Abstract
Objective(s):
Some studies suggest that chronic low-grade inflammation is involved in insulin resistance in polycystic ovary syndrome (PCOS). This study assessed possible involvement of alteration in expression of two pro-inflammatory factors, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in adipose tissues of PCOS rats in the impairment of insulin actions. Also, effects of resveratrol as an anti-inflammatory agent were investigated.
Materials and Methods:
Fifteen female Wistar rats (21 days old) were divided into three groups (n=5): I) Control, II) PCO-model-saline: served as PCOS rats and to induce PCOS, received subcutaneously testosterone enanthate 1 mg/100 g body weight subcutaneously for 35 days, III) PCO-model-resveratrol, after receiving testosterone, received resveratrol 10 mg/kg intraperitoneally for 28 days. The expression of Tnf-α and Il-6 mRNAs in adipose tissues was determined by the qRT-PCR method.
Results:
The Il-6 mRNA expression in the visceral adipose tissue of PCOS rats was increased in comparison to controls (P<0.05). Tnf-α and Il-6 mRNA expression in visceral and subcutaneous adipose tissues of polycystic ovarian rats was similar to controls. The expression of Tnf-α mRNA in subcutaneous adipose tissue and Tnf-α and Il-6 mRNAs in the visceral adipose tissue of the PCO-model-resveratrol group were lower than PCOS rats (P<0.05).
Conclusion:
Increased expression of Il-6 mRNA in the visceral adipose tissue of polycystic ovarian rats may be one cause of insulin resistance observed in them and resveratrol as an anti-inflammatory and anti-hyperglycemic agent may decrease the risk of diabetes by reduction of expression of pro-inflammatory cytokines TNF-α and IL-6 in PCOS patients.
Keywords: Adipose tissue IL-6, Polycystic ovary – syndrome, Rat, Resveratrol, TNF-α
Introduction
Polycystic ovary syndrome (PCOS) is one of the most frequent endocrine disorders among women in child bearing age and its prevalence is between 6%–15% (1). This syndrome may be associated with chronic anovulation and excess levels of androgen and these complications may lead to infertility. This syndrome affects various reproductive, endocrine, and metabolic functions. The patients afflicted by this syndrome may show an increased prevalence of obesity, insulin resistance (approximately 50%–70% of all PCOS patients have insulin resistance (2)), dyslipidemia, endothelial dysfunction, metabolic syndrome, and risk factors for type 2 diabetes mellitus (DM), but the etiology of this heterogonous syndrome is unknown (3, 4). Recently, some researchers have suggested pro-inflammatory mediators are involved in the pathophysiology of PCOS. They believe PCOS is a chronic, low-grade inflammatory disorder without
IL-6 is an adipokine that is produced by adipocytes and stromal cells of the fat tissue and some evidence shows IL-6 may play a role in insulin resistance (11). Moreover, mediators of chronic low-grade inflammation such as IL-6 are predictors of risk of type 2 diabetes (12). It has been presented that in PCOS women, IL-6 levels are increased (13, 14). Several studies have suggested that IL-6 increment may be related to hyperandrogenism and insulin resistance in PCOS patients (12, 15). However, the findings on effects of IL-6 on insulin resistance are different (16-18). Some evidence has shown that insulin resistance in PCOS patients is due to defects in insulin signaling pathway (19, 20) and in PCOS, adipocyte-derived factors such as free fatty acids and TNF-α affect insulin signaling in the skeletal muscles (21, 22). These factors may cause phosphorylation of IRS-1 by intercellular serine kinases that lead to impairment of signaling events that are mediated by tyrosine phosphorylation such as PI-3 kinase activation (23).
Resveratrol (trans-3, 4’, 5-trihydroxystilbene) is a phytochemical that is found in various components of the diet, such as mulberries, peanuts, grapes, and red wines. Recent studies have shown that resveratrol has various biologic effects such as anti-cancer, anti-inflammatory, antioxidant, anti-aging, and cardio-protective actions. Furthermore, it has been demons-trated that resveratrol has advantageous properties against metabolic disorders (24). Considering recent studies that have shown PCOS is a low-grade chronic inflammatory condition, this natural anti-inflammatory agent may be able to decrease inflammatory activity and may be useful against metabolic disorders that usually are associated with PCOS. Due to previous studies that have indicated TNF-α and IL-6 may be involved in the impairment of insulin function (11, 12, 15) and most PCOS patients have low-grade inflammation (25, 26), in the present study, for the first time, we evaluated whether Tnf-α and Il-6 mRNAs expression in adipose tissues of PCO induced rats is altered in comparison with control rats. This study was carried out on a rat model of PCOS. In this animal model of PCOS that has been introduced by Beloosesky and colleagues (27), some features of PCOS such as insulin resistance and polycystic ovaries have been established. Also, we investigated whether treatment with resveratrol as an anti-inflammatory agent can reduce Tnf-α and Il-6 mRNAs expression in the adipose tissues of these animal models of PCOS.
Materials and Methods
Experimental animals and induction of polycystic ovarian syndrome phenotype and preparation of serum and tissue samples
This study was performed on 21 day old immature female Wistar rats (19–33 g). The study protocols were confirmed by the Local Ethics Committee for Animal Research studies at the Institutional Animal Care and Ethical Committee of Biological Sciences of Razi University (no: 396-2-017). Approved standards of animal care were considered. Animals were housed in the controlled conditions at 20–22 °C with a 12-hr light-dark cycle and had access to water and standard rodent chow pellet (Behparvar, Tehran, Iran) ad libitum. Beginning at 21 days of age the experimental animals were weighed and randomly divided into three groups as follows:
I) The control group (n=5). These animals had no treatment.
