ABSTRACT
Introduction: The efficacy of single injections of abobotulinumtoxinA (Dysport) is established in adults with upper limb spasticity. In this study we assessed the effects of repeated injections of abobotulinumtoxinA over 1 year. Methods: Patients (n = 258, safety population) received 500 U, 1,000 U, or 1,500 U (1,500‐U dose included 500‐U shoulder injections) for up to 4 or 5 treatment cycles. Assessments included treatment‐emergent adverse events (TEAEs), muscle tone, passive and active range of motion (XV1, XA), angle of catch (XV3), Disability Assessment Scale (DAS) score, Modified Frenchay Scale (MFS) score, and Physician Global Assessment (PGA) score. Results: The incidence of TEAEs decreased across cycles. Muscle tone reduction and XV1 remained stable across cycles, whereas XV3 and XA continued to improve at the finger, wrist, and elbow flexors. DAS and PGA improved across cycles. MFS improved best with 1,500 U. Discussion: A favorable safety profile and continuous improvements in active movements and perceived and active function were associated with repeated abobotulinumtoxinA injections in upper limb muscles. Muscle Nerve 57: 245–254, 2018
Keywords: active function, botulinum toxin, open label, stroke, traumatic brain injury, upper limb spasticity
Abbreviations
- AE
adverse event
- BoNT‐A
botulinum toxin A
- DAS
Disability Assessment Scale
- ECG
electrocardiogram
- EQ‐5D
5‐dimension EuroQoL
- MAS
Modified Ashworth Scale
- MFS
Modified Frenchay Scale
- PGA
Physician Global Assessment
- PTMG
primary target muscle group
- QoL
quality of life
- SAE
serious adverse event
- SD
standard deviation
- SF‐36
36‐item Short Form Health Survey
- TBI
traumatic brain injury
- TEAE
treatment‐emergent adverse events
- XV1
passive range of motion
- XV3
angle of catch
- XA
active range of motion
- XN
“normal” maximal anatomical angle
In hemiparesis after stroke or traumatic brain injury (TBI), passive and active antagonist muscle resistance may cause reduced active movement, impaired function, and abnormal limb postures.1, 2
Botulinum toxin A (BoNT‐A) is a first‐line treatment for muscle overactivity in spastic paresis,3, 4, 5 and coupling injections with neurorehabilitation may improve outcomes.6, 7, 8 Although numerous studies have reported post‐stroke or ‐TBI benefits of abobotulinumtoxinA (Dysport; Ipsen) on muscle tone4, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 and passive function4, 9, 14, 17 for up to 24 weeks after a single injection, its effects on active function remained uncertain.3, 4, 19, 21, 22 Few studies have addressed the safety and efficacy of repeated BoNT‐A injections and none have shown effects on active movement.19, 20, 23, 24
The present study is an open‐label extension of a double‐blind study that first demonstrated improvements in active range of motion after a single abobotulinumtoxinA injection.25 This was observed alongside benefits for muscle tone, spasticity, and perceived function; however, no significant changes in active function were seen.25 Herein we assessed 1‐year safety and efficacy of abobotulinumtoxinA in adults with upper limb spasticity over repeated treatment cycles, using multiple outcomes, including active function, with active range of motion, spasticity, perceived function, and muscle tone.
METHODS
Study Population
This open‐label extension included rollover patients from the double‐blind trial (abobotulinumtoxinA and placebo groups)25 and newly recruited patients receiving from 1 to 4 or 5 treatment cycles, respectively, ≥12 weeks apart over a fixed duration of 15 months per patient. The number of treatment cycles received depended on treatment interval duration, which varied according to each patient's individual needs. Patients' disposition data are shown in Figure 1.
Figure 1.
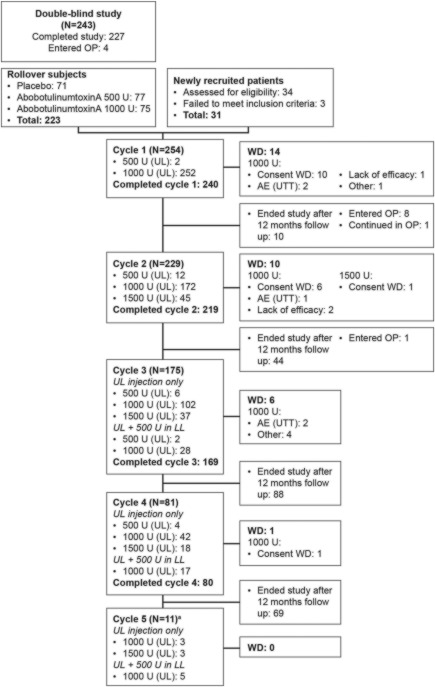
Disposition of patients. Differences in patient numbers between cycles due to patients entering the observational phase (OP) or ending the study after 12 months of follow‐up. Patients in the OP were followed up every 4 weeks until they required reinjection or completed 12 months of follow‐up. aIndicates newly recruited patients only. UL, upper limb; LL, lower limb; AE (UTT), adverse event (unrelated to treatment); WD, withdrawn.
Open‐label cycle 1 includes newly recruited and rollover patients, representing the second abobotulinumtoxinA injection for double‐blind study patients from the abobotulinumtoxinA group.
All double‐blind study patients were eligible to be rollover patients providing they completed the double‐blind study without major protocol deviations and/or ongoing adverse events (AEs) of unacceptable risk. Inclusion criteria for newly recruited patients were the same as for the double‐blind study (refer to Supplementary Material available online).25
The primary target muscle group (PTMG; extrinsic finger, wrist, or elbow flexors) was selected by the investigators at baseline (rollover) or inclusion (newly recruited) as the muscle group with the highest Modified Ashworth Scale (MAS)26 score. The PTMG remained constant throughout the study.
Study Objectives and Assessments
The primary objective was to assess safety of repeated abobotulinumtoxinA treatment cycles over 1 year in hemiparetic patients with upper limb spasticity post‐stroke or ‐TBI. Treatment‐emergent AEs (TEAEs) were elicited by direct, non‐leading questioning or spontaneous reports from study consent to study end. Other safety parameters were vital signs (blood pressure, heart rate), laboratory data, electrocardiogram (ECG) analysis, and binding and neutralizing antibody analysis.
