Abstract
Objective(s):
Varicocele is an abnormal dilation in the testicular vein, which can cause hypoxia, reactive oxygen species accumulation, elevation in testicular temperature, and promote apoptosis and increase proinflammatory cytokine production. According to the varicocele pathophysiology, it is possible that a group of cytosolic receptors called nucleotide oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NLRP3) inflammasomes also involve in varicocele pathogenesis. Due to the important role of antioxidant in decreasing the testis tissue damage, in this study we investigated the protective effect of resveratrol (RES) on NLRP3 complex and apoptosis in experimental varicocele rats.
Materials and Methods:
In this study, 40 male Wistar rats were randomly divided into 5 groups (8 rats in each group): Control, experimental left varicocele (ELV), ELV + ethanol, ELV + 20 mg/kg RES and ELV + 50 mg/kg RES. Varicocele was induced by partial ligation of the left renal vein. Three months after varicocele induction, RESwas orally administered to rats for 1 month. The expression levels of NLRP3, apoptosis associated speck-like protein (ASC), caspase-1, Bax and Bcl2 were analyzed using real time PCR.
Results:
Our results showed that RESat both doses significantly (P≤ 0.05) decreased the gene expression levels of ASC, NLRP3, caspase-1 and Bax and increased Bcl2 gene expression at high dose.
Conclusions:
RESby reducing inflammatory factors and decreasing apoptosis might be used as adjuvant therapy to reduce varicocele complication.
Keywords: Apoptosis, Inflammasomes, Resveratrol, Rat, Varicocele
Introduction
Varicocele that is the most common cause of male infertility occurs in 4.4-22% of male population (1, 2). Varicocele is an abnormal enlargement of the testicular vein plexus (especially in the left testis) that results in some pathological problems in the testis tissue. Three different theories have been mentioned on the causes of varicoceles: 1) the angle of drainage of the testicular vein in the right side is straight while the left side is curved. 2) Venous valves in the left internal spermatic vein are absent or inefficient. 3) Location of the left renal vein between the aorta and superior mesenteric artery inserts a partial obstruction (3). This abnormal dilatation of the pampiniform plexus leads to hypoxia, blood stasis, testicular temperature elevation, apoptosis, increased oxidative stress and reactive oxygen spices (ROS) accumulation. These pathological events in testis tissue impair spermatogenesis, sperm parameters and reduce male fertility (3, 4).
Varicocele induces inflammatory responses in the testis tissue (5). Inflammation is the first immunological response against tissue damage (6).
During inflammation, multiprotein complexes, named inflammasomes are activated (7). In our previous study (data not shown), we suggested that among different members of inflammasome (NLRP1, NLRP2, NLRP3 and AIM), the nucleotide oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NALP3) inflammasome is involved in varicocele pathogenesis. The NLRP3 inflammasome is a complex of NLRP3, apoptosis associated speck-like protein (ASC) and caspase-1 (8). Once NLRP3 is activated, it cleaves procaspse-1 to caspase-1 and then caspase-1 converts the pro-interleukin-1β and 18 to their active forms (7, 9). Accumulation of these pro-inflammatory cytokines in the testis could disrupt testicular function, spermatogenesis and androgen production (10).
Antioxidant therapy is one of the appropriate treatment modality for reducing the effects of varicocele on male fertility (11).
Resveratrol (RES) (3,5,4’-trihydoxy-trans-stilbene) that is found in some plants such as mulberries, peanuts and grapeshas a widespread biological activities such as antioxidant, anti-inflammatory and anti-apoptosis(12). Previous studies demonstrated that RES can improve sperm parameters and histological changes occurred in the testis tissue of the experimental left varicocele rats (13, 14). In the current study, we investigated the protective effect of RESagainst inflammation and apoptosis induced by varicocele in rats.
Materials and Methods
Animals
Research and animal care were approved by the Ethics Committee of Arak University of Medical Sciences. In vivo experiments were performed on 14-weeks old adult male Wistar rats (250±20 g, Pasteur, Iran). Animals were housed at 24°C and controlled conditions with free access to water and food.
