Abstract
The role of vascular endothelial growth factor (VEGF), including in retinal vascular diseases, has been well studied, and pharmacological blockade of VEGF is the gold standard of treatment for neovascular age‐related macular degeneration, retinal vein occlusion and diabetic macular oedema. Placental growth factor (PGF, previously known as PlGF), a homologue of VEGF, is a multifunctional peptide associated with angiogenesis‐dependent pathologies in the eye and non‐ocular conditions. Animal studies using genetic modification and pharmacological treatment have demonstrated a mechanistic role for PGF in pathological angiogenesis. Inhibition decreases neovascularization and microvascular abnormalities across different models, including oxygen‐induced retinopathy, laser‐induced choroidal neovascularization and in diabetic mice exhibiting retinopathies. High levels of PGF have been found in the vitreous of patients with diabetic retinopathy. Despite these strong animal data, the exact role of PGF in pathological angiogenesis in retinal vascular diseases remains to be defined, and the benefits of PGF‐specific inhibition in humans with retinal neovascular diseases and macular oedema remain controversial. Comparative effectiveness research studies in patients with diabetic retinal disease have shown that treatment that inhibits both VEGF and PGF may provide superior outcomes in certain patients compared with treatment that inhibits only VEGF. This review summarizes current knowledge of PGF, including its relationship to VEGF and its role in pathological angiogenesis in retinal diseases, and identifies some key unanswered questions about PGF that can serve as a pathway for future basic, translational and clinical research.
Keywords: angiogenesis, diabetic retinopathy, neovascularization, placental growth factor, retina, vascular endothelial growth factor
Introduction
Angiogenesis, the growth of new blood vessels from preexisting ones (Cao et al. 2011), is an important biological mechanism governing various physiological processes, including fetal development (Otrock et al. 2007), corpus luteum formation (De Falco et al. 2002), the response of cardiac and skeletal muscles to physical exercise (De Falco et al. 2002) and wound healing (Witmer et al. 2003). Aberrant angiogenesis is involved in a number of pathological conditions, including cancer (Fischer et al. 2008), obesity (Fischer et al. 2008), rheumatoid arthritis (Yoo et al. 2009) and vascular disorders of the choroid [i.e. age‐related macular degeneration (AMD)] and the retina [i.e. diabetic retinopathy (DR)] (Kowalczuk et al. 2011).
The process of angiogenesis is complex and involves a multistep cascade of molecular and cellular events (Adams & Alitalo 2007). In brief, sprouting of new vessels begins with the activation by growth factors of quiescent endothelial cells from venules, selection of endothelial tip cells regulated by delta‐like ligand 4, multiple Notch receptors and vascular endothelial growth factor (VEGF) and its receptors (VEGFRs). Following this selection of cells, other ligand/receptor interactions (VEGF/VEGFR‐2; semaphorin/neuropilin/plexin; netrin/UNC5B, SLIT/ROBO4) guide elongation and outgrowth of the vascular sprout. Integrins, Rho GTPase CDC42 and Rac1 as well as pressure‐induced inverse membrane blebbing are involved in sprout fusion and lumen formation (Gebala et al. 2016). Vascular maturation, mediated by platelet‐derived growth factor, EGFL7 and pericytes, then occurs, accompanied by a restoration of the dominant angioinhibitory phenotype of the neovascular network and perfusion (Adams & Alitalo 2007; Wietecha et al. 2013).
Among the many angiogenic factors identified in vascular regulation, the VEGF superfamily and the VEGFRs play a decisive role in both physiological and pathological angiogenesis, and, in particular, vascular permeability (Nagy et al. 2008). The VEGF family of growth factors consists of several homologues [VEGF‐A, VEGF‐B, VEGF‐C, VEGF‐D, VEGF‐E and placental growth factor (PGF, previously known as PlGF)]. It is well known that VEGF‐A (commonly referred to as VEGF), the first member of the VEGF family to be identified (Ferrara & Henzel 1989; Plouet et al. 1989; Nagy et al. 2007), contributes to angiogenesis, activating quiescent endothelial cells and promoting vascular permeability through VEGFR‐1 (also known as Flt1) binding, and stimulating cell proliferation through VEGFR‐2 (also known as Flk1) binding (Carmeliet et al. 2001; Autiero et al. 2003; Adams & Alitalo 2007). Vascular endothelial growth factor‐A also plays a role in mobilizing and recruiting endothelial progenitor cells to sites of neovascularization and tissue regeneration (Beaudry et al. 2007).
Placental growth factor (PGF), a homologous factor to VEGF‐A, is also implicated in pathological angiogenesis, especially in retinal disorders, although its function is less well understood. Animal models have suggested that PGF is not essential for physiological angiogenesis but plays a role in pathological angiogenic conditions (Carmeliet et al. 2001; De Falco et al. 2002; Autiero et al. 2003; Rakic et al. 2003; Fischer et al. 2007, 2008; Otrock et al. 2007; Kowalczuk et al. 2011; Tarallo et al. 2011; Yao et al. 2011; De Falco 2012; Papadopoulos et al. 2012; Zheng et al. 2012). This review summarizes what is currently known about PGF, its relationship to VEGF and its role in pathological angiogenesis in retinal disorders; identifies gaps in knowledge; and proposes future directions for research and potential clinical applications.
Subjects and Methods
VEGF in the normal retina
At least six retinal cell types [retinal pigment epithelium, astrocytes, Müller cells, vascular endothelium, ganglion cells (Penn et al. 2008) and microglial cells (Krause et al. 2014)] have been studied for their ability to produce and secrete VEGF. In normal retinal vasculature, expression of the receptor VEGFR‐1 is predominant compared with VEGFR‐2 (Takagi et al. 1996), and it is found widely distributed in both vascular endothelial cells and pericytes. By contrast, VEGFR‐2 exhibits a highly restricted pattern and is primarily expressed in non‐vascular photoreceptors and ganglion cells (Cao et al. 2010). There is currently a lack of consensus regarding whether and how VEGFRs are expressed in the neuronal components of the retina. Some studies have shown that all three VEGFRs (‐1, ‐2 and ‐3) have been localized in neuronal elements of the inner retina, suggesting that VEGF exerts biological functions on non‐vascular cells, including a direct neuroprotective role (Witmer et al. 2003). In other studies, long‐term transgenic expressions of a VEGF inhibitor (soluble Flt‐1) or chronic pharmacological inhibition of VEGF‐R activity in mice did not lead to morphological or functional deficits (Ueno et al. 2008; Miki et al. 2010). These results are consistent with human clinical experience, including the recent finding that neuronal function of the retina is improved by the administration of VEGF inhibitors in patients with diabetic macular oedema (DMO) (Gonzalez et al. 2015) and AMD (Sulzbacher et al. 2015), as determined by microperimetry.