Secondary objectives were assessment of efficacy of repeated abobotulinumtoxinA treatment over 1 year on:
Muscle tone (MAS) for finger, wrist, and elbow flexors, and shoulder extensors.
Steps 2, 3, and 4 of the Five‐Step Assessment [including the Tardieu Scale with passive range of motion (XV1) and angle of catch (XV3), as well as active range of motion (XA); see below],27 for finger, wrist, and elbow flexors; in shoulder extensors only XV1 and XV3 were assessed.
Perceived function and pain [Disability Assessment Scale (DAS)].28
Active upper limb function [Modified Frenchay Scale (MFS)]29, 30 rated by assessors independent of the study.
Physician Global Assessment (PGA) of treatment response, assessed using a 9‐point scale from –4 (markedly worse) to 4 (markedly improved) and rated by an assessor other than the MAS assessor.
Patient‐reported ease of applying splint.31
Quality of life (QoL), using the 36‐item Short Form Health Survey (SF‐36; perceived health score)32 and EuroQoL 5‐Dimension Questionnaire (EQ‐5D).33
In the Five‐Step Assessment, steps 2, 3, and 4 evaluate angles of antagonist resistance. XV1 (angle of arrest upon slow and strong passive stretch) reflects passive extensibility of the muscle–tendon complex and spastic dystonia. XV1 is interpreted with respect to XN (normal expected amplitude) using a coefficient of shortening, CSH = (XN – XV1) / XN (finger, wrist, and elbow flexors, and shoulder extensors: XN = 300°, 180°, 180°, and 180°, respectively). A higher coefficient of shortening indicates stiffer muscles.29 XV3 (angle of catch upon fast stretch) reflects spasticity. XA (maximal active range of motion against antagonist) reflects passive (stiffness) and active (spastic cocontraction) resistance to agonist effort.27, 29 Reliability of XV1, XV3, and XA measurements has been demonstrated in children and adults.34, 35, 36 Numerous studies support the value of XV1 and XV3 (Tardieu Scale) as compared with the MAS.37, 38
Study Interventions
At cycle 1, all patients received 1,000 U abobotulinumtoxinA, except those who had TEAEs during the double‐blind study who received 500 U abobotulinumtoxinA, based on the investigator's judgment. From cycle 2, patients received 500 U, 1,000 U, or 1,500 U injections at the investigator's discretion per cycle. For patients receiving 1,500 U abobotulinumtoxinA it was required that 500 U be injected into shoulder extensors (maximum 1,000 U across finger, wrist, and elbow flexors). Patients receiving <1,500 U total dose could receive shoulder muscle injections, providing PTMGs were injected with required doses. From cycle 3, patients could receive concomitant injections (500 U) into ≥1 calf muscle, providing the total dose was ≤1,500 U. To ensure optimal targeting of the injection, the protocol mandated that injections were administered using electrical stimulation (technique using an injection needle to deliver electrical pulses, which causes specific muscles to contract and thus ensures injection into the correct muscle).39, 40 At each visit from week 12 onward, patients were assessed to determine whether reinjection was required, based on the investigator's clinical judgment.
Recommended injection volumes for individual muscle groups are summarized in Table S1 (refer to Supplementary Material online). The rationale for total injection volumes and dilutions used is also presented in the Supplementary Material.
No standardized physiotherapy regimen was associated with this protocol, but community physiotherapy initiated before study enrollment remained unchanged whenever possible until study end.
Statistical Analysis
No formal statistical comparisons were performed owing to the open‐label study design. A descriptive analysis is presented for safety and efficacy parameters, and for comparisons of MAS in PTMG between toxin‐naive and non–toxin‐naive patients as well as between patients undergoing physiotherapy at baseline and those who were not. As patients could move between dose groups at each cycle, technical efficacy results for individual muscles (XV1, XV3, and XA) were presented for all doses pooled; for comparison, a subanalysis of these parameters was performed for 27 patients who received a constant 1,000 U dose throughout the double‐blind study to cycle 3. A post‐hoc analysis assessed mean (± standard deviation [SD]) coefficients of shortening at baseline.
An additional post‐hoc analysis exploring linear relationships between composite XA (sum of XA against finger, wrist, and elbow flexors) and MFS was performed using Pearson correlation coefficients at baseline and week 4 of each cycle.
Standard Protocol Approval, Registration, and Patient Consent
Our study was performed in compliance with the Declaration of Helsinki, good clinical practice guidelines, and local regulatory requirements, and with approval from all relevant institutional review boards and ethics committees. All patients signed site‐specific, approved, written informed consent forms before study entry.
RESULTS
Baseline Characteristics and Exposure
In total, 258 patients (227 rollover and 31 newly recruited patients; 4 rollover patients entered the observation phase before cycle 1 and were not injected in the open‐label phase) were enrolled over 34 sites in Belgium, Czech Republic, France, Italy, Poland, Russia, Slovakia, and the USA. Age was 52.4 ± 13.9 years, 64.3% were male, and 45.0% were toxin‐naive in the paretic limb. Hemiparesis was stroke‐induced in 89.1% of patients and TBI‐induced in 10.9%; time since event was 5.1 ± 4.2 years and 9.9 ± 8.0 years, respectively. The proportion of patients undergoing physiotherapy before the study was 48.4%; this proportion remained similar throughout the study.
Extrinsic finger flexors were selected as PTMG in 56.6% of patients, wrist flexors in 16.7%, and elbow flexors in 26.7%. Coefficients of shortening at baseline29 were 34.5 ± 16.4% for extrinsic finger flexors (n = 225), 20.7 ± 16.2% for wrist flexors (n = 186), 3.9 ± 7.2% for elbow flexors (n = 185), and 25.4 ± 14.1% for shoulder extensors (n = 31).29 Principal target of treatment for DAS was limb position in 44.6% of patients, dressing in 26.4%, hygiene in 22.1%, and pain in 6.6%.