The animals were divided randomly into following groups prior to the operation procedure (8 rats in each group): Control, Experimental leftvaricocele induction, Experimental leftvaricocele induction + RES20 treatment (ELV + 20 mg/kg RES), Experimental left varicocele induction + RES50 treatment (ELV + 50 mg/kg RES), Experimental left varicocele induction + vehicle control (ELV + 10 % ethanol).
Surgical procedure
Rats were anaesthetized with intraperitoneal (IP) injection of 100 mg/kg ketamine and 10 mg/kg xylozine (both from Alfasan, Iran). After shaving and cleaning the surgical area, a midline incision was performed and the left renal and spermatic veins were dissected from around tissue. A 0.85 mm wire was placed parallel to the left renal vein and a 4-0 silk suture was used for ligation around the wire and left renal vein proximal to the Inferior vena cava (IVC). Then the wire was carefully removed and the abdominal wall was sutured (15).
Resveratrol administration
3 months after surgery, RESwas given to the animals (20 and 50 mg/kg/daily by gavage) for one month. The animals were sacrificed under deep anesthesia and immediately transcardinally perfused with 150-200 ml phosphate-buffered saline (PBS; Sigma, Germany). Testis was removed and transferred to the liquid nitrogen and stored at -70°C until further use.
RNA isolation and cDNA synthesis
After sampling, the expression of NLRP3, ASC, caspase-1, Bax and Bcl2 genes in all groups was studied by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Total RNA was extracted using peqGold RNA TriFast (PeqLab, Germany) according to the manufacturer’s instructions. The RNA pellet was dissolved in diethylpyrocarbonate-treated water (DEPC treated water; SinaClon, Iran) and quantified spectrophotometrically at 260 nmwavelength. The integrity of the extracted total RNA was assessed by agarose gel electrophoresis and verified by the presence of the 28S and 18S rRNA bands. Immediately after RNA preparation, 2 μg of total RNA was used for cDNA synthesis in a total volume of 20 μl by using RevertAid ™ First Strand cDNA Synthesis Kit (Aryatous, Iran). The cDNA was stored at -80°C until use.
Quantitative RT-PCR
qRT-PCR was carried out using the Life Cycle Real time PCR (Roche, USA). qRT-PCR was performed in a total volume of 20 μl containing 2 μl of cDNA (5-fold diluted), 0.5 μl of 5 mmol/l solutions of each of the forward and reverse primers, and 10 μl of 2x SYBR green DNA PCR Master Mix. Each sample was loaded in duplicate.
The primer sequences used for amplifications are summarized in Table 1.
Table 1.
Primer sets used for amplification
| Genes | Primers sequences (5’ to 3’) | Product length (bp) |
|---|---|---|
| NLRP3 | Forward: TCTGTTCATTGGCTGCGGAT Reverse: GCCTTTTTCGAACTTGCCGT | 314 |
| ASC | Forward: GCTGCAGATGGACCCCATAG Reverse: ACATTGTGAGCTCCAAGCCA | 80 |
| Caspase-1 | Forward: CACGAGACCTGT GCGATCAT Reverse: CTTGAGGGAACCACTCGGTC | 212 |
| Bax | Forward: GCTACAGGGTTTCATCCAG Reverse: TCCACATCAGCAATCATCC | 174 |
| Bcl2 | Forward: AGCGTCAACAGGGAGATG Reverse: CCACAAAGGCATCCCAG | 118 |
| Cyclo A | Forward: GGCAAATGCTGGACCAAACAC Reverse: TTAGAGTTGTCCACAGTCGGAGATG | 196 |
NLRP3: Nucleotide oligomerization domain (NOD)-like receptor family pyrin domain containing 3, ASC: Apoptosis associated speck-like protein
Melt curve analysis was performed after each run to check for the presence of non-specificPCR pro-ducts and primer dimers. All samples were normalized against Cyclo A (internal control) using the comparative CT method (ΔΔCT).
