Abstract
Lactoferrin is a multifunctional glycoprotein with therapeutic potential for bone tissue engineering. The aim of this study was to assess the efficacy of local application of lactoferrin on bone regeneration. Five‐millimetre critical‐sized defects were created over the right parietal bone in 64 Sprague–Dawley rats. The rats were randomized into four groups: group 1 (n = 20) had empty defects; group 2 (n = 20) had defects grafted with collagen gels (3 mg/ml); group 3 (n = 20) had defects grafted with collagen gels impregnated with bovine lactoferrin (10 μg/gel); and group 4 (n = 4) had sham surgeries (skin and periosteal incisions only). The rats were sacrificed at 4 or 12 weeks post‐operatively, and the calvaria were excised and evaluated with micro‐CT (Skyscan 1172) followed by histology. The bone volume fraction (BV/TV) was higher in lactoferrin‐treated animals at both timepoints, with groups 1, 2, 3 and 4 measuring 10.5 ± 1.1%, 8.6 ± 1.4%, 16.5 ± 0.6% and 24.27 ± 2.6%, respectively, at 4 weeks (P < 0.05); and 12.2 ± 1.3%, 13.6 ± 1.5%, 21.9 ± 1.2% and 29.3 ± 0.8%, respectively, at 12 weeks (P < 0.05). Histological analysis revealed that the newly formed bone within the calvarial defects of all groups was a mixture of woven and lamellar bone, with more bone in the group treated with lactoferrin at both timepoints. Our study demonstrated that local application of lactoferrin significantly increased bone regeneration in a rat critical‐sized calvarial defect model. The profound effect of lactoferrin on bone regeneration has therapeutic potential to improve the poor clinical outcomes associated with bony non‐union. LF In Vivo JTERM Authors Contributions. Copyright © 2016 The Authors Journal of Tissue Engineering and Regenerative Medicine Published by John Wiley & Sons, Ltd.
Keywords: lactoferrin, bone regeneration, bone healing, calvarial defect
1. Introduction
Bone grafting is one of the most common surgical procedures performed worldwide, and its indications range from reconstructive orthopaedic and maxillofacial surgery to spinal fusions. Globally, over two million bone grafting procedures are performed annually, and this number is projected to increase exponentially as a result of population aging (Brounts et al., 2013; Giannoudis et al., 2005).
Clinically, autologous bone remains the ‘gold standard’ graft choice for bone grafting. Whilst effective, the amount of autologous bone that can be safely harvested is limited, and the procedure can be associated with unacceptably high complication rates and donor site morbidity (Almaiman et al., 2013; Scheerlinck et al., 2013; Vura et al., 2013). Alternative graft choices, including allografts, xenografts and synthetic bone graft substitutes, have failed to perform as well as autologous bone graft. In addition, the use of allografts and xenografts can be limited due to the risk of disease transmission and foreign body responses (Shegarfi and Reikeras, 2009). In recent years, commercial forms of recombinant bone morphogenetic protein (rhBMP) have shown clinical efficacy, and are available for specific clinical applications including spinal fusions and treatment of long bone non‐unions (Kanakaris et al., 2008; Malham et al., 2015; Papanna et al., 2012). However, serious adverse effects and safety concerns have been reported in relation to the use of rhBMP, and its high cost can be prohibitive for widespread clinical use (Carragee et al., 2011; Comer et al., 2012; Epstein, 2013). Due to the intrinsic limitations associated with currently available bone grafting options, and the rising incidence of bony defects that exceeds the body's regenerative capabilities, the need to find improved bone graft materials has never been greater.
Lactoferrin, belonging to the transferrin family, is an iron‐binding glycoprotein present in milk, as well as other bodily secretions. This multifunctional glycoprotein plays a pivotal role in a range of biological processes, and has anti‐microbial, anti‐neoplastic, anti‐inflammatory and immunomodulatory activities (Amini and Nair, 2011; Gonzalez‐Chavez et al., 2009; Vogel, 2012). Furthermore, lactoferrin has been found to be a potent anabolic factor for bone growth. In vitro, lactoferrin dose‐dependently enhances the proliferation and differentiation of bone‐forming cells (osteoblasts), and potently inhibits the formation of bone‐resorbing cells (osteoclasts; Cornish et al., 2004). While in vivo, local injection of lactoferrin over the calvarium of mice resulted in a profound increase in bone formation compared with vehicle controls (Cornish et al., 2004). However, from a tissue engineering point of view, the effect of local application of lactoferrin on bone regeneration remains unclear. To our knowledge, only three studies have investigated the effect of local application of lactoferrin on bone regeneration. All three studies utilized calvarial defect models, with varying degrees of success. In both rat and pig calvarial defects, lactoferrin increased bone regeneration (Gormez et al., 2015; Takaoka et al., 2011), yet in a rabbit calvarial defect model, augmentation of a bovine bone graft (Bio‐Oss) with lactoferrin did not result in any significant improvement in bone regeneration (Paknejad et al., 2012).
