-4. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy J, Korn N, Thurston R. Prostaglandin levels in seminal plasma and sperm extracts of the domestic turkey, and the effects of cyclooxygenase inhibitors on sperm mobility. Reprod Biol Endocrinol. 2003;1:74. doi: 10.1186/1477-7827-1-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripiciano A, Filippini A, Ballarini F, Palombi F. Contractile response of peritubularmyoid cells to prostaglandin F2alpha. Mol Cell Endocrinol. 1998;138:143–150. doi: 10.1016/s0303-7207(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 8.Qstensen M, Khamashta M, Lokshin M, Parke A, Brucato A, Carp H, et al. Anti- inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8:209. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez M, Greenberg E. More aspirin for less cancer? J Natl Cancer Inst. 2007;99:582–583. doi: 10.1093/jnci/djk147. [DOI] [PubMed] [Google Scholar]
- 10.Didolkar A, Patel P, Roychowdhury D. Effect of aspirin on spermatogenesis in mature and immature rats. Int J Androl. 1980;3:585–593. doi: 10.1111/j.1365-2605.1980.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 11.Didolkar A, Gurjar A, Joshi U, Sheth A, Roychowdhury D. Effects of aspirin on blood plasma levels of testosterone, LH and FSH in maturing male rats. Int J Androl. 1980;3:312–318. doi: 10.1111/j.1365-2605.1980.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 12.Gwayi N, Bernard R. The effects of melatonin on sperm motility in vitro in Wistar rats. Andrologia. 2002;34:391–396. doi: 10.1046/j.1439-0272.2002.00522.x. [DOI] [PubMed] [Google Scholar]
- 13.Hemadi M, Saki G, Shokri S, Ghasemi F. Follicular dynamics in neonate vitrified ovarian grafts after host treatment with melatonin. Folia Morphol. 2011;70:18–23. [PubMed] [Google Scholar]
- 14.Stutz G, Zamudio J, Santillán M, Vincenti L, de Cuneo M, Ruiz R. The effect of alcohol, tobacco, and aspirin consumption on seminal quality among healthy young men. Arch Environ Health. 2004;59:548–552. doi: 10.1080/00039890409603432. [DOI] [PubMed] [Google Scholar]
- 15.Polat A, Emre M. Influence of melatonin and acetylsalicylic acid on lipid peroxidation and antioxidant enzyme activities in gastric mucosa. J Gastroenterol. 2006;41:507–508. doi: 10.1007/s00535-006-1837-7. [DOI] [PubMed] [Google Scholar]
- 16.Hussein M, Abu-Dief E, Abou El-Ghait A, Adly M, Abdelraheem M. Morphological evaluation of the radioprotective effects of melatonin against X-ray-induced early and acute testis damage in Albino rats: an animal model. Int J Exp Pathol. 2006;87:237–250. doi: 10.1111/j.1365-2613.2006.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter R, Tan D, Manchester L, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 18.Altintas R, Polat A, Parlakpinar H, Vardi N, Beytur A, Oguz F, et al. The effect of melatonin on acetylsalicylic acid-induced kidney and testis damage. Hum Exp Toxicol. 2014;33:383–395. doi: 10.1177/0960327113506240. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organisation. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO; 2010. pp. 7–37. [Google Scholar]
- 20.Shokri S, Aitken R, Abdolvahhabi M, Abolhasani F, Ghasemi F, Kashani I, et al. Exercise and supraphysiological dose of nandrolonedecanoate increase apoptosis in spermatogenic cells. Basic Clin Pharmacol Toxicol. 2010;106:324–330. doi: 10.1111/j.1742-7843.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 21.Fakoya F, Caxton-Martins E. Morphological alterations in the seminiferous tubules of adult Wistar rats: the effects of prenatal ethanol exposure. Folia Morphol. 2004;63:195–202. [PubMed] [Google Scholar]