Statistical analysis
The results are expressed as mean±standard deviation (SD). The statistical significance between the mean values was determined by one-way analysis of variance (ANOVA) followed by a Tukey’s post-hoc test with P≤0.05 as the statistically significant criterion.
Results
Resveratrol down-regulates NLRP3 inflammasome components in varicocelized rats
To verify the anti-inflammatory effects of RES-treated animals, testis tissue was analyzed for gene expression of NLRP3 using real time PCR. As expected, experimental varicocele induced a significant up-regulation of NLRP3 gene expression 3 months after partial ligation of left renal vein (P≤0.05). The mRNA level of NLRP3 was significantly lower in RES20 (P≤0.05)and RES50 (P<0.01)treated rats compared with the varicocelized and ethanol-gavaged animals (Figure 1). In the line with this finding, the gene expression of ASC and caspase-1 revealed (i) higher mRNA level of these genes in varicocele-induced and vehicle-treated animals and (ii) lower gene expression in RES-treated animals (Figures 2 and 3). Although western blot was not performed, RESpossibly ameliorates varicocele-induced inflammation in this model.
Figure 1.
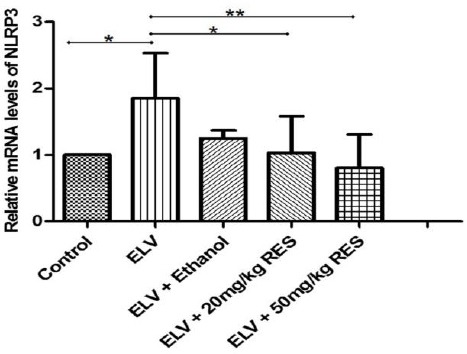
The expression level of nucleotide oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NLRP3) gene in the testis tissue as determined by real time PCR is presented in different groups. Higher level of NLRP3 expression in the varicocele and vehicle groups and low expression level of this gene after resveratrol administration is shown. *: P≤0.05, **: P≤0.01
Figure 2.
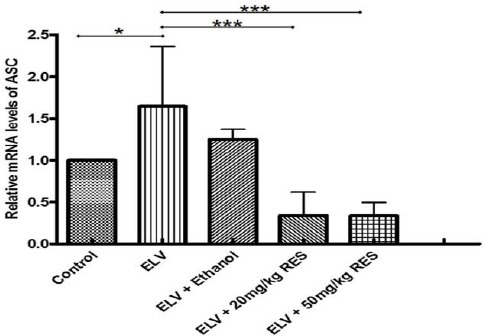
The expression level of apoptosis associated speck-like protein (ASC) gene in the testis tissue as determined by real time PCR is presented in different groups. Higher level of ASC expression 3 months after varicocele induction in the varicocele and vehicle groups and low expression level of this gene after one month resveratrol administration is demonstrated. *: P≤0.05, ***: P≤0.001
Figure 3.
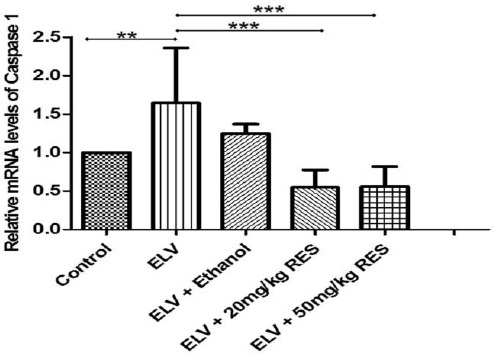
The expression level of caspase-1 gene in the testis tissue as determined by real time PCR is presented in different groups. The significant increase of caspase-1 mRNA level in the varicocele and vehicle groups is shown, and resveratrol administration decreased significantly caspase-1 gene expression in treated animals. **: P≤0.01, ***: P≤0.001
Resveratrol prevents cell apoptosis
In varicocele, both death receptor and mitochondrial-mediate apoptosis are responsible for testis tissue apoptosis (16). We evaluated whether different doses of REScould prevent apoptosis. For this purpose, the gene expression of Bax (a pro-apoptotic member of Bcl2 family) and Bcl2 (an anti-apoptotic gene) was analyzed in the testis tissue. The real time PCR analysis revealed that mRNA level of Bax was significantly lower in the RES20 (P<0.001) and RES50 (P<0.001) groups compared with the varicocelized and ethanol-gavaged animals (Figure 4), and Bcl2 gene expression was meaningfully higher only in the RES50 treated rats (P<0.01) (Figure 5). These data strongly implicated that RESprevents apoptosis after varicocele in a dose dependent manner.