VEGF as a therapeutic target
In 1956, scientists hypothesized the existence of a then‐unknown vasoformative ‘Factor X’ that develops in ischaemic retinal tissue and stimulates neovascularization (Wise 1956). Vascular endothelial growth factor (VEGF) was subsequently identified as ‘Factor X’ based, in part, on tumour models showing the hypoxia‐driven association between VEGF and angiogenesis (Plate et al. 1992; Shweiki et al. 1992). Further evidence supporting the role of VEGF in ocular neovascularization was reported in 1994, when neovascularization of the iris and elevated levels of VEGF mRNA and protein were discovered in non‐human primate eyes in which ischaemia had been induced by laser photocoagulation (Miller et al. 1994). Elevated levels of VEGF in ocular fluids of patients with active retinal and corneal neovascular ocular disease, but not in patients without new vessel growth, further implicated the growth factor (Aiello et al. 1994; Malecaze et al. 1994). Direct causal association was shown when VEGF protein was injected into the eyes of non‐human primates, leading to both retinal angiogenesis and hyperpermeability of newly formed vessels (Tolentino et al. 1996).
Anti‐VEGF therapy: a gold standard for retinal disorders
Concurrent with the successful development of VEGF‐targeting agents for cancer, a number of anti‐VEGF agents have become new standards of care for vascular ocular disease. The first anti‐VEGF agent to be approved for the treatment of neovascular AMD was pegaptanib, a pegylated aptamer that selectively binds to and neutralizes VEGF‐A165, but not VEGF‐A121 (Papadopoulos et al. 2012). Subsequent anti‐VEGF drugs include bevacizumab, ranibizumab and intravitreal aflibercept. Bevacizumab is a recombinant, humanized monoclonal antibody that binds all isoforms of VEGF‐A and is approved to treat cancer (Papadopoulos et al. 2012). Ranibizumab is an affinity‐matured antigen‐binding fragment derived from bevacizumab but with a higher affinity for VEGF‐A (Papadopoulos et al. 2012). Aflibercept is a fusion protein comprising elements of VEGFR‐1 and VEGFR‐2 that acts as a decoy receptor, binding not only to multiple isoforms of VEGF‐A but also to VEGF‐B and PGF isoforms (Papadopoulos et al. 2012; Deissler et al. 2014). Randomized controlled clinical trials have demonstrated remarkable efficacy in reducing vision loss through intravitreal injection of ranibizumab and aflibercept for neovascular AMD (Table S1) (Rosenfeld et al. 2006; Brown et al. 2009; Schmidt‐Erfurth et al. 2014), macular oedema following branch retinal vein occlusion (Table S2) (Campochiaro et al. 2010, 2015; Clark et al. 2015), macular oedema following central retinal vein occlusion (Table S3) (Campochiaro et al. 2011; Heier et al. 2014; Ogura et al. 2014) and DMO (Table S4) (Nguyen et al. 2012; Brown et al. 2013, 2015; Korobelnik et al. 2014). Furthermore, a recent DRCR.net non‐inferiority study demonstrated that long‐term anti‐VEGF blockade may be a reasonable alternative to panretinal laser photocoagulation, at least through 2 years, for the treatment of proliferative DR (Gross et al. 2015).
Ocular adverse events such as increased intraocular pressure, endophthalmitis, uveitis or intraocular inflammation, retinal detachment, retinal tear, vitreous haemorrhage and traumatic lens damage have been reported in some patients treated with anti‐VEGF drugs (Schmucker et al. 2011, 2012; Falavarjani & Nguyen 2013). A review of five randomized controlled trials (RCTs) of ranibizumab showed low rates of serious ocular adverse events, but a significantly increased relative harm compared with controls. In addition, the authors also concluded that the safety profile of bevacizumab could not be adequately assessed due to the poor quality of adverse event monitoring and reporting in the trials (Schmucker et al. 2012).
In the oncology setting, systemic administration of anti‐VEGF drugs has been associated with an increased risk of adverse events, including arterial thromboembolic events (Avery et al. 2014). The doses of these agents used in ophthalmology are substantially lower; however, for many patients with neovascular retinal diseases, frequent injections of anti‐VEGF agents over prolonged periods of time may be required to obtain optimal functional and morphological outcomes. As a result, closer examination of the systemic safety of intravitreal anti‐VEGF drugs has been undertaken. A pooled analysis of key arterial non‐fatal thromboembolic effects in the ANCHOR and MARINA trials of ranibizumab indicated a possible safety signal, and the incidence of serious non‐ocular haemorrhage was higher in the patients treated with ranibizumab compared with control groups in ANCHOR, MARINA and PIER (Schmucker et al. 2011, 2012). Data regarding the systemic safety risks of bevacizumab are inconclusive (Falavarjani & Nguyen 2013). More recently, a review of the literature concluded that there is no study showing a significant increase in the incidence of myocardial infarction in patients treated with ranibizumab (Gibson & Gibson 2014), and a meta‐analysis of 10 phase 2 and 3 studies in wet AMD found no meaningful differences between adverse event rates for intravitreal aflibercept and controls, although the authors acknowledged that continued surveillance is needed to evaluate the potential that systemic adverse events may be related to anti‐VEGF therapy (Kitchens et al. 2016).
Also in neovascular AMD, RCTs have shown that monthly dosing is required to obtain optimal visual outcomes with ranibizumab (Rosenfeld et al. 2006; Brown et al. 2009), a dosing regimen that may be burdensome for patients and healthcare providers. Studies such as AURA have demonstrated that, in real‐life clinical practice, ranibizumab is typically administered less frequently, resulting in visual gains that are lower than those seen in RCTs (Holz et al. 2015). The treatment burden associated with intravitreal aflibercept in neovascular AMD may be less than that of ranibizumab, as intravitreal aflibercept given every 8 weeks has been shown to produce similar visual gains compared with monthly ranibizumab (Heier et al. 2012). Furthermore, vision gains in real‐world studies of intravitreal aflibercept were comparable to those seen in RCTs (Talks et al. 2016).