Duration of repeated abobotulinumtoxinA treatment was 54.0 ± 9.9 weeks for rollover (including double‐blind study) patients and 55.8 ± 11.9 weeks for newly recruited patients. Overall, 240 patients completed cycle 1, 219 completed cycle 2, 169 completed cycle 3, 80 completed cycle 4, and 11 completed cycle 5 (Fig. 1). Injection doses and prevalence by muscle group (PTMG and overall) are summarized in Table S2 (Supplementary Material). At cycle 1, 99.2% of patients received 1,000 U abobotulinumtoxinA in the upper limb. Across cycles, a trend toward using higher doses was observed: 1,500 U abobotulinumtoxinA was used in 19.7% of patients at cycle 2 and 43.2% by cycle 4 (Fig. 1). The proportion of patients injected in shoulder extensors (regardless of dose) progressed from 13.8% (cycle 1) to 40.7% (cycle 4). Affected lower limbs were injected in 17.1% and 21.0% of patients during cycles 3 and 4, respectively.
Safety Results
Figure 1 shows most patients who left the study between cycles did so because they reached maximum treatment duration (15 months), due to injection intervals of >12 weeks, and not because of AEs. Under these circumstances, overall incidence of TEAEs decreased across subsequent treatment cycles for all doses combined, from 40.2% (cycle 1) to 13.6% (cycle 4). Similarly, TEAEs considered treatment‐related decreased from 7.1% to 2.5% (Table 1). Most TEAEs were of mild to moderate intensity. The most frequently reported TEAEs were focal muscle weakness, falls, and pain in extremities. Over all open‐label cycles, muscle weakness was reported in 11 (4.3%) and 3 (5.0%) patients after 1,000 U and 1,500 U injections, respectively. After injections of 500 U, 1,000 U, and 1,500 U, falls occurred in 2 (11.8%), 15 (5.9%), and 4 (6.7%) patients, respectively, and pain in extremities occurred in 1 (5.9%), 11 (4.3%), and 4 (6.7%) patients, respectively. In cycles 3 and 4 (when lower limb injections were permitted), occurrence rates of falls were 5.5% (n = 2 of 36) in patients receiving lower limb injections and 3.6% (n = 5 of 139) in patients receiving upper limb injections only. None of these falls at cycles 3 and 4 were considered treatment‐related by the investigator and none led to fractures.
Table 1.
Summary of treatment emergent adverse events by treatment cycle and dose.
| Events |
Double‐blind (N = 154) |
Cycle 1 (N = 254) |
Cycle 2 (N = 229) |
Cycle 3 (N = 175) |
Cycle 4 (N = 81) |
|---|---|---|---|---|---|
| Summary of TEAEs by treatment cycle | |||||
| aboBoNT‐A 500 U (UL) | n = 78 | n = 2 | n = 12 | n = 8 | n = 4 |
| TEAEs | 32 (41.0) [89] | 1 (50.0) [2] | 3 (25.0) [10] | 1 (12.5) [2] | 0 |
| Related TEAEs | 6 (7.7) [11] | 0 | 1 (8.3) [1] | 0 | 0 |
| AESI | 4 (5.1) [7] | 0 | 0 | 0 | 0 |
| SAEs | 3 (3.8) [3] | 0 | 0 | 0 | 0 |
| aboBoNT‐A 1,000 U (UL) | n = 76 | n = 252 | n = 172 | n = 130 | n = 59 |
| TEAEs | 32 (42.1) [64] | 101 (40.1) [192] | 45 (26.2) [81] | 31 (23.8) [83] | 10 (16.9) [25] |
| Related TEAEs | 7 (9.2) [10] | 18 (17.1) [26] | 4 (2.3) [5] | 3 (2.3) [4] | 2 (3.4) [3] |
| AESI | 7 (9.2) [7] | 14 (5.6) [15] | 2 (1.2) [2] | 2 (1.5) [3] | 1 (1.7) [1] |
| SAEs | 2 (2.6) [2] | 10 (4.0) [12] | 6 (3.5) [10] | 5 (3.8) [10] | 1 (1.7) [3] |
| aboBoNT‐A 1,500 U (UL) | NA | NA | n = 45 | n = 37 | n = 18 |
| TEAEs | — | — | 14 (31.1) [29] | 15 (40.5) [22] | 1 (5.6) [1] |
| Related TEAEs | — | — | 3 (6.7) [3] | 2 (5.4) [2] | 0 |
| AESI | — | — | 3 (6.7) [3] | 1 (2.7) [1] | 0 |
| SAEs | — | — | 0 | 1 (2.7) [1] | 0 |
| All doses (UL+LL) | n = 154 | n = 254 | n = 229 | n = 175 | n = 81 |
| TEAEs | 64 (41.6) [153] | 102 (40.2) [194] | 62 (27.1) [120] | 47 (26.9) [107] | 11 (13.6) [26] |
| Related TEAEs | 13 (8.4) [21] | 18 (7.1) [26] | 8 (3.5) [9] | 5 (2.9) [6] | 2 (2.5) [3] |
| AESI | 11 (7.1) [14] | 14 (5.5) [15] | 5 (2.2) [5] | 3 (1.7) [4] | 1 (1.2) [1] |
| SAEs | 5 (3.2) [5] | 10 (3.9) [12] | 6 (2.6) [10] | 6 (3.4) [11] | 1 (1.2) [3] |
| Preferred terma | |||||
| All aboBoNT‐A doses | n = 154 | n = 254 | n = 229 | n = 175 | n = 81 |
| Fall | 3 (1.9) [3] | 9 (3.5) [9] | 7 (3.1) [7] | 6 (3.4) [7] | 1 (1.2) [2] |
| Muscle weakness | 6 (3.9) [6] | 9 (3.5) [9] | 2 (0.9) [2] | 2 (1.1) [2] | 1 (1.2) [1] |
| Pain in extremity | 0 | 6 (2.4) [6] | 6 (4.6) [8] | 2 (1.1) [2] | 2 (2.5) [2] |
| Nasopharyngitis | 8 (5.2) [8] | 4 (1.6) [5] | 0 | 2 (1.1) [2] | 0 |
| Bronchitis | 2 (1.3) [2] | 0 | 1 (0.4) [1] | 4 (2.3) [4] | 0 |
| Blood triglycerides increased | 4 (2.6) [4] | 0 | 1 (0.4) [1] | 0 | 0 |
Data expressed as: n (%) [number of events]. Results are from patients injected with active treatment only, and placebo group is not shown. Patients are grouped according to dose received in the UL, regardless of dose injected in the lower limb. aboBoNT‐A, abobotulinumtoxinA; AESI, adverse event of special interest; LL, lower limb; N, number of patients in group; n, number of patients with data; NA, not applicable; SAE, serious adverse event; TEAE, treatment‐emergent adverse event; UL, upper limb.
