Abstract
Objective(s):
Heparin-binding hemagglutinin (HBHA), a mycobacterial cell surface protein, mediates adhesion to nonphagocytic cells and the dissemination of Mycobacterium tuberculosis (M. tuberculosis) from the site of primary infection. Superior expression systems are required to obtain abundant M. tuberculosis proteins for the purpose of diagnosing M. tuberculosis infection or for the immunization. Here, HBHA was expressed by Pichia pastoris (P. pastoris) GS115 strain , and the immunogenicity of HBHA was evaluated.
Materials and Methods:
The HBHA gene of M. tuberculosis was cloned into the pPIC9K plasmid, which was good for electroporation into P. pastoris GS115 strain. Unlabeled HBHA protein was purified using a Sepharose CL-6B column, and its expression was confirmed using anti-HBHA polyclonal antibody from mouse serum. We injected C57BL/6 mice with HBHA/ dimethyldioctadecylammonium/trehalose 6,6’-dibehenate (HBHA/DDA/TDB) to investigate the immunogenicity of this potential vaccine.
Results:
The results demonstrated that HBHA/DDA/TDB has the ability to induce high levels of HBHA-specific IgG antibody and its subclasses, as well as interferon-gamma, compared with injection of phosphate-buffered saline, DDA/TDB alone and Bacillus Calmette-Guérin (BCG) controls (P<0.05). Moreover, the ratio of IgG2a/IgG1 of the HBHA/DDA/TDB group was higher than that of the BCG group (P<0.05).
Conclusion:
HBHA with no label has excellent immunogenicity, and is suitable for evaluating the effectiveness to prevent M. tuberculosis infection.
Keywords: DDA, Heparin-binding-hemagglutinin, Mycobacterium, Pichia pastoris GS115, Tuberculosis, TDB
Introduction
Mycobacterium tuberculosis (M. tuberculosis) is one of the most important morbigenous microorganisms, causing tuberculosis in humans. In 2015, 10.4 million new tuberculosis cases were estimated worldwide, and about 1.4 million cases died (1). The development of novel and effective vaccines and methods of diagnosis have provided new strategies to combat this ancient infectious disease. To date, many of the proteins and molecular components of M. tuberculosis involved in the pathogenesis have been identified through genomics, proteomics, and other techniques (2, 3). One of these components is heparin-binding hemagglutinin (HBHA), and HBHA-targeted tools are effectively used to defend against M. tuberculosis infection (4-6).
HBHA is a kind of surface protein, which is expressed by many members of the M. tuberculosis complex (7). It has the ability to adhere to nonphagocytic cells such as epithelial cells, endothelial cells and fibroblasts, and to participate in the extrapulmonary dissemination of M. tuberculosis (6, 7). HBHA was found to be a protective factor in a mouse aerosol challenge model of M. tuberculosis, and could induce much more interferon-gamma (IFN-γ) among latent TB infections (LTBIs) (8-12). Therefore, HBHA has attracted increasing attention as a potentially powerful new TB vaccine and diagnostic marker (13). Thus far, HBHA has been derived from many bacterial strains, including Escherichia coli (E. coli), Bacillus Calmette-Guérin (BCG), and Mycobacterium smegmatis (M. smegmatis) (4, 7, 14). However, the GC content of E. coli genes is relatively low, and E. coli has no efficient machinery to express M. tuberculosis genes with a higher GC content (15). In addition, the amount of proteins produced by E. coli is not adequate. M. smegmatis and BCG are not conventional systems used to produce high levels of proteins, and are associated with bio-safety concerns when their genes are transfected with respect to virulence. In this sense, these systems are not suitable for the large-scale industrial production of M. tuberculosis proteins. Therefore, efficient expression systems are needed to obtain M. tuberculosis proteins for the diagnosis of M. tuberculosis infection or large-scale immunization.
The yeast strain Pichia pastoris (P. pastoris) GS115 has high GC-rich preferred codon usage, suggesting that the transcription and translation of M. tuberculosis GC-rich genes may be improved when using this biont as a host (16-19). In this study, we utilized the P. pastoris GS115 strain to produce extracellular secreted HBHA with no label, and evaluated the immunogenicity of this HBHA protein with the adjuvant dimethyldioctadecy- lammonium/trehalose 6,6’-dibehenate (DDA/TDB) (20).