Finally, there are some patients who either fail to respond during the induction phase or experience a decreased response to treatment over time. For these patients, switching therapy from one drug to another has proven to be an effective treatment strategy. For example, a recent meta‐analysis showed that patients with wet AMD switched from ranibizumab to intravitreal aflibercept experienced statistically significant visual and anatomic improvements (Seguin‐Greenstein et al. 2016).
As a result of the visual outcomes seen in numerous clinical trials, and in spite of the potential negative aspects of treatment, VEGF blockade using intravitreal aflibercept or ranibizumab is currently the gold standard therapeutic strategy for treating these conditions.
Description of PGF
All members of the VEGF family are characterized by their cysteine knot motif, and the three‐dimensional structure of PGF is strikingly similar to that of VEGF‐A, although the two proteins only share 42% amino acid sequence identity (Fig. 1) (De Falco et al. 2002; Autiero et al. 2003; De Falco 2012). Alternative splicing generates four isoforms that differ in size (PGF‐1, ‐2, ‐3 and ‐4). Placental growth factor‐1 (PGF‐1) and PGF‐2 are the major isoforms, consisting of 131 and 152 amino acid residues, respectively. Placental growth factor (PGF) is a dimeric protein whose monomers are held together by disulphide bonds. If expressed by the same cell, PGF and VEGF‐A can form a heterodimer (Autiero et al. 2003; Otrock et al. 2007; Tarallo et al. 2011, 2012; De Falco 2012). Placental growth factor (PGF) specifically binds to the receptor VEGFR‐1, but not to VEGFR‐2 (Carmeliet et al. 2001; De Falco et al. 2002; Autiero et al. 2003; Witmer et al. 2003; Roy et al. 2006; Fischer et al. 2007, 2008; Otrock et al. 2007; Van de Veire et al. 2010; Huang et al. 2011; Kowalczuk et al. 2011; Tarallo et al. 2011, 2012; Yao et al. 2011; De Falco 2012; Papadopoulos et al. 2012; Chen et al. 2013). Additionally, the isoforms carrying heparin‐binding domain bind to neuropilin (De Falco et al. 2002; Roy et al. 2006; Fischer et al. 2007, 2008; Otrock et al. 2007; Kowalczuk et al. 2011; Tarallo et al. 2011, 2012; De Falco 2012). Studies have demonstrated that neuropilin‐1 is expressed in angiogenic vessels in animal models (Ishihama et al. 2001; Lanahan et al. 2013; Gelfand et al. 2014) as well as in humans (Cui et al. 2003; Lim et al. 2005). Based on their research using neonatal mouse retinas, Pan et al. hypothesize that blocking neuropilin‐1 inhibits vascular remodelling, thereby rendering vessels more susceptible to treatment with anti‐VEGF agents (Pan et al. 2007). The PGF/VEGF‐A heterodimer binds VEGFR‐1 or induces VEGFR‐1/VEGFR‐2 dimerization (Tarallo et al. 2010).
Figure 1.
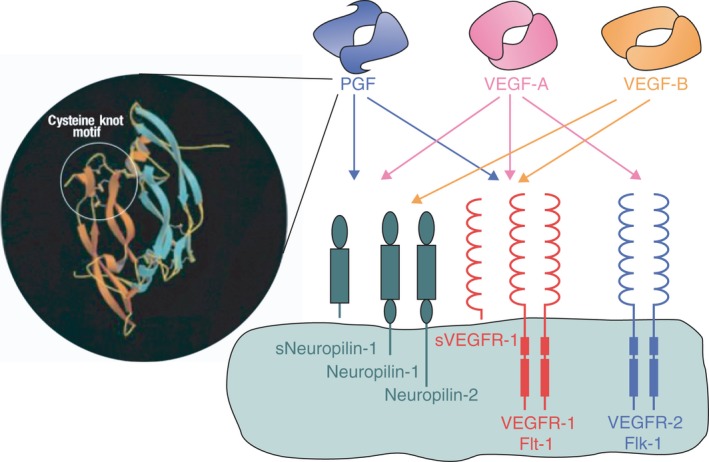
Structural model of human placental growth factor (PGF)‐1 and schematic representation of the binding of homodimers of PGF, vascular endothelial growth factor (VEGF)‐A, and VEGF‐B to related receptors. Adapted from De Falco S, Gigante B & Persico MG (2002): Structure and function of placental growth factor. Trends Cardiovasc Med 12: 241–246.
Placental growth factor (PGF) stimulates endothelial cell migration via VEGFR‐1, and it recruits cells that play an active role in angiogenesis (e.g. monocyte macrophages, smooth muscle cells and pericytes) via VEGFR‐1 (De Falco 2012; Cicatiello et al. 2015). Placental growth factor (PGF) may indirectly stimulate angiogenesis by binding to VEGFR‐1 and thereby freeing up more VEGF‐A to activate VEGFR‐2, or it may signal endothelial cells to undergo angiogenesis through direct VEGFR‐1 binding (Fig. 2) (Carmeliet et al. 2001; Autiero et al. 2003; Feeney et al. 2003; Witmer et al. 2003; Fischer et al. 2008; De Falco 2012). In addition, leucocyte infiltration is connected with angiogenesis. VEGFR‐1 ligands are monocyte attractants and contribute to the recruitment of leucocytes. These recruited leucocytes provide an indirect (VEGF‐independent) pathway of angiogenesis through the secretion of proangiogenic factors (Mantovani et al. 2008).
Figure 2.
