Summary
Early-onset acromegaly causing gigantism is often associated with aryl-hydrocarbon-interacting receptor protein (AIP) mutation, especially if there is a positive family history. A15y male presented with tiredness and visual problems. He was 201 cm tall with a span of 217 cm. He had typical facial features of acromegaly, elevated IGF-1, secondary hypogonadism and a large macroadenoma. His paternal aunt had a history of acromegaly presenting at the age of 35 years. Following transsphenoidal surgery, his IGF-1 normalized and clinical symptoms improved. He was found to have a novel AIP mutation destroying the stop codon c.991T>C; p.*331R. Unexpectedly, his father and paternal aunt were negative for this mutation while his mother and older sister were unaffected carriers, suggesting that his aunt represents a phenocopy.
Learning points:
Typical presentation for a patient with AIP mutation with excess growth and eunuchoid proportions.
Unusual, previously not described AIP variant with loss of the stop codon.
Phenocopy may occur in families with a disease-causing germline mutation.
Background
Early-onset acromegaly causing gigantism is associated with mutations in the aryl-hydrocarbon-interacting receptor protein (AIP) gene in 30–40% of the cases (1, 2), but chances are even higher if there is a positive family history of acromegaly. Here, we present a case with typical clinical features with a novel AIP mutation but unexpected genetic testing results in the family.
Case presentation
A 15-year-old male was referred to the endocrinology clinic for excessive fatigue and sleepiness. He was born through normal vaginal delivery and his birth weight was 3.5 kg. He grew normally until he was 11, after which he experienced an accelerated height velocity and increase in weight. He developed voice change around the age of 12 years and began growing pubic hair when he was 13 years. He developed left hip pain when he was 14 and was found to have mild scoliosis and his right leg was 2.5 cm shorter than the left. He noticed tingling of both hands and gradual loss of vision over 6 months, which rapidly deteriorated about 2 weeks prior to his presentation. His mother mentioned that he snored heavily during sleep and slept on a semi-reclined couch because he was unable to lay flat.
His father was First Nation’s descent and mother was Caucasian. Mid-parental height was 179.2 cm (50th–75th percentile). His parents and older sister had normal height and no medical concerns. His paternal aunt had undergone surgery for a growth hormone-producing adenoma at the age of 35 years.
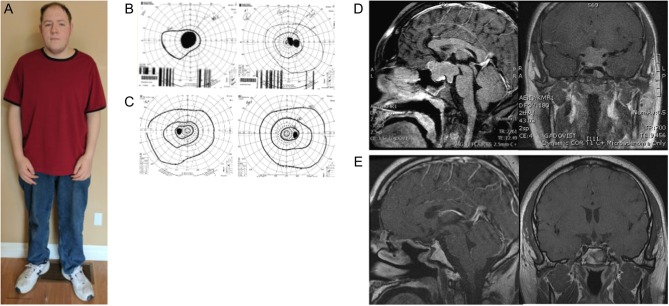
On examination, his height was 201.2 cm and weight was 126.2 kg (BMI = 31.3 kg/m2) (Fig. 1A). His shoe size was American 16 EEE (extra-wide). His sitting height was 100 cm, arm span was 217 cm and bone age was between 14.5 and 15 years. He had evidence of scoliosis. Goldmann visual fields showed bilateral hemianopia (Fig. 1B). His skin was pale and greasy, facial features were coarse with significant prognathism and widely spaced teeth. Tinel’s sign was negative.
Figure 1.
(A) Patient 5 years after surgery; Goldmann visual field test before (B) and after (C) surgery; MRI images of the adenoma before (D) and after surgery (E).
Investigation
Goldmann visual field tests revealed bilateral hemianopia (Fig. 1B). His baselines endocrine investigations showed a 09:00 h cortisol of 98 nmol/L ((normal range (NR) = 145–612), TSH: 2.37 IU/L (NR = 0.35–5.4), fT4: 8.8 pmol/L (NR = 11–19), prolactin: 15.4 µg/L (NR = 2.1–17.7), random GH: 13.4 µg/L (NR < 3.0), IGF-1: 1600 µg/L (NR = 232–1077 for his age and sex), testosterone <0.3 nmol/L (NR = 8.0–32), FSH: 0.5 IU/L (NR = 1.5–9.3), LH: 0.4 IU/L (NR = 1.4–18.1), total calcium: 2.31 mmol/L (NR = 2.23–2.58) and nadir GH after 75 g oral glucose tolerance test of 7.9 µg/L. His MRI scan showed a 4.0 × 3.3 × 2.8 cm pituitary macroadenoma with bilateral cavernous sinus and suprasellar extension, which was hyperintense on T2 weighted images (Fig. 1C). The X-ray of the hips did not show any radiological evidence of slipped femoral epiphyses or avascular necrosis.