II) The PCO-model-saline group (n=5). These animals served as a model of PCOS. To induce polycystic ovarian syndrome phenotype, testosterone enanthate 1 mg/100 g body weight, dissolved in sesame oil, was injected into them subcutaneously for 35 days, once a day (27). Then, saline 1 ml/kg body weight was injected into them intraperitoneally for 28 days (4 weeks).
III) The PCO-model-resveratrol group (n=5). After induction of polycystic ovary phenotype by injection of testosterone enanthate (1 mg/100 g body weight subcutaneously for 35 days) in this group, resveratrol (Sigma Aldrich, Missouri) 10 mg/kg body weight (28) was injected into the rats of this group intraperitoneally for 28 days (4 weeks) once a day; resveratrol was dissolved in saline (Figure 1).
Figure 1.
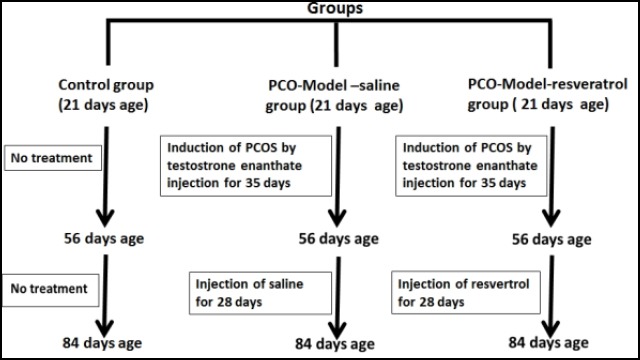
The flow chart of the experimental protocol. The abbreviations represent the following: Control: Normal control rats; PCO-model-saline: these rats received testosterone enanthate 1 mg/100 g body weight subcutaneously for 35 days once daily. Then, saline was injected into them intraperitoneally for 28 days once daily; PCO-model-resveratrol: these rats received testosterone enanthate 1 mg/100 g body weight subcutaneously for 35 days once daily. Then, resveratrol 10 mg/kg was injected into them intraperitoneally for 28 days once daily
At the end of the treatments, the overnight fasted animals were anesthetized by intraperitoneal injection of a combination of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (7 mg/ kg). The blood sample of each rat was obtained by cardiac puncture, allowed to clot for 10 min at room temperature, centrifuged at 3000 RPM for 20 min. The serum samples were prepared and stored at -20 ºC until biochemical analysis. Besides, the samples of retroperitoneal visceral adipose tissue were removed and weighed and the ratios of visceral adipose tissue weight to the body weight (partial weights of these sections) were calculated and shown as percentages. Moreover, the samples of parametrial visceral adipose tissue were removed and weighed and partial weights of these sections were determined; this fat is attached to each ovary and uterus. Also, samples of subcutaneous adipose from the inguinal fat tissues were removed and weighed. The adipose tissue samples were immediately frozen in liquid nitrogen, and stored at -80 °C for further analysis. To evaluate signs of PCO in rats, the left ovary from each rat was removed and fixed in formaldehyde 4%, embedded in paraffin and 5 µm slides were prepared. After hematoxylin and eosin staining, histomorphology of ovaries was evaluated.
Total RNA isolation, cDNA synthesis, and real-time qPCR
Isolation of total RNAs from samples of inguinal subcutaneous adipose tissues and parametrial visceral tissue was performed using the TRIzol reagent (Life Technologies, U.S.A.). Total RNA was extracted from these tissue samples according to the manufacturer’s protocol. After isolation, the RNA concentrations were measured spectrophotometrically using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). Each sample was treated with DNase I (Ferementase, California, U.S.A.) to eliminate any possible DNA contamination. The expression of Tnf-α and Il-6 mRNAs was measured by using a quantitative real time-polymerase chain reaction (qRT-PCR) method. Total RNA, oligo (dT) primers (Denazist, Mashhad, Iran) and RT-Enzyme (Thermo Scientific, USA) were used to synthesize cDNA. Reverse transcription reactions were carried out in a DNA thermal cycler (Techne, U.S.A.) at 42 °C for 60 min and 70 °C for 10 min and the cDNA samples were stored at −20 °C. In order to determine gene expressions, 2 μl cDNA, 3 μl water, and 6 μl 2 X SYBR Green Master mixes (SYBR premix Ex Taq ™ II (Takara Holdings Inc., Kyoto, Japan) and primer pairs at 5 pmol concentrations in a final volume of 12 μl were mixed.
The Allele ID 6 software was used to design primers and all primers were examined by NCBI BLAST in order to make certain that they were special for the target mRNA transcript. The sequences of primers are presented in Table 1.
Table 1.