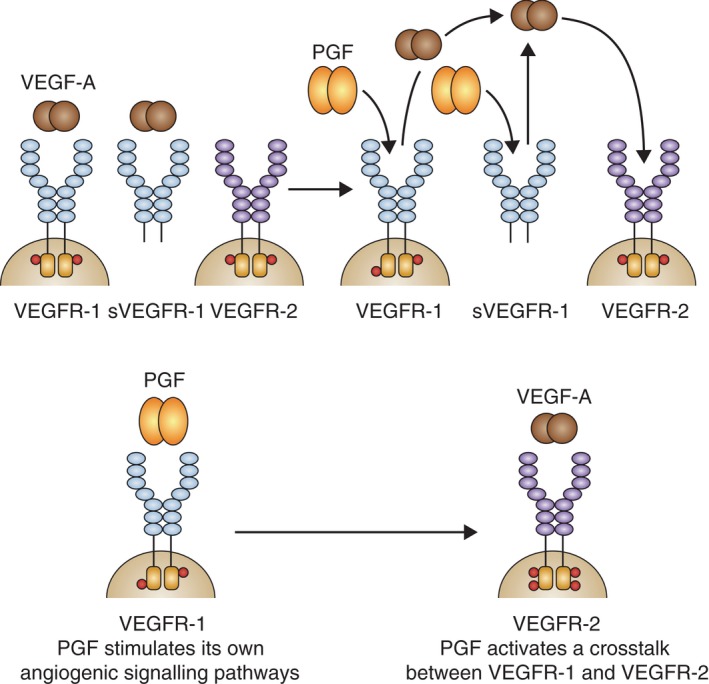
Molecular mechanisms of placental growth factor (PGF). VEGF = vascular endothelial growth factor; VEGFR = VEGF receptor. Adapted from Fischer C, Mazzone M, Jonckx B & Carmeliet P (2008): FLT1 and its ligands VEGFB and PGF: drug targets for anti‐angiogenic therapy? Nat Rev Cancer 8: 942–956.
Role of PGF — animal models
A number of animal models have been used to examine the role of PGF in pathological angiogenesis of the retina. Mice in which the gene expressing PGF (Plgf) was inactivated were exposed to 80% oxygen for 5 days [oxygen‐induced retinopathy (OIR)]. After normal oxygen levels were restored, there was decreased neovascularization, venous dilatation and arterial tortuosity observed in Plgf‐deficient mice compared with wild‐type mice (Carmeliet et al. 2001). Another study using the OIR model demonstrated that intravitreal administration of PGF prior to hyperoxic exposure protected the retinal vessels from hyperoxia‐induced vaso‐attenuation without increasing neovascularization (Shih et al. 2003). Somewhat surprisingly, PGF apparently does not lead to increased retinal angiogenesis in response to hypoxia (Penn et al. 2008). In a rat model where rat PGF had been overexpressed using non‐viral gene transfer, vessel tortuosity and dilation, vascular abnormalization and leaky microaneurysms were observed without true preretinal neovascularization, suggesting that PGF could play a role in the early vascular telangiectasia and aneurysms observed in retinal occlusive diseases associated with macular oedma (Kowalczuk et al. 2011).
The role of PGF has been studied in an animal model of laser‐induced choroidal neovascularization (CNV). Induction of CNV using argon lasers resulted in an almost complete absence of neovascularization in mice that were genetically modified (Rakic et al. 2003) or pharmacologically treated (Van de Veire et al. 2010) to be PGF deficient, compared with their wild‐type counterparts. While these models are currently used in the preclinical development of antiangiogenic compounds, they recapitulate more a wound repair angiogenesis with a strong inflammatory component, rather than a model of neovascular AMD.
The effects of pharmacological inhibition of PGF on retinal vascular development have also been studied. Neonatal mice injected with an anti‐mouse VEGF‐A polyclonal antibody showed a significant reduction in the hyaloid and vascular structure, whereas mice injected with a PGF‐neutralizing antibody showed marked persistence of the hyaloid without significant effect on the developing retinal vessels (Feeney et al. 2003).
Placental growth factor (PGF) activates VEGFR‐1, meriting a review of VEGFR‐1 activation in pathological retinal angiogenesis. A statistically significant reduction of laser‐induced CNV formation occurred in wild‐type mice injected with anti‐VEGFR‐1 antibody compared with control animals (Rakic et al. 2003). The expression of VEGFR‐1 without tyrosine kinase activity (Flt1‐TK −/− ) reduced CNV in mice (Van de Veire et al. 2010), indicating the importance of VEGFR signalling. A monoclonal antibody directed against VEGFR‐1 suppressed VEGF‐driven neovascularization in mice cornea and in subcutaneous Matrigel implants, and also blocked neovascularization in the ischaemic retina. Immunostaining demonstrated reduction in vessel density and in size of tumours implanted in mice treated with anti‐VEGFR‐1, compared with controls (Luttun et al. 2002). The VEGFR‐1 antagonist iVR1 inhibited tumour growth and neovascularization in syngenic and xenograft models of colorectal cancer, as well as neovascularization in laser‐induced CNV (Cicatiello et al. 2015). The blockade of VEGFR‐1 and VEGFR‐2 by neutralizing antibodies for mouse VEGFR‐1 and VEGFR‐2 resulted in suppression of the development of CNV in both the OIR and laser CNV models. Vascular endothelial growth factor receptor‐1 (VEGFR‐1) blockade also inhibits the expression of genes known to be involved in angiogenesis, such as CXCR‐4, Ang2, Tie2 and EpoR (Huang et al. 2011).
Perhaps the most elegant and compelling demonstration of PGF function in diabetic retinal pathology employed a novel animal model in which a PGF−/− mouse was crossed with the Akita diabetic mouse. Akita mice were crossed with PGF−/− mice in a C57BL/6J background for two generations to give birth to the progeny with the genotype of Akita.PGF−/−. Additional mating of Akita.PGF−/− and PGF−/− was performed to fix the PGF mutation to homozygosity while maintaining the Akita mutation in the heterozygous state. The genotyping was performed as described in the literature. The progeny (Akita.PGF−/−) were diabetic but lacked PGF. Molecular and histological changes in the retina were compared between Akita.PGF−/− mice and wild‐type mice, Akita diabetic mice and PGF−/− mice. Placental growth factor deficiency in the Akita.PGF−/− mouse reduced diabetes‐associated retinal cell death and decreased retinal capillary degeneration and pericyte death. Moreover, while diabetes caused a significant vascular leakage in Akita mice compared with non‐diabetic control mice, the deletion of PGF in Akita.PGF−/− mice markedly reduced diabetes‐related vascular leakage (Huang et al. 2015). These findings suggest that PGF may be significant mediator in diabetes of hallmarks of DR, such as retinal hyperpermeability, retinal capillary degeneration and pericyte loss.
Together, these findings suggest that PGF as well as VEGF, and their shared receptors VEGFR‐1 and NP‐1, are potential targets for retinal and choroidal vascular diseases in which both factors are expressed. While these animal studies may show somewhat counterintuitive results with respect to PGF‐related neovascularization and protection, it is likely that these findings highlight a yet‐to‐be‐identified, intricate interplay with PFG and VEGF/VEGFR‐1 in balancing protection and restoration of vessels, without aberrant neovascularization, in a hypoxic retina. Emerging data from human studies support the findings from animal models. High levels of PGF have been measured in the vitreous humour (Khaliq et al. 1996, 1998; Mitamura et al. 2002; Rakic et al. 2003; Miyamoto et al. 2008; Kowalczuk et al. 2011; Chen et al. 2013) and in retinal tissue (Spirin et al. 1999; Rakic et al. 2003; Kowalczuk et al. 2011; Chen et al. 2013) of eyes with DR.