Preferred term for TEAEs displayed by ≥2% of patients at any cycle.
Three fatalities occurred due to serious AEs (SAEs), all following injections of 1,000 U; none were considered treatment‐related by the investigator and all were considered most likely to be related to underlying clinical conditions. These were due to metastatic cancer, cardiopulmonary arrest, and myocardial infarction. In addition, 2 TEAE cases led to withdrawal, both after 1,000 U injections: 1 patient had emotional lability reported as an SAE, which was considered unrelated to treatment; and 1 patient had a pregnancy after initial open‐label injection, after which she gave birth to a full‐term, healthy neonate.
Although none of the AEs of special interest were assessed by investigators as causally related to abobotulinumtoxinA, the sponsor's evaluation identified 2 cases (constipation and diplopia) in which possible remote spread of toxin effect could not be ruled out. No cases of generalized muscular weakness, dysphagia, or dysphonia were seen as remote spread effects. In addition, no AE terms were assessed as suggestive of hypersensitivity‐type reactions.
At baseline, 4 patients had neutralizing antibodies. Overall, 11 patients (4.3% of enrolled patients) converted for neutralizing antibodies during the study. Of these, 3 had transient seroconversion while continuously injected, returning to negative by study end, and 8 seroconverted for neutralizing antibodies up to study end. These 11 patients received various doses of abobotulinumtoxinA, in a proportion comparable to that of the whole study population. There was no suggestion of reduced abobotulinumtoxinA efficacy in patients with neutralizing antibodies at baseline or throughout the study.
No clinically significant differences in clinical hematology or biochemistry parameters occurred at any dose or cycle from baseline to last visit. Mean changes in systolic and diastolic blood pressure and heart rate between baseline and week 4 were within clinically normal ranges and, despite slight increases in blood pressure and heart rate, these were not considered significant for any dose or cycle. ECG analysis showed no cardiac safety risk at any dose or cycle.
Efficacy Results
Muscle tone improvements (MAS) after initial injection into PTMGs in the double‐blind study from baseline (3.9 ± 0.4) to week 4 (2.7 ± 1.1)25 remained stable across cycles (cycle 4 week 4, 2.6 ± 1.1) (see Table S3 in Supplementary Material). Effects on muscle tone were similar between toxin‐naive and non–toxin‐naive patients, and between patients who underwent physiotherapy and those who did not.
XV1, XV3, and XA improved at week 4 of each cycle in PTMGs and shoulder extensors (Fig. 2). In PTMGs, XV1 remained relatively stable across cycles, whereas XV3 and XA continued to improve (Fig. 2). For extrinsic finger flexors, XV1 was maintained across cycles (between 207.6 ± 47.6° at cycle 4 and 217.2 ± 40.7° at cycle 2; baseline 193.1 ± 49.6°), whereas XV3 and XA continued to improve up to 162.2 ± 50.1° (baseline 98.6 ± 53.2°) and 91.4 ± 56.2° (baseline 41.9 ± 48.9°), respectively (Fig. 2A). Comparable results were observed for wrist and elbow flexors (Fig. 2B and C, respectively). In elbow flexors, XA was noted to consistently reach higher values than XV3 (Fig. 2C). In shoulder extensors, improvements in XV1 and XV3 were observed over cycles with increases in mean change from baseline, from +3.2 ± 8.0° (double‐blind) to +10.8 ± 26.1° (cycle 4) for XV1, and +11.4 ± 18.3° (double‐blind) to +18.0 ± 19.6° (cycle 4) for XV3 (see Table S3 in Supplementary Material). Results for XV1, XV3, and XA in 27 patients injected with a constant 1,000‐U dose throughout double‐blind to cycle 3 were comparable.
Figure 2.
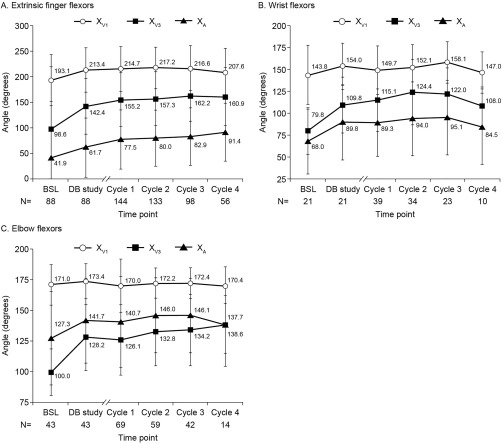
Passive range of motion (XV1), angle of catch (XV3), and active range of motion (XA) at baseline and week 4 of each cycle in each primary target muscle group for all patients and all doses pooled. Values are presented as mean (standard deviation). BSL, baseline of double‐blind study; DB, double‐blind.
For perceived function (DAS), percentage of responders (≥1 grade reduction from baseline) at week 4 progressively increased in all 4 domains from double‐blind to cycle 4 (Fig. 3). Increases for all doses combined were observed from 45.4% to 53.1% for limb position, 32.9% to 53.1% for dressing, 34.9% to 46.9% for hygiene, and 28.3% to 48.1% for pain. Figure 3 suggests more marked effects at cycles 3 and 4 with 1,500 U of abobotulinumtoxinA compared with 1,000 U for limb position (70.3% vs. 56.9% and 61.1% vs. 49.2%, respectively) and dressing (59.5 vs. 42.3% and 61.1 vs. 47.5%, respectively). Results for patients injected with 500 U are not presented, as very few patients received this dose.
Figure 3.
