Abstract
Objective(s):
Tanacetum species are traditionally used as insecticide, and externally wound healer as well as for anti-inflammatory and antihistaminic properties. The in vivo wound-healing and anti-inflammatory potential of four Tanacetum species, Tanacetum argenteum (Lam.) Willd. subsp. argenteum (TA), Tanacetum heterotomum (Bornm.) Grierson (TH), Tanacetum densum (Lab.) Schultz Bip. subsp. sivasicum (TD), and Tanacetum vulgare L. (TV) was investigated.
Materials and Methods:
The chloroform (CHCl3) and methanol:water (80:20) extracts were prepared from the aerial parts of each plant. For assessment of the wound-healing activity, linear incision on rats and circular excision on mice wound models were used and histopathological analyses were conducted on the tissues treated with the test materials. For the evaluation of the anti-inflammatory activity, Whittle Method based on the inhibition of the acetic acid-induced increase in capillary permeability was used. In order to elucidate the phytochemical contents of the extracts, HPLC profiles of active fractions were screened and quantitative analysis was conducted within the scope of HPLC analysis.
Results:
The CHCl3 extracts of TD, TA and TV were found to have significant wound healing activity (37.1%, 30.8% and 26.1% tensile strength; 88.05%, 72.93% and 44.88% contraction values, respectively) and anti-inflammatory activities (31.5% and 26.6% inhibition values for TD and TA). Parthenolide content of the CHCl3 extracts of TA, TH and TV were found 242.66±1.53, 190.16±5.62 and 177.51±3.73 µg/100 mg plant material, respectively.
Conclusion:
According to the results, the other secondary metabolites present in the aerial parts of the Tanacetum species possibly exerted synergistic effects on the observed healing of the wounds.
Keywords: Asteraceae, Anti-inflammatory, Excision, Incision, Tanacetum, Wound-healing
Introduction
The genus Tanacetum L., which belongs to the Asteraceae family and grows in the temperate regions of Europe and West Asia, comprises about 200 species. The species of this genus are traditionally used for anti-inflammatory, antihistaminic, and stomachic properties and as insecticide (1-3). In addition, these species are used against psoriasis, migraine, nausea, vomiting, tinnitus, dizziness, hysteria, neuralgia, asthma, kidney weakness, constipation, gynecological disorders, and emmenagogue as an anthelmintic food additive and are reported to be used externally as a poultice to heal eruptive skin diseases (4-10). The leaves of Tanacetum vulgare L. were reported to be utilized for wound-healing (11) and the infusion prepared from the aerial parts of Tanacetum densum (Labill.) Sch. Bip. subsp. densum is used for the treatment spelling (12, 13). These species contain sterols, essential oil components, sesquiterpene lactones, resins, bitter substances, acetylenes, flavonoids, coumarins and tannic acid (6, 9, 14-17). Several biological activity studies have also been conducted on Tanacetum species revealing their analgesic, antipyretic, antitumor, antioxidant, cardiotonic, spasmolytic, hypoglycemic, diuretic, laxative, acaricidal, antileishmanial, antifungal, antibacterial and herbicidal activities (6-10, 18-24). However, there has been no study on the assessment of the wound-healing activity of Tanacetum species despite their previously reported traditional utilizations.
The aim of the present study is to evaluate the in vivo wound-healing and anti-inflammatory potentials of four Tanacetum species namely Tanacetum argenteum (Lam.) Willd. subsp. argenteum, Tanacetum heterotomum (Bornm.) Grierson, Tanacetum densum subsp. (Lab.) Schultz Bip. sivasicum and T. vulgare. Among them, T. densum subsp. sivasicum and T. argenteum subsp. argenteum are endemic to Turkey. In order to elucidate the phytochemical contents of the extracts, HPLC profiles of these species were screened and quantitative analysis of three flavonoid aglycones; apigenol, quercetin, kaempferol and a sesquiterpene lactone; parthenolide were conducted within the scope of HPLC analysis.
Materials and Methods
Plant material
Tanacetum species were collected from different regions of Anatolia (Table 1). Taxonomic identification of the plants was confirmed by Assistant Professor Mehmet Tekin (Department of Pharmaceutical Botany, Faculty of Pharmacy, Trakya University). Voucher specimens were stored in the Herbarium of Cumhuriyet University, Faculty of Science (CUFH).
Table 1.
Locality of the plant samples of different Tanacetum species
| Plant species | Locality | Altitude | Date | Herbarium No | |
|---|---|---|---|---|---|
| T. argenteum subsp. argenteum (TA) | Böğrüdelik village, Sivas | 1845 m | 2012 | M.Tekin 1255 | |
| T. heterotomum (TH) | Ziyarettepe, Sivas | 1402 m | 2012 | M.Tekin 1315 | |
| T. densum subsp. sivasicum (TD) | Böğrüdelik village, Sivas | 1850 m | 2012 | M.Tekin 1257 | |
| T. vulgare (TV) | Karaçayır, Sivas | 1440 m | 2012 | M.Tekin 1313 | |
Dried and powdered aerial parts of each plant (30 g) were extracted with 300 ml CHCl3 during 8 hr for 3 days. Residues were dried and extracted with methanol: water (MeOH:H2O) (80:20) at room temperature during 8 hr for 3 days by continuous stirring. The extracts were filtered and concentrated using evaporator to yield dry crude extracts.
