Abstract
Objective(s):
Multidrug resistance (MDR) is a major obstacle in the successful chemotherapy of ovarian cancer. Inhibition of P-glycoprotein (P-gp), a member of ATP-binding cassette (ABC) transporters, is a well-known strategy to overcome MDR in cancer. The aim of this study was to investigate the efficiency and ability of CRISPR/Cas9 genome editing technology to knockdown ABCB1 gene expression in adriamycin resistant (A2780/ADR) ovarian cancer cell line and evaluate the sensitivity changes to doxorubicin.
Materials and Methods:
Three single-guide RNAs (sgRNAs) targeting the fourth and fifth exons of human ABCB1 gene were designed in this study. Expression level of ABCB1 was detected using quantitative real time PCR (qRT-PCR) after co-transfection of all three sgRNAs into A2780/ADR cell line and subsequent antibiotic selection. Drug sensitivity to doxorubicin was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Results:
The results showed that CRISPR/Cas9 system could significantly reduce the expression of P-gp. The dramatic decline in ABCB1 gene expression was associated with increased sensitivity of cells transfected with sgRNAs to doxorubicin.
Conclusion:
Based on the results of this study, it is concluded that the CRISPR-based systems, used in the present study, effectively down-regulated the target gene and acted as an ideal and cost-effective tool for gene editing of A2780/ADR cell line resulting in restoration of nonmalignant phenotype.
Keywords: CRISPR, Doxorubicin, Drug resistance, Ovarian cancer, P-glycoprotein
Introduction
Epithelial ovarian cancer is the sixth most common cancer and most lethal gynecological malignancy among women (1, 2). Due to the location of the ovaries within the pelvis, ovarian cancer does not represent specific symptoms in the early stages. Therefore, two thirds of the patients are diagnosed at the advanced stages and 5-year survival rate is estimated to be less than 25% (3–5). Surgery and chemotherapy are recommended as initial treatment and taxane therapy with a platinum analog is the backbone of chemotherapy (6, 7). Despite these strategies, recurrence often occurs within 2 years. These relapsing tumors often exhibit chemo-resistance towards several anti-cancer agents with various structures and mechanisms of action (3, 8). MDR in cancer is generally the result of increased expression of membrane efflux proteins which belong to the ATP-binding cassette (ABC) transporters superfamily. Consequently, cancer cells actively transfer a wide variety of pharmaceutical compounds, including chemotherapeutic agents out of the cells, resulting in reduced intracellular drug accumulation in resistant cells (9). Classical multidrug resistance to chemotherapeutic agents is attributed to the overexpression of ABCB1 gene (known as P-glycoprotein (P-gp) or MDR1) (10). It has been documented that overexpression of P-gp is the key factor for reduced chemo-sensitivity in a wide range of tumors, including ovarian cancer (11, 12). Couple of studies have indicated that several signal transduction pathways are involved in dysregulation of ABCB1 gene. According to this, it seems that targeting ABCB1, as a downstream object in multiple pathways, is the ideal choice to restore drug sensitivity in MDR cancer cells (9,13). Over the past decade, a revolutionary technology named genome editing has been developed that provides unique opportunities for researchers to manipulate genome in a wide range of cell types and organisms. This new approach is based on the application of engineered chimeric nucleases that consist of sequence-specific DNA-binding domains that fuse to a nonspecific DNA cleavage module (nuclease) (14). These tools include zinc-finger nucleases (ZFNs), transcription-activator-like effector nucleases (TALENs) and most recently clustered regularly-interspaced short palindromic repeats (CRISPR)-Cas9 system. In comparison to the ZFNs and TALENs technologies that require protein–DNA interactions to recognize the target sequence, locus targeting by CRISPR/Cas9 system acts through RNA-DNA interactions and since the synthesis of RNA and its entry into the cell is easier than protein domains, designing and reprogramming CRISPR/Cas9 are simplified (15). Desired genetic modifications could be achieved by introducing a double-strand break (DSB) at a specific target sequence followed by stimulation of cellular DNA repair pathways, including error-prone non-homologous end joining (NHEJ) and the homologous recombination (HR) (14). Following triggering the NHEJ pathway, the expression of the target gene can be interrupted by frameshift mutations in the coding regions (16). Since the overexpression of P-gp is found in primary and metastatic ovarian cancer cells and is considered as a major obstacle to chemotherapy (17), the aim of the present study was to investigate the potential application of CRISPR/Cas9 system, as a novel genome-editing tool, to knockdown ABCB1 gene expression in adriamycin resistant cell line A2780/ADR in order to reinforce sensitivity to doxorubicin.