Given the limited and inconsistent evidence on the effect of local application of lactoferrin for bone regeneration, we designed this study to further evaluate the effect of local application of lactoferrin on bone regeneration using a rat critical‐sized calvarial defect model. We hypothesized that local application of lactoferrin leads to increased bone regeneration.
2. Materials and Methods
Collagen/lactoferrin gel preparation.
Collagen gels (100 μl) were prepared in a sterile fashion in a tissue culture hood by neutralizing sterile rat collagen type I (In Vitro Technology, Auckland, New Zealand) with 2.3 μl of 1 m NaOH. The gels were diluted to a final concentration of 3 mg/ml using phosphate‐buffered saline. The gels were allowed to set at 37 °C for at least an hour before surgical implantation into the calvarial defects. Matthews et al. have previously utilized this type of collagen gel to assess its effect on osteoblastogenesis. In their study, the collagen gels remained intact after 24 days of culture (Matthews et al., 2014). In vivo, the degradation time of the type of collagen gels used in our study is unclear. For preparation of lactoferrin‐impregnated collagen gels, bovine lactoferrin (Fonterra Co‐operative Group, New Zealand) was incorporated into the 100 μl collagen gels at a concentration of 100 μg/ml. The bovine lactoferrin was thoroughly mixed with the collagen gel by pipetting the solution up and down 10 times. The gels were allowed to set at 37 °C for at least an hour before surgical implantation into the calvarial defects.
2.1. Surgical procedure
Ethical approval was obtained from the University of Auckland Animal Ethics Committee. In total, 64 sexually mature, similarly aged, male Sprague–Dawley rats weighing more than 250 g were bred for this study. Pre‐operatively, the rats were checked for general health, weighed and randomized into four groups.
Empty defects (n = 20): 5‐mm, bi‐cortical, extra‐dural defect created over the right parietal bone and left empty.
Collagen carrier group (n = 20): 5‐mm, bi‐cortical, extra‐dural defect created over the right parietal bone, which was grafted with a collagen gel (3 mg/ml).
Lactoferrin‐treated group (n = 20): 5‐mm, bi‐cortical, extra‐dural defect created over the right parietal bone, which was grafted with a collagen gel impregnated with bovine lactoferrin (10 μg/gel).
Sham surgery (n = 4): midline incision through skin and periosteum down to parietal bone without creation of a bony defect.
Two hours prior to surgery, the rats were pre‐medicated with a subcutaneous injection of a non‐steroidal anti‐inflammatory analgesic, carprofen (10 μl/g; Norbrook, New Zealand). Anaesthetic induction was performed in a rat induction box with continuous isoflurane, and anaesthesia was maintained through a specialized nose cone (5% isoflurane with 2 l oxygen for induction and 2.5% isoflurane with 2 l oxygen for maintenance). A 2.5‐ml subcutaneous injection of normal saline was administered immediately following induction to account for fluid losses during surgery.
Once the rats were appropriately anaesthetized, they were placed prone with the calvaria shaved and disinfected with 2% chlorhexidine and 70% ethanol (NZHealthe, New Zealand). The surgical area was draped using OpSite Incise Drape (Smith&Nephew, New Zealand). An incision centred over the sagittal suture was made down to the periosteum. The periosteum was divided in line with the skin incision and elevated as a single flap. A 5‐mm‐diameter, bicortical, extra‐dural defect was created over the right parietal bone using a trephine burr (Komet Trephine, HenrySchein, New Zealand) attached to a hand‐held dental drill (Saeshin Thumb Set & Mini Contra Angle Hand Piece, HenrySchein, New Zealand). The dental drill was set at 10 000 revolutions per minute (RPM) and the trephine tip was cooled with normal saline flushes during the burring process. The periosteal flap was then reflected over the defects and sutured to the contralateral side using a 4/0 Monocryl suture (Amtech Medical, New Zealand). The periosteal flap functioned as an envelope to contain the implants (Figure 1). The skin was then closed using continuous subcuticular 4/0 Monocryl sutures (Amtech Medical, New Zealand). Following skin closure, 0.2 ml of marcain (1.25 mg/ml solution) was infiltrated around the surgical site for post‐operative analgesia (Amtech Medical, New Zealand).