Materials and Methods
Media and strains
Middlebrook 7H11 agar, supplemented with 10% Albumin-Dextrose-Catalase (ADC), 0.5% glycerol and 0.05% Tween 80, was used for the culture of Mycobacterium bovis (M. bovis) BCG China. Luria-Bertani medium (1% peptone, 1% yeast extract and 0.5% NaCl) was used to nourish Top 10 E. coli strains, and if required, a final concentration of 100 μg/ml ampicillin was added. Yeast potato dextrose (YPD) medium, minimal dextrose (MD), buffered complex glycerol medium (BMGY), and buffered complex methanol medium (BMMY) were used for the culture of P. pastoris GS115 strain and prepared using the multi-copy Pichia expression kit (Invitrogen) in accordance with the manufacturer instructions.
Construction of the recombinant pPIC9K-heparin-binding hemagglutinin plasmid
Because of two Xho I restriction enzyme site in the sequence of pPIC9K plasmid, Xho I is not suitable for directly attaching to pPIC9K. Therefore, pPIC9 is needed as transition. HBHA was mutated (at the Xho I restriction enzyme site: GAG→GAA) and cloned into the pET30b plasmid, designated pET30b-HBHA (Life Invitrogen, Shanghai, China). According to the HBHA sequence and the multiple cloning sites of pPIC9, HBHA-Fwd (CCTCGAGAAAAGAGAGGCTGAAGCTATGGCTGAAAACTCGAAC) was designed with Xho I (CTCGAG) restriction enzyme site, Kex2 signal cleavage (AAAAGA) and Ste13 signal cleavage (GAGGCTGAAGCT), and HBHA-Rev (GGAATTCTTACTTCTGGGTGACCTTCTTGGC) include EcoR I (GAATTC) restriction enzyme site. The coding sequence of HBHA was amplified by polymerase chain reaction (PCR) with their respective primers under the following conditions: 94°C for 5 mins; 30 cycles of 94 °C for 50 sec, 60 °C for 40 sec, 72 °C for 50 sec; 72 °C for 10 mins; and held at 4 °C. The products were cloned into the Xho I and EcoR I sites of the pPIC9 plasmid, designated pPIC9-HBHA.
The pPIC9-HBHA recombinant plasmid was then digested by BamH I and EcoR I, and the small fragment was cloned into pPIC9K, designated pPIC9K-HBHA.
Transformation and screening of multi-copy transformants
The pPIC9K-HBHA (8 μg) plasmid was linearized by Sac I, and transformed into the P. pastoris GS115 strain by electrotransformation at 1.5 kV, 25 μF, and 200 Ω for 4.8 msec. Transformants were selected on MD plates, and then His+ transformants were selected through YPD plates with 1–5 mg/ml Geneticin 418 (G418). PCR, with the preexisting conditions, was used to verify the positive resistant strains under a G418 selective pressure of 5 mg/ml.
Expression and purification of heparin-binding hemagglutinin in Pichia pastoris GS115 strain
The positive colonies resistant to 5 mg/ml G418 were inoculated in 100 ml of BMGY at 30°C with constant shaking at 250 rpm until the optical density at 600 nm reached 3.0. The sediment of P. pastoris was resuspended in 20 ml of BMMY, and continuously induced for 96 hrs at 30 °C with shaking at 250 rpm. Methanol was maintained at a concentration of 1% (v/v). Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed to confirm that HBHA was expressed successfully.
The supernatant was separated from the culture, and the purification was completed using a Sepharose CL-6B column (GE Healthcare, Somerset, NJ, USA). HBHA protein was lyophilized, diluted in phosphate-buffered saline (PBS) using pyrogen-free reagents, aliquoted, and stored at −20°C. The protein concentration was determined using a bicinchoninic acid (BCA) Protein Assay Kit (Beyotime, Shanghai, China). The purified protein was confirmed by western blotting with anti-HBHA protein mouse serum (diluted 1/800) as the primary antibody and peroxidase-conjugated goat anti-mouse IgG (diluted 1/5000; Proteintech Biotech, Wuhan, China) as the secondary antibody. The immunoblots were visualized using enhanced chemiluminescence technology (Tiangen Biotech, China).