HPLC analysis
Quercetin, kaempferol, apigenin and parthenolide contents of CHCl3 extracts of Tanacetum species were investigated using HPLC analysis. HPLC analyses were carried out using Agilent LC 1200 model chromatograph (Agilent Technologies, California, USA). Separation was carried out by using gradient elution on ACE 5 C18 (250 mm× 4.6 mm; 5 µm) column. 0.2% phosphoric acid in water (A) and acetonitrile (B) were used in gradient elution as the mobile phase. The analysis was started with the ratio of A:B 90:10, v/v. Afterwards, the ratio of A:B was linearly changed to 0:100 in 36 min. During the last 4 min of the analysis, the solvent ratio was isocratic at the rate of A:B 0:100, v/v. The analyses were conducted with the flow rate of 1 ml/min and the injection volume was 10 µl. The column temperature was kept at 40 °C during the analyses. Parthenolide and flavonoid aglycones were analyzed at 214 nm and 330 nm, respectively. Peak areas were integrated automatically by computer using Agilent Software.
Preparation of standard solutions and calibration
The stock solution for the reference compound was prepared at the concentration of 0.1 mg/ml. The compound was weighed and dissolved with methanol in volumetric flask and the final volume was adjusted to 10 ml. The stock solution was diluted to obtain the concentration levels between 0.0005 mg/ml and 0.05 mg/ml. Triplicate analyses were carried out for each concentration level and the calibration curve was obtained using peak areas against concentration.
Optimization of the sample extraction procedure and preparation of samples
Aerial parts of the four different Tanacetum species were used in this experiment. CHCl3 and MeOH:H2O (80:20) mixture were used successively for the extraction of plant samples. For HPLC analysis, CHCl3 extracts were prepared from plant material to obtain total extract. Ten mg of CHCl3 extracts of four species were weighed in 10 ml volumetric flask, dissolved in MeOH and adjusted to the final volume separately, and each extract was filtered through 0.45 µm membrane filter after adjusting to a final volume of 10 ml with same solvent. Triplicate 10 µl injections were performed for plant samples.
Validation procedure-Limit of detection and quantification
The injections were repeated 9 times to verify the values of limit of detection (LOD) and limit of quantification (LOQ).
Biological activity tests
Animals
Male Sprague Dawley rats (160-180 g) and Swiss albino mice (20–25 g) were provided from Laboratory of Experimental Animals, Kobay, Turkey. Each group consisted of six animals. Before the experiments, the animals were left 3 days for acclimatization at room temperature, standard humidity and light-controlled (12 hr light/12 hr dark) conditions. The animals were maintained on standard pellet diet and water ad libitum. Animals were processed according to the suggested European ethical guidelines for the care of laboratory animals. The study was performed according to the international rules considering the animal experiments and biodiversity rights (Gazi University Ethical Council Project Number: G.U.ET-10.027).
Preparation of test samples for bioassay
For the assessment of wound-healing activity, the test samples were prepared in an ointment base (glycol stearate, 1,2 propylene glycol, liquid paraffin, 3:6:1) in 1% concentration and applied 0.5 g onto the wounded sites. Madecassol® (Bayer) was used as the reference ointment. For the evaluation of anti-inflammatory activity, extract suspensions were prepared as described previously (25).
For the anti-inflammatory activity evaluation, test samples were administered orally to the test animals after suspending in a mixture of distilled H2O and 0.5% sodium carboxy methyl cellulose (CMC). The control group animals received vehicle only (26). Indomethacin (10 mg/kg) in 0.5% CMC was used as a reference drug (27).
Wound-healing activity
Linear incision wound model
Linear incision wound model was performed on the rats according to the model, which was previously described by Lodhi et al. 2006 (28), and Suguna et al. 2002 (29) with some modifications (25). Two linear incisions were made on the dorsal part of the rat. Tensile strength of the treated skin was measured with a tensiometer (Zwick/Roell Z 0.5, Germany)
Circular excision wound model
A circular wound was created on the dorsal region of each mouse according to the method described by Süntar et al. 2013 (25). After treatment of the wounds, wound contraction was calculated as percentage of the reduction. A specimen sample of tissue was taken in order to be analyzed histopathologically (30).
Histopathology
The tissues were stained and histopathologically examined (25).
Anti-inflammatory activity
Acetic acid-induced increase in capillary permeability
Inhibitory activity of the test samples on the increased vascular permeability induced by acetic acid in mice was evaluated according to Whittle method (31) with some modifications (32).