Figure 1.

Creation of calvarial defect. (A) Incision through the skin over the saggital suture. (B) A 5‐mm‐diameter trephine burr was used to create the bicortical defect. (C) A 5‐mm‐diameter bicortical right parietal bone calvarial defect shown with overlying periosteal envelope
Post‐operatively, the rats were housed singularly and transferred to a warming cabinet overnight for recovery. Carprofen (10 μl/g) and normal saline (2 ml) were administered subcutaneously twice daily for 48 h post‐operatively for analgesia and fluid replacement. The rats were weighed daily and monitored for signs of illness, infection, pain or distress twice daily for the first two post‐operative days. Following this, they were weighed and checked daily until post‐operative day 7. They were then weighed and monitored on a weekly basis.
2.2. Tissue processing
At the end of the experimental procedures, the rats were euthanised with CO2 inhalation. The calvaria containing both the parietal lobes and parts of the frontal and occipital lobes were excised. The calvaria were immediately fixed in 10% neutral buffered formalin and stored at 4 °C. After 3 days, the calvaria were transferred to 70% ethanol for storage at 4 °C until analysis by micro‐CT.
2.3. Micro‐CT
All the specimens were scanned using a Skyscan 1172 micro‐CT scanner (X‐ray voltage 50 kV, 1.0 mm aluminium filter, isotropic voxel size 12 μm). After standardized reconstruction using NRecon software, the datasets were analysed using CTAn software (Bruker micro‐CT, Belgium). To quantitatively evaluate newly formed bone, a cylindrical volume of interest with a diameter of 5 mm and a height of 2.196 mm was concentrically positioned over the defect site in the axial plane. Outcome measures included the bone volume fraction (BV/TV) and tissue mineral density (TMD).
2.4. Histology
Following micro‐CT, the specimens were decalcified using 10% formic acid for 1 week. After decalcification, the specimens were paraffin embedded in a Leica APS 300S auto processor. Histological sections (10 μm in thickness) were prepared from the mid‐point of the defects in the coronal plane using a Leica Microtome RM 2145. Two sections per sample were placed on Leica ApexTM Superior adhesive slides and stained with haematoxylin and eosin (H&E). Digital images of the stained sections were obtained using the Olympus BX50F microscope with an Olympus DP72 digital colour camera (Olympus, Japan). All the H&E‐stained sections were evaluated by a blinded musculoskeletal pathologist.
2.5. Statistical analysis
All data were statistically analysed by analysis of variance (anova) with post hoc Tukey's test, and expressed as mean ± standard error of the mean (SEM). Graph pad prism 6 software (Graph pad software, USA) was used for statistical analysis.
3. Results
Two lactoferrin‐treated specimens from the 4‐week cohort were excluded from analysis. One rat died on the third post‐operative day due to bowel obstruction and the other specimen was excluded due to damage to the calvarial bone during tissue excision. All other animals tolerated the procedure well and made satisfactory progress post‐operatively. There was no evidence of local or systemic infection in any of the animals throughout the peri‐operative period.
3.1. Micro‐CT results
At the 4‐week timepoint, bone regeneration, as measured by the bone volume fraction (BV/TV), was significantly higher in the lactoferrin‐treated animals (16.5 ± 0.6%) compared with group 1 (10.5 ± 1.1%) and group 2 (8.6 ± 1.4%; P < 0.05). By the 12 week post‐operative mark, the bone volume fraction increased in all four experimental groups compared with the 4‐week timepoint (Figure 2). However, the increase was much more profound in the lactoferrin‐treated group (21.9 ± 1.2%) compared with group 1 (12.2 ± 1.3%) and group 2 (13.6 ± 1.5%; P < 0.05). The animals in group 4 (sham surgeries) had the highest BV/TV compared with the other three groups at both 4 weeks (24.27 ± 2.6%) and 12 weeks (29.3 ± 0.8%). There was no difference in TMD between the groups at either the 4 or 12 week post‐operative mark.