Immunization of mice
The adjuvant DDA/TDB was prepared in the same manner as previously described for DDA/ monophosphoryl lipid A/TDB (DDA/MPL/TDB, DMT) (20). Two hundred microliters of HBHA/DDA/TDB contained 20 μg/100 μl of HBHA emulsified in 100 μl of DDA/TDB adjuvant. Female C57BL/6 (H-2b) mice at 6–8 weeks old were purchased from the Center for Animal Experiment of Wuhan University (Wuhan, China) and maintained in a biosafety laboratory on standard laboratory chow. The mice were immunized subcuta-neously (SC) three times with 0.2 ml of HBHA/ DDA/TDB at 3-week intervals.
The mice were also SC-vaccinated with the BCG China strain as a positive control, once at the proximal end of the tail with approximately 1 × 106 colony-forming units in a final volume of 200 μl of PBS. Control mice were treated with 200 μl of PBS only. Mice experiments were repeated twice. Animal experiments were performed on the basis of the policies of the Chinese Council on Animal Care, and approved by the Committee on the Ethics of Animal Experiments of Wuhan University (Wuhan, China).
Heparin-binding hemagglutinin-specific antibody titers and interferon-gamma secretion
Nine weeks after the first immunization, the presence of HBHA-specific IgG, IgG1, and IgG2a (replaced by IgG2c) antibodies, existed in the mouse serum were tested by enzyme-linked immunosorbent assay (ELISA) as previously described (20). At the same time, the spleen cells were obtained from each mouse, counted, and placed in 24-well plates in triplicate at 2.5 × 106 cells/well and incubated with HBHA (10 μg/ml) and RPMI1640 medium at 37 °C under 5% CO2. After 72 hrs, the culture supernatants were harvested to test IFN-γ levels using ELISA kits as previously described (20). The data are shown as mean±SEM log10 endpoint titers per group, the ratio of IgG2a/IgG1 of different groups (n = 3), and the mean±SD (pg/ml) per group (n = 3). These experiments were repeated twice with similar results.
Statistical analysis
Statistical analysis was performed with SPSS 17.0 software, and one-way ANOVA analysis was used to show the difference among groups. P<0.05 was considered significant.
Results
Successful construction of the recombinant pPIC9K-heparin-binding hemagglutinin plasmid
In order to utilize the P. pastoris GS115 strain for the production of HBHA, pPIC9K was used as the expression vector of HBHA. The pPIC9 plasmid was used as a transition vector, and the recombinant pPIC9K-HBHA plasmid was successfully constructed with the help of BamH I and EcoR I digestion (Figure 1).
Figure 1.
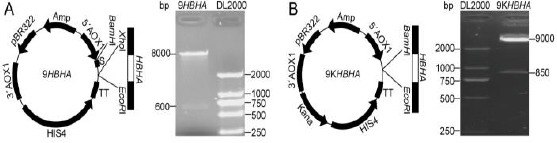
Construction of pPIC9-heparin-binding hemagglutinin (HBHA) (A) and pPIC9K- HBHA plasmids (B). The HBHA gene was connected into pPIC9 plasmid with Xho I and EcoR I restriction enzymes (A), and the construction of pPIC9K-HBHA plasmid depended on BamH I and EcoR I restriction enzymes (B)
Successful expression and purification of heparin-binding hemagglutinin in Pichia pastoris GS115 strain
The PCR results confirmed that the pPIC9K-HBHA plasmid was successfully integrated into the genome of P. pastoris under pressure of 5 mg/ml G418, and the size of the HBHA gene was 600 bp (Figure 2A). SDS-PAGE analysis showed that HBHA was expressed with an apparent molecular weight of about 22 kDa (Figure 2B). The HBHA concentrate in the culture supernatant was directly purified in one step, and confirmed by western blotting (Figure 2C).
Figure 2.