Sequences of primers used in quantitative real time-polymerase chain reaction (qRT-PCR) experiments, the annealing temperatures for PCR amplification of each gene that were investigated, and the amplicon lengths
| SYBR Green RT-PCR Primers | Sense primer(5’ toward 3’) | Anti-sense primer(5’ toward 3’) | Annealing Temperature (°C) | Amplicon length (bp) |
|---|---|---|---|---|
| β-actin | CACACCCGCCACCAGTTC | GACCCATACCCACCATCACAC | 60 | 166 |
| Tnf-α | AAATGGGCTCCCTCTCATCAGTTC | TCCGCTTGGTGGTTTGCTACGAC | 60 | 180 |
| Il-6 | GACTTCCAGCCAGTTGCCTTCTTG | TGGTCTGTTGTGGGTGGTATCCTC | 60 | 111 |
The primers are shown for the sequences of rat Il-6: interleukin-6; TNF-α: tumor necrosis factor-α, β-actin, and beta-actin. β-actin was selected as the reference gene
The qRT-PCR was done by a Corbett Research RG 3000 thermal cycler (CR CORBETT, Australia) as follows: The initial denaturation at 95 °C for 2 min, denaturation at 95 °C for 10 sec, annealing at 60 °C for 30 sec with 45 repeated thermal cycles measuring the green fluorescence at the end of each extension step. The triplicate PCRs were performed on each sample of cDNA synthesized from the adipose of each rat. The comparative threshold cycle (CT) method was used for gene expression analysis as follows:
The mean CT of treated and control rats from the triplicate PCRs for the target and internal control gene were determined. The mean ± SEM for the relative amount of target genes in three groups of rats using the 2-ΔCT method were calculated (29). The difference in expression of the target genes in the adipose tissues of various groups was determined by the one-way ANOVA test. The melting analysis confirmed the specificity of PCR products. The relative expression of genes with respect to internal control β-actin was estimated.
Insulin and glucose assay and estimation of the insulin sensitivity), insulin resistance, and β-cell function
The serum insulin concentrations were assessed using a Mercodia rat insulin ELISA kit (Mercodia AB, Sylveniusgatan 8A, SE-754 50 Uppsala, Sweden). The Coefficient of variation within assay was 3.1% and between assays was 4.4%. Absorbance at 490 nm was measured with a model EL 800 universal microplate reader (Bio-TEK instruments, INC, USA). The serum glucose concentrations were assessed using the GOD- PAP/Endpoint method with a commercial kit (Zistchemi, Tehran, Iran) according to the kit protocol. To prepare the standard curve absorbance value of the standard insulin versus the respondent concentration and absorbance value of the standard glucose versus the corresponding concentration were plotted. The following equations were used to estimate the insulin sensitivity (HOMA-IS), insulin resistance (HOMA-IR), and the beta-cell function (HOMA-β Cell Function):
HOMA-IS= 10000/fasting insulin× fasting glucose
HOMA-IR= fasting insulin× fasting glucose/405
HOMA-β cell function= 20× fasting insulin/fasting glucose−3.5 (30)
Statistical analysis
Values are shown as mean ± standard error of the mean. The data analysis was done using SPSS statistical software (SPSS Inc., Chicago, IL, U.S.A.) and one-way ANOVA followed by the Post hoc test (Tukey’s honestly significant difference) and P-values less than 0.05 were considered statistically significant.
Results
Effects of PCO induction by testosterone enanthate and resveratrol on body composition
At beginning of treatments, at 21 days of age, the body weights of animals of all groups (control, PCO-model-saline, and PCO-model-resveratrol groups) were similar statistically (P<0.05). Also, the body weights of animals of the PCO-model-saline and the PCO-model-resveratrol groups at beginning of treatment with saline and resveratrol (at 56 days of age), were similar statistically and were higher than the control group (P<0.05) (Table 2).
Table 2.
Effects of PCO induction by testosterone enanthate and resveratrol on body composition
| Groups | Control (n= 5) | PCO-model-saline (n= 5) | PCO-model-resveratrol (n= 5) |
|---|---|---|---|
| Body weight at the beginning of treatment with testosterone enanthate (g) (At 21 days of age) | 24.40±1.631 | 25.80±2.518 | 27.00±1.581 |
| Initial body weight at the beginning of treatment with resveratrol or saline (g) (At 56 days of age) | 166.8±5.543 | 201.6±7.174** | 207.0±3.647*** |
| Final body weight at the end of treatment with resveratrol or saline (g) (At 84 days of age) | 204.8± 5.044 | 205.8± 9.124 | 224.2± 5.380Ψ |
| Weights of parametrial fat depots (g)(At 84 days of age) | 3.434±0.1192 | 5.710±0.07085*** | 5.196± 0.1143***, Ψ |
| Partial weights of parametrial fat depots (%) (g) (At 84 days of age) | 1.684±0.09373 | 2.810±0.08620*** | 2.319±0.02848***, ΨΨ |
| Weights of retroperitoneal fat depots (g) (At 84 days age) | 2.134±0.07646 | 3.180±0.08854*** | 3.130±0.1242*** |
| Partial weights of retroperitoneal fat depots (%) (At 84 days age) | 1.040±0.02168 | 1.553±0.06112*** | 1.398±0.05152*** |
The data are expressed as mean±SEM. The data analysis was done using one-way ANOVA followed by the Tukey’s Post hoc test. P-values less than 0.05 were considered statistically significant. The abbreviations represent the following: Control: Normal control rats; PCO-Model-Saline: these rats received testosterone enanthate 1 mg/100 g body weight subcutaneously for 35 days once daily. Then, saline was injected into them intraperitoneally for 4 weeks once daily; PCO-Model resveratrol: these rats received testosterone enanthate 1 mg/100 g body weight subcutaneously for 35 days once daily. Then, resveratrol 10 mg/kg was injected into them intraperitoneally for 28 days once daily.
: P<0.01 vs. Control;
: P<0.001 vs. Control;
: P<0.05 vs. PCO-model-saline;
: P<0.05 vs. PCO-model-saline
In order to prove that treatment with testosterone enanthate induced PCOS in the rats of this study, the histopathological alterations in the ovarian sections of rats in the PCO-model-saline group were assessed and compared with the ovarian sections of rats in the control group. In the ovaries of rats in the PCO-model-saline group, cystic follicles and atretic follicles with no corpora lutea were seen (Figure 2A), whereas the ovaries of the control group were normal with corpora lutea and small and medium-sized antral follicles (Figure 2B). Because of the goals of the present study, data about the evaluation of the signs of PCO aren’t presented here.