Treatment
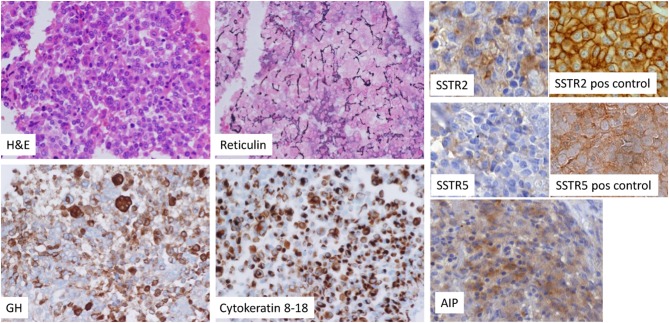
A diagnosis of gigantism with eunuchoid proportions and associated hypopituitarism was made on the basis of typical clinical features, elevated serum IGF-1 and non-suppressed GH after oral glucose. Pituitary replacement therapy was initiated with oral hydrocortisone, thyroxine and long-acting injectable testosterone. He underwent transsphenoidal excision of the pituitary tumor and the pathology confirmed a sparsely granulated eosinophilic somatotroph adenoma (Fig. 2) with a Ki-67 index of 5% and positive immunostaining for GH as well as scattered staining for prolactin and TSH. SSTR2 and SSTR5 staining did not show characteristic membranous staining. AIP staining showed faint positivity. Surgery led to normalization of GH and IGF-1 with post-OGTT GH of 0.2 µg/L and IGF-1 of 302 µg/L. Post-surgery MRI is shown in Fig. 1D.
Figure 2.
Haematoxylin & eosin (H&E), reticulin, GH, cytokeratin 8–18 staining, AIP (Novus, 1:1000), SSTR2 (Abcam, 1:500) and SSTR5 (Abcam, 1:100) staining (10). For the latter two stainings positive controls are provided (images adjusted from (10).
Outcome and follow-up
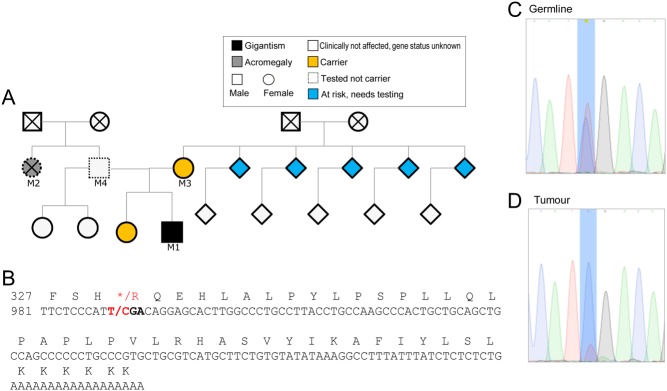
His visual fields normalized with complete restoration of vision after surgery (Fig. 1C) and the tingling of his hands also improved. He remains in remission 5 years after surgery with serum IGF-1 of 225 µg/L (NR = 147–283) and fasting GH of 0.26 µg/L as well as pituitary replacement therapy. Based on the family history, invasive macroadenoma, male gender and young age at presentation, he was offered genetic testing. The test identified an aryl-hydrocarbon-interacting receptor protein (AIP) variant c.991T>C; p.*331R (Fig. 3), which has not been previously described. Sequencing of the AIP gene in the tumor DNA suggest the presence of loss of heterozygosity (Fig. 3C and D), although some normal tissue was present in the sample. Subsequent family screening showed the same variant in her mother and sister (Fig. 3A); physical examination and radiological or biochemical assessments showed no abnormalities. Siblings of the patient’s mother were invited for genetic testing. The proband’s father was negative for the mutation. We also tested a paraffin block available from his paternal aunt and no mutation was detected, suggesting that her case was a phenocopy.
Figure 3.
(A) Family tree and C-terminal sequence of AIP showing amino acids from codon 327 and (B) nucleotides from c.981 based on NM_003977 coding cDNA. The stop codon TGA (*) is mutated to CGA, which is coding for arginine (R). No stop codons are present in the rest of the RNA. Germline (C) and tumor (D) DNA sequence of the proband, showing reduced height for ‘T’ (normal sequence) allele. The lack of complete absence of the T allele corresponds to the pathological report suggesting some normal tissue in the sample.