Materials and Methods
Cell culture
Adriamycin resistant (A2780/ADR) ovarian cancer cell line was obtained from European Collection of Authenticated Cell Cultures (ECACC), No. 93112520, UK. This cell line was cultured in RPMI 1640 medium (Sigma-Aldrich, Germany) supplemented with 10% FBS (Gibco, UK), 100 µg/ml streptomycin and 100 U/ml penicillin (Sigma-Aldrich, Germany) and was incubated in a 5% CO2 atmosphere at 37°C. Based on the recommendations of the Cell Bank (ECACC) to maintain high P-gp levels, 10μM Doxorubicin (Tedec-Meiji Farma, S.A, Spain) was added to the culture medium once a week, usually after each passage.
Design and construction of sgRNA/Cas9 expression vectors
Three custom-designed sgRNA/Cas9 expression vectors, targeting the fourth and fifth exons of ABCB1 gene (Accession No NM_000927.3), were designed using CRISPR DESIGN (http://crispr.mit.edu/) tool and were then synthesized by Guangzhou FulenGen Company (P R China). Moreover, a scramble (null) vector was designed as the negative control. After transformation of the constructs into the Escherichia coli TOP10 as the host and plasmid extraction to confirm the integrity of the amplified plasmids, digestion was performed with EcoRI and BamHI restriction enzymes.
Transfection of cells
A total number of 6×105 A2780/ADR cells at passage 3 or 4 (after thawing of the cells) were transfected with 2.5 μg of all three Cas9-sgRNA plasmids (HCP213100-CG01-3a, HCP213100-CG01-3b and HCP213100-CG01-3c) using Lipofectamine® 2000 reagent and Opti-MEM I reduced serum medium (Invitrogen Life Technologies) following the manufacturer’s instructions. Furthermore, scramble vector was transfected into the cells as the negative control. Cells were treated with 600 μg/ml G418 (Neomycine-Roche Life Science, Penzberg, Upper Bavaria, Germany) for three days, 48 hr after being transfected, to remove the untransfected cells and selection of the resistant cells.
Genomic DNA extraction and Surveyor mismatch cleavage assay
Genomic DNA was extracted from transfected and control cells using PrimePrep TM Genomic DNA Isolation Kit (GenetBioInc, Korea). Three target sites of sgRNA were amplified by Pfu DNA Polymerase (Maxcell, Iran) and using specific primers (designed with Primer3web version 4) complementary to the upstream and downstream of these regions, including F: 5´-GGTGTCTTGGACTAGGTTGG-3´ and R: 5´-TCTGCTGGCACTTCAGTTG-3´ as well as F: 5´-CTTTAGTGGGATCTTGGAGTG-3´ and R: 5´-TTTGGCTGCTTTCATTGTCA-3´, to target sites located on fourth and fifth exons, respectively. The PCR products were then purified using Prime Prep PCR Purification Kit (GenetBioInc, Korea). Surveyor mismatch cleavage assay (Transgenomic SURVEYOR mutation detection kit for standard gel electrophoresis) was used to scan indel mutations induced by Cas9 nuclease. To generate heteroduplex pairs, equal amounts (400 ng) of mutant and wild-type purified PCR products were mixed with 1x PCR buffer (Bioron-Rheinhorststra, Ludwigshafen, German) and water to a final volume of 20 µl. The mixture was denatured and reannealed in a thermocycler (95°C, 10 min; 95-85°C at -2°C/sec; 85-25°C at -0.1°C/sec; hold at 4°C) (18). Subsequently, 1 µl of both the surveyor nuclease and surveyor enhancer were added to the hybridized PCR products to a total volume of 20 µl in separated nuclease-free 0.2 ml tubes (on ice). The reaction mixture was incubated at 42°C for 20 min after being gently mixed and was stopped by adding 2 µl of the stop solution. Finally, the digested products were separated on a 2.5% agarose gel electrophoresis.