Figure 4.
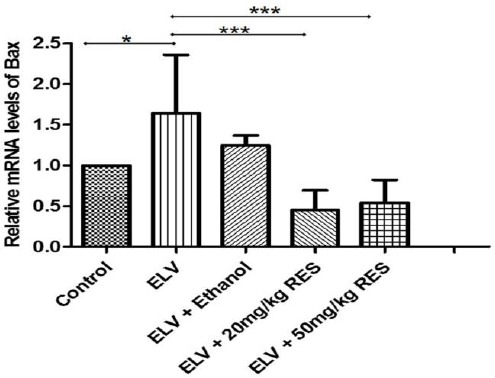
Apoptosis determined by evaluating gene expression level of a pro-apoptotic member of Bcl2 family (Bax) using real time PCR is presented in different groups. Lower gene expression level in resveratrol 20 mg/kg (RES20)and RES50 treated animals compared with the varicocelized and ethanol gavaged animals. *: P≤0.05, ***: P≤0.001
Figure 5.
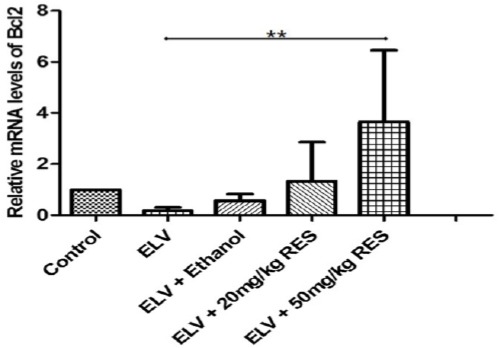
The expression level of Bcl2 gene in the testis tissue is presented in different groups. The expression level of Bcl2 is significantly potentiated in resveratrol 50 mg/kg (RES50)compared to other treatment group. **: P≤0.01
Discussion
The pathogenesis of varicocele, one of the common causes of male infertility, is not well understood and findings show that it is multifactorial (17). Due to the limitation in study design for investigation of human testis structure during varicocele, animal models of varicocele are valuable tools in this regard (15). In the present study, varicocele was induced by partial ligation of the left renal artery, a common model of varicocele, which mimics the compression of the left renal vein between the aorta and the superior mesenteric artery (18).
Varicocele by producing stress oxidative and ROS accumulation in the testis tissue impairs spermatogenesis and male fertility (19). Therefore, antioxidant therapy has been considered as a treatment modality for varicocele(20). In this study, we used RES, a natural polyphenolic compound derived from grape (21) for reducing inflammation and apoptosis in the testis tissue during varicocele.