Discussion
This patient had a typical clinical course of an AIP mutation-related pituitary adenoma with early-onset disease causing gigantism not just due to the high levels of GH but also due to the hypogonadism-induced growth causing eunuchoid proportions: his span was 16 cm larger than height, although the scoliosis and the shorter leg on one side might have confounded these measurements.
The variant identified in this patient is unusual as it disrupts the stop codon and replaces it with an arginine. There is no stop codon in the 3′UTR sequence of the cDNA (Fig. 3). This change is predicted to cause a lengthened protein, which might be misfolded and therefore rapidly degraded (3). Stop loss mutations are generally considered damaging (4). Although the tumor sample contained some normal tissue, the presence of loss of heterozygosity supports the pathogenicity of this variant. AIP staining is known to be unreliable for distinguishing AIP mutation-positive tumors from negative ones (5, 6).
It was quite unexpected that the patient’s father and the sample from paternal aunt did not carry the mutation despite our initial anticipation that the paternal aunt’s disease was also due to this AIP mutation. Phenocopies, microprolactinoma or acromegaly has previously been described in families with AIP mutations (7, 8, 9).
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written, informed consent has been obtained from the patient for the publication of this article.
Author contribution statement
S A I, K A A, L P and D B C cared for the patient, S E C performed pathologic analysis, D C and D I performed experiments and S A I and M K wrote the paper.
References
- 1.Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, et al Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocrine-Related Cancer 2015. 22 745–757. ( 10.1530/ERC-15-0320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacovazzo D, Caswell R, Bunce B, Jose S, Yuan B, Hernandez-Ramirez LC, Kapur S, Caimari F, Evanson J, Ferrau F, et al Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study. Acta Neuropathologica Communications 2016. 4 56 ( 10.1186/s40478-016-0328-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández-Ramírez LC, Martucci F, Morgan RM, Trivellin G, Tilley D, Ramos-Guajardo N, Iacovazzo D, D’Acquisto F, Prodromou C, Korbonits M, et al. Rapid proteasomal degradation of mutant proteins is the primary mechanism leading to tumorigenesis in patients with missense AIP mutations. Journal of Clinical Endocrinology and Metabolism 2016. 101 3144–3154. ( 10.1210/jc.2016-1307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernansaiz-Ballesteros RD, Salavert F, Sebastian-Leon P, Aleman A, Medina I, Dopazo J. Assessing the impact of mutations found in next generation sequencing data over human signaling pathways. Nucleic Acids Research 2015. 43 W270–W275. ( 10.1093/nar/gkv349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leontiou CA, Gueorguiev M, van der Spuy J, Quinton R, Lolli F, Hassan S, Chahal HS, Igreja SC, Jordan S, Rowe J, et al The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. Journal of Clinical Endocrinology and Metabolism 2008. 93 2390–2401. ( 10.1210/jc.2007-2611) [DOI] [PubMed] [Google Scholar]
- 6.Jaffrain-Rea ML, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen JF, D’Innocenzo E, Barlier A, Giangaspero F, Esposito V, et al. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocrine-Related Cancer 2009. 16 1029–1043. ( 10.1677/ERC-09-0094) [DOI] [PubMed] [Google Scholar]
- 7.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, et al Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 2006. 312 1228–1230. ( 10.1126/science.1126100) [DOI] [PubMed] [Google Scholar]
- 8.Williams F, Hunter S, Bradley L, Chahal HS, Storr H, Akker SA, Kumar AV, Orme SM, Evanson J, Morrison PJ, et al. Clinical experience in the screening and management of a large kindred with familial isolated pituitary adenoma due to an aryl hydrocarbon receptor interacting protein (AIP) mutation. Journal of Clinical Endocrinology and Metabolism 2014. 99 1122–1131. ( 10.1210/jc.2013-2868) [DOI] [PubMed] [Google Scholar]
- 9.Niyazoglu M, Sayitoglu M, Firtina S, Hatipoglu E, Gazioglu N, Kadioglu P. Familial acromegaly due to aryl hydrocarbon receptor-interacting protein (AIP) gene mutation in a Turkish cohort. Pituitary 2014. 17 220–226. ( 10.1007/s11102-013-0493-1) [DOI] [PubMed] [Google Scholar]
- 10.Iacovazzo D, Carlsen E, Lugli F, Chiloiro S, Piacentini S, Bianchi A, Giampietro A, Mormando M, Clear AJ, Doglietto F, et al Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: an immunohistochemical study. European Journal of Endocrinology 2016. 174 241–250. ( 10.1530/EJE-15-0832) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a