Figure 2.
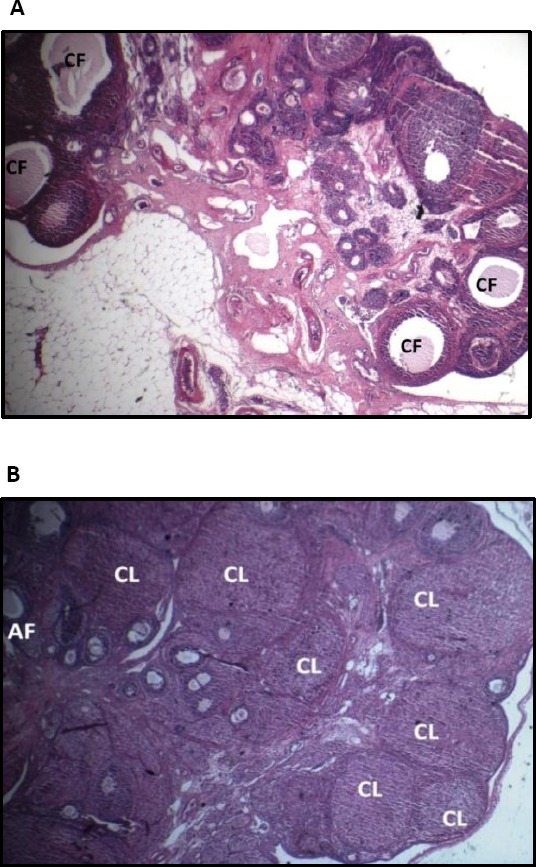
The ovarian sections of rats in the PCO-model-saline groups (A) and in the control group (B) (magnification ×40). The abbreviations represent the following: control: Normal control rats; PCO-model-saline: these rats served as models of PCOS and received testosterone enanthate 1 mg/100 g body weight subcutaneously for 35 days once daily. Then, saline was injected into them intraperitoneally for 28 days once daily. The ovaries in the control group had normal morphology with corpora lutea and antral follicles. The ovaries in PCO-model-saline group displayed cystic follicles and atretic follicles with no corpora lutea. CL: Corpus luteum; CF: Cystic follicle; AF: Antral follicle
At the end of treatments (at 84 days of age) the mean of weights of parametrial fat depots and weights of retroperitoneal fat depots and their partial weights in animals of PCO-model-saline and PCO-model-resveratrol groups were higher than the control group (P<0.001). In the PCO-model-resveratrol group, the mean of parametrial fat depots weights and partial weights of parametrial fat depots were lower than the PCO-model-saline group (P<0.0.05) (Table 2).
Effect of induction of PCOS by testosterone enanthate and resveratrol on gene expression of Tnf-α and Il-6 mRNAs in adipose tissues
In the visceral adipose tissue, mRNA expression of Tnf-α in PCO-model-saline group was higher than the control group but not significantly (P= 0.205) and mRNA expression of Tnf-α in PCO-model-resveratrol group were lower than the PCO-model-saline group (P=0.006) (Figure 3A). The relative expression levels of Il-6 mRNAs in the visceral adipose tissue of the PCO-model-saline group were increased when compared to the control group (P= 0.000) and the relative expression levels of Tnf-α mRNAs in the visceral adipose tissue of the PCO-model-resveratrol group were decreased in comparison with the PCO-model-saline group (P=0.000) (Figure 3B).
Figure 3.
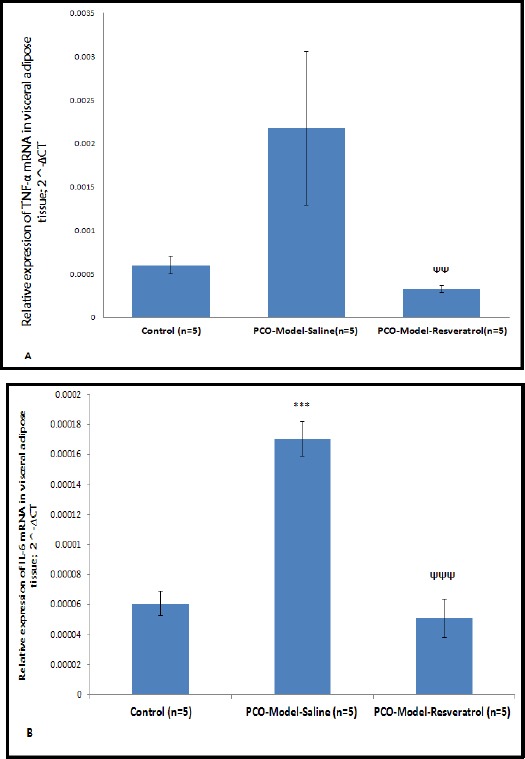
The relative expression levels of Tnf-α (A) and Il-6 (B) mRNAs in the visceral adipose tissue of three groups. The expression values were normalized to β-actin and were compared using the 2^-ΔCT method. The data are represented as means for 5 animals per group with the standard errors of the means. Statistics were by one-way ANOVA followed by the Tukey’s post hoc test. P-values less than 0.05 were considered statistically significant. ΨΨ: P<0.01 vs. the PCO-model-saline group. ***: P<0.001 vs. the control group; ΨΨΨ: P<0.001 vs. the PCO-model-saline group
As presented in Figure 4A, the relative expression levels of Tnf-α mRNAs in the subcutaneous adipose tissue of the PCO-model-saline group were increased in comparison with the control group but not significantly and the relative expression levels of Tnf-α mRNAs in the subcutaneous adipose tissue of the PCO-model-resveratrol group were lower than the PCO-model-saline group (P= 0.029) (Figure 4A). In the control, PCO-model-saline, and PCO-model-resveratrol groups, the expression of Il-6 mRNA was not different significantly (P<0.05) (Figure 4B).