Clinical Applications of PGF Targeting
Despite emerging research, the exact contribution of PGF in pathological ocular neovascularization in humans has not been fully elucidated, and its role as a potential primary therapeutic target is not well understood. The question remains regarding whether PGF targeting is responsible for the improved vision outcomes following inhibition of both VEGF‐A/‐B and PGF using intravitreal aflibercept, compared with ranibizumab, which targets VEGF‐A alone. The recent Protocol T study compared the efficacy and safety of intravitreal aflibercept (2.0 mg) with ranibizumab (0.3 mg) or off‐label bevacizumab (1.25 mg) in eyes with DMO. From baseline to 1 year, the mean visual acuity improved by 13.3 letters with intravitreal aflibercept, by 9.7 letters with bevacizumab and by 11.2 letters with ranibizumab (p < 0.001 for intravitreal aflibercept versus bevacizumab and p = 0.03 for intravitreal aflibercept versus ranibizumab); however, the difference was driven by the eyes with worse visual acuity at baseline (p < 0.001 for interaction). Among eyes with initial visual acuity of <69 letters (approximately 20/50 or worse), the mean improvement at 1 year was 18.9 letters with intravitreal aflibercept, 11.8 with bevacizumab and 14.2 with ranibizumab (p < 0.001 for intravitreal aflibercept versus bevacizumab, p = 0.003 for intravitreal aflibercept versus ranibizumab and p = 0.21 for ranibizumab versus bevacizumab). There were no significant differences among the study groups in the rates of serious adverse events (p = 0.40), hospitalization (p = 0.51), death (p = 0.72) or major cardiovascular events (p = 0.56) (Wells et al. 2015). Although there remain many unanswered questions about the role of PGF in DMO, the results of this study may suggest a possible advantage to dual blockade, as intravitreal aflibercept‐treated eyes with a baseline vision of 20/50 or worse achieved significantly greater visual improvement compared with eyes treated with ranibizumab or bevacizumab. This is the first clinical evidence that targeting an angiogenic factor beyond VEGF (i.e. PGF) may translate into additional clinical benefit. Alternatively, the apparently superior efficacy of intravitreal aflibercept in this subpopulation could also be due to its substantially higher affinity for VEGF‐A (Papadopoulos et al. 2012). Further, prospective analyses are required to determine whether PGF plays a role in the superior efficacy of intravitreal aflibercept in this subpopulation.
Currently, there are no antiangiogenic agents with a primary target of PGF for use in retinal disease; however, in a phase 1 study of TB‐403 (RO 5323441), a novel antiangiogenic agent directed against PGF in patients with advanced solid tumours, no dose‐limiting toxicities were observed, and a maximum tolerated dose was not reached (Lassen et al. 2012). The safety and tolerability of intravitreal aflibercept, which inhibits PGF in addition to VEGF‐A, for the treatment of retinal diseases has been well documented. A recent meta‐analysis concluded that the rates of ocular and systemic adverse events were similar in eyes treated with intravitreal aflibercept compared with controls, and were similar across disease states studied (Kitchens et al. 2016). Further research is needed to confirm the safety of PGF inhibition, either alone or in conjunction with VEGF inhibition, particularly during long‐term exposure.
Recent studies have shown that switching from one anti‐VEGF agent to another, and in particular, switching from ranibizumab and/or bevacizumab to intravitreal aflibercept, which binds not only to VEGF‐A but also to VEGF‐B and PGF, can be effective in the treatment of refractory patients (Bakall et al. 2013; Ho et al. 2013; Kumar et al. 2013; Yonekawa et al. 2013; Chang et al. 2014; Wykoff et al. 2014). Further research is needed to define the mechanisms of the differential response to treatment. Examination of data and specimens from patients who have had different responses to anti‐VEGF therapy may help to determine whether there is a correlation between the response and the properties of the pharmacological agent.
Future Directions of PGF Inhibition in Retinal Vascular Disorders
Despite the existence of animal models, important questions remain regarding the clinical role of PGF inhibition in humans. For example, it is not known whether PGF inhibition has an effect on outer retinal barrier permeability (subretinal fluid, outer retinal oedema). The effects of PGF inhibition on abnormal retinal vessels are also unknown. It is also unclear whether PGF inhibition could block the activation of microglia/macrophages and subretinal inflammation and subsequent chronic subretinal remodelling.
There is a need for assessment of the possible role of PGF and its stimulation/inhibition in the management of retinal vascular disorders. Future research could assess the role of VEGF/PGF heterodimers in physiological versus pathological activity (permeability and neovascularization), as well as their relationship in the context of pure VEGF‐A blockade versus VEGF‐A + PGF blockade. Anti‐PGF treatment may work well in patients who are refractory to or who have incomplete response to anti‐VEGF therapy; however, additional research is needed to further elucidate the multiple mechanisms by which PGF appears to modulate VEGF‐driven angiogenesis.
Conclusion
The role of VEGF‐A in both physiological and pathological angiogenesis has been well studied in laboratory and clinical settings. Treatment with VEGF inhibition is the standard of care in retinal vascular diseases such as AMD, DMO and retinal vein occlusion. Animal models show that PGF mediates both permeability and neovascularization, as well as inflammation. Additional translational research, including investigation of PGF‐associated biomarkers and evaluation of inflammatory cytokines, is needed to further elucidate the role of PGF in pathological retinal angiogenesis in humans. Understanding these mechanisms may shed light on how PGF inhibition may offer advantages over sole VEGF blockade to improve vision in patients with retinal vascular disorders, especially for those individuals who are refractory or incompletely responsive to anti‐VEGF monotherapy.
Supporting information
Table S1. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in neovascular age‐related macular degeneration.
Table S2. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in branch retinal vein occlusion.
Table S3. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in central retinal vein occlusion
Table S4. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in diabetic macular oedema.