Statistical analysis of the data
One-way analysis of variance (ANOVA) and Students-Newman-Keuls post hoc tests were used to analyze the data. The values of P≤ 0.05 were considered statistically significant. No statistical tests were performed for histopathological data, which were considered to be nonparametric.
Results
In the present study, in vivo wound-healing and anti-inflammatory activities of various Tanacetum species were investigated. Among these species, T. densum subsp. sivasicum, T. vulgare and T. argenteum subsp. argenteum were found to have wound-healing activity potential in both wound models. The highest activity was observed for the CHCl3 extract of TD, with the tensile strength value of 37.1% in linear incision wound model (Table 2) and with the contraction value of 88.05% in circular excision wound model (Table 3). Histopathological findings also supported the biological activity results. Phases in wound-healing processes (inflammation, proliferation, and remodeling) were observed within the experimental groups with different degree (Table 4). Comparing the other experimental groups, best remodeling was observed in the reference group and then in the T. densum subsp. sivasicum CHCl3 extract group. Delayed wound-healing processes were observed in the negative control and vehicle groups. Histopathological results are shown in Figure 1, which stained with hematoxylin & eosin (HE) and Van Gieson (VG).
Table 2.
Effects of the test materials on linear incision wound model
| Material | Extract type | Tensile strength ± SEM | (%Tensile strength) |
|---|---|---|---|
| Vehicle | 15.42 ± 2.18 | 7.2 | |
| Negative Control | 14.39± 2.21 | - | |
| T. argenteum subsp. argenteum | CHCl3 | 20.17 ± 1.65 | 30.8** |
| MeOH:H2O | 18.41 ± 1.79 | 19.4 | |
| T. heterotomum | CHCl3 | 18.26 ± 1.94 | 18.4 |
| MeOH:H2O | 17.46 ± 1.78 | 13.2 | |
| T. densum subsp. sivasicum | CHCl3 | 21.14 ± 1.89 | 37.1** |
| MeOH:H2O | 15.32 ± 2.25 | - | |
| T. vulgare | CHCl3 | 19.44 ± 1.86 | 26.1* |
| MeOH:H2O | 15.95 ± 2.52 | 3.3 | |
| Madecassol® | 23.16 ± 1.50 | 50.2*** |
: P<0.05;
: P<0.01;
: P<0.001;
SEM.: Standard error of the mean, percentage of the tensile strength values: The vehicle group was compared to the negative control group; The test materials and the reference material were compared to vehicle group
Table 3.
Effects of the test materials on circular excision wound model
| Material | Extract type | Wound area (mm2) ± SEM (Contraction%) | |||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | ||
| Vehicle | 20.25±3.24 | 18.32±2.45 (2.86) | 15.49±2.08 (3.61) | 10.24±1.69 (9.70) | 6.56±1.89 (7.48) | 4.10±0.47 (6.18) | |
| Negative Control | 21.16±3.31 | 18.86±2.78 | 16.07±2.16 | 11.34±1.79 | 7.09±0.91 | 4.37±0.66 | |
| T. argenteum subsp. argenteum | CHCl3 | 22.24±3.83 | 15.52±1.68 (15.28) | 12.29±1.84 (20.66) | 7.71±1.92 (24.71) | 3.86±0.69 (41.16)* | 1.11±0.55 (72.93)*** |
| MeOH:H2O | 20.75±2.54 | 16.92±1.95 (7.64) | 13.88±1.99 (10.39) | 8.91±1.36 (12.98) | 5.45±0.99 (16.92) | 3.49±0.70 (14.88) | |
| T. heterotomum | CHCl3 | 21.19±3.04 | 15.98±1.75 (12.77) | 13.13±1.92 (15.24) | 8.35±1.86 (18.45) | 4.64±0.75 (29.26) | 2.81±0.39 (21.71) |
| MeOH:H2O | 19.96±3.15 | 18.42±2.25 - | 14.04±2.22 (9.36) | 8.91±1.74 (12.99) | 5.54±0.91 (15.54) | 3.03±0.52 (26.09) | |
| T. densum subsp. sivasicum | CHCl3 | 21.47±2.99 | 15.56±1.93 (15.07) | 12.08±1.79 (22.01) | 7.11±1.33 (30.57)* | 2.55±0.81 (61.13)** | 0.49±0.48 (88.05)*** |
| MeOH:H2O | 20.68±2.90 | 17.31±2.10 (5.51) | 14.50±2.09 (6.39) | 8.99±1.50 (12.21) | 5.73±0.91 (12.65) | 1.76±0.61 (8.29) | |
| T. vulgare | CHCl3 | 21.55±3.31 | 17.91±2.07 (2.23) | 14.32±2.11 (7.55) | 8.45±2.01 (17.48) | 4.02±1.10 (38.71)* | 2.26±0.43 (44.88)* |
| MeOH:H2O | 20.52±3.41 | 15.77±2.17 (13.91) | 13.38±2.07 (13.62) | 8.49±1.70 (17.08) | 4.98±0.98 (24.08) | 2.66±0.59 (22.92) | |
| Madecassol® | 20.44±2.67 | 15.07±1.91 (17.74) | 11.27±1.71 (27.24) | 5.29±1.12 (48.34)* | 2.01±0.32 (69.36)** | 0.00±0.00 (100.00)*** | |
: P<0.05;
: P<0.01;
: P<0.001;
SEM: Standard error of the mean; percentage of the tensile strength values: The vehicle group was compared to the negative control group; The test materials and the reference material were compared to vehicle group
Table 4.