Figure 4.
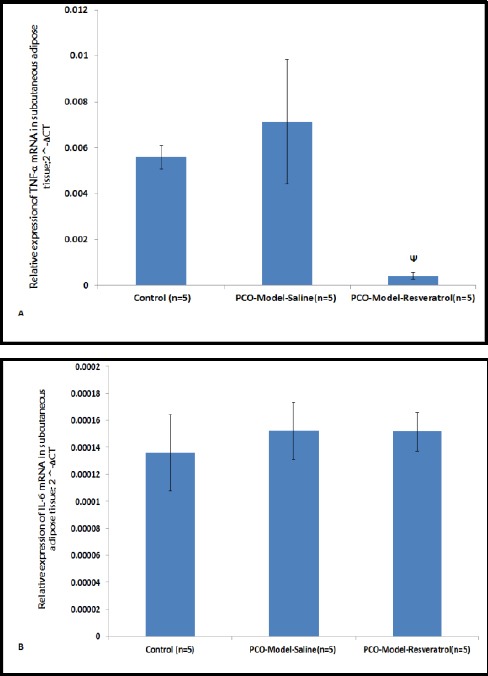
The relative expression levels of Tnf-α (A) and Il-6 (B) mRNAs in subcutaneous adipose tissues of three groups. The expression values were normalized to β-actin and were compared using the 2^-ΔCT method. The data are represented as means for 5 animals per group with the standard errors of the means. Statistics were by one-way ANOVA followed by the Tukey’s Post hoc test. P_values less than 0.05 were considered statistically significant. Ψ: P<0.05 vs. the PCO-model-saline group
Effect of induction of PCOS by testosterone enanthate and resveratrol on insulin sensitivity, β-cell function, and insulin resistance
As shown in Table 3, the mean of fasting serum glucose levels was higher in the PCO induced rats when compared with the control untreated group (P=0.008) and in PCO induced rats that were treated with resveratrol, it was decreased in comparison with PCO induced rats (P=0.011) (Table 3). Table 2 shows that in three groups, the levels of fasting serum insulin were not statistically different (P<0.05).
In the PCO-model-saline group, the insulin sensitivity (HOMA-IS) levels were found to be significantly decreased when compared to the control group (P=0.002) and in the PCO-model-resveratrol, HOMA-IS levels were lower than the PCO-model-saline group (P=0.000) (Table 3).
In the PCO-model-saline group, the mean of levels of insulin resistance (HOMA-IR) was higher than the control group (P=0.014) and in the PCO-model-resveratrol group, it was lower than the PCO-model-saline group (P=0.008) (Table 3). As presented in Table 2, β-cell function (HOMA-β cell function) levels were not different statistically between various groups (P<0.05) (Table 3).
Discussion
In the present study on a rat model of the polycystic ovary, HOMA-IR level was found to be significantly increased in comparison with the control rats. The insulin resistance observed in PCO induced rats is in agreement with previous studies that have demonstrated that PCOS is often associated with insulin resistance (31). Also, in PCO induced rats, insulin sensitivity (HOMA-IS) was decreased in comparison to the control rats. This finding is in accordance with other studies that showed that in PCOS patients, insulin sensitivity was reduced by 35%–40% and their results showed the level of such a decrease is similar to that observed in type 2 diabetes mellitus (32).
Previous studies have shown that the central body fat distribution pattern, known as visceral obesity, is frequently associated with insulin resistance (2). In our study in the PCO induced rats, the mean of parametrial and retroperitoneal visceral fat depots weights and their partial weight percents were higher than those of control rats. Our results are in agreement with findings of previous studies and this finding may be a cause for insulin resistance observed in PCOS. However, it has been reported that normoweight PCOS patients have lower subcutaneous fat in the gluteofemoral area (33). In the present study, we didn’t evaluate the subcutaneous fat distribution and more research is needed. However, in most of PCOS women insulin resistance is often independent of fatness (2).
In the present study, for the first time, we investigated whether Tnf-α and Il-6 mRNAs are produced in adipose tissues of PCO induced rats. The inflammation in PCOS may be due to PCOS condition per se or due to excess adipose tissue that is seen in PCOS patients because 52%–64% women with PCOS have abdominal obesity (34, 35).