In the present study, we showed that three months after varicocele induction, the levels of NLRP3 inflommasome components including NLRP3, ASC and caspase-1were up-regulated and RES administration for one month could decrease elevated levels of these genes. NLRP3 inflammasome is activated by pathological stress including stroke (22), spinal cord injury (23), and diabetic mellitus (24). Previous studies have shown that varicocele increases pro-inflammatory and inflammatory cytokines such as interleukin-1 (25), and 6 as well as tumor necrosis factor (26) and hypoxia induced factor (27, 28). In this study, we showed that RES by its anti-inflammatory properties decreases NLRP3 inflammasome activity in the testis tissue. RES, by activation of sirtuin-1(Sirt-1) signaling, can inhibit nuclear factor kappa beta (NF-κb) and then NLRP3 inflammasome activity (29). The dose dependent anti-apoptotic effect of RESon varicocelized testis was another finding of this study. Our study revealed that the apoptosis rate decreases by using high RES concen-trations. Low and high doses of RESwas effective to decrease Bax gene expression. However, Bcl2 gene expression was higher at high dose compared to low dose. RES has been used in different doses in various studies. Liu et al. showed that 200 mg/kg administration of RES could decrease apoptosis induced by the spinal cord injury in rats (30), and Mendes et al. claimed that RES at the concentration of 300 mg/kg was able to improve sperm quality and decrease TUNEL positive cells in the experimental left varicocele rats; however, this dose could not decrease testicular levels of malondialdehyde(13). On the other hand, Orique et al.observed that intraperitoneal injection of RES at the doses of 1 and 10 mg/kg improved sperm motility in the hyperthyroid rats (31). As mentioned previously, RES is a Sirt-1 activator and this activation could repress p53-dependent apoptosis. By this mechanism, RES can prevent cardiomyocytes from apoptosis induced by hypoxia (32). This polyphenol also exerts its anti-apoptotic properties by inhibiting caspase-7 activity in neuroblastoma cell line exposed to paclitaxel (33). The involvement of these pathways in varicocele needs more investigation.
Conclusion
In summary, our results suggest that RES might be an adjuvant therapeutic option in patients with varicocele by decreasing inflammatory events and apoptosis. Further studies are required to show if RES increase fertility outcomes in patients with varicocele.
Acknowledgment
This study emanated from an MSc thesis (No. 2408) approved and supported by the Arak University of Medical Sciences, Arak, Iran.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Pasqualotto F, Agarwal A. Varicocele and male infertility: an evidence based review. Arch Med Sci. 2009;5:S20–27. [Google Scholar]
- 2.Galan J, De Felici M, Buch B, Rivero M, Segura A, Royo J, et al. Association of genetic markers within the KIT and KITLG genes with human male infertility. Hum Reprod. 2006;21:3185–3192. doi: 10.1093/humrep/del313. [DOI] [PubMed] [Google Scholar]
- 3.Krishna Reddy S. Varicocele and Male Infertility: Current Issues in Management-A Review. Med Surg Urol. 2014;3:137–142. [Google Scholar]
- 4.Sandlow J. Pathogenesis and treatment of varicoceles: Controversy still surrounds surgical treatment. BMJ. 2004;328:967–968. doi: 10.1136/bmj.328.7446.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habibi B, Seifi B, Mougahi S-H, Ojaghi M, Sadeghipour H. Increases in interleukin-6 and interferon-gamma levels is progressive in immature rats with varicocele. Ir J Med Sci (1971-) 2015;184:531–537. doi: 10.1007/s11845-014-1167-3. [DOI] [PubMed] [Google Scholar]
- 6.Zedler S, Faist E. The impact of endogenous triggers on trauma-associated inflammation. Curr Opin Crit Care. 2006;12:595–601. doi: 10.1097/MCC.0b013e3280106806. [DOI] [PubMed] [Google Scholar]