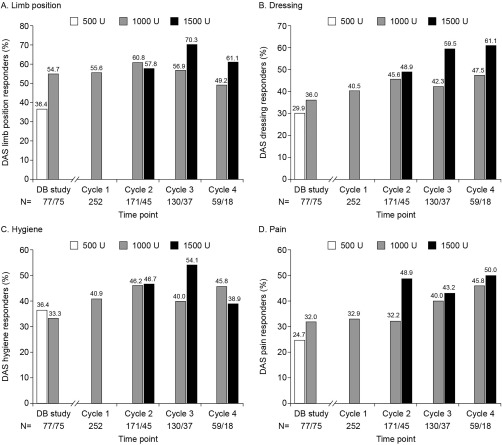
Disability Assessment Scale responders for each domain by abobotulinumtoxinA dose at week 4 of each cycle. Data for 500 U are not presented for cycles 1–4 due to small patient numbers, which provide little statistical value. N values are presented in order of doses shown (500 U, 1,000 U, 1,500 U). DAS, Disability Assessment Scale; DB, double‐blind.
For active function, mean change from baseline in global MFS score across cycles peaked at +0.43 ± 0.82 (cycle 2) after 1,000 U injections and +0.76 ± 0.67 (cycle 2) after 1,500 U injections (Fig. 4, see also Table S3 in Supplementary Material). There was high correlation between composite XA and MFS at each of these study visits (Table 2).
Figure 4.
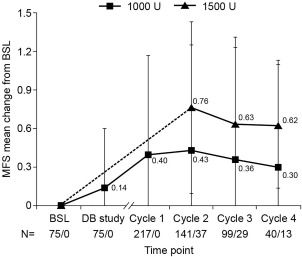
Mean change in Modified Frenchay Scale overall score by abobotulinumtoxinA dose at week 4 of each cycle. Values are presented as mean (standard deviation). Data for 500 U are not presented due to small patient numbers, which provide little statistical value. N values are presented as those for 1,000 U and 1,500 U. The dotted line indicates change from baseline to cycle 2 for 1,500 U, as patients could not receive this dose in the DB study and at cycle 1. BSL, baseline of DB study; DB, double‐blind; MFS, Modified Frenchay Scale.
Table 2.
Correlation between Modified Frenchay Scale and composite active range of motion (XA) at week 4 of each cycle (absolute values).
| Visit | Placebo | aboBoNT‐A 1,000 U | aboBoNT‐A 1,500 U |
|---|---|---|---|
| Baseline (n) | 37 | 43 | — |
| Correlation coefficient | 0.6600 | 0.6486 | — |
| P‐value | <0.0001 | <0.0001 | — |
| Double‐blind (n) | 36 | 43 | — |
| Correlation coefficient | 0.6077 | 0.5632 | — |
| P‐value | <0.0001 | <0.0001 | — |
| Cycle 1 (n) | — | 119 | — |
| Correlation coefficient | — | 0.4987 | — |
| P‐value | — | <0.0001 | — |
| Cycle 2 (n) | — | 83 | 23 |
| Correlation coefficient | — | 0.5156 | 0.3905 |
| P‐value | — | <0.0001 | 0.0654 |
| Cycle 3 (n) | — | 49 | 19 |
| Correlation coefficient | — | 0.5407 | 0.5368 |
| P‐value | — | <0.0001 | 0.0178 |
| Cycle 4 (n) | — | 16 | 10 |
| Correlation coefficient | — | 0.3082 | 0.6654 |
| P‐value | — | 0.2456 | 0.0358 |
Composite XA equals the sum of XA for extrinsic finger, wrist, and elbow flexors. Coefficients presented are Pearson correlations. Data for 500 U are not presented due to small patient numbers, providing little statistical value. aboBoNT‐A, abobotulinumtoxinA; XA, active range of motion.
PGA scores (1.5 at double‐blind week 4) were 1.7, 1.9, 1.9, and 2.0 for all doses at cycle 1 to cycle 4, respectively. Mean scores for ease of applying a splint (improved during the double‐blind study) remained stable across open‐label cycles at week 4. Slightly positive changes in SF‐36 or EQ‐5D scores were observed from baseline to final visit: SF‐36 physical component, +1.07 ± 6.76; SF‐36 mental component, +0.96 ± 11.1; and visual analog scale of EQ‐5D, +2.8 ± 19.0.
Across open‐label cycles, a large proportion of patients did not require reinjection at week 12 (all doses combined). For patients who received a second treatment cycle in the open‐label phase, 35% were reinjected at week 16 or later (20% at week 16, 7.0% at week 20, 8% at week 24 or later). For those who received a third treatment cycle in the OL phase, 24% were reinjected at week 16 or later (19% were retreated at week 16, 3% at week 20, 2% at week 24 or later).
DISCUSSION
This open‐label extension to a double‐blind study in 223 rollover and 31 newly recruited hemiparetic adults with upper limb spasticity showed that repeated abobotulinumtoxinA injections (500 U, 1,000 U, and 1,500 U), administered using electrical stimulation, were well tolerated over 1 year. Injections were associated with further improvements in active range of motion and active function, despite stabilization of tone and passive range of motion.
Safety of Repeated AbobotulinumtoxinA Injections over 1 Year
For patients remaining in the study there were decreasing reports of TEAEs across cycles. As many patients completed 15 months of follow‐up before study end (Fig. 1), it cannot be concluded definitively that the incidence of TEAEs decreased across repeated injection cycles. These reports of decreasing TEAEs are still remarkable as patients present at later cycles received more injection cycles and tended to be injected with higher doses, yet reported fewer TEAEs. Possible explanations for increasingly higher injection doses across cycles, despite gradual spasticity reduction (Fig. 2), include patients developing a tolerance to side effects as well as the option to use the 1,500 U total dose to include shoulder muscles and then lower limb injections after cycles 2 and 3, respectively. Thus, no cumulative detrimental effect on safety was suggested over several injections cycles.
A slightly higher percentage of falls (none considered treatment‐related) was observed in patients receiving lower limb injections compared with those receiving upper limb injections only at cycles 3 and 4. This observation is difficult to interpret as the expected risk of falls would be higher in patients with higher levels of lower limb overactivity, which may have led the investigator to include the lower limb into the injection plan.
Slight increases in blood pressure and heart rate were not associated with clinical cardiac events or cardiac safety risk and were most likely related to underlying clinical conditions of the patient. Finally, the way TEAEs were elicited (non‐leading questioning and spontaneous reports) may be open to risks of underreporting, as many leading questioning result in overreporting. Yet, TEAE elicitation methods remained consistent across treatment cycles, where reduced incidence of TEAEs was observed.