In the present study, Il-6 mRNA expression in visceral fat of PCO induced animals was more than the levels of the visceral adipose tissue of control rats. Cytokine IL-6 inhibits lipoprotein lipase, but it doesn’t stimulate lipolysis. IL-6 secretion in adipocytes of obese individuals and diabetic patients was high and this was associated with insulin resistance (11). It has been shown that IL-6 induces intracellular expression of Socs3 mRNA and as a result, insulin signaling is inhibited (36). The Socs3 expression in adipocytes of obese diabetic animals is increased and IL-6 stimulates Socs3 expression in adipocytes (37). Some studies suggest that inflammation in PCOS is due to obesity associated with PCOS rather than PCOS condition because it has been shown that TNF α, IL-6, and markers of inflammation in obese PCOS patients didn’t have a significant difference in comparison with obese controls. Most of PCOS patients are obese and it has been demonstrated that visceral fat plays a significant role in pro-inflammation in PCOS (38). Moreover, Tnf-α mRNA level of visceral fat of PCO induced rats was higher than control animals; albeit, this difference wasn’t statistically significant. This result might be ascribed to the small number of animals that were studied. In the present study, Tnf-α and Il-6 mRNAs expression in the subcutaneous adipose tissue of PCO induced rats was not significantly different from control rats. Our finding on subcutaneous fat may be due to the low number of samples. The finding of our study was in keeping with one study that showed serum TNF-α were similar in PCOS patients and control subjects (39). However in their study IL-6 was significantly higher in insulin-resistant PCOS groups in comparison with control subjects. Our results suggest that in PCOS, the visceral adipose tissue may secret more IL-6 in comparison with subcutaneous adipose tissue. In this regards, one study showed that the levels of pro-inflammatory cytokine IL-6 were significantly increased in PCOS patients (40). Some studies suggest that insulin resistance may change milieu of monocytes from insulin-resistant subjects so that it causes an increase in production of IL-6 by these cells. IL-6 can induce hepatic production of CRP, so it can result in further inflammation (41). One study showed that fatness, not insulin resistance is responsible for serum inflammatory cardiovascular risk markers in pre-menopausal women (26). In their study serum inflammatory markers such as CRP, TNF-α, and interleukin-6 were measured. They suggested their results may be due to increase in the mass of adipose tissue. There is some evidence that demonstrated insulin resistance and hyperandrogenism may alter adipocyte function (2). Moreover, it has been indicated that TNF-α by serine phosphorylation of IRS-1 decreases insulin sensitivity. In the present study insulin sensitivity in PCO induced rats was lower than control rats. Because mRNA expression of TNF-α by visceral and subcutaneous adipose tissues in PCO induced rats of the present study were not different from control rats, the insulin resistance observed in the PCO induced rats may be due to increase in the mass of adipose tissue because relative weights of visceral tissues were increased in the PCO induced rats and as a consequent, TNF-α secretion in the PCO rats might be increased; therefore, it might be a cause for the insulin resistance observed in PCO rats. In addition to interfering with insulin signaling that leads to decreased expression of GLUT 4(42), the overproduction of TNF-α by adipose tissue via other ways may be involved in the pathogenesis of insulin resistance. TNF-α by stimulation of lipolysis in adipocytes and increasing circulating non-stratified free fatty acids may cause insulin resistance (43). Besides, TNF-α by increasing ceramide production can cause induction of insulin resistance in the muscle cells, white adipocytes, and brown adipocytes (44). In addition, ceramides can cause apoptosis of β-cells in the pancreas (45). Various studies have suggested that the activation of NADPH oxidase in the macrophages, which are resident in the adipose tissue during the development of excess fat tissue, by induction of oxidative stress can activate the nuclear factor kappa B (NFκB). NFκB is an inflammation transcription factor. This factor, in turn, activates transcription of TNF-α and IL-6 (17, 46) and these factors may finally cause inflammation and insulin resistance. In the present study, the increased activity of NADPH oxidase in PCOS may be a cause for insulin resistance observed in PCO induced animals.
In the present study, for the first time, we assessed effects of resveratrol, a natural important anti-inflammatory agent, on the pro-inflammatory genes Tnf-α and Il-6 mRNAs expression in the visceral and subcutaneous adipose tissues of PCO induced rats. Our results showed that the relative expression levels of Tnf-α and Il-6 mRNAs in the visceral adipose tissue and expression levels of Tnf-α mRNAs in the subcutaneous adipose tissue of PCO induced rats treated with resveratrol were decreased when compared to polycystic ovarian rats that didn’t receive resveratrol. Besides, our results showed that resveratrol possesses hypoglycemic effects in the PCO induced rats. It decreased levels of fasting serum glucose and insulin resistance and increased insulin sensitivity. Our findings are in accordance with previous work which has shown that in the rats with a high-fat diet, infusion of resveratrol resulted in improvement of insulin sensitivity and increase in glycogen production by the liver (47). There is some evidence that shows resveratrol may inhibit the inflammatory transcription factor NFκB signaling (48). This ability of resveratrol makes it an ideal candidate for inhibiting NFκB activities and inflammation in PCOS. It has been shown that resveratrol reduced IL-6 levels in the blood and decreased TNF-α expression in the vessel walls of diabetic rats (49). Moreover, one study by Brasnyó et al. has reported that in the diabetic patients, resveratrol improved insulin resistance and reduced blood glucose and oxidative stress levels. They reported that resveratrol by activation of Akt signaling pathway, an essential stage in insulin signaling, reduced insulin resistance (24). The mechanisms of anti-diabetic effects of resveratrol are unknown. In our PCO induced rats that received resveratrol, the mean of fasting serum insulin levels and β-cell function indices were not changed in comparison with PCO induced rats, which suggests effects of resveratrol were not due to stimulation of secretion of insulin by the pancreatic β-cells. Our findings are in agreement with one study that showed the anti-hyperglycemic effect of resveratrol is not through insulin (50). Resveratrol may decrease the release of glucose by the liver or may increase glucose usage by the various tissues by potentiation of insulin signaling or by direct effects (51).