The authors wish to acknowledge the contributions of the late Moritz A. Koenerdig (Institut fur funktionnelle und klinische Anatomie, University of Mainz, Mainz, Germany), who reviewed an early draft of the manuscript before his death in 2015. Medical writing support was provided by Corey Eagan, MPH, of PAREXEL, and was funded by Bayer Pharmaceuticals.
Quan Dong Nguyen serves on the Scientific Advisory Boards for Astellas, Bayer, Genentech, Oligasis and Regeneron. He chairs the Steering Committees for the RISE and RIDE studies, as well as the EYEGUARD, SAKURA and VISUAL studies and is on the Steering Committee for the VIVID and VISTA studies. Francine Behar‐Cohen is a consultant for Bayer. Wai‐Ching Lam serves on the Scientific Advisory Boards for Novartis, Bayer, Allergan and Alcon. He has received research grants from Novartis, Bayer and Allergan; travel grants from Novartis and Bayer; and honoraria from Bayer. Jennifer Pluim is an employee of Bayer Pharmaceuticals. Federico Ricci serves as an advisor for Bayer, Allergan, Novartis, Alcon and Ophtotech. Sandro De Falco, Xuri Li, William Li and Nadine Reichhart have no conflict of interests to disclose.
References
- Adams RH & Alitalo K (2007): Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG et al. (1994): Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- Autiero M, Luttun A, Tjwa M & Carmeliet P (2003): Placental growth factor and its receptor, vascular endothelial growth factor receptor‐1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost 1: 1356–1370. [DOI] [PubMed] [Google Scholar]
- Avery RL, Castellarin AA, Steinle NC et al. (2014): Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98: 1636–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR & Mahajan VB (2013): Aflibercept therapy for exudative age‐related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 156: 15–22. [DOI] [PubMed] [Google Scholar]
- Beaudry P, Hida Y, Udagawa T et al. (2007): Endothelial progenitor cells contribute to accelerated liver regeneration. J Pediatr Surg 42: 1190–1198. [DOI] [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP & Ianchulev T (2009): Ranibizumab versus verteporfin photodynamic therapy for neovascular age‐related macular degeneration: two‐year results of the ANCHOR study. Ophthalmology 116: 57–65. [DOI] [PubMed] [Google Scholar]
- Brown DM, Nguyen QD, Marcus DM et al. (2013): Long‐term outcomes of ranibizumab therapy for diabetic macular edema: the 36‐month results from two phase III trials: RISE and RIDE. Ophthalmology 120: 2013–2022. [DOI] [PubMed] [Google Scholar]
- Brown DM, Schmidt‐Erfurth U, Do DV et al. (2015): Intravitreal aflibercept for diabetic macular edema: 100‐week results from the VISTA and VIVID studies. Ophthalmology 122: 2044–2052. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY & Rubio RG (2010): Ranibizumab for macular edema following branch retinal vein occlusion: six‐month primary end point results of a phase III study. Ophthalmology 117: 1102–1112. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, Murahashi WY & Rubio RG (2011): Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve‐month outcomes of a phase III study. Ophthalmology 118: 2041–2049. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Clark WL, Boyer DS et al. (2015): Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24‐week results of the VIBRANT study. Ophthalmology 122: 538–544. [DOI] [PubMed] [Google Scholar]
- Cao R, Xue Y, Hedlund EM et al. (2010): VEGFR1‐mediated pericyte ablation links VEGF and PlGF to cancer‐associated retinopathy. Proc Natl Acad Sci USA 107: 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Arbiser J, D'Amato RJ et al. (2011): Forty‐year journey of angiogenesis translational research. Sci Transl Med 3: 114rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A et al. (2001): Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7: 575–583. [DOI] [PubMed] [Google Scholar]
- Chang AA, Li H, Broadhead GK, Hong T, Schlub TE, Wijeyakumar W & Zhu M (2014): Intravitreal aflibercept for treatment‐resistant neovascular age‐related macular degeneration. Ophthalmology 121: 188–192. [DOI] [PubMed] [Google Scholar]
- Chen X, Li J, Li M et al. (2013): KH902 suppresses high glucose‐induced migration and sprouting of human retinal endothelial cells by blocking VEGF and PlGF. Diabetes Obes Metab 15: 224–233. [DOI] [PubMed] [Google Scholar]
- Cicatiello V, Apicella I, Tudisco L et al. (2015): Powerful anti‐tumor and anti‐angiogenic activity of a new anti‐vascular endothelial growth factor receptor 1 peptide in colorectal cancer models. Oncotarget 6: 10563–10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WL, Boyer DS, Heier JS et al. (2015): Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52‐week results of the VIBRANT study. Ophthalmology 122: 538–544. [DOI] [PubMed] [Google Scholar]
- Cui JZ, Hinz BJ, Greve MD, Potter MJ, Hornan D, Samad A, To E & Matsubara JA (2003): Expression of neuropilin‐1 in choroidal neovascular membranes. Can J Ophthalmol 38: 41–45. [DOI] [PubMed] [Google Scholar]
- De Falco S (2012): The discovery of placenta growth factor and its biological activity. Exp Mol Med 44: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Falco S, Gigante B & Persico MG (2002): Structure and function of placental growth factor. Trends Cardiovasc Med 12: 241–246. [DOI] [PubMed] [Google Scholar]
- Deissler HL, Lang GK & Lang GE (2014): Capacity of aflibercept to counteract VEGF‐stimulated abnormal behavior of retinal microvascular endothelial cells. Exp Eye Res 122: 20–31. [DOI] [PubMed] [Google Scholar]
- Falavarjani KG & Nguyen QD (2013): Adverse events and complications associated with intravitreal injection of anti‐VEGF agents: a review of literature. Eye (Lond) 27: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney SA, Simpson DA, Gardiner TA, Boyle C, Jamison P & Stitt AW (2003): Role of vascular endothelial growth factor and placental growth factors during retinal vascular development and hyaloid regression. Invest Ophthalmol Vis Sci 44: 839–847. [DOI] [PubMed] [Google Scholar]
- Ferrara N & Henzel WJ (1989): Pituitary follicular cells secrete a novel heparin‐binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161: 851–858. [DOI] [PubMed] [Google Scholar]
- Fischer C, Jonckx B, Mazzone M et al. (2007): Anti‐PlGF inhibits growth of VEGF(R)‐inhibitor‐resistant tumors without affecting healthy vessels. Cell 131: 463–475. [DOI] [PubMed] [Google Scholar]
- Fischer C, Mazzone M, Jonckx B & Carmeliet P (2008): FLT1 and its ligands VEGFB and PlGF: drug targets for anti‐angiogenic therapy? Nat Rev Cancer 8: 942–956. [DOI] [PubMed] [Google Scholar]