Limitations of the Efficacy Study
Patient numbers per dose varied at each cycle, as patients moved between doses. All doses are thus presented together for changes in XV1, XV3, and XA; however, results for 27 patients receiving a constant dose (1,000 U) throughout double‐blind study to cycle 3 proved comparable with the whole group for these parameters. Not all patients required reinjection at week 12 of each cycle; thus, time intervals between injections varied. This is to be considered for Figure 2, in which the slight downward trend for XV3 and XA by cycle 4 for wrist and elbow flexors corresponded to the limited number of patients injected at cycle 4. At cycle 4, the 10 patients injected into wrist flexors as PTMG (Fig. 2B) and 14 patients injected into elbow flexors as PTMG (Fig. 2C) are those patients injected most often, and therefore likely to have been more severe or less responsive to treatment compared with other patients. XA was measured in PTMGs only, thus active shoulder flexion was not measured; this may have given specific insight into potential benefit of injections targeting shoulder extensors. Finally, goal attainment measurements could have added patient‐centered reporting of outcomes, in addition to assessment of perceived function (DAS), as patient goals may vary across cycles.9, 17 In the present study, PTMGs remained constant throughout cycles, which may constitute a relative weakness of the study in terms of adjusting objectives and reaching maximal efficacy with each injection.9, 17
Cumulative Effect of Repeated AbobotulinumtoxinA Injections on Active Movements
Although the improvements in muscle tone and XV1 observed after initial injection25 were maintained across cycles, progressive improvements in XV3 (reflecting muscle activation upon fast stretch at rest) and XA (reflecting muscle activation upon spastic cocontraction during voluntary command) continued for all PTMGs across injection cycles. For example, active finger extension more than doubled by cycle 4 from double‐blind baseline (increase of about 50°), potentially allowing for sufficient active hand opening for some grasping tasks, as suggested by the positive MFS results. Similar to cumulative spasticity reduction (XV3), a large amount of descending motor unit recruitment in spastic cocontraction41 remaining despite a first injection may be further diminished through additional injection cycles.
Interestingly, elbow flexors were the sole PTMG where XA consistently surpassed XV3. This may relate to particularly low coefficients of shortening in elbow flexors (3.9%), which are likely related to fewer shortening positions being imposed on this muscle group compared with other upper limb muscles (finger flexors, wrist flexors, and shoulder extensors) during acute and subacute stroke care.29 A low coefficient of shortening may allow for reduced tension during elbow extension, generating less afferent volley and therefore less spastic cocontraction in elbow flexors.41 In addition, the method of measuring active elbow extension (downward movement not requiring shoulder flexor recruitment)25 may have placed elbow flexors at an additional advantage in terms of cocontraction.42
Improvement of Perceived and Active Function across Cycles
Progressive increases in perceived function (DAS responders) occurred across cycles, particularly for dressing, a domain mostly involving active upper limb movements.
Active function improvement on MFS across cycles was at least double that of the double‐blind study (+0.14),25 reaching a level considered clinically meaningful (>0.5 increase in mean change from baseline).30 Although improvements in perceived function (DAS responders) have been demonstrated,10 prior studies have not provided evidence supporting the benefit of BoNT‐A injections in active function.10, 21
For perceived and active function, responder rates were higher after 1,500 U injections compared with 1,000‐U injections, which may suggest the importance of injecting the shoulder muscles. Indeed, shoulder extensor injections may have improved active shoulder flexion (not assessed here), a joint movement required in most daily activities,43 as tested in the DAS and MFS. However, the present data could not demonstrate that higher responder rates were not simply due to higher doses injected, regardless of shoulder targeting. Patients injected with 1,500 U had notably lower baseline MFS scores (3.17 ± 0.96 vs. 3.94 ± 1.50; see Table S3 in Supplementary Material), which may explain why they received the highest dose and shoulder injections. Interestingly, MFS improvement patterns paralleled those of XA, and strong correlations were demonstrated between active function and composite active motion at each visit. Yet, these functional improvements were paralleled by positive but small changes in SF‐36 and EQ‐5D scores,44, 45 which could suggest functional upper limb changes of a greater magnitude may be needed for a meaningful impact on quality of life or perceived health scores. Overall, the results of the present study seem more positive than those obtained by Shaw et al. or Lagalla et al.,19, 20 which may be due to a number of factors, including the electrical stimulation localization method systematically used to target muscles in our study, overall doses injected, and the outcome measures used. MFS (using visual analog rating for each task instead of an ordinal scale) and XA measurements used here may be more sensitive to change than the Action Research Arm Test,19 or Frenchay Arm Test.20
In this study we obtained favorable safety data in hemiparetic adults with upper limb spasticity after repeated abobotulinumtoxinA injections over 1 year. Across cycles, efficacy on muscle tone and passive range of motion (XV1) was maintained in the elbow, wrist, and extrinsic finger flexors, whereas angle of catch (XV3) and active range of motion (XA) continued to progress. Shoulder extensors also showed improvements in XV1 and XV3. Perceived function (DAS) improved over repeated cycles. Higher efficacy was suggested when injecting 1,500 U vs. 1,000 U abobotulinumtoxinA for perceived and active function, possibly indicating the importance of shoulder muscle injections. In addition, the retreatment intervals of >12 weeks observed in many patients could potentially reduce the burden associated with frequency of injections for patients and their caregivers/families. This also highlights the need for a tailored approach in the treatment of patients with upper limb spasticity. As these results were obtained without a systematic standardized rehabilitation protocol, it may be worth exploring if repeated BoNT injections in combination with an aggressive, individually tailored rehabilitation program would yield greater improvements over time in hemiparetic patients.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information
ACKNOWLEDGMENTS
Medical writing and submission support, under the direction of the authors, were provided by Jacqueline Harte and Germanicus Hansa‐Wilkinson of Watermeadow Medical, an Ashfield Company, funded by Ipsen.
Funding: This study was sponsored by Ipsen Innovation, Les Ulis, France.