In the present study in the subcutaneous adipose tissue of the PCO-model-resveratrol group, the relative expression levels of Tnf-α mRNAs also were lower than those of the PCO-model-saline, but the expression levels of Il-6 mRNAs were not different. The special site for the effects of resveratrol is not clear. As we mentioned above, resveratrol is an NFκB signaling inhibitor and effects of resveratrol may be due to inhibition of NFκB activity. Moreover, resveratrol is a powerful antioxidant and it may scavenge reactive oxygen species (50). In PCOS, hyperglycemia may cause induction of oxidative stress and inflammatory response independently from obesity. In PCOS patients, expression of P47phox, which is a cytosolic part of NADPH oxidase, is high. The oxidation of NADPH by NADPH oxidase via production of reactive oxygen species (ROS) may induce oxidative stress in PCO induced rats (52). Because oxidative stress can activate NFκB as an inflammatory transcription factor that may stimulate transcription of TNF-α and IL-6, and because resveratrol is an antioxidant agent, the effects of resveratrol in our study may be due to its antioxidant properties. In this regards, it has been shown that in rats with diabetes induced by streptozotocin, resveratrol reduced oxidative stress and improved disorders related to diabetes (53, 54). Our findings are in agreement with previous studies that show inflammation mediators such as IL-6 and TNF-α may be involved in insulin resistance in PCOS. In PCOS, hyperandrogenemia may pre-activate mononuclear cells and cause induction of inflammation by hyper-glycemia or in an opposite manner, inflammation, that is induced by increase in the blood glucose level, causes more production of androgens by ovaries (8). The strategies to improve inflammation can be useful in the treatment of PCOS and metabolic disorders associated with it and our results showed that resveratrol can be useful in the management of PCOS. Further investigations are needed with larger samples of animals and humans to clarify the effects of resveratrol on the IL-6 and TNF-α levels and inflammation in PCOS.
Conclusion
To sum up, the present study was performed in order to shed light on the causes that can explain the insulin resistance in PCOS and the expression of Tnf-α and Il-6 mRNAs in adipose tissues of polycystic ovary induced rats. The novel findings of this study are in accordance with previous studies suggesting that visceral adipose tissue may play a more important role in pro-inflammation in PCOS. Also, this evidence supports that resveratrol as an anti-inflammatory and anti-hyperglycemic agent may decrease the risk of diabetes in the PCOS patients by reduction of expression of pro-inflammatory cytokines TNF-α and IL-6 and it can be considered as an important agent in research on PCOS and treatment of PCOS patients. Due to differences between humans and animal models more research is needed.
Acknowledgment
The results presented in this paper were part of a Ph.D. student thesis. This study was supported by Shahid Beheshti University, Tehran, Iran.
Conflicts of interest
The authors declare that no conflict of interest exists.
References
- 1.Johansson J, Stener-Victorin E. Polycystic ovary syndrome: effect and mechanisms of acupuncture for ovulation induction. Evid Based Complement Alternat Med. 2013;2013:762615. doi: 10.1155/2013/762615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sathyapalan T, Atkin SL. Mediators of inflammation in polycystic ovary syndrome in relation to adiposity. Mediat Inflamm. 2010;2010:758654. doi: 10.1155/2010/758656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 4.Desai V, Prasad NR, Manohar SM, Sachan A, Narasimha SRPVL, Bitla ARR. Oxidative stress in non-obese women with polycystic ovarian syndrome. J Clin Diagn Res. 2014;8:CC01–CC03. doi: 10.7860/JCDR/2014/8125.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95:1048–1058. e1–2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med. 2014;2014:719050. doi: 10.1155/2014/719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong Y-l, Liang X-y, Yang X, Li Y, Wei L-n. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Euro J Ob Gyn Repro Biol. 2011;159:148–150. doi: 10.1016/j.ejogrb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 8.González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77:300–305. doi: 10.1016/j.steroids.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahorska-Markiewicz B, Janowska J, Olszanecka-Glinianowicz M, Zurakowski A. Serum concentrations of TNF-[alpha] and soluble TNF-[alpha] receptors in obesity. Int J Obes Relat Metab Disord. 2000;24:1392–1395. doi: 10.1038/sj.ijo.0801398. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann C, Lorenz K, Braithwaite S, Colca J, Palazuk B, Hotamisligil G, et al. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994;134:264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- 11.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 12.Mohlig M, Spranger J, Osterhoff M, Ristow M, Pfeiffer A, Schill T, et al. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol. 2004;150:525–532. doi: 10.1530/eje.0.1500525. [DOI] [PubMed] [Google Scholar]
- 13.Lin YS, Tsai SJ, Lin MW, Yang CT, Huang MF, Wu MH. Interleukin-6 as an early chronic inflammatory marker in polycystic ovary Syndrome with insulin receptor substrate-2 polymorphism. Am J Reprod Immunol. 2011;66:527–533. doi: 10.1111/j.1600-0897.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- 14.Escobar-Morreale HF, Calvo RM, Villuendas G, Sancho J, Millán JL. Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obes Res. 2003;11:987–996. doi: 10.1038/oby.2003.136. [DOI] [PubMed] [Google Scholar]
- 15.González F, Nair KS, Daniels JK, Basal E, Schimke JM, Blair HE. Hyperandrogenism sensitizes leukocytes to hyperglycemia to promote oxidative stress in lean reproductive-age women. J Clin Endocrinol Metab. 2012;97:2836–2843. doi: 10.1210/jc.2012-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franckhauser S, Elias I, Sopasakis VR, Ferre T, Nagaev I, Andersson CX, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. 2008;51:1306–1316. doi: 10.1007/s00125-008-0998-8. [DOI] [PubMed] [Google Scholar]