Figure 2.
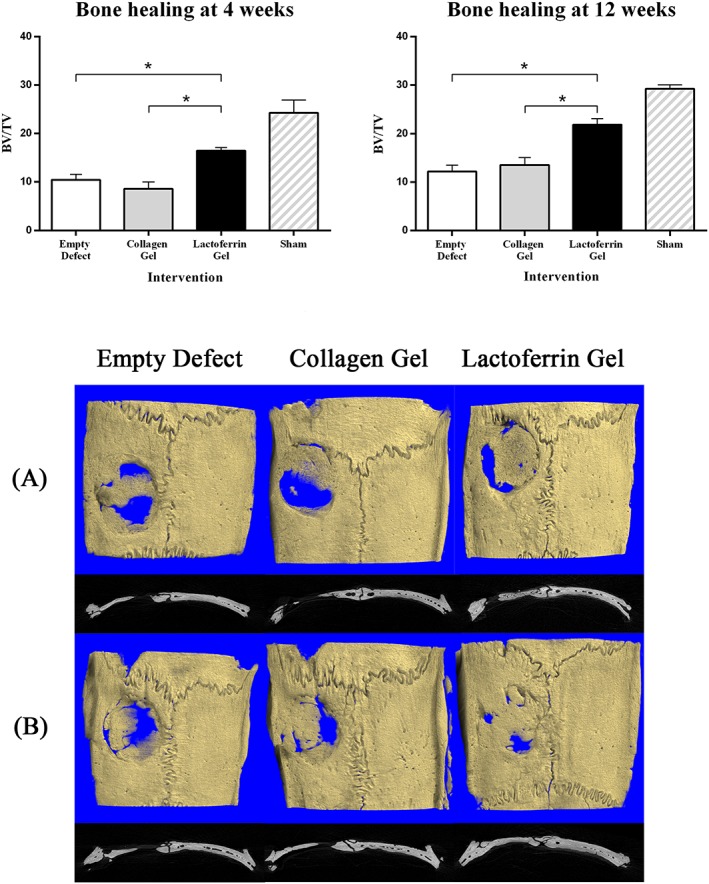
Top: bone volume fraction at 4 weeks (left) and 12 weeks (right). Bottom: representative 3D images of calvarial defects at 4 weeks (A) and 12 weeks (B). Treatment groups include empty defects (left); defects treated with a 100 μl collagen gel (3 mg/ml; middle); and defects treated with a 100 μl collagen gel containing bovine lactoferrin (10 μg; right)
3.2. Histology
Histological assessment by a musculoskeletal pathologist blinded to the treatment groups revealed that there was no evidence of residual gels or infection in any of the specimens, nor was there excessive presence of inflammatory cells in any of the specimens at both timepoints.
In keeping with the micro‐CT findings, the lactoferrin‐treated group had greater amounts of regenerated bone at both 4 and 12 weeks (Figure 3A). At higher magnification, large amounts of fibrous tissue were seen in the empty defect and collagen‐treated groups. A mixture of lamellar and woven bone were identified without any differences in the types of bone formed between the three groups at both timepoints (Figure 3B).
Figure 3.
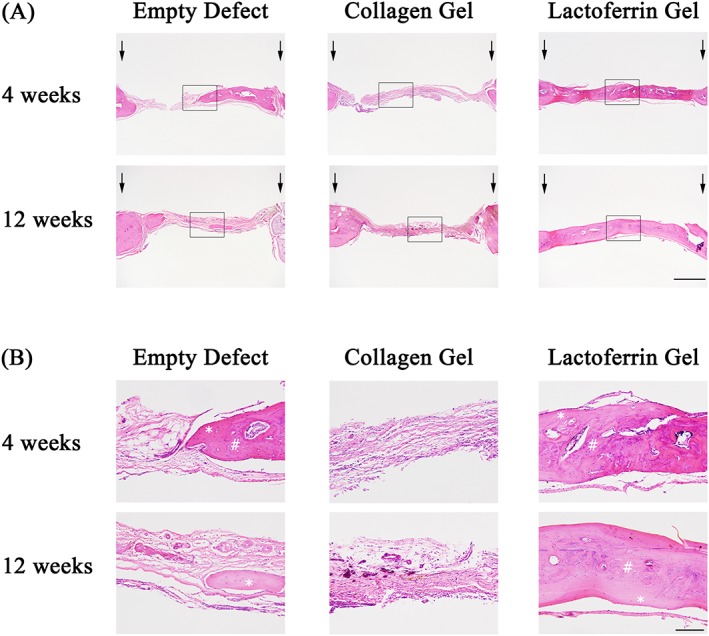
Histological assessment of bone regeneration at 4 and 12 weeks post‐treatment. Coronal sections through the mid‐point of the defects were prepared from de‐calcified specimens and stained with haematoxylin and eosin (H&E). (A) Coronal sections demonstrating the calvarial defects with arrows identifying the original defect edge. Scale bar: 1 mm. (B) Coronal images taken from within the calvarial defects corresponding to the rectangular box from (A). #Represents woven bone; *represents lamellar bone. Scale bar: 150 μm
3.2.1. Discussion
Here, we have shown that local application of lactoferrin significantly improved bone regeneration in a rat critical‐sized calvarial defect model. This study represents the largest in vivo study evaluating the role of local application of lactoferrin as an anabolic growth factor in the context of bone regeneration.