- Gebala V, Collins R, Geudens I, Phng LK & Gerhardt H (2016): Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo . Nat Cell Biol 18: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand MV, Hagan N, Tata A et al. (2014): Neuropilin‐1 functions as a VEGFR2 co‐receptor to guide developmental angiogenesis independent of ligand binding. Elife 3: e03720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JM & Gibson SJ (2014): A safety evaluation of ranibizumab in the treatment of age‐related macular degeneration. Expert Opin Drug Saf 13: 1259–1270. [DOI] [PubMed] [Google Scholar]
- Gonzalez VH, Boyer DS, Schmidt‐Erfurth U et al. (2015): Microperimetric assessment of retinal sensitivity in eyes with diabetic macular edema from a phase 2 study of intravitreal aflibercept. Retina 35: 687–694. [DOI] [PubMed] [Google Scholar]
- Gross JG, Glassman AR, Jampol LM et al. (2015): Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 314: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V et al. (2012): Intravitreal aflibercept (VEGF trap‐eye) in wet age‐related macular degeneration. Ophthalmology 119: 2537–2548. [DOI] [PubMed] [Google Scholar]
- Heier JS, Clark WL, Boyer DS et al. (2014): Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two‐year results from the COPERNICUS study. Ophthalmology 121: 1414–1420. [DOI] [PubMed] [Google Scholar]
- Ho VY, Yeh S, Olsen TW, Bergstrom CS, Yan J, Cribbs BE & Hubbard GB III(2013): Short‐term outcomes of aflibercept for neovascular age‐related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol 156: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S et al. (2015): Multi‐country real‐life experience of anti‐vascular endothelial growth factor therapy for wet age‐related macular degeneration. Br J Ophthalmol 99: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shen J & Vinores SA (2011): Blockade of VEGFR1 and 2 suppresses pathological angiogenesis and vascular leakage in the eye. PLoS One 6: e21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, He J, Johnson D et al. (2015): Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha‐VEGF pathway inhibition. Diabetes 64: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama H, Ohbayashi M, Kurosawa N, Kitsukawa T, Matsuura O, Miyake Y & Muramatsu T (2001): Colocalization of neuropilin‐1 and Flk‐1 in retinal neovascularization in a mouse model of retinopathy. Invest Ophthalmol Vis Sci 42: 1172–1178. [PubMed] [Google Scholar]
- Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H & Ahmed A (1996): Localisation of placenta growth factor (PlGF) in human term placenta. Growth Factors 13: 243–250, color. [DOI] [PubMed] [Google Scholar]
- Khaliq A, Foreman D, Ahmed A, Weich H, Gregor Z, McLeod D & Boulton M (1998): Increased expression of placenta growth factor in proliferative diabetic retinopathy. Lab Invest 78: 109–116. [PubMed] [Google Scholar]
- Kitchens JW, Do DV, Boyer DS et al. (2016): Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology 123: 1511–1520. [DOI] [PubMed] [Google Scholar]
- Korobelnik JF, Do DV, Schmidt‐Erfurth U et al. (2014): Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121: 2247–2254. [DOI] [PubMed] [Google Scholar]
- Kowalczuk L, Touchard E, Omri S et al. (2011): Placental growth factor contributes to micro‐vascular abnormalization and blood‐retinal barrier breakdown in diabetic retinopathy. PLoS One 6: e17462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause TA, Alex AF, Engel DR, Kurts C & Eter N (2014): VEGF‐production by CCR2‐dependent macrophages contributes to laser‐induced choroidal neovascularization. PLoS One 9: e94313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Marsiglia M, Mrejen S, Fung AT, Slakter J, Sorenson J & Freund KB (2013): Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age‐related macular degeneration. Retina 33: 1605–1612. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Zhang X, Fantin A et al. (2013): The neuropilin 1 cytoplasmic domain is required for VEGF‐A‐dependent arteriogenesis. Dev Cell 25: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen U, Nielsen DL, Sorensen M et al. (2012): A phase I, dose‐escalation study of TB‐403, a monoclonal antibody directed against PlGF, in patients with advanced solid tumours. Br J Cancer 106: 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JI, Spee C, Hangai M, Rocha J, Ying HS, Ryan SJ & Hinton DR (2005): Neuropilin‐1 expression by endothelial cells and retinal pigment epithelial cells in choroidal neovascular membranes. Am J Ophthalmol 140: 1044–1050. [DOI] [PubMed] [Google Scholar]
- Luttun A, Tjwa M, Moons L et al. (2002): Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti‐Flt1. Nat Med 8: 831–840. [DOI] [PubMed] [Google Scholar]
- Malecaze F, Clamens S, Simorre‐Pinatel V, Mathis A, Chollet P, Favard C, Bayard F & Plouet J (1994): Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor‐like activity in proliferative diabetic retinopathy. Arch Ophthalmol 112: 1476–1482. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A & Balkwill F (2008): Cancer‐related inflammation. Nature 454: 436–444. [DOI] [PubMed] [Google Scholar]
- Miki A, Miki K, Ueno S et al. (2010): Prolonged blockade of VEGF receptors does not damage retinal photoreceptors or ganglion cells. J Cell Physiol 224: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Adamis AP, Shima DT et al. (1994): Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol 145: 574–584. [PMC free article] [PubMed] [Google Scholar]
- Mitamura Y, Tashimo A, Nakamura Y, Tagawa H, Ohtsuka K, Mizue Y & Nishihira J (2002): Vitreous levels of placenta growth factor and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetes Care 25: 2352. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, de Kozak Y, Normand N, Courtois Y, Jeanny JC, Benezra D & Behar‐Cohen F (2008): PlGF‐1 and VEGFR‐1 pathway regulation of the external epithelial hemato‐ocular barrier. A model for retinal edema. Ophthalmic Res 40: 203–207. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Dvorak AM & Dvorak HF (2007): VEGF‐A and the induction of pathological angiogenesis. Annu Rev Pathol 2: 251–275. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Benjamin L, Zeng H, Dvorak AM & Dvorak HF (2008): Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QD, Brown DM, Marcus DM et al. (2012): Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119: 789–801. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Roider J, Korobelnik JF et al. (2014): Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18‐month results of the phase 3 GALILEO study. Am J Ophthalmol 158: 1032–1038. [DOI] [PubMed] [Google Scholar]
- Otrock ZK, Makarem JA & Shamseddine AI (2007): Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis 38: 258–268. [DOI] [PubMed] [Google Scholar]