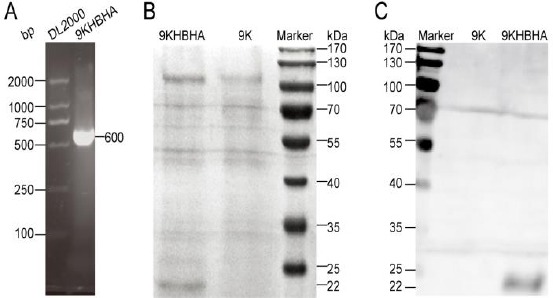
Expression and purification of heparin-binding hemagglutinin (HBHA). PCR demonstrated that pPIC9K-HBHA plasmid was integrated into the genome of Pichia pastoris under pressure of 5 mg/ml G418 (A). SDS-PAGE analysis showed that HBHA was successfully expressed (B). The purification of HBHA was confirmed by western blotting with anti-HBHA protein mouse serum (diluted 1/800) (C)
Heparin-binding hemagglutinin induced a Th1 immune response in immunized mice
To evaluate the immunogenicity of HBHA/DDA/TDB in mice, the presence of HBHA- specific antibodies was determined by ELISA at nine weeks after the first immunization (Figure 3). Mice vaccinated with HBHA/DDA/TDB produced higher levels of HBHA-specific IgG, IgG1, and IgG2a antibodies, compared with BCG− and DDA/TDB−vaccinated controls (P<0.05), whereas the antibody levels were higher in the BCG-treated mice than in the DDA/TDB control. In addition, the ratio of IgG2a/IgG1 of the HBHA/DDA/TDB group was higher than that of the BCG group (P<0.05) (Figure 3). Furthermore, PBS and DDA/TDB controls had the lowest levels of HBHA-specific IFN-γ, and HBHA/DDA/TDB induced higher IFN-γ levels than the BCG control (P < 0.05, Figure 3).
Figure 3.
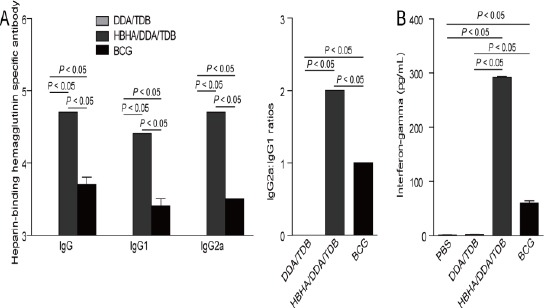
Heparin-binding hemagglutinin (HBHA)-specific antibody titers and interferon-gamma (IFN-γ) secretion in different groups (n = 3). Nine weeks after first immunization, ELISA was used to test the HBHA-specific IgG, IgG1, and IgG2a (replaced by IgG2c) antibody titers in single samples belonging to different vaccinated C57BL/6 mice (A and B). The results are shown as mean±SEM log10 endpoint titers and the ratio of IgG2a/IgG1 of different groups (n = 3). Nine weeks after first immunization, the spleen cells of every mouse were obtained, counted, and placed in triplicate at 2.5 × 106 cells/well and incubated with HBHA (10 μg/ml) in 24-well plates at 37 °C under 5% CO2 (C). After 72 hrs, the culture supernatants were harvested, and ELISA was used to detect the concentration of IFN-γ in the suspension. The results of IFN-γ concentration are shown as mean±SD (pg/ml). These experiments were repeated twice with similar results
Discussion
P. pastoris GS115 strain and the pPIC9K plasmid were used to obtain unlabeled HBHA protein for the screening of M. tuberculosis infection or large-scale immunization. HBHA with no label was successfully expressed in the P. pastoris GS115 strain and purified, and showed good ability to induce a strong Th1 immune response in mice.
HBHA has been previously shown to induce a Th1 immune response (13, 21). In our study, HBHA/DDA/TDB induced abundant production of HBHA-specific IFN-γ and high levels of HBHA-specific IgG antibody and its subclasses. BCG prime-HBHA boost, nanoparticle-Ag85B-HBHA vaccine, HBHA-cholera toxin and MPL-formulated HBHA were also reported to enhance cellular immune responses against M. tuberculosis infection compared with the control, and induced HBHA-specific IFN-γ (22-25). Another vaccine, developed with an M. smegmatis strain expressing the fusion protein HBHA-interleukin-12, was shown to enhance immunogenicity by improving the Th1 immune response against TB (26). In addition, HBHA-specific CD8+ T cells express memory cell markers and show all three effector functions involved in CD8+ T cells-mediated protective immunity mechanisms (12). It is worth mentioning that patients with pulmonary TB also develop a strong humoral response specific to HBHA (5, 27). One study pointed out that local and systemic humoral immunity induced by a mucosal vaccine based on HBHA impaired extrapulmonary dissemination (28).