Using micro‐CT, the bone volume fractions (BV/TV) in all three groups were able to be quantified, and the results demonstrated a profound increase in bone regeneration in response to targeted, local lactoferrin treatment at both the early (4 week) and late (12 week) timepoints. The increase in bone volume fraction in the lactoferrin group was not accompanied by any increase in TMD, suggesting that the degrees of mineralization of newly formed bone were similar between the three goups (Bouxsein et al., 2010). Microscopically, increased bone regeneration was observed in the group treated with lactoferrin compared with the other two groups. Evidence of remodelling was seen in the form of a mixture of lamellar and woven bone in all treatment groups at both timepoints. There was no difference between the groups in terms of the types of bone formed or the degree of inflammation, suggesting that the anabolic effect of lactoferrin was unlikely to be related to its well‐known immune modulatory properties (Adlerova et al., 2008; Gonzalez‐Chavez et al., 2009; Malet et al., 2011; Vogel, 2012).
The role of lactoferrin as an anabolic agent for bone health was first published over a decade ago (Cornish et al., 2004). In this study, the positive effect of lactoferrin on bone health was attributed, at least in part, to the synergistic ability of this molecule to enhance the survival and differentiation of osteoblasts and inhibit osteoclastogenesis (Cornish et al., 2004). In the ensuing period, a number of studies have investigated the mechanism through which lactoferrin functions to improve bone healing and regeneration. At a molecular level, the anabolic effect of lactoferrin on bone regeneration has been attributed to its mitogenic effect on host osteoblasts, an effect mediated through the low‐density lipoprotein receptor‐related protein‐1 (LRP1) and activation of p42/44 mitogen‐activated protein kinase (MAPK) signalling and the PI3‐kinase‐dependent phosphorylation of Akt in osteoblasts (Cornish et al., 2006; Grey et al., 2004; Naot et al., 2011).
From an osteoporosis point of view, a number of in vivo studies have reiterated the beneficial role of lactoferrin on systemic bone health in osteoporotic animal models. Malet et al. (2011) investigated the effect of oral bovine lactoferrin on bone health in ovariectomized mice. The authors showed that the improvement of bone status by oral lactoferrin is mediated through modulation of antigen presentation properties, T‐cell activation and other immunological functions (Malet et al., 2011). The result from this study is supported by three other studies demonstrating that systemic administration of bovine lactoferrin resulted in improved bone mass and bone microarchitecture in ovariectomized rats or mice (Blais et al., 2009; Guo et al., 2009; Hou et al., 2012).
In the context of bone tissue engineering, evidence has emerged demonstrating the therapeutic potential of systemic administration of lactoferrin for bone tissue regeneration. Oral administration of lactoferrin has been shown to increase bone regeneration in a rabbit distraction osteogenesis model (Li et al., 2015). In this study, the authors postulated that the beneficial role of systemic administration of lactoferrin on bone regeneration was due to its ability to increase the expression of osteoprotegrin (OPG) and decrease the expression of receptor activator of nuclear factor‐kappa‐B ligand (RANKL) in the distraction gap (Li et al., 2015). In bone formation and remodelling, RANKL/RANK signalling regulates the formation and survival of osteoclasts. OPG, on the other hand, functions as a decoy receptor to RANKL, hence OPG protects the skeleton from excessive bone resorption. Thus, by modulating the RANKL/RANK/OPG pathway in a favourable manner, Li et al. showed a plausible mechanism through which lactoferrin increases bone regeneration in a distraction osteogenesis model. Lactoferrin delivered locally to a site of bony defect has the potential to modulate the host osteoblastic gene expression and the RANKL/RANK/OPG pathway in a favourable pattern. Further studies are warranted to explore this potential mechanism of action.