RNA isolation and SYBR-based qRT-PCR analysis
Total RNA was obtained using the RNX-Plus (SinaClon Co Iran) kit. DNase I treatment of RNA (Qiagen, Germany) was subsequently performe toremove contaminatingDNA. For this purpose, RNA samples were mixed with distilled water, buffer as well as DNase I and were incubated at 37°C for 30 min. In order to inactivate DNaseI, EDTA was added to the digestion reactions to chelate ions in the digestion buffer. The mixtures were then incubated at 65°C for 5 min. PrimeScript RT reagent Kit (TAKARA Bio Inc. Japan) was used to synthesize cDNA using 50 ng of total RNA at a volume of 10 μl and RealQ Plus 2x master mix green (Ampliqon, Denmark) was applied for real-time qPCR following the manufacture’s protocols using an ABI StepOnePlus™ Real-Time system (Applied Biosystems, USA). The details of the sequence-specific primers are illustrated in Table 1. Since no suitable reference gene has been identified in gynecological cancer cell lines, including human ovarian cancer (19, 20), seven housekeeping genes, including GAPDH, β-actin, β2M, 18SrRNA, SDHA, PPIA and RPS13, were selected in this study for identification of the most stable reference gene to normalize the target gene. The amplification efficiency was determined by serial dilutions. Target amplification efficiency was equal to that of the reference genes. Gene expression was analyzed using the relative quantification (RQ) method by standard formula: 2-ΔΔCT (16).
Table 1.
Details of oligonucleotide sequences used for Q-PCR analysis
| Gene | Accession No | Forward and Reverse primers | Product size (bp) |
|---|---|---|---|
| ABCB1 | NM_000927.3 | GAAAAGAAACCAACTGTCAGTG | 249 |
| CATGTCTTCCTCCAGATTCATG | |||
| GAPDH | NM_002046.2 | GTCCACTGGCGTCTTCAC | 161 |
| AGGCATTGCTGATGATCTTGA | |||
| β-Actin | NM_001101.2 | GAAGATCAAGATCATTGCTCCT | 168 |
| AAGTCATAGTCCGCCTAGAAG | |||
| β2M | NM_004048.2 | AGGACTGGTCTTTCTATCTCTT | 156 |
| CGGCATCTTCAAACCTCCAT | |||
| 18S rRNA | NR_003286.2 | TAGTCGCCGTGCCTACCA | 102 |
| TGCTGCCTTCCTTGGATGT | |||
| SDHA | NM_004168 | GCGAAAGGTTTATGGAGCGA | 121 |
| TGATCTTTCTCAGGGCCACA | |||
| PPIA | NM_021130 | CCTGGTGGTGCATGCCTAGT | 71 |
| CTCACTCTAGGCTCAAGCAATCC | |||
| RPS13 | NM_001017 | ACATCTGACGACGTGAAGGAG | 121 |
| TGCCTGTCACAAAACGTACTTG |
Cell viability assay
Cells transfected with Scramble and sgRNA vectors as well as the untransfected control cells were plated into 96-well plates after antibiotic selection (1×104 cells/well) and were subsequently exposed to increasing concentrations of doxorubicin (0.2, 1, 5, 25 µM) while being incubated at 37°C for 48 hr (21). Following this 48 hr incubation period, 20µl of colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, USA) was added to each well. The supernatant was gently removed after 4 hr incubation at 37°C and replaced with 150 μl dimethylsulfoxide (DMSO; Sigma- Aldrich, USA) (21). Plates were placed on a mechanical plate shaker for 10 min prior to measuring the absorbance of each well at a wavelength of 490 nm using a spectrophotometer (PG Instrument T80, PG Instrument, England). The cell viability rate was normalized by the following formula (absorbance of experimental group/absorbance of control group) ×100. All experiments were performed in triplicate.
Statistical analysis
Data were analyzed using IBM SPSS 20 (IBM, NY, USA) statistics program. The ANOVA was used to determine differences of ABCB1 expression between the groups. Two-way ANOVA was also used to determine the differences of MTT assay between the groups. A P-value of <0.001 was considered statistically significant. All data were presented as the mean ± standard error (SE).