Wound healing processes and healing phases of the experimental groups
| Groups | Extract type | Wound Healing Processes | Healing Phases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | U | RE | FP | CD | MNC | PMN | NV | I | P | R | ||
| Vehicle | +++ | +++ | - | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | |
| Negative Control | +++ | +++ | - | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | |
| T. argenteum subsp. argenteum | CHCl3 | ++/+++ | ++ | - | +++ | +++ | +++ | +++ | +++ | ++/+++ | ++/+++ | - |
| MeOH:H2O | ++/+++ | ++ | - | ++/+++ | ++/+++ | +++ | +++ | +++ | ++/+++ | ++/+++ | - | |
| T. heterotomum | CHCl3 | ++/+++ | ++ | - | +++ | +++ | +++ | +++ | +++ | ++/+++ | ++/+++ | - |
| MeOH:H2O | +++ | +++ | - | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | |
| T. densum subsp. sivasicum | CHCl3 | + | - | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| MeOH:H2O | +++ | +++ | - | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | |
| T. vulgare | CHCl3 | ++ | ++ | - | ++/+++ | ++/+++ | +++ | ++/+++ | ++/+++ | ++/+++ | ++/+++ | - |
| MeOH:H2O | +++ | +++ | - | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | |
| Madecassol® | ++ | - | +/++ | ++ | ++ | ++ | ++ | ++ | ++ | ++/+++ | +/++ | |
Hematoxylin & eosin (HE) and Van Gieson (VG) stained sections were scored as mild (+), moderate (++) and severe (+++) for epidermal and/or dermal re-modeling. S: Scab, U: Ulcus, RE: Re-epithelization, FP: Fibroblast proliferation, CD: Collagen depositions, MNC: Mononuclear cells, PMN: Polymorphonuclear cells, NV: Neovascularization, I: Inflammation phase, P: Proliferation phase, R: Re-modeling phase
Figure 1.
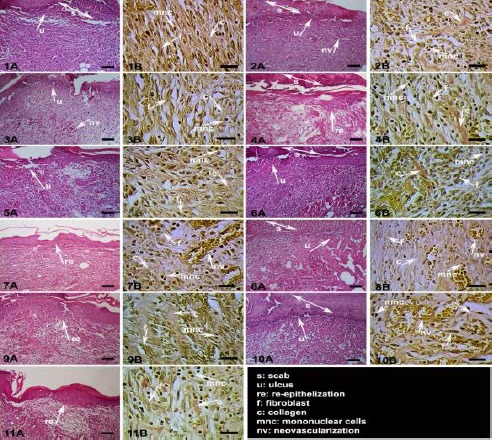
Histopathological view of treated tissues; Skin sections show the hematoxylin & eosin (HE) stained epidermis and dermis in A, and the dermis stained with Van Gieson (VG) in B. The original magnification was x 100 and the scale bars represent 120 µm for figures in A, and the original magnification was x 400 and the scale bars represent 40 µm for B. Data are representative of 6 animal per group. 1) Vehicle group; 2) Negative control group (untreated group); 3) T. argenteum subsp. argenteum MeOH:H2O extract group; 4) T. argenteum subsp. argenteum CHCl3 extract group; 5) T. heterotomum MeOH:H2O extract group; 6) T. heterotomum CHCl3 extract group;7) T.densum subsp. sivasicum MeOH:H2O extract group; 8) T.densum subsp. sivasicum CHCl3 extract group; 9) T. vulgare MeOH:H2O extract group; 10) T. vulgare CHCl3 extract group; 11) Reference group (wound tissue treated with Madecassol®); Arrows pointing events during wound healing; s: scab, u: ulcus, re: re-epithelization, f: fibroblast, c: collagen, mnc: mononuclear cells, nv: neovascularization
The results of the anti-inflammatory activity assessment revealed that CHCl3 extracts of T. densum subsp. sivasicum and T. argenteum subsp. argenteum possess anti-inflammatory activity in Whittle Method by displaying 31.5% and 26.6% inhibition values, respectively (Table 5).
Table 5.