HBHA can be used as a biomarker of anti-TB protective immunity and LTBI, and has the ability to provide protection against M. tuberculosis infection (21). HBHA induced specific CD4+ T cells, and this response was significantly higher in patients with LTBI (29). HBHA promoted a potent IFN-γ response in LTBI, and multifunctional IFN-γ+IL-2+IL-17+CD4+ T cells in household contacts (30). An IFN-γ release assay showed that HBHA had comparable diagnostic capacity for recent and remote LTBI, and was complementary to the QuantiFERON-TB Gold In-Tube test (QFT-GIT) for the screening of latent TB in HIV-infected patients (31, 32). Another study indicated that the T cell response to HBHA produced by M. smegmatis was useful for differentiating between active and non-active TB for positive QFT-GIT results in a whole blood system (33). As a TB vaccine, HBHA boost and BCG prime reduced the bacterium carrying capacity by 0.7 log compared to BCG alone (34, 35).
Many proteins of M. tuberculosis have been expressed through P. pastoris expression system. Vaccines containing these proteins possess good immunogenicity and protection. ESAT6:HspX:Fcγ2a, CFP-10:HspX:Fcγ2a, CFP-10:HspX:His, ESAT-6:CFP-10:Fcγ2a, ESAT-6:CFP-10:His, ESAT-6:Fcγ2a, ESAT-6: His, CFP-10:Fcγ2a and CFP-10:His could induce good Th1 response, characterized with high levels of IL-12 and IFN-γ (36-40). mBNBD4 and mBNBD5 play important roles in controlling intracellular survival of mycobacteria and in inhibiting mycobacterial growth (41, 42). Sialylated recombinant human lactoferrin generated improved antigen-specific recall responses to BCG antigens (43). Furthermore, BCG with sialylated lactoferrin adjuvant resulted in significant reduction in associated pathology following challenge with virulent organisms (43). Recombinant VP1 and a synthetic multi-epitope FMDV (EG), fusion with HSP70, enhanced both the humoral- and cell-mediated immune responses (44). rCFP32 had the ability to react with the sera of individuals with tuberculosis and enhance serum immunoreactivity (45, 46). Vp1-HSP70 fusion protein could elicit specific humoral- and cellular- immune responses (47). In our research, HBHA could also induce a strong Th1 immune response in immunized mice.
Conclusion
In summary, unlabeled HBHA expressed in the P. pastoris GS115 strain showed favorable immunogenicity, and induced high levels of HBHA-specific antibodies and IFN-γ. These results may be beneficial for further development of HBHA with no label as a candidate vaccine against TB. In future studies, we intend to assess the effectiveness of the TB vaccine HBHA/DDA/TDB to protect C57BL/6 mice against aerosol M. tuberculosis infection.
Acknowledgment
This work was supported by the science and technology plan project of General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (No. 2017IK092).