Results
ABCB1 knockdown by CRISPR/Cas9
To knockdown ABCB1 gene, 3 sgRNAs targeting human ABCB1 gene were selected. The sgRNA sequences, in all the vectors, were regulated by U6 promoter. Cas9 was regulated by CMV and T7 promoters. Furthermore, mCherry, a red fluorescent protein, was regulated by SV40 promoter (Figure 1-A). Two of the target sequences were located on the sense strands of the fourth and fifth exons, respectively (5’-TGACAAGTTGTATATGGTGG-3’ & 5’-CTAGGTGATATCAATGATAC-3’) and one was located on the antisense strand of the forth exon (5’-CCAAACACCAGCATCATGAG-3’) (Figure 1-B). A2780/ADR cells were transfected with all three vectors carrying the sgRNAs/Cas9, simultaneously (Figure 2), scrambled transfected cells were kept as control. Treatment with G418 was adopted in order to select transfected cells.
Figure 1.
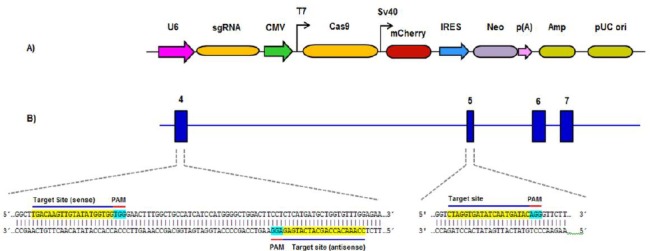
The linear map of CRISPR/Cas9 vector (A) and the locations and sequences of the three sgRNAs targeting the fourth and fifth exons of ABCB1 gene (B)
Figure 2.
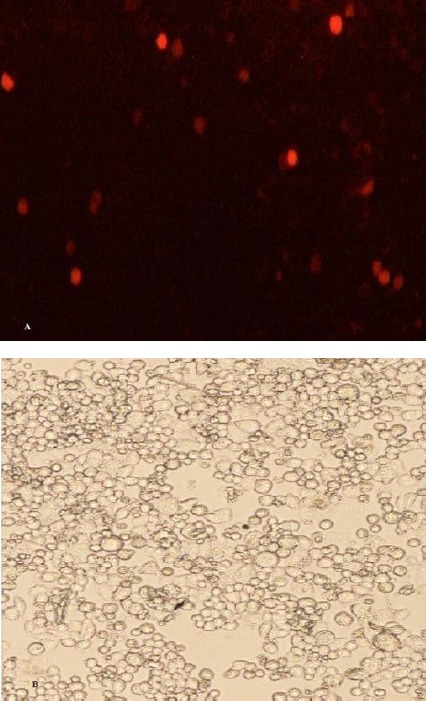
A2780/ADR cells transfected with all 3 CRISPR plasmids. The expression of mCherry (red fluorescent protein) in the cells was examined 72 hr post-transfection. Images were acquired using a fluorescent microscope (A) and a light microscope (B)
Cells were harvested, total RNA was extracted and ABCB1 gene expression levels were evaluated, 72 hr post-treatment.
ABCB1 gene expression in knockdown cells
Among the candidate reference genes, expression of GAPDH and β-Actin showed minor variation across the samples. Therefore, expression level of ABCB1, as the target gene, was normalized to an average of both reference genes (GAPDH/β-Actin). As shown in Figure 3, ABCB1 gene expression was considerably downregulated (fold change 0.001) in cells transfected with sgRNAs ABCB1 compared to that in the cells transfected with scramble vector and untransfected control cells (***, P<0.001).
Figure 3.