Conflicts of Interest: J.‐M.G. served as a consultant on advisory boards for and received research grant support from Allergan, Ipsen, and Merz. M.O.D. has received grants from and served on the scientific advisory board for Ipsen. M.V. received compensation from Ipsen through his university hospital institution for conducting this clinical trial. P.H. has served on the scientific advisory board of the Spastic Paraplegia Foundation and International Essential Tremor Foundation; has received travel/speaker honoraria from Teva Neuroscience and Lundbeck; has received research support from the National Institute of Neurological Disorders and Stroke; has served as Editor‐in‐chief for the Journal of Parkinsonism and Restless Legs Syndrome; has served on the editorial boards of Neurology, Parkinsonism and Related Disorders; and has received royalty payments from Elsevier. S.K. has received honoraria from Ipsen for serving as a trainer in multiple programs and as a lecturer. S.K. has also received honoraria from Allergan and Merz for serving as a trainer. M.R.‐B. serves as a primary investigator for Teva Pharmaceutical Industries, Ltd.; Pfizer, Inc.; Acorda Therapeutics, Inc.; and Kyowa Kirin Pharmaceutical Development, Inc.; and receives research support from the Medical University of Silesia and the CHDI Foundation, Inc. B.R. has received research support and speaking and consulting fees from Allergan, Ipsen, and Merz, and physician training for Allergan. S.L.T. has received speaker fees and meeting sponsorship from Ipsen and Merz. A.L. served as a sub‐investigator in clinical trials sponsored by Ipsen, and has received honoraria from Allergan, Merz, and Ipsen for serving as a trainer. F.C.B. served on the scientific advisory board for Ipsen (DOSIS project) and Medtronic, and received research support from the French Muscular Dystrophy Association for developing PRO in muscular dystrophy patients. All non‐Ipsen authors (J.‐M.G., M.O.D., M.V., P.H., S.K., M.R.‐B., B.R., S.L.T., A.L., F.C.B.) received compensation from Ipsen for conducting this clinical trial. A.‐S.G., C.V., and P.P. are employees of Ipsen.
REFERENCES
- 1. Gracies JM. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005;31:535–551. [DOI] [PubMed] [Google Scholar]
- 2. Gracies JM. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 2005;31:552–571. [DOI] [PubMed] [Google Scholar]
- 3. Sheean G, Lannin NA, Turner‐Stokes L, Rawicki B, Snow BJ, Cerebral Palsy Institute. Botulinum toxin assessment, intervention and after‐care for upper limb hypertonicity in adults: international consensus statement. Eur J Neurol 2010;17(suppl 2):74–93. [DOI] [PubMed] [Google Scholar]
- 4. Wissel J, Ward AB, Erztgaard P, Bensmail D, Hecht MJ, Lejeune TM, et al European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med 2009;41:13–25. [DOI] [PubMed] [Google Scholar]
- 5. Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016;86:1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu X, Tong KY, Song R, Tsang VS, Leung PO, Li L. Variation of muscle coactivation patterns in chronic stroke during robot‐assisted elbow training. Arch Phys Med Rehabil 2007;88:1022–1029. [DOI] [PubMed] [Google Scholar]
- 7. Kinnear BZ, Lannin NA, Cusick A, Harvey LA, Rawicki B. Rehabilitation therapies after botulinum toxin‐A injection to manage limb spasticity: a systematic review. Phys Ther 2014;94:1569–1581. [DOI] [PubMed] [Google Scholar]
- 8. Wang YH, Meng F, Zhang Y, Xu MY, Yue SW. Full‐movement neuromuscular electrical stimulation improves plantar flexor spasticity and ankle active dorsiflexion in stroke patients: a randomized controlled study. Clin Rehabil 2016;30:577–586. [DOI] [PubMed] [Google Scholar]
- 9. Ashford S, Fheodoroff K, Jacinto J, Turner‐Stokes L. Common goal areas in the treatment of upper limb spasticity: a multicentre analysis. Clin Rehabil 2016;30:617–622. [DOI] [PubMed] [Google Scholar]
- 10. Dashtipour K, Chen JJ, Walker HW, Lee MY. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil 2015;94:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jost WH, Hefter H, Reissig A, Kollewe K, Wissel J. Efficacy and safety of botulinum toxin type A (Dysport) for the treatment of post‐stroke arm spasticity: results of the German–Austrian open‐label post‐marketing surveillance prospective study. J Neurol Sci 2014;337:86–90. [DOI] [PubMed] [Google Scholar]
- 12. McCrory P, Turner‐Stokes L, Baguley IJ, De Graaff S, Katrak P, Sandanam J, et al Botulinum toxin A for treatment of upper limb spasticity following stroke: a multi‐centre randomized placebo‐controlled study of the effects on quality of life and other person‐centred outcomes. J Rehabil Med 2009;41:536–544. [DOI] [PubMed] [Google Scholar]
- 13. Rosales RL, Chua‐Yap AS. Evidence‐based systematic review on the efficacy and safety of botulinum toxin‐A therapy in post‐stroke spasticity. J Neural Transm (Vienna) 2008;115:617–623. [DOI] [PubMed] [Google Scholar]
- 14. Simpson DM, Gracies JM, Graham HK, Miyasaki JM, Naumann M, Russman B, et al Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence‐based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;70:1691–1698. [DOI] [PubMed] [Google Scholar]
- 15. Smith SJ, Ellis E, White S, Moore AP. A double‐blind placebo‐controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin Rehabil 2000;14:5–13. [DOI] [PubMed] [Google Scholar]
- 16. Suputtitada A, Suwanwela NC. The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil Rehabil 2005;27:176–184. [DOI] [PubMed] [Google Scholar]
- 17. Turner‐Stokes L, Fheodoroff K, Jacinto J, Maisonobe P. Results from the Upper Limb International Spasticity Study‐II (ULISII): a large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real‐life clinical management. BMJ Open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosales RL, Kong KH, Goh KJ, Kumthornthip W, Mok VC, Delgado‐De Los Santos MM, et al Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: a randomized controlled trial. Neurorehabil Neural Repair 2012;26:812–821. [DOI] [PubMed] [Google Scholar]