References
- 1.World Health Organization. Global tuberculosis report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 2.de Souza GA, Wiker HG. A proteomic view of Mycobacteria. Proteomics. 2011;11:3118–3127. doi: 10.1002/pmic.201100043. [DOI] [PubMed] [Google Scholar]
- 3.Ernst JD, Trevejo-Nunez G, Banaiee N. Genomics and the evolution, pathogenesis, and diagnosis of tuberculosis. J Clin Invest. 2007;117:1738–1745. doi: 10.1172/JCI31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menozzi FD, Bischoff R, Fort E, Brennan MJ, Locht C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci U S A. 1998;95:12625–12630. doi: 10.1073/pnas.95.21.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masungi C, Temmerman S, Van Vooren JP, Drowart A, Pethe K, Menozzi FD, et al. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J Infect Dis. 2002;185:513–520. doi: 10.1086/338833. [DOI] [PubMed] [Google Scholar]
- 6.Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, et al. The heparin-binding haemagglutinin of Mtuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 7.Menozzi FD, Rouse JH, Alavi M, Laude-Sharp M, Muller J, Bischoff R, et al. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med. 1996;184:993–1001. doi: 10.1084/jem.184.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parra M, Pickett T, Delogu G, Dheenadhayalan V, Debrie AS, Locht C, et al. The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis. Infect Immun. 2004;72:6799–6805. doi: 10.1128/IAI.72.12.6799-6805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrero GG, Locht C. Recombinant HBHA boosting effect on BCG-induced immunity against Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:730702. doi: 10.1155/2011/730702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temmerman S, Pethe K, Parra M, Alonso S, Rouanet C, Pickett T, et al. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat Med. 2004;10:935–941. doi: 10.1038/nm1090. [DOI] [PubMed] [Google Scholar]
- 11.Hougardy JM, Schepers K, Place S, Drowart A, Lechevin V, Verscheure V, et al. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One. 2007;2:e926. doi: 10.1371/journal.pone.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temmerman ST, Place S, Debrie AS, Locht C, Mascart F. Effector functions of heparin-binding hemagglutinin-specific CD8+T lymphocytes in latent human tuberculosis. J Infect Dis. 2005;192:226–232. doi: 10.1086/430930. [DOI] [PubMed] [Google Scholar]
- 13.Belay M, Legesse M, Mihret A, Ottenhoff TH, Franken KL, Bjune G, et al. IFN-gamma and IgA against non-methylated heparin-binding hemagglutinin as markers of protective immunity and latent tuberculosis: results of a longitudinal study from an endemic setting. J Infect. 2016;72:189–200. doi: 10.1016/j.jinf.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 14.Delogu G, Bua A, Pusceddu C, Parra M, Fadda G, Brennan MJ, et al. Expression and purification of recombinant methylated HBHA in Mycobacterium smegmatis. FEMS Microbiol Lett. 2004;239:33–39. doi: 10.1016/j.femsle.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Andersson GE, Sharp PM. Codon usage in the Mycobacterium tuberculosis complex. Microbiology. 1996;142:915–925. doi: 10.1099/00221287-142-4-915. [DOI] [PubMed] [Google Scholar]
- 16.Cregg JM, Cereghino JL, Shi J, Higgins DR. Recombinant protein expression in Pichia pastoris. Mol Biotechnol. 2000;16:23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- 17.Giga-Hama Y, Kumagai H. Foreign gene expression in fission yeast S. pombe. Seikagaku. 1998;70:300–304. [PubMed] [Google Scholar]
- 18.Lin-Cereghino GP, Stark CM, Kim D, Chang J, Shaheen N, Poerwanto H, et al. The effect of alpha-mating factor secretion signal mutations on recombinant protein expression in Pichia pastoris. Gene. 2013;519:311–317. doi: 10.1016/j.gene.2013.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22:249–270. doi: 10.1002/yea.1208. [DOI] [PubMed] [Google Scholar]