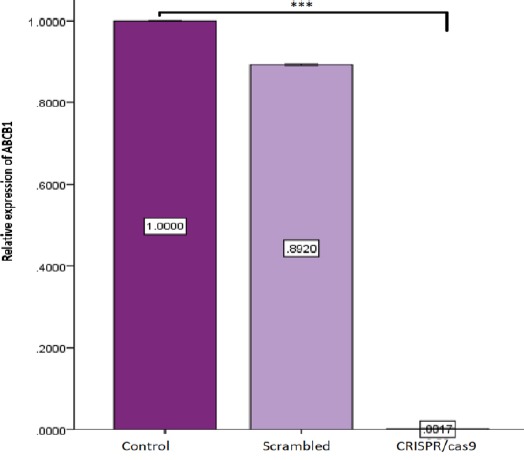
Real-time Q-PCR analysis of expression of ABCB1. The mRNA levels of ABCB1 were analyzed by real-time PCR, 72 hr after antibiotic selection. Expression levels of ABCB1 were normalized to an average of two reference genes, GAPDH and β-Actin. A2780/ADR cells transfected with CRISPR/Cas9 showed a remarkable decrease in ABCB1 expression level compared to the control and scramble groups; bars: SE (***, P< 0.001)
Insertion/deletion (indel) scanning using Surveyor mismatch cleavage assay
To assess indel generated by CRISPR/Cas9 system, A2780/ADR cells were transfected with individual sgRNAs. Untransfected cells were removed by neomycin treatment, genomic DNA was extracted, genomic regions containing sgRNA target sites were amplified, and finally Surveyor mismatch cleavage assay was performed. As shown in Figure 4, the predicted indel sites were cleaved by Surveyor nuclease and generated several bands compared to the control target site. These DNA fragments illustrated the existence of indels in target sites as well as confirming the efficiency of CRISPR/Cas9 system.
Figure 4.
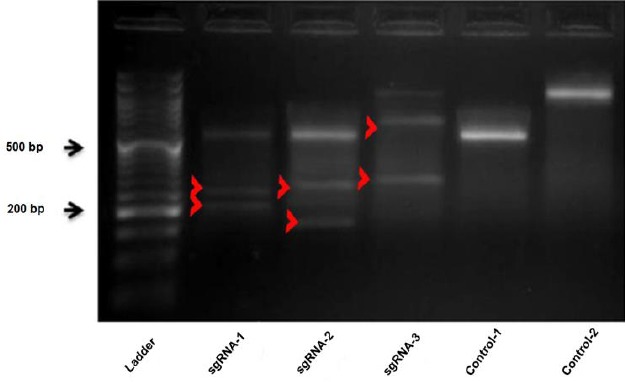
Detection of indels generated by CRISPR/Cas9 using surveyor assay. Red arrowheads indicate predicted Cas9 cutting sites of ABCB1 PCR products., sgRNA-1: PCR product size: 510 bp, predicted cleavage size: 220 & 290 bp, sgRNA-2: PCR product size: 510 bp, predicted cleavage size: 170 & 340 bp, sgRNA-3: PCR product size: 952 bp: Predicted cleavage size: 335 & 617 bp, Control-1: 510 bp, Control-2: 952 bp
Sensitivity to doxorubicin in ABCB1 knockdown cells
Reinforcement of sensitivity to doxorubicin was monitored by MTT assay using transfected A2780/ADR, seventy-two hr post-antibiotic selection. The results clearly showed that down-regulation of P-gp in cells transfected with CRISPR/Cas9 constructs was associated with a significant increase in sensitivity to doxorubicin compared to the control cells. As the percentage of viable cells transfected with CRISPR/Cas9 was less than 50% of that of the control group, 48 hr post-treatment with various concentrations of doxorubicin (0.2, 1, 5, 25 µM) (***, P<0.001) (Figure 5).
Figure 5.