- 7.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y, Wang Y, Du L, Xu C, Cao J, Fan T, et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int J Mol Sci. 2013;14:14105–14118. doi: 10.3390/ijms140714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58:1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 11.Kefer JC, Agarwal A, Sabanegh E. Role of antioxidants in the treatment of male infertility. Int J Urol. 2009;16:449–457. doi: 10.1111/j.1442-2042.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendes TB, Paccola CC, de Oliveira Neves FM, Simas JN, da Costa Vaz A, Cabral REL, et al. Resveratrol improves reproductive parameters of adult rats varicocelized in peripuberty. Reproduction. 2016;152:23–35. doi: 10.1530/REP-16-0025. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Dayem M. Histological and immunohistochemical changes in the adult rat testes after left experimental varicocele and possible protective effects of resveratrol. Egypt J Histol. 2009;32:81–90. [Google Scholar]
- 15.Turner T. The study of varicocele through the use of animal models. Hum Reprod Update. 2001;7:78–84. doi: 10.1093/humupd/7.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Ku JH, Shim HB, Kim SW, Paick JS. The role of apoptosis in the pathogenesis of varicocele. BJU Int. 2005;96:1092–1096. doi: 10.1111/j.1464-410X.2005.05807.x. [DOI] [PubMed] [Google Scholar]
- 17.Schoor RA, Elhanbly SM, Niederberger CS. The pathophysiology of varicocele-associated male infertility. Curr Urol Rep. 2001;2:432–436. doi: 10.1007/s11934-001-0035-7. [DOI] [PubMed] [Google Scholar]
- 18.Paduch DA, Skoog SJ. Current management of adolescent varicocele. Rev Urol. 2001;3:120–133. [PMC free article] [PubMed] [Google Scholar]
- 19.Romeo C, Santoro G. Free radicals in adolescent varicocele testis. Oxid Med Cell Longev. 2014:2014. doi: 10.1155/2014/912878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semercioz A, Onur R, Ogras S, Orhan I. Effects of melatonin on testicular tissue nitric oxide level and antioxidant enzyme activities in experimentally induced left varicocele. Neuro Endocrinol Lett. 2003;24:86–90. [PubMed] [Google Scholar]
- 21.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 22.Fann DY-W, Lee S, Manzanero S, Tang S-C, Gelderblom M, Chunduri P, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790. doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Ibrahim E, de Rivero Vaccari JP, Lotocki G, Aballa TC, Dietrich WD, et al. Involvement of the inflammasome in abnormal semen quality of men with spinal cord injury. Ferti Steril. 2013;99:118–124. e112. doi: 10.1016/j.fertnstert.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Sulaiman M, Matta MJ, Sunderesan N, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;298:H833–H843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin Z, Celik-Ozenci C, Akkoyunlu G, Korgun ET, Acar N, Erdogru T, et al. Increased expression of interleukin-1αand interleukin-1βis associated with experimental varicocele. Ferti Steril. 2006;85:1265–1275. doi: 10.1016/j.fertnstert.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Nallella KP, Allamaneni SS, Pasqualotto FF, Sharma RK, Thomas AJ, Agarwal A. Relationship of interleukin-6 with semen characteristics and oxidative stress in patients with varicocele. Urology. 2004;64:1010–1013. doi: 10.1016/j.urology.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 27.Kilinc F, Kayaselcuk F, Aygun C, Guvel S, Egilmez T, Ozkardes H. Experimental varicocele induces hypoxia inducible factor-1α, vascular endothelial growth factor expression and angiogenesis in the rat testis. J Urol. 2004;172:1188–1191. doi: 10.1097/01.ju.0000135455.97627.15. [DOI] [PubMed] [Google Scholar]
- 28.Lee J-D, Jeng S-Y, Lee T-H. Increased expression of hypoxia-inducible factor-1αin the internal spermatic vein of patients with varicocele. J Urol. 2006;175:1045–1048. doi: 10.1016/S0022-5347(05)00417-9. [DOI] [PubMed] [Google Scholar]
- 29.Shao B-Z, Xu Z-Q, Han B-Z, Su D-F, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262–270. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 31.Ourique GM, Finamor IA, Saccol EM, Riffel AP, Pês TS, Gutierrez K, et al. Resveratrol improves sperm motility, prevents lipid peroxidation and enhances antioxidant defences in the testes of hyperthyroid rats. Rep Toxicol. 2013;37:31–39. doi: 10.1016/j.reprotox.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Chen C-J, Yu W, Fu Y-C, Wang X, Li J-L, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1–FoxO1 pathway. Biochem Biophys Res Commun. 2009;378:389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 33.Nicolini G, Rigolio R, Miloso M, Bertelli AA, Tredici G. Anti-apoptotic effect of trans-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett. 2001;302:41–44. doi: 10.1016/s0304-3940(01)01654-8. [DOI] [PubMed] [Google Scholar]