- 20.Teng X, Tian M, Li J, Tan S, Yuan X, Yu Q, et al. Immunogenicity and protective efficacy of DMT liposome-adjuvanted tuberculosis subunit CTT3H vaccine. Hum Vaccin Immunother. 2015;11:1456–1464. doi: 10.1080/21645515.2015.1037057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locht C, Hougardy JM, Rouanet C, Place S, Mascart F. Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis (Edinb) 2006;86:303–309. doi: 10.1016/j.tube.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Fukui M, Shinjo K, Umemura M, Shigeno S, Harakuni T, Arakawa T, et al. Enhanced effect of BCG vaccine against pulmonary Mycobacterium tuberculosis infection in mice with lung Th17 response to mycobacterial heparin-binding hemagglutinin adhesin antigen. Microbiol Immunol. 2015;59:735–743. doi: 10.1111/1348-0421.12340. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero GG, Debrie AS, Locht C. Boosting with mycobacterial heparin-binding haemagglutinin enhances protection of Mycobacterium bovis BCG-vaccinated newborn mice against Mtuberculosis. Vaccine. 2010;28:4340–4347. doi: 10.1016/j.vaccine.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 24.Stylianou E, Diogo GR, Pepponi I, van Dolleweerd C, Arias MA, Locht C, et al. Mucosal delivery of antigen-coated nanoparticles to lungs confers protective immunity against tuberculosis infection in mice. Eur J Immunol. 2014;44:440–449. doi: 10.1002/eji.201343887. [DOI] [PubMed] [Google Scholar]
- 25.Verwaerde C, Debrie AS, Dombu C, Legrand D, Raze D, Lecher S, et al. HBHA vaccination may require both Th1 and Th17 immune responses to protect mice against tuberculosis. Vaccine. 2014;32:6240–6250. doi: 10.1016/j.vaccine.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S, Zhao Y, Mao F, Zhang C, Bai B, Zhang H, et al. Protective and therapeutic efficacy of Mycobacterium smegmatis expressing HBHA-hIL12 fusion protein against Mycobacterium tuberculosis in mice. PLoS One. 2012;7:e31908. doi: 10.1371/journal.pone.0031908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanetti S, Bua A, Delogu G, Pusceddu C, Mura M, Saba F, et al. Patients with pulmonary tuberculosis develop a strong humoral response against methylated heparin-binding hemagglutinin. Clin Diagn Lab Immunol. 2005;12:1135–1138. doi: 10.1128/CDLI.12.9.1135-1138.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohama H, Umemura M, Okamoto Y, Yahagi A, Goga H, Harakuni T, et al. Mucosal immunization with recombinant heparin-binding haemagglutinin adhesin suppresses extrapulmonary dissemination of Mycobacterium bovis bacillus Calmette-Guerin (BCG) in infected mice. Vaccine. 2008;26:924–932. doi: 10.1016/j.vaccine.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Hutchinson P, Barkham TM, Tang W, Kemeny DM, Chee CB, Wang YT. Measurement of phenotype and absolute number of circulating heparin-binding hemagglutinin, ESAT-6 and CFP-10, and purified protein derivative antigen-specific CD4 T cells can discriminate active from latent tuberculosis infection. Clin Vaccine Immunol. 2015;22:200–212. doi: 10.1128/CVI.00607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loxton AG, Black GF, Stanley K, Walzl G. Heparin-binding hemagglutinin induces IFN-gamma(+) IL-2(+) IL-17(+) multifunctional CD4(+) T cells during latent but not active tuberculosis disease. Clin Vaccine Immunol. 2012;19:746–751. doi: 10.1128/CVI.00047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyndham-Thomas C, Corbiere V, Dirix V, Smits K, Domont F, Libin M, et al. Key role of effector memory CD4+T lymphocytes in a short-incubation heparin-binding hemagglutinin gamma interferon release assay for the detection of latent tuberculosis. Clin Vaccine Immunol. 2014;21:321–328. doi: 10.1128/CVI.00651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyndham-Thomas C, Dirix V, Schepers K, Martin C, Hildebrand M, Goffard JC, et al. Contribution of a heparin-binding haemagglutinin interferon-gamma release assay to the detection of Mycobacterium tuberculosis infection in HIV-infected patients: comparison with the tuberculin skin test and the QuantiFERON-TB Gold In-tube. BMC Infect Dis. 2015;15:59. doi: 10.1186/s12879-015-0796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delogu G, Chiacchio T, Vanini V, Butera O, Cuzzi G, Bua A, et al. Methylated HBHA produced in Msmegmatis discriminates between active and non-active tuberculosis disease among RD1-responders. PLoS One. 2011;6:e18315. doi: 10.1371/journal.pone.0018315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman MJ, Fernandez C. Neonatal vaccination with Mycobacterium bovis BCG: potential effects as a priming agent shown in a heterologous prime-boost immunization protocol. Vaccine. 