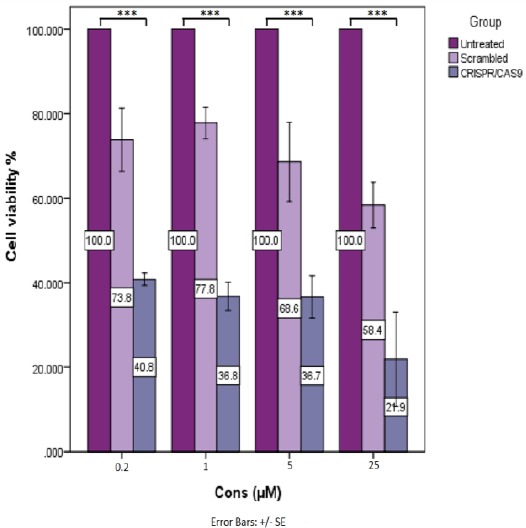
Doxorubicin chemosensitivity. MTT assay was performed to evaluate A2780/ADR cells viability, transfected and untransfected cells (control), 48 hr post-treatment with increasing concentrations of doxorubicin. The optical density of each well was measured with a microplate spectrophotometer at 490 nm. The viability of cells transfected with CRISPR/Cas9 was dramatically decreased compared to the control and scrambled transfected cells. In all groups, the proliferation rate of the cells was decreased with increasing concentrations of doxorubicin. The greatest decrease in proliferation rate was observed in A2780/ADR cells transfected with CRISPR/Cas9 compared to other two groups. Each condition was repeated three times (***, P<0.001)
Discussion
Genome editing tools, including ZFNs, TALENs and CRISPR/cas9, are considered as robust approaches to manipulate targeted genomes in a wide range of cell types and organisms (14). Compared to custom-designed nucleases, including ZNF and TALEN, RNA-guided DNA endonucleases from the type II CRISPR system are more efficient, flexible and less costly (14). The ability of multiplex genome editing via introduction of multiple sgRNAs into a single CRISPR array and its convenient design indicates that CRISPR technology is easily programmable and widely applicable as a gene editing tool. Due to these advantages, CRISPR/Cas-mediated gene therapy has created more hopes for possible editing and amendments of the gene mutations involved in human disease as well as targeted disruption of disease-relevant genes (22, 23). Furthermore, some studies have clearly demonstrated the ability of CRISPR/Cas systems to regulate gene expression via various mechanisms (24). Overexpression of one or more ATP-binding cassette (ABC) drug transporters is a common phenomenon in cancer cells which has been associated with MDR in solid tumors and is responsible for chemo-resistance phenotype in cancers. Several studies have tried to investigate the relevance of P-gp expression in various tumors osteosarcoma cell lines for example, but the results remain controversial (25, 26). A previous study showed there was no correlation between MDR1 mRNA expression and disease progression in patients with osteosarcoma (27). However, another study found positive immuno-staining for P-gp is an independent risk factor for a poor outcome of osteosracoma patients (28). In ovarian cancer, up-regulation of ABCB1/P-gp, a member of the ABC transporter family, is strongly associated with poor response to the routinely prescribed chemotherapeutic agents (9, 29). Simoff et al. (2016) have reported complete knockout of endogenous ABCB1 in MDCK cells by CRISPR/Cas9 system (30). Many groups have used CRISPR/Cas9 system for precisely and rapidly engineering both loss-of-function and gain-of-function mutations in tumor-suppressor genes, oncogenes and other regulator genes of cellular transformation or drug response. However, the CRISPR/Cas system to knockout ABCB1 gene expression in adriamycin resistant cell line A2780/ADR is yet to be investigated. Accordingly, drug-resistant ovarian cancer cell line A2780/ADR was selected, in the present study, as the target cells with increased P-gp expression. CRISPR/Cas9 gene editing system was used to downregulate ABCB1 gene expression and its consequences were evaluated. CRISPR/Cas9 system, via creating DSB upstream of the protospacer adjacent motif (PAM) sequence within the selected exons and activating NHEJ repair pathway, results in imposing small insertions or deletions (indels) around the DSB. Small indels in target sites may induce frameshift mutations and premature stop codons, leading to expression of a truncated protein of target gene (31). In the present study, CRISPR/Cas9 efficiency to create indels in target sites was confirmed by Surveyor nuclease assay. Real-time PCR data revealed that ABCB1 gene expression level was dramatically down-regulated as the result of indel mutation. Subsequently, down-regulation of ABCB1 led to increased doxorubicin drug sensitivity in A2780/ADR cancer cells. MTT assay was used to assess viability of CRISPR/Cas9 treated cells in the presence of various concentrations of doxorubicin. The results of MTT assay confirmed a≥50 % re-sensitization to doxorubicin in cells transfected with CRISPR/Cas9 against P-gp compared to the control cells. These results are consistent with the findings of the recent studies conducted by Yang et al. and Ha et al (26, 32).