Despite the pre‐clinical evidence demonstrating therapeutic potential of systemic administration of lactoferrin in improving bone health and bone tissue regeneration, concerns remain regarding the poor bioavailability of orally administered lactoferrin in humans (Kuwata et al., 2001; Mikogami et al., 1994; Yao et al., 2015). Evidence shows that lactoferrin is prone to enzymatic degradation in the human gastrointestinal tract, severely limiting the bioavailability of this molecule (Yao et al., 2015). In order to circumvent the potential problem with poor oral bioavailability, a more appropriate route of delivery of lactoferrin may be in the form of a local agent directly to the site of skeletal defect. Clinically, targeted delivery of growth factors directly to the site of a skeletal defect has several clear advantages over systemic administration, and may therefore be a preferred route of administration. Firstly, a desired therapeutic effect can often be achieved with a lower dosage when a growth factor is given locally compared with systemically. Reducing the drug dosage not only lowers the manufacturing costs but also minimizes the chance of causing adverse drug reactions. Secondly, locally active growth factors can be combined with orthopaedic implants or scaffolds to act synergistically to improve osteointegration and ultimately lead to better clinical outcomes. Despite the clear advantages of local delivery, the evidence on the therapeutic potential of lactoferrin as a local growth factor is sparse. Only three in vivo studies have evaluated the effect of lactoferrin as a local growth factor for bone regeneration. All three studies utilized calvarial defect models and their results have been inconsistent (Gormez et al., 2015; Paknejad et al., 2012; Takaoka et al., 2011). In one study, a combination of bovine lactoferrin‐loaded gelatin microspheres and bovine bone promoted bone regeneration around dental implants in a swine calvarial defect model (Gormez et al., 2015). However, this study did not include a delivery control group involving defects treated with gelatin microsphere alone, making it difficult to attribute the improved bone regeneration to the effect of lactoferrin alone (Gormez et al., 2015). In another study utilizing a rat calvarial defect model, the authors showed that local application of a lactoferrin gelatin hydrogel significantly improved bone regeneration (Takaoka et al., 2011). However, the small number of animals in each treatment arm (n = 3) limited the study conclusions. The final study investigating the effect of local application of lactoferrin on bone regeneration did not show any advantage of combining lactoferrin with an anorganic bovine bone graft in a rabbit calvarial defect model (Paknejad et al., 2012). In this study, four defects were created in each animal, which may lead to problems with cross‐contamination when different materials are grafted into each defect. It is conceivable that lactoferrin grafted into one defect may leak into surrounding defects and affect the outcome of the study.
Given the paucity of published data and the intrinsic limitations with existing evidence on the effect of lactoferrin on bone regeneration, our study was performed to further define the role of local application of lactoferrin on bone regeneration. The results from our study provide further support to show that lactoferrin is an effective local growth factor for bone tissue regeneration. The strengths of our study include the large sample size (n = 64) and having both early (4 week) and late (12 week) timepoints to delineate any potential temporal effect of lactoferrin on bone healing. In addition, we created a single defect in each animal to eliminate potential problems with cross‐contamination if multiple defects were created in the same animal. One potential limitation to our study is the retained periosteum, which may overestimate the osteogenic potential of lactoferrin. However, the authors believe that any confounding effect of the retained periosteum is likely to be small given the randomization process and that a single surgeon performed the operations in all animals. The rationale for retaining the periosteum was so that it could function as a biological mechanical sleeve to prevent migration of the implants away from the calvarial defect. Another inherent limitation of our study is the calvarial defect model itself, which does not permit physiological biomechanical loading to occur. Nevertheless, our findings, viewed in the context of the existing literature, should encourage future studies utilizing more clinically relevant, load‐bearing models such as spinal fusion and long bone defect models. Future studies should focus on delineating the mechanisms by which lactoferrin functions to increase bone regeneration in vivo.