- 22.Aral F, Karaçal F, Baba F. The effect of enrofloxacin on sperm quality in male mice. Res Vet Sci. 2008;84:95–99. doi: 10.1016/j.rvsc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Mohamadghasemi F, Faghani M, Jahromi SK, Bahadori M, Nasiri E, Hemadi M. Effect of Melatonin on proliferative activity and apoptosis in spermatogenic cells in mouse under chemotherapy. J Reprod Contracept. 2010;21:79–94. [Google Scholar]
- 24.Dare W, Noronha C, Kusemiju O, Okanlawon O. The effect of ethanol on spermatogenesis and fertility in male Sprague-Dawley rats pretreated with acetylsalicylic acid Niger. Postgrad Med J. 2002;9:194–198. [PubMed] [Google Scholar]
- 25.Vyas A, Ram H, Purohit A, Jatwa R. Adverse Effects of Subchronic Dose of Aspirin on Reproductive Profile of Male Rats. J Pharm. 2016:6585430. doi: 10.1155/2016/6585430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyedeji K, Bolarinwa A, Adigun A. Effect of aspirin on reproductive functions in male albino rats. Res J Pharm. 2013;7:16–20. [Google Scholar]
- 27.Vane J, Botting R. The mechanism of action of aspirin. Trombosis Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 28.Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother. 2006;60:97–108. doi: 10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Shang X, Huang Y, Ye Z, Yu X, Gu W. Protection of melatonin against damage of sperm mitochondrial function induced by reactive oxygen species. Zhonghua Nan KeXue. 2004;10:604–607. [PubMed] [Google Scholar]
- 30.Kokolis N, Theodosiadou E, Tsantarliotou M, Rekkas C, Goulas P, Smokovitis A. The effect of melatonin implants on blood testosterone and acrosin activity in spermatozoa of the ram. Andrologia. 2000;32:107–114. doi: 10.1046/j.1439-0272.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 31.Roodbari F, Abedi N, Talebi AR. Early and late effects of Ibuprofen on mouse sperm parameters, chromatin condensation, and DNA integrity in mice. Iran J Reprod Med. 2015;13:703–710. [PMC free article] [PubMed] [Google Scholar]
- 32.Shefi S, Turek P. Definition and current evaluation of subfertile men. Int Braz J Urol. 2006;32:385–397. doi: 10.1590/s1677-55382006000400002. [DOI] [PubMed] [Google Scholar]
- 33.Evenson D. The sperm chromatin structure assay (SCSA) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016;169:56–75. doi: 10.1016/j.anireprosci.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Tanyildizi S, Bozkurt T. Effects of acetylsalicylic acid and metamizol on hyaluronidase activity and sperm characteristics in rams. Anim Reprod Sci. 2003;76:195–204. doi: 10.1016/s0378-4320(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 35.Asok K, Chinoy N. Effects of acetylsalicylic acid on reproductive organs of adolescent male rats. Endocrinol Exp. 1988;22:187–195. [PubMed] [Google Scholar]
- 36.Ateşşahin A, Sahna E, Türk G, Ceribaşi AO, Yilmaz S, Yüce A, et al. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res. 2006;41:21–27. doi: 10.1111/j.1600-079X.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 37.Mohammadghasemi F, Jahromi SK, Hajizadeh H, Homafar MA, Saadat S. The Protective Effects of Exogenous Melatonin on nicotine-induced changes in mouse ovarian follicles. J Reprod Infertil. 2012;13:143–150. [PMC free article] [PubMed] [Google Scholar]
- 38.Saadat S, Mohammadghasemi F, Jahromi SK, Homafar M, Haghiri M. Melatonin protects uterus and oviduct exposed to nicotine in mice. Interdiscip Toxicol. 2014;7:41–46. doi: 10.2478/intox-2014-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brzozowski T, Konturek P, Zwirska-Korczala K, Konturek S, Brzozowska I, Drozdowicz D, et al. Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J Pineal Res. 2005;39:375–385. doi: 10.1111/j.1600-079X.2005.00264.x. [DOI] [PubMed] [Google Scholar]