Activation of a variety of signaling pathways, including FOXO3a, PI3K/Akt, NF-κB, extracellular signal-regulated kinase (ERK) as well as HSP27 depletion pathways, are involved in up-regulation of ABC transporters (33). Therefore, targeting this efflux pump, as a downstream target in multiple signal transduction pathways, is one of the most direct routes to repress up-regulated ABCB1 and overcome the MDR phenotype in cancer cells. Over the past three decades, considerable variety of ABCB1 inhibitors have been emerged; however, none of them have been approved for clinical use. The main drawback of these ABCB1 inhibitors was non-specific toxicity as well as toxic interactions between the inhibitor and chemotherapeutic agents used for treatment (10, 34). Nevertheless, genetic tools to combat drug resistance in cancers presented promising approaches with desirable targeted silencing. In the field of post-transcriptional gene silencing, RNAi is considered as a leading tool for targeted gene knockdown; however, delivery limitations, low stability in the blood stream, unpredictable off-target effects and finally immune stimulation are the main limiting factors in the field of therapeutic applications (14, 35, 36). Fortunately, development of Cas9-based tools has provided a suitable tool for precise modification of the target sites in the desired gene (37). In this study, complete sensitivity to doxorubicin did not occur despite the dramatic reduction in expression of P-gp. To enhance the outcome of drug sensitivity, it is suggested that multiple sgRNAs be designed and experimentally evaluated for simultaneous targeting of multiple important transporters.
Conclusion
The present study assessed the potential of CRISPR/cas9 to reverse MDR in adriamycin resistant ovarian cancer cell line. The results of this study introduce CRISPR/cas9 technology as an innovative and robust strategy to manipulate the genome in order to achieve the desired objectives. However, further studies are required to investigate the rate of off-target effects especially in new variants of CRISPR/Cas systems as alternatives to wild type as well as the capability of multiplexing sgRNAs to generate stronger results.
Acknowledgment
This study was partly supported by grants (No 656) from Cellular and Molecular Research Center of Qazvin University of Medical Sciences, Qazvin, Iran. We are grateful to Department of Genetics and Molecular Biology as well as Regenerative Medicine Center of Isfahan University of Medical Sciences, Isfahan, Iran for their kind cooperation during this research. The results described in this paper were part of student thesis.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pract Res Clin Obster. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Beaufort CM, Helmijr JC, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N, et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014;9:e103988. doi: 10.1371/journal.pone.0103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi A, Yokoyama Y, Osawa Y, Miura R, Mizunuma H. Gene therapy for ovarian cancer using carbonyl reductase 1 DNA with a polyamidoamine dendrimer in mouse models. Cancer Gene Ther. 2016;23:24–28. doi: 10.1038/cgt.2015.61. [DOI] [PubMed] [Google Scholar]
- 4.Cho KR, Shihle M. Ovarian Cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinartz S, Finkernage F, Adhikary T, Rohnalter V, Schumann T, Schober Y, et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol. 2016;17:108. doi: 10.1186/s13059-016-0956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res. 2013;19:961–968. doi: 10.1158/1078-0432.CCR-12-2243. [DOI] [PubMed] [Google Scholar]
- 7.Markman M. Current standards of care for chemotherapy of optimally cytoreduced advanced epithelial ovarian cancer. Gynecol Oncol. 2013;131:241–245. doi: 10.1016/j.ygyno.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–316. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- 9.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1:93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets. 2013;13:326–346. doi: 10.2174/15680096113139990076. [DOI] [PubMed] [Google Scholar]
- 11.Follit CA, Brewer FK, Wise JG, Vogel PD. In silico identified targeted inhibitors of P-glycoprotein overcome multidrug resistance in human cancer cells in culture. Pharmacol Res Perspect. 2015;3:e00170. doi: 10.1002/prp2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 13.Sui H, Fan ZZ, Li Q. Signal transduction pathways and transcriptional mechanisms of ABCB1/Pgp-mediated multiple drug resistance in human cancer cells. J Int Med Res. 2012;40:426–435. doi: 10.1177/147323001204000204. [DOI] [PubMed] [Google Scholar]