- 17.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, et al. Insulin/IGF-1 and TNF-αstimulate phosphorylation of IRS-1 at inhibitory Ser 307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes AG, Mesa JL, Neill BA, Chung J, Carey AL, Steinberg GR, et al. Prolonged interleukin-6 administration enhances glucose tolerance and increases skeletal muscle PPARαand UCP2 expression in rats. J Endocrinol. 2008;198:367–374. doi: 10.1677/JOE-08-0113. [DOI] [PubMed] [Google Scholar]
- 19.Ciaraldi TP, el-Roeiy A, Madar Z, Reichart D, Olefsky JM, Yen S. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab. 1992;75:577–583. doi: 10.1210/jcem.75.2.1322430. [DOI] [PubMed] [Google Scholar]
- 20.Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS) Am J Physiol Endocrinol Metab. 2001;281:E392–E399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- 21.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 22.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 23.Tanti J-F, Gremeaux T, Van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 24.Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–441. doi: 10.1016/s0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 26.Escobar-Morreale H, Villuendas G, Botella-Carretero J, Sancho J, San Millan J. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003;46:625–633. doi: 10.1007/s00125-003-1090-z. [DOI] [PubMed] [Google Scholar]
- 27.Beloosesky R, Gold R, Almog B, Sasson R, Dantes A, Land-Bracha A, et al. Induction of polycystic ovary by testosterone in immature female rats: modulation of apoptosis and attenuation of glucose/insulin ratio. Int J Mol Med. 2004;14:207–216. doi: 10.3892/ijmm.14.2.207. [DOI] [PubMed] [Google Scholar]
- 28.Ergenoglu M, Yildirim N, Yildirim AGS, Yeniel O, Erbas O, Yavasoglu A, et al. Effects of resveratrol on ovarian morphology, plasma anti-mullerian hormone, IGF-1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod Sci. 2015;22:942–947. doi: 10.1177/1933719115570900. [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Aref A-BM, Ahmed OM, Ali LA, Semmler M. Maternal rat diabetes mellitus deleteriously affects insulin sensitivity and Beta-cell function in the offspring. J Diabetes Res. 2013:2013. doi: 10.1155/2013/429154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. Clin Endocrinol Metab. 2006;91:336–340. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 32.O'Driscoll J, Mamtora H, Higginson J, Pollock A, Kane J, Anderson D. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol (Oxf) 1994;41:231–236. doi: 10.1111/j.1365-2265.1994.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 33.Horejsi R, Möller R, Rackl S, Giuliani A, Freytag U, Crailsheim K, et al. Android subcutaneous adipose tissue topography in lean and obese women suffering from PCOS: comparison with type 2 diabetic women. Am J Phys Anthropol. 2004;124:275–281. doi: 10.1002/ajpa.10364. [DOI] [PubMed] [Google Scholar]
- 34.Goodarzi MO, Korenman SG. The importance of insulin resistance in polycystic ovary syndrome. Fertil Steril. 2003;80:255–258. doi: 10.1016/s0015-0282(03)00734-9. [DOI] [PubMed] [Google Scholar]
- 35.ESHRE TR Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose Expression of Tumor Necrosis Factor- : Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 37.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 38.Orio Jr F, Palomba S, Cascella T, Milan G, Mioni R, Pagano C, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2619–2623. doi: 10.1210/jc.2002-022033. [DOI] [PubMed] [Google Scholar]
- 39.Fulghesu AM, Sanna F, Uda S, Magnini R, Portoghese E, Batetta B. IL-6 serum levels and production is related to an altered immune response in polycystic ovary syndrome girls with insulin resistance. Mediators Inflamm. 2011;2011:389317. doi: 10.1155/2011/389317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heutling D, Schulz H, Nickel I, Kleinstein J, Kaltwasser P, Krzyzanowska K, et al. Endothelial, inflammatory and endocrine markers in women with PCOS before and after metformin treatment. Exp Clin Endocrinol Diabetes. 2006;114:15–195. [Google Scholar]
- 41.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens J, Pekala P. Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulations is coordinate and independent of protein synthesis. J Biol Chem. 1992;267:13580–13584. [PubMed] [Google Scholar]
- 43.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 44.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-αin brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 45.Shimabukuro M, Zhou Y-T, Levi M, Unger RH. Fatty acid-induced βcell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes PJ, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 47.Côté CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M, et al. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med. 2015;21:498–505. doi: 10.1038/nm.3821. [DOI] [PubMed] [Google Scholar]
- 48.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 49.Zheng X, Zhu S, Chang S, Cao Y, Dong J, Li J, et al. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur J Pharmacol. 2013;720:147–157. [PubMed] [Google Scholar]
- 50.Su H-C, Hung L-M, Chen J-K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 51.Soufi FG, Mohammad-Nejad D, Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress–nuclear factor κB–apoptosis pathway. Pharmacol Rep. 2012;64:1505–1514. doi: 10.1016/s1734-1140(12)70948-9. [DOI] [PubMed] [Google Scholar]
- 52.Chanock SJ, El Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;270:24519–2422. [PubMed] [Google Scholar]
- 53.Sharma S, Anjaneyulu M, Kulkarni S, Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology. 2006;76:69–75. doi: 10.1159/000089720. [DOI] [PubMed] [Google Scholar]
- 54.Kumar A, Kaundal RK, Iyer S, Sharma SS. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007;8013:1236–1244. doi: 10.1016/j.lfs.2006.12.036. [DOI] [PubMed] [Google Scholar]