In conclusion, this study demonstrated that local application of lactoferrin significantly increased bone regeneration in a rat critical‐sized calvarial defect model. The profound anabolic effect of lactoferrin was evident as early as 4 weeks and persisted until 12 weeks post‐implantation. The additional putative roles of lactoferrin as an anti‐microbial, anti‐inflammatory and immuno‐modulatory agent make this naturally occurring glycoprotein an attractive growth factor for bone regeneration.
Sponsors
EU FP7‐ ‘SkelGEN’ under grant agreement number 318553; New Zealand Health Research Council Clinical Research Training Fellowship; New Zealand Orthopaedic Association Research Foundation; New Zealand Wishbone Trust.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1. Supporting info item
Acknowledgements
Dr Ryan Gao was funded by a Clinical Research Training Fellowship by The New Zealand Health Research Council. This study was partially funded by New Zealand Orthopaedic Association Research Foundation, New Zealand Wishbone Trust and supported by the European Union Seventh Framework Programme skelGEN (FP7/2007‐2013) under grant agreement no. (318553).
Gao, R. , Watson, M. , Callon, K. E. , Tuari, D. , Dray, M. , Naot, D. , Amirapu, S. , Munro, J. T. , Cornish, J. , and Musson, D. S. (2018) Local application of lactoferrin promotes bone regeneration in a rat critical‐sized calvarial defect model as demonstrated by micro‐CT and histological analysis. J Tissue Eng Regen Med, 12: e620–e626. doi: 10.1002/term.2348.
References
- Adlerova L, Bartoskova A, Faldyna M. 2008; Lactoferrin: a review. Vet Med 53: 457–468. [Google Scholar]
- Almaiman M, Al‐Bargi HH, Manson P. 2013; Complication of anterior iliac bone graft harvesting in 372 adult patients from may 2006 to may 2011 and a literature review. Craniomaxillofac Trauma Reconstr 6: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini AA, Nair LS. 2011; Lactoferrin: a biologically active molecule for bone regeneration. Curr Med Chem 18: 1220–1229. [DOI] [PubMed] [Google Scholar]
- Blais A, Malet A, Mikogami T, Martin‐Rouas C, Tome D. 2009; Oral bovine lactoferrin improves bone status of ovariectomized mice. Am J Physiol Endocrinol Metab 296: E1281–E1288. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. 2010; Guidelines for assessment of bone microstructure in rodents using micro‐computed tomography. J Bone Miner Res 25: 1468–1486. [DOI] [PubMed] [Google Scholar]
- Brounts SH, Lee JS, Weinberg S, Lan Levengood SK, Smith EL, Murphy WL. 2013; High affinity binding of an engineered, modular peptide to bone tissue. Mol Pharm 10: 2086–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. 2011; A critical review of recombinant human bone morphogenetic protein‐2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11: 471–491. [DOI] [PubMed] [Google Scholar]
- Comer GC, Smith MW, Hurwitz EL, Mitsunaga KA, Kessler R, Carragee EJ. 2012; Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein‐2 augmentation: a 10‐year cohort controlled study. Spine J 12: 881–890. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Naot D et al 2004; Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 145: 4366–4374. [DOI] [PubMed] [Google Scholar]
- Cornish J, Palmano K, Callon KE et al 2006; Lactoferrin and bone; structure‐activity relationships. Biochem Cell Biol 84: 297–302. [DOI] [PubMed] [Google Scholar]
- Epstein NE. 2013; Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int 4: S343–S352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, Tsiridis E. 2005; Bone substitutes: an update. Injury 36 Suppl 3: S20–S27. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Chavez SA, Arevalo‐Gallegos S, Rascon‐Cruz Q. 2009; Lactoferrin: structure, function and applications. Int J Antimicrob Agents 33: 301.e1‐301.e8. [DOI] [PubMed] [Google Scholar]
- Gormez U, Kurkcu M, Benlidayi M, Ulubayram K, Sertdemir Y, Daglioglu K. 2015; Effects of bovine lactoferrin in surgically created bone defects on bone regeneration around implants. J Oral Sci 57: 7–15. [DOI] [PubMed] [Google Scholar]