Inhibitory effect of the test materials on acetic acid-induced increase in capillary permeability
| Material | Extract type | Dose (mg/kg) | Evans blue concentration (μg/ml) ± SEM | Inhibition (%) |
|---|---|---|---|---|
| Control | 12.01 ± 2.18 | - | ||
| T. argenteum subsp. argenteum | CHCl3 | 100 | 8.82 ± 0.91 | 26.6* |
| MeOH:H2O | 100 | 10.91± 1.35 | 9.2 | |
| T. heterotomum | CHCl3 | 100 | 11.58 ± 1.23 | 3.6 |
| MeOH:H2O | 100 | 10.94 ± 0.97 | 8.9 | |
| T.densum subsp. sivasicum | CHCl3 | 100 | 8.23 ± 0.79 | 31.5** |
| MeOH:H2O | 100 | 9.59 ± 1.44 | 20.2 | |
| T. vulgare | CHCl3 | 100 | 10.39 ± 1.46 | 13.5 |
| MeOH:H2O | 100 | 9.65 ± 1.81 | 19.7 | |
| Indomethacin | 10 | 5.97 ± 0.51 | 50.3*** |
SEM: Standard error of the mean;
: P<0.05.
: P<0.01.
: P<0.001 significant from the control
In the present study, the quantification of three flavonoid aglycones and a sesquiterpene was conducted on the same Tanacetum species by using HPLC method. Selected HPLC method was determined by comparing the chromatographic profile and data obtained from the standards and samples, considering the following parameters; retention time, analyzing time for samples, separation, peak shapes and maximum UV absorption of the standards. In order to optimize the suitable chromatographic separation of four compounds in four different Tanacetum species, many different isocratic and gradient elution were investigated. Phosphoric acid was also used as modifier. Furthermore, different temperatures (30°C, 35°C and 40°C) and different flow rates (1.2 ml/min, 1 ml/min, 0.8 ml/min) were tested. Finally good separations for the extracts of Tanacetum species have been achieved under 40°C temperature, 1 ml/min flow rate and gradient elution of water (containing 0.2% phosphoric acid) (A), and acetonitrile (B). The chromatograms were obtained at 214 nm and 330 nm for standards and samples. Identification of the peaks was confirmed by comparison of the retention times and UV absorption spectra with acquired standards (Figure 2).
Figure 2.
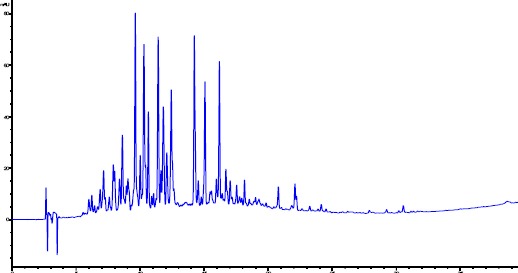
HPLC chromatogram of T. argenteum subsp. Argenteum (214 nm)
The highest content of the parthenolide was detected in T. argenteum subsp. argenteum CHCl3 extract. The parthenolide contents of the CHCl3 extracts of T. argenteum subsp. argenteum, T. vulgare and T. heterotomum were found as 242.66±1.53 µg/100 mg; 177.51±3.73 µg/100 mg and 190.16±5.62 µg/100 mg, respectively. Parthenolide was not detected in T. densum subsp. sivasicum CHCl3 extract. Furthermore, quercetin, kaempferol and apigenin have also been investigated in CHCl3 extracts. Quercetin and kaempferol has not been detected even at LOD levels. However, T. heterotomum was determined as the only species containing apigenin in quite small amount. According to the HPLC chromatograms and UV absorbances of the peaks, investigated Tanacetum species have flavonoids in varying amounts together with other compounds. LOD and LOQ levels of parthenolide were determined as 0.4139 µg/ml and 1.3793 µg/ml, respectively.
Discussion
Tanacetum species are popular among the people living in rural areas evidenced by the previously published ethnomedicinal data (1-10). Among Tanacetum species, Tanacetum parthenium (L.) Schultz-Bip. (Feverfew) is popular for its use in migraine prophylaxis (5, 9). The activity potential was confirmed by several clinical trials, which demonstrated its lowering effect on the intensity and frequency of headache, visual disturbance, nausea and vomiting induced by migraine (33-35). The secondary metabolites present in the leaves have been shown to exert the activity synergistically and the whole leaf extract has been suggested to be used for the prevention of migraine (5, 36). In vivo and in vitro studies revealed that parthenolide-depleted feverfew extract possessed antioxidant activity. According to the in vitro studies, the extract decreased cigarette smoke-mediated damage, UV-induced hydrogen peroxide and pro-inflammatory cytokine release (37). Wound-healing and anti-inflammatory activity results exhibited herein could be related to potential antioxidant activity of the tested Tanacetum species. The phytochemical studies yielded the chemical constituents of T. parthenium as volatile oil components, sesquiterpene lactones, coumarin derivatives and flavonoids (mainly kaempferol, quercetin, apigenin and luteolin derivatives) (9, 14). Besides T. parthenium, another species such as T. vulgare is also a well-known folk remedy that is externally used as poultice to heal some eruptive skin diseases, sprains, gout, contusions and scabies, or to kill lice, and fleas (4). Previous study by Brown et al. (1997) demonstrated that T. vulgare, Tanacetum ptarmiciflorum (Webb & Berth.) Schultz. Bip. and Tanacetum niveum (Lagasca) Schultz-Bip. contain parthenolide and these species exhibit in vitro anti-inflammatory activity. However, lower parthenolide contents of T. vulgare and T. ptarmiciflorum proved that parthenolide was not the only constituent responsible for such activity (4). T. vulgare was further reported to have antibacterial and antihelmintic compounds as well as polysaccharides (6-8, 10). The oil obtained from T. vulgare is used by applying on skin as repellent against insects and the common tick Ixodes ricinus (1, 15). T. vulgare comprises of sesquiterpenes and sesquiterpene lactones, flavonoid derivatives, hydroxycoumarins, sterols, tannic acid, resins and essential oil components (6, 15-17).