2009;27:4038–4046. doi: 10.1016/j.vaccine.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Rouanet C, Debrie AS, Lecher S, Locht C. Subcutaneous boosting with heparin binding haemagglutinin increases BCG-induced protection against tuberculosis. Microbes Infect. 2009;11:995–1001. doi: 10.1016/j.micinf.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Soleimanpour S, Farsiani H, Mosavat A, Ghazvini K, Eydgahi MR, Sankian M, et al. APC targeting enhances immunogenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol. 2015;99:10467–10480. doi: 10.1007/s00253-015-6952-z. [DOI] [PubMed] [Google Scholar]
- 37.Mosavat A, Soleimanpour S, Farsiani H, Sadeghian H, Ghazvini K, Sankian M, et al. Fused Mycobacterium tuberculosis multi-stage immunogens with an Fc-delivery system as a promising approach for the development of a tuberculosis vaccine. Infect Genet Evol. 2016;39:163–172. doi: 10.1016/j.meegid.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Farsiani H, Mosavat A, Soleimanpour S, Sadeghian H, Akbari EM, Ghazvini K, et al. Fc-based delivery system enhances immunogenicity of a tuberculosis subunit vaccine candidate consisting of the ESAT-6: CFP-10 complex. Mol Biosyst. 2016;12:2189–2201. doi: 10.1039/c6mb00174b. [DOI] [PubMed] [Google Scholar]
- 39.Kebriaei A, Derakhshan M, Meshkat Z, Eidgahi MR, Rezaee SA, Farsiani H, et al. Construction and immunogenicity of a new Fc-based subunit vaccine candidate against Mycobacterium tuberculosis. Mol Biol Rep. 2016;43:911–922. doi: 10.1007/s11033-016-4024-9. [DOI] [PubMed] [Google Scholar]
- 40.Baghani AA, Soleimanpour S, Farsiani H, Mosavat A, Yousefi M, Meshkat Z, et al. CFP10: mFc?2 as a novel tuberculosis vaccine candidate increases immune response in mouse. Iran J Basic Med Sci. 2017;20:122–130. doi: 10.22038/ijbms.2017.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang JJ, Lyu Y, Zhao DM, Tian LH, Yin XM, Yang LF, et al. Antimicrobial activity of recombinant mature bovine neutrophil beta-defensin 4 on mycobacterial infection. Int J Tuberc Lung Dis. 2015;19:711–716. doi: 10.5588/ijtld.13.0272. [DOI] [PubMed] [Google Scholar]
- 42.Kang J, Zhao D, Lyu Y, Tian L, Yin X, Yang L, et al. Antimycobacterial activity of Pichia pastoris -derived mature bovine neutrophil beta-defensins 5. Eur J Clin Microbiol Infect Dis. 2014;33:1823–1834. doi: 10.1007/s10096-014-2152-5. [DOI] [PubMed] [Google Scholar]
- 43.Hwang SA, Wilk K, Kruzel ML, Actor JK. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine. 2009;27:3026–3034. doi: 10.1016/j.vaccine.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su C, Duan X, Wang X, Wang C, Cao R, Zhou B, et al. Heterologous expression of FMDV immunodominant epitopes and HSP70 in Ppastoris and the subsequent immune response in mice. Vet Microbiol. 2007;124:256–263. doi: 10.1016/j.vetmic.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 45.Benabdesselem C, Barbouche MR, Jarboui MA, Dellagi K, Ho JL, Fathallah DM. High level expression of recombinant Mycobacterium tuberculosis culture filtrate protein CFP32 in Pichia pastoris. Mol Biotechnol. 2007;35:41–49. doi: 10.1385/mb:35:1:41. [DOI] [PubMed] [Google Scholar]
- 46.Benabdesselem C, Fathallah DM, Huard RC, Zhu H, Jarboui MA, Dellagi K, et al. Enhanced patient serum immunoreactivity to recombinant Mycobacterium tuberculosis CFP32 produced in the yeast Pichia pastoris compared to Escherichia coli and its potential for serodiagnosis of tuberculosis. J Clin Microbiol. 2006;44:3086–3093. doi: 10.1128/JCM.02672-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su CX, Duan XG, Wang XQ, Ren XF, Cao RB, Zhou B, et al. Fusion expression of O type foot-and-mouth diseases virus VP1 gene and HSP70 gene and induction of immune responses in mice. Sheng Wu Gong Cheng Xue Bao. 2006;22:733–736. [PubMed] [Google Scholar]