- Grey A, Banovic T, Zhu Q et al 2004; The low‐density lipoprotein receptor‐related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol 18: 2268–2278. [DOI] [PubMed] [Google Scholar]
- Guo HY, Jiang L, Ibrahim SA et al 2009; Orally administered lactoferrin preserves bone mass and microarchitecture in ovariectomized rats. J Nutr 139: 958–964. [DOI] [PubMed] [Google Scholar]
- Hou JM, Xue Y, Lin QM. 2012; Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via OPG/RANKL/RANK pathway. Chung Kuo Yao Li Hsueh Pao 33: 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakaris NK, Calori GM, Verdonk R et al 2008; Application of BMP‐7 to tibial non‐unions: a 3‐year multicenter experience. Injury 39 Suppl 2: S83–S90. [DOI] [PubMed] [Google Scholar]
- Kuwata H, Yamauchi K, Teraguchi S et al 2001; Functional fragments of ingested lactoferrin are resistant to proteolytic degradation in the gastrointestinal tract of adult rats. J Nutr 131: 2121–2127. [DOI] [PubMed] [Google Scholar]
- Li W, Zhu S, Hu J. 2015; Bone regeneration is promoted by orally administered bovine lactoferrin in a rabbit tibial distraction osteogenesis model. Clin Orthop Relat Res 473: 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malet A, Bournaud E, Lan A, Mikogami T, Tome D, Blais A. 2011; Bovine lactoferrin improves bone status of ovariectomized mice via immune function modulation. Bone 48: 1028–1035. [DOI] [PubMed] [Google Scholar]
- Malham GM, Giles GG, Milne RL, Blecher CM, Brazenor GA. 2015; Bone morphogenetic proteins in spinal surgery: what is the fusion rate and do they cause cancer? Spine (Phila Pa 1976) 40: 1737–1742. [DOI] [PubMed] [Google Scholar]
- Matthews BG, Naot D, Callon KE et al 2014; Enhanced osteoblastogenesis in three‐dimensional collagen gels. Bonekey Rep 3: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikogami T, Heyman M, Spik G, Desjeux JF. 1994; Apical‐to‐basolateral transepithelial transport of human lactoferrin in the intestinal cell line HT‐29cl.19A. Am J Phys 267: G308–G315. [DOI] [PubMed] [Google Scholar]
- Naot D, Chhana A, Matthews BG et al 2011; Molecular mechanisms involved in the mitogenic effect of lactoferrin in osteoblasts. Bone 49: 217–224. [DOI] [PubMed] [Google Scholar]
- Paknejad M, Rokn AR, Yaraghi AA, Elhami F, Kharazifard MJ, Moslemi N. 2012; Histologic and histomorphometric evaluation of the effect of lactoferrin combined with anorganic bovine bone on healing of experimentally induced bony defects on rabbit calvaria. Dent Res J (Isfahan) 9: S75–S80. [PMC free article] [PubMed] [Google Scholar]
- Papanna MC, Al‐Hadithy N, Somanchi BV et al 2012; The use of bone morphogenic protein‐7 (OP‐1) in the management of resistant non‐unions in the upper and lower limb. Injury 43: 1135–1140. [DOI] [PubMed] [Google Scholar]
- Scheerlinck LM, Muradin MS, van der Bilt A, Meijer GJ, Koole R, Van Cann EM. 2013; Donor site complications in bone grafting: comparison of iliac crest, calvarial, and mandibular ramus bone. Int J Oral Maxillofac Implants 28: 222–227. [DOI] [PubMed] [Google Scholar]
- Shegarfi H, Reikeras O. 2009; Review article: bone transplantation and immune response. J Orthop Surg (Hong Kong) 17: 206–211. [DOI] [PubMed] [Google Scholar]
- Takaoka R, Hikasa Y, Hayashi K, Tabata Y. 2011; Bone regeneration by lactoferrin released from a gelatin hydrogel. J Biomater Sci Polym Ed 22: 1581–1589. [DOI] [PubMed] [Google Scholar]
- Vogel HJ. 2012; Lactoferrin, a bird's eye view. Biochem Cell Biol 90: 233–244. [DOI] [PubMed] [Google Scholar]
- Vura N, Reddy KR, Sudhir R, Rajasekhar G, Kaluvala VR. 2013; Donor site evaluation: anterior iliac crest following secondary alveolar bone grafting. J Clin Diagn Res 7: 2627–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Bunt C, Cornish J, Quek SY, Wen J. 2015; Oral delivery of bovine lactoferrin using pectin‐ and chitosan‐modified liposomes and solid lipid particles: improvement of stability of lactoferrin. Chem Biol Drug Des 86: 466–475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting info item