Anti-inflammatory activity studies have been conducted on Tanacetum microphyllum DC., which is traditionally used for inflammatory conditions and rheumatic diseases. In vivo trials showed that flavonoids; 5,7,3’-trihydroxy-3,6,4’-trimethoxyflavone (centaureidin) and 5,3’-dihydroxy-4’-methoxy-7-carbomethoxyflavonol as well as a sesquiterpene lactone, and hydroxyachillin isolated from the aerial parts of this species also exhibited these activities (38, 39). Anti-inflammatory activity of the plant was also confirmed by in vivo studies. The flavonoid derivatives; ermanin and 5,3’-dihydroxy-4’-methoxy-7-methoxycarbonylflavonol isolated from T. microphyllum were reported to inhibit inducible nitric oxide synthase and cyclooxygenase-2, which were assumed to be the mechanisms of their anti-inflammatory activity (40). Tanacetum larvatum (Griseb. ex Pant.) Kanitz is another species that was reported to exhibit anti-inflammatory and antiulcerogenic activity. The mechanism of such activity was supposed to be due to the inhibition of DNA binding of the transcription factor NF-κB (21). In the present study, T. densum subsp. sivasicum and T. argenteum subsp. argenteum demonstrated significant anti-inflammatory activity.
The essential oil of Tanacetum santolinoides (DC.) Feinbr. and Fertig was found to possess antimicrobial activity on Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Candida albicans (19). According to the other records, significant antimicrobial effects of Tanacetum balsamita L., Tanacetum aucherianum (DC.) Schultz. Bip. and Tanacetum chiliophyllum (Fisch. Et Mey.) Schultz. Bip. var. chiliophyllum were demonstrated (22, 41). Antimicrobial activity tests were conducted with the aerial part extract of T. densum subsp. sivasicum and the extract was found to be active against B. subtilis and Klebsiella pneumoniae (42). Evaluation of antioxidant activities of T. densum subsp. sivasicum, T. densum subsp. eginense and T. densum subsp. amani revealed that the most active subspecies was T. densum subsp. sivasicum in compliance with its higher phenolic content (43). The wound-healing activity of T. densum subsp. sivasicum demonstrated in the present study, could be related to its both antimicrobial and antioxidant effects, which were previously reported.
In previous studies, the secondary metabolites present in the leaves of T. parthenium have been shown to exert the analgesic activity synergistically and thus the whole leaf extract has been suggested to be used for the prevention of migraine (5, 36).
Tanacetum species were found to contain sterols, essential oil components, sesquiterpene lactones, resins, bitter substances, acetylenes, flavonoids, coumarins and tannic acid (6,9, 14-17). According to the current results, wound-healing activity does not seem to be in accordance with parthenolide content. In this case, flavonoid aglycones and terpenic compounds that may be found in CHCl3 fraction are supposed to be responsible for such an activity.
In addition, studies on T. vulgare and T. ptarmiciflorum have shown that parthenolide was not the only constituent responsible for anti-inflammatory activity (4).
Conclusion
Parthenolide appears not to be the only principle compound responsible for the wound-healing activity but other secondary metabolites present in the aerial parts of the Tanacetum species studied possibly exerted synergistic effects on the observed healing of the wounds.