- 19. Shaw LC, Price CI, van Wijck FM, Shackley P, Steen N, Barnes MP, et al Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: effect on impairment, activity limitation, and pain. Stroke 2011;42:1371–1379. [DOI] [PubMed] [Google Scholar]
- 20. Lagalla G, Danni M, Reiter F, Ceravolo MG, Provinciali L. Post‐stroke spasticity management with repeated botulinum toxin injections in the upper limb. Am J Phys Med Rehabil 2000;79:377–384. [DOI] [PubMed] [Google Scholar]
- 21. Rosales RL, Efendy F, Teleg ES, Delos Santos MM, Rosales MC, Ostrea M, et al Botulinum toxin as early intervention for spasticity after stroke or non‐progressive brain lesion: a meta‐analysis. J Neurol Sci 2016;371:6–14. [DOI] [PubMed] [Google Scholar]
- 22. Foley N, Pereira S, Salter K, Fernandez MM, Speechley M, Sequeira K, et al Treatment with botulinum toxin improves upper‐extremity function post stroke: a systematic review and meta‐analysis. Arch Phys Med Rehabil 2013;94:977–989. [DOI] [PubMed] [Google Scholar]
- 23. Elovic EP, Brashear A, Kaelin D, Liu J, Millis SR, Barron R, et al Repeated treatments with botulinum toxin type a produce sustained decreases in the limitations associated with focal upper‐limb poststroke spasticity for caregivers and patients. Arch Phys Med Rehabil 2008;89:799–806. [DOI] [PubMed] [Google Scholar]
- 24. Gordon MF, Brashear A, Elovic E, Kassicieh D, Marciniak C, Liu J, et al Repeated dosing of botulinum toxin type A for upper limb spasticity following stroke. Neurology 2004;63:1971–1973. [DOI] [PubMed] [Google Scholar]
- 25. Gracies JM, Brashear A, Jech R, McAllister P, Banach M, Valkovic P, et al Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double‐blind randomised controlled trial. Lancet Neurol 2015;14:992–1001. [DOI] [PubMed] [Google Scholar]
- 26. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–207. [DOI] [PubMed] [Google Scholar]
- 27. Gracies JM, Bayle N, Vinti M, Alkandari S, Vu P, Loche CM, et al Five‐step clinical assessment in spastic paresis. Eur J Phys Rehabil Med 2010;46:411–421. [PubMed] [Google Scholar]
- 28. Brashear A, Zafonte R, Corcoran M, Galvez‐Jimenez N, Gracies JM, Gordon MF, et al Inter‐ and intrarater reliability of the Ashworth Scale and the Disability Assessment Scale in patients with upper‐limb poststroke spasticity. Arch Phys Med Rehabil 2002;83:1349–1354. [DOI] [PubMed] [Google Scholar]
- 29. Gracies JM. Coefficients of impairment in deforming spastic paresis. Ann Phys Rehabil Med 2015;58:173–178. [DOI] [PubMed] [Google Scholar]
- 30. Baude M, Mardale V, Loche CM, Hutin E, Gracies JM, Bayle N. Intra‐ and inter‐rater reliability of the Modified Frenchay Scale to measure active upper limb function in hemiparetic patients. Ann Phys Rehabil Med 2016;59s:e59–e60. [Google Scholar]
- 31. Kanovsky P, Slawek J, Denes Z, Platz T, Comes G, Grafe S, et al Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post‐stroke upper limb spasticity. J Rehabil Med 2011;43:486–492. [DOI] [PubMed] [Google Scholar]
- 32. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36‐Item Short‐Form Health Survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–263. [DOI] [PubMed] [Google Scholar]
- 33. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 34. Gracies JM, Burke K, Clegg NJ, Browne R, Rushing C, Fehlings D, et al Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch Phys Med Rehabil 2010;91:421–428. [DOI] [PubMed] [Google Scholar]
- 35. Ben‐Shabat E, Palit M, Fini NA, Brooks CT, Winter A, Holland AE. Intra‐ and interrater reliability of the Modified Tardieu Scale for the assessment of lower limb spasticity in adults with neurologic injuries. Arch Phys Med Rehabil 2013;94:2494–2501. [DOI] [PubMed] [Google Scholar]
- 36. Baude M, Loche CM, Gault‐Colas C, Pradines M, Gracies JM. Intra‐ and inter‐rater reliabilities of a stepped clinical assessment of chronic spastic paresis in adults. Ann Phys Rehabil Med 2015:e4–e5. [Google Scholar]
- 37. Patrick E, Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin Rehabil 2006;20:173–182. [DOI] [PubMed] [Google Scholar]
- 38. Fleuren JF, Voerman GE, Erren‐Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, et al Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 2010;81:46–52. [DOI] [PubMed] [Google Scholar]
- 39. Chin TY, Nattrass GR, Selber P, Graham HK. Accuracy of intramuscular injection of botulinum toxin A in juvenile cerebral palsy: a comparison between manual needle placement and placement guided by electrical stimulation. J Pediatr Orthop 2005;25:286–291. [DOI] [PubMed] [Google Scholar]
- 40. Picelli A, Lobba D, Midiri A, Prandi P, Melotti C, Baldessarelli S, et al Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: a randomized controlled trial comparing three injection techniques. Clin Rehabil 2014;28:232–242. [DOI] [PubMed] [Google Scholar]
- 41. Vinti M, Couillandre A, Hausselle J, Bayle N, Primerano A, Merlo A, et al Influence of effort intensity and gastrocnemius stretch on co‐contraction and torque production in the healthy and paretic ankle. Clin Neurophysiol 2013;124:528–535. [DOI] [PubMed] [Google Scholar]
- 42. Sukal‐Moulton T, Krosschell KJ, Gaebler‐Spira DJ, Dewald JP. Motor impairments related to brain injury timing in early hemiparesis. Part II: Abnormal upper extremity joint torque synergies. Neurorehabil Neural Repair 2014;28:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schurr K, Ada L. Observation of arm behaviour in healthy elderly people: implications for contracture prevention after stroke. Aust J Physiother 2006;52:129–133. [DOI] [PubMed] [Google Scholar]
- 44. Chen P, Lin KC, Liing RJ, Wu CY, Chen CL, Chang KC. Validity, responsiveness, and minimal clinically important difference of EQ‐5D‐5L in stroke patients undergoing rehabilitation. Qual Life Res 2016;25:1585–1596. [DOI] [PubMed] [Google Scholar]
- 45. Wyrwich KW, Metz SM, Kroenke K, Tierney WM, Babu AN, Wolinsky FD. Triangulating patient and clinician perspectives on clinically important differences in health‐related quality of life among patients with heart disease. Health Serv Res 2007;42:2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information