- 14.Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Ruiz R, Rodriguez-Perales S. CRISPR-Cas9: A revolutionary tool for cancer modelling. Int J Mol Sci. 2015;16:22151–22168. doi: 10.3390/ijms160922151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang H, Yi B, Ma R, Zhang X, Zhao H, Xi Y. CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo. Sci Rep. 2016;6:22312. doi: 10.1038/srep22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Wang J, Cai K, Jiang L, Zhou D, Yang C, et al. Downregulation of gene MDR1 by shRNA to reverse multidrug-resistance of ovarian cancer A2780 cells. J Cancer Res Ther. 2012;8:226–231. doi: 10.4103/0973-1482.98975. [DOI] [PubMed] [Google Scholar]
- 18.Guschin DY, Waite AJ, Katibah GE, Miller JC, Holmes MC, Rebar EJ. A rapid and general assay for monitoring endogenous gene modification. Methods Mol Biol. 2010;649:247–256. doi: 10.1007/978-1-60761-753-2_15. [DOI] [PubMed] [Google Scholar]
- 19.Li YL, Ye F, Hu Y, Lu WG, Xie X. Identification of suitable reference genes for gene expression studies of human serous ovarian cancer by real-time polymerase chain reaction. Anal Biochem. 2009;394:110–116. doi: 10.1016/j.ab.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Jacob F, Guertler R, Naim S, Nixdorf S, Fedier A, Hacker NF, et al. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS One. 2013;8:e59180. doi: 10.1371/journal.pone.0059180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Z, Cao L, Zhang J. miR-21 modulates paclitaxel sensitivity and hypoxia-inducible factor-1-alpha expression in human ovarian cancer cells. Oncol Lett. 2013;6:795–800. doi: 10.3892/ol.2013.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome editing using CRISPR/Cas system. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savic N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15–21. doi: 10.1016/j.trsl.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Bikard D, Marraffini LA. Control of gene expression by CRISPR-Cas systems. F1000 Prime Rep. 2013;5:47. doi: 10.12703/P5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Li Zh, Zhang Q, De Amorim Bernstein K, Lozano-Calderon S, Choy E, et al. Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR-Cas9 system to reverse drug resistance. Oncotarget. 2016;7:83502–83513. doi: 10.18632/oncotarget.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Qiu JG, Li Y, Di JM, Zhang WJ, Jiang QW, et al. Targeting ABCB1-mediated tumor multidrug resistance by CRISPR / Cas9-based genome editing. Am J Transl Res. 2016;8:3986–3994. [PMC free article] [PubMed] [Google Scholar]
- 27.Wunder JS, Bull SB, Aneliunas V, Lee PD, Davis AM, Beauchamp CP, et al. MDR1 gene expression and outcome in osteosarcoma: a prospective, multicenter study. J Clin Oncol. 2000;18:2685–2694. doi: 10.1200/JCO.2000.18.14.2685. [DOI] [PubMed] [Google Scholar]
- 28.Baldini N, Scotlandi K, Barbanti-Bròdano G, Manara MC, Maurici D, Bacci G, et al. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med. 1995;333:1380–1385. doi: 10.1056/NEJM199511233332103. [DOI] [PubMed] [Google Scholar]
- 29.Jekerle V, Klinkhammer W, Scollard DA, Breitbach K, Reilly RM, Piquette-Miller M, et al. In vitro and in vivo evaluation of WK-X-34, a novel inhibitor of P-glycoprotein and BCRP using radio imaging techniques. Int J Cancer. 2006;119:414–422. doi: 10.1002/ijc.21827. [DOI] [PubMed] [Google Scholar]
- 30.Simoff I, Karlgren M, Backlund M, Lindstrom AC, Gaugaz FZ, Matsson P, et al. Complete knockout of endogenous Mdr1 (Abcb1) in MDCK cells by CRISPR-Cas9. J Pharm Sci. 2016;105:1017–1021. doi: 10.1016/S0022-3549(15)00171-9. [DOI] [PubMed] [Google Scholar]
- 31.Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet. 2015;16:299–311. doi: 10.1038/nrg3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ha JS, Byun J, Ahn DR. Overcoming doxorubicin resistance of cancer cells by Cas9-mediated gene disruption. Sci Rep. 2016;6:22847. doi: 10.1038/srep22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sims JT, Ganguly SS, Bennett H, Friend JW, Tepe J, Plattner R. Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-kB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS One. 2013;8:e55509. doi: 10.1371/journal.pone.0055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaghan R, Luk F, Bebawy M. Inhibition of the multidrug resistance P-glycoprotein: time for a change of strategy? Drug Metab Dispos. 2014;42:623–631. doi: 10.1124/dmd.113.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaymaz BT, Kosova B. Advances in therapeutic approaches using RNA interference as a gene silencing tool. Adv Tech Biol Med. 2013;1:108. [Google Scholar]
- 36.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porteus MH. Towards a new era in medicine: therapeutic genome editing. Genome Biol. 2015;16:286. doi: 10.1186/s13059-015-0859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