Acknowledgment
Throughout the experiments, animals were processed according to the suggested European ethical guidelines for the care of laboratory animals. The present study was performed according to the international rules considering the animal experiments and biodiversity rights (Gazi University Ethical Council Project Number: G.U.ET-10.027).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Başer HCB, Demirci B, Tabanca N, Özek T, Gören N. Composition of the essential oils of Tanacetum armenum (DC.) Schultz Bip T. balsamita L T. chiliophyllum (Fisch.&Mey.) Schultz Bip. var chiliophyllum and T. haradjani (Rech. Fil.) Grierson and the enantiomeric distribution of camphor and carvone. Flavour Fragr. 2001;16:195–200. [Google Scholar]
- 2.Kılıç Ö. Essential oil composition of four endemic Tanacetum L. (Asteraceae) taxa from Turkey and a chemotaxonomic approach. J Agric Sci Technol. 2014;4:197–202. [Google Scholar]
- 3.Susurluk H, Çalışkan Z, Gürkan O, Kırmızıgül S, Gören N. Antifeedant activity of some Tanacetum species and bioassay guided isolation of the secondary metabolites of Tanacetum cadmeum subsp cadmeum (Compositae) Ind Crops Prod. 2007;26:220–228. [Google Scholar]
- 4.Brown AMG, Edwards CM, Davey MR, Power JB, Lowe KC. Effects of extracts of Tanacetum species on human polymorphonuclear leucocyte activity in vitro. Phytother Res. 1997;11:479–484. [Google Scholar]
- 5.Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.). An update of a systematic review. Public Health Nutr. 2000;3:509–514. doi: 10.1017/s1368980000000598. [DOI] [PubMed] [Google Scholar]
- 6.Lahlou S, Israili ZH, Lyoussi B. Acute and chronic toxicity of a lyophilised aqueous extract of Tanacetum vulgare leaves in rodents. J Ethnopharmacol. 2008;117:221–227. doi: 10.1016/j.jep.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Lahlou S, Tahraoui A, Israili Z, Lyoussi B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J Ethnopharmacol. 2007;110:458–463. doi: 10.1016/j.jep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Mantle D, Eddeb F, Pickering AT. Comparison of relative antioxidant activities of British medicinal plant species in vitro. J Ethnopharmacol. 2000;72:47–51. doi: 10.1016/s0378-8741(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 9.Pareek A, Suthar M, Rathore GS, Bansal V. Feverfew (Tanacetum parthenium L.). A systematic review. Pharmacogn Rev. 2011;5:103–110. doi: 10.4103/0973-7847.79105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie G, Schepetkin IA, Quinn MT. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. Int Immunopharmacol. 2007;7:1639–1650. doi: 10.1016/j.intimp.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza GC, Haas AP, von Poser GL, Schapoval EE, Elisabetsky E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol. 2004;90:135–143. doi: 10.1016/j.jep.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Arnold N, Baydoun S, Chalak L, Raus T. A contribution to the flora and ethnobotanical knowledge of Mount Hermon, Lebanon. Fl Medit. 2015;25:13–55. [Google Scholar]
- 13.Baydoun S, Lamis C, Helena D, Nelly A. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J Ethnopharmacol. 2015;173:139–156. doi: 10.1016/j.jep.2015.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Long C, Sauleau P, David B, Lavaud C, Cassabois V, Ausseil F, et al. Bioactive flavonoids of Tanacetum parthenium revisited. Phytochemistry. 2003;64:567–569. doi: 10.1016/s0031-9422(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 15.Palsson K, Jaenson TG, Baeckstrom P, Borg-Karlson AK. Tick repellent substances in the essential oil of Tanacetum vulgare. J Med Entomol. 2008;45:88–93. doi: 10.1603/0022-2585(2008)45[88:TRSITE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Sanz JF, Marco JA. NMR studies of tatridin a and some related sesquiterpene lactones from Tanacetum vulgare. J Nat Prod. 1991;54:591–596. [Google Scholar]
- 17.Schinella GR, Giner RM, Recio MC, Mordujovich de Buschiazzo P, Rios JL, Manez S. Anti-inflammatory effects of South American Tanacetum vulgare. J Pharm Pharmacol. 1998;50:1069–1074. doi: 10.1111/j.2042-7158.1998.tb06924.x. [DOI] [PubMed] [Google Scholar]
- 18.Chiasson H, Belanger A, Bostanian N, Vincent C, Poliquin A. Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. J Econ Entomol. 2001;94:167–171. doi: 10.1603/0022-0493-94.1.167. [DOI] [PubMed] [Google Scholar]
- 19.El-Shazly A, Dorai G, Wink M. Composition and antimicrobial activity of essential oil and hexane-ether extract of Tanacetum santolinoides (dc.) Feinbr. and Fertig. Z Naturforsch. 2002;C57:620–623. doi: 10.1515/znc-2002-7-812. [DOI] [PubMed] [Google Scholar]
- 20.Jain NK, Kulkarni SK. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol. 1999;68:251–259. doi: 10.1016/s0378-8741(99)00115-4. [DOI] [PubMed] [Google Scholar]