- Pan Q, Chanthery Y, Liang WC et al. (2007): Blocking neuropilin‐1 function has an additive effect with anti‐VEGF to inhibit tumor growth. Cancer Cell 11: 53–67. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N, Martin J, Ruan Q et al. (2012): Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15: 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW & Hartnett ME (2008): Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27: 331–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate KH, Breier G, Weich HA & Risau W (1992): Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo . Nature 359: 845–848. [DOI] [PubMed] [Google Scholar]
- Plouet J, Schilling J & Gospodarowicz D (1989): Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT‐20 cells. EMBO J 8: 3801–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic JM, Lambert V, Devy L et al. (2003): Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 44: 3186–3193. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY & Kim RY (2006): Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- Roy H, Bhardwaj S & Yla‐Herttuala S (2006): Biology of vascular endothelial growth factors. FEBS Lett 580: 2879–2887. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik JF et al. (2014): Intravitreal aflibercept injection for neovascular age‐related macular degeneration: ninety‐six‐week results of the VIEW studies. Ophthalmology 121: 193–201. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Loke YK, Ehlken C, Agostini HT, Hansen LL, Antes G & Lelgemann M (2011): Intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) for the treatment of age‐related macular degeneration: a safety review. Br J Ophthalmol 95: 308–317. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Ehlken C, Agostini HT, Antes G, Ruecker G, Lelgemann M & Loke YK (2012): A safety review and meta‐analyses of bevacizumab and ranibizumab: off‐label versus goldstandard. PLoS One 7: e42701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin‐Greenstein S, Lightman S & Tomkins‐Netzer O (2016): A meta‐analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant neovascular age‐related macular degeneration following switching to treatment with aflibercept. J Ophthalmol 2016: 4095852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SC, Ju M, Liu N & Smith LE (2003): Selective stimulation of VEGFR‐1 prevents oxygen‐induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest 112: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D & Keshet E (1992): Vascular endothelial growth factor induced by hypoxia may mediate hypoxia‐initiated angiogenesis. Nature 359: 843–845. [DOI] [PubMed] [Google Scholar]
- Spirin KS, Saghizadeh M, Lewin SL, Zardi L, Kenney MC & Ljubimov AV (1999): Basement membrane and growth factor gene expression in normal and diabetic human retinas. Curr Eye Res 18: 490–499. [DOI] [PubMed] [Google Scholar]
- Sulzbacher F, Roberts P, Munk MR, Kaider A, Kroh ME, Sacu S & Schmidt‐Erfurth U (2015): Relationship of retinal morphology and retinal sensitivity in the treatment of neovascular age‐related macular degeneration using aflibercept. Invest Ophthalmol Vis Sci 56: 1158–1167. [DOI] [PubMed] [Google Scholar]
- Takagi H, King GL & Aiello LP (1996): Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes 45: 1016–1023. [DOI] [PubMed] [Google Scholar]
- Talks JS, Lotery AJ, Ghanchi F et al. (2016): First‐Year visual acuity outcomes of providing aflibercept according to the view study protocol for age‐related macular degeneration. Ophthalmology 123: 337–343. [DOI] [PubMed] [Google Scholar]
- Tarallo V, Vesci L, Capasso O et al. (2010): A placental growth factor variant unable to recognize vascular endothelial growth factor (VEGF) receptor‐1 inhibits VEGF‐dependent tumor angiogenesis via heterodimerization. Cancer Res 70: 1804–1813. [DOI] [PubMed] [Google Scholar]
- Tarallo V, Tudisco L & De FS (2011): A placenta growth factor 2 variant acts as dominant negative of vascular endothelial growth factor A by heterodimerization mechanism. Am J Cancer Res 1: 265–274. [PMC free article] [PubMed] [Google Scholar]
- Tarallo V, Bogdanovich S, Hirano Y, Tudisco L, Zentilin L, Giacca M, Ambati J & De FS (2012): Inhibition of choroidal and corneal pathologic neovascularization by Plgf1‐de gene transfer. Invest Ophthalmol Vis Sci 53: 7989–7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N & Adamis AP (1996): Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol 114: 964–970. [DOI] [PubMed] [Google Scholar]
- Ueno S, Pease ME, Wersinger DM et al. (2008): Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J Cell Physiol 217: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Veire S, Stalmans I, Heindryckx F et al. (2010): Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 141: 178–190. [DOI] [PubMed] [Google Scholar]
- Wells JA, Glassman AR, Ayala AR et al. (2015): Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietecha MS, Cerny WL & DiPietro LA (2013): Mechanisms of vessel regression: toward an understanding of the resolution of angiogenesis. Curr Top Microbiol Immunol 367: 3–32. [DOI] [PubMed] [Google Scholar]
- Wise GN (1956): Retinal neovascularization. Trans Am Ophthalmol Soc 54: 729–826. [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GF, van Noorden CJ & Schlingemann RO (2003): Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22: 1–29. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Brown DM, Maldonado ME & Croft DE (2014): Aflibercept treatment for patients with exudative age‐related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial). Br J Ophthalmol 98: 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Wu X, Zhuang G et al. (2011): Expression of a functional VEGFR‐1 in tumor cells is a major determinant of anti‐PlGF antibodies efficacy. Proc Natl Acad Sci USA 108: 11590–11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa Y, Andreoli C, Miller JB et al. (2013): Conversion to aflibercept for chronic refractory or recurrent neovascular age‐related macular degeneration. Am J Ophthalmol 156: 29–35. [DOI] [PubMed] [Google Scholar]
- Yoo SA, Yoon HJ, Kim HS, Chae CB, De FS, Cho CS & Kim WU (2009): Role of placenta growth factor and its receptor flt‐1 in rheumatoid inflammation: a link between angiogenesis and inflammation. Arthritis Rheum 60: 345–354. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Gu Q & Xu X (2012): Inhibition of ocular neovascularization by a novel peptide derived from human placenta growth factor‐1. Acta Ophthalmol 90: e512–e523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in neovascular age‐related macular degeneration.
Table S2. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in branch retinal vein occlusion.
Table S3. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in central retinal vein occlusion
Table S4. Pivotal randomized clinical trials of anti‐vascular endothelial growth factor drugs in diabetic macular oedema.