- 21.Petrovic SD, Dobric S, Bokonjic D, Niketic M, Garcia-Pineres A, Merfort I. Evaluation of Tanacetum larvatum for an anti-inflammatory activity and for the protection against indomethacin-induced ulcerogenesis in rats. J Ethnopharmacol. 2003;87:109–113. doi: 10.1016/s0378-8741(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 22.Salamcı E, Kordalı S, Kotan R, Cakır A, Kaya Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucherianum and Tanacetum chiliophyllum var. chiliophyllum. Biochem Syst Ecol. 2007;35:569–581. [Google Scholar]
- 23.Tiuman TS, Ueda-Nakamura T, Garcia Cortez DA, Dias Filho BP, Morgado-Diaz JA, de Souza W, et al. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob Agents Chemother. 2005;49:176–182. doi: 10.1128/AAC.49.11.176-182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C, Chen F, Wang X, Kim HJ, He GQ, Haley-Zitlin V, et al. Antioxidant constituents in ferverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem. 2006;96:220–227. [Google Scholar]
- 25.Suntar I, Kupeli Akkol E, Keles H, Yesilada E, Sarker SD. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J Ethnopharmacol. 2013;149:103–110. doi: 10.1016/j.jep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Küpeli Akkol E, Suntar I, Keles H, Yesilada E. The potential role of female flowers inflorescence of Typha domingensis Pers. in wound management. J Ethnopharmacol. 2011;133:1027–1032. doi: 10.1016/j.jep.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Küpeli Akkol E, Bahadır Acıkara Ö, Suntar I, Ergene B, Saltan Citoglu G. Ethnopharmacological evaluation of some Scorzonera species In vivo anti-inflammatory and antinociceptive effects. J Ethnopharmacol. 2012;140:261–270. doi: 10.1016/j.jep.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Lodhi S, Pawar RS, Jain AP, Singhai AK. Wound healing potential of Tephrosia purpurea (Linn.) Pers. in rats. J Ethnopharmacol. 2006;108:204–210. doi: 10.1016/j.jep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother Res. 2002;16:227–231. doi: 10.1002/ptr.827. [DOI] [PubMed] [Google Scholar]
- 30.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid ul Z. Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in guinea pigs. J Ethnopharmacol. 2006;107:161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Whittle BA. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Br J Pharmacol Chemother. 1964;22:246–253. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yesilada E, Kupeli E. Clematis vitalba L. aerial part exhibits potent anti-inflammatory, antinociceptive and antipyretic effects. J Ethnopharmacol. 2007;110:504–515. doi: 10.1016/j.jep.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Johnson ES, Kadam NP, Hylands DM, Hylands PJ. Efficacy of feverfew as prophylactic treatment of migraine. Br Med J. 1985;291:569–573. doi: 10.1136/bmj.291.6495.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy JJ, Heptinstall S, Mitchell JR. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet. 1988;2:189–192. doi: 10.1016/s0140-6736(88)92289-1. [DOI] [PubMed] [Google Scholar]
- 35.Palevitch D, Earon G, Carasso R. Feverfew (Tanacetum parthenium) as a prophylactic treatment for migraine: A double-blind placebo-controlled study. Phytother Res. 1997;11:508–511. [Google Scholar]
- 36.Awang DWC. Prescribing therapeutic feverfew (Tanacetum parthenium (L.) Schultz Bip., syn Chrysanthemum parthenium (L.) Bernh.) Integr Med. 1998;1:11–13. [Google Scholar]
- 37.Martin K, Sur R, Liebel F, Tierney N, Lyte P, Garay M, et al. Parthenolide-depleted Feverfew (Tanacetum parthenium) protects skin from UV irradiation and external aggression. Arch Dermatol Res. 2008;300:69–80. doi: 10.1007/s00403-007-0818-x. [DOI] [PubMed] [Google Scholar]
- 38.Abad MJ, Bermejo P, Villar A, Valverde S. Anti- inflammatory activity of two flavonoids from Tanacetum microphyllum. J Nat Prod. 1993;56:1164–1167. doi: 10.1021/np50097a022. [DOI] [PubMed] [Google Scholar]
- 39.Abad MJ, Bermejo P, Valverde S, Villar A. Anti-inflammatory activity of hydroxyachillin, a sesquiterpene lactone from Tanacetum microphyllum. Planta Med. 1994;60:228–231. doi: 10.1055/s-2006-959464. [DOI] [PubMed] [Google Scholar]
- 40.Guerra JA, Molina M, Abad MJ, Villar AM, Paulina B. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids isolated from Tanacetum microphyllum. Int Immunopharmacol. 2006;6:1723–1728. doi: 10.1016/j.intimp.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Kubo A, Kubo I. Antimicrobial agents from Tanacetum balsamita. J Nat Prod. 1995;58:1565–1569. [Google Scholar]
- 42.Goren N, Bozokjohansson C, Jakupovic J, Lin LJ, Shieh HL, Cordell GA, et al. Sesquiterpene lactones with antibacterial activity from Tanacetum densum subsp sivasicum. Phytochemistry. 1992;31:101–104. [Google Scholar]
- 43.Tepe B, Sokmen A. Secreening of the antioxidative properties and total phenolic contents of three endemic Tanacetum subspecies from Turkish flora. Bioresour Technol. 2007;98:3076–3079. doi: 10.1016/j.biortech.2006.10.019. [DOI] [PubMed] [Google Scholar]


