Abstract
Background
CD39 and CD73 are two novel cell surface markers of CD25highFoxp3+ regulatory T‐cells (Tregs). Concordant expression of these two ectoenzymes not only discriminate Tregs from other cell populations, but also generates pericellular adenosine, which has been reported to suppress proliferation of activated T effector (Teff) cells. Because it is currently unclear whether human ectoenzymes (CD39/CD73) are involved in the impaired suppressive activity of Tregs in psoriasis, we examined the frequencies and phenotypes of CD39/CD73‐expressing Tregs and related receptor adenosine receptor 2A (A2A R) in peripheral blood of patients with different types of psoriasis.
Methods
Peripheral blood mononuclear cells (PMBC) were prepared from patients with three different types of psoriasis (psoriasis vulgaris, pustular psoriasis and erythrodermic psoriasis). CD4+ cells were separated from PBMC by negative selection on midiMACS columns, and the frequencies and phenotypes of CD39 and CD73 expressing Tregs, and A2A R expressing Teff were all determined by flow cytometry analysis. Blood from healthy volunteers served as controls.
Results
The expression of single CD73+ Tregs was markedly reduced (approximately 50%) in psoriasis vulgaris, compared to normal controls. In pustular psoriasis, the mean numbers of CD39+ Tregs and A2AR+ Teff was significantly lower than in normal controls. Among three different types of psoriasis, CD39 expression was strikingly reduced in the blood Treg population of pustular psoriasis patients. Decreased CD73+ Tregs levels were observed in psoriasis vulgaris compared to pustular psoriasis and erythrodermic psoriasis.
Conclusions
The differences in the expression of CD39− and CD73− Tregs may be a factor in the pathogenesis of psoriasis.
Keywords: A2AR, CD39, CD73, ectoenzymes, psoriasis, Tregs
Abbreviations
- A2AR
adenosine 2a receptor
- AMP
adenosine monophosphate
- cAMP
cyclic adenosine monophosphate
- mAb
monoclonal antibody
- PBMC
peripheral blood mononuclear cell
- PP
pustular psoriasis
- PE
erythrodermic psoriasis
- PV
psoriasis vulgaris
- Teff
T effector cells
- Treg
T regulatory cells
Introduction
CD4+CD25highFoxp3+ regulatory T‐cells (Tregs) are believed to be the key lymphocyte sub‐population in the maintenance of immunological self‐tolerance by suppressing immune responses.1, 2, 3 The study of Su and colleagues4 focused on their specific surface markers, which have now been expanded to include CC chemokine receptor type 4 (CCR4), CCR5, CTLA‐4 and CD127, has allowed a better understanding of the immunosuppressive mechanisms of Treg cells.
CD39/ectonucleoside triphosphate diphosphohydrolase and CD73/ecto‐5′‐nucleotidase are two novel cell surface markers identified in mouse Tregs.5 The concordant expression of these two ectoenzymes not only differentiates Tregs from other cell populations but also generates pericellular adenosine. Adenosine generation and its consequent effects, mediated by adenosine 2a receptors (A2A R) on T effector cells (Teff), play a critical role in the blockade of the activated Teff proliferation and pro‐inflammatory cytokine secretion (Fig. 1).6, 7 Conversion of extracellular adenosine triphosphate to adenosine by pericellular adenosinergic pathway (CD39, CD73 and A2A R) has already been demonstrated as a key anti‐inflammatory mechanism of Tregs with intriguing implications in immune‐mediated human diseases such as, multiple sclerosis,8 squamous cell carcinoma9 and arthritis.10 Psoriasis is a common, chronic autoimmune disease of the skin that has serious effects on the patient's physical and mental health.11 The pathogenesis of psoriasis is complex and involves genetic, immunological and even neurological factors. It is now widely accepted that a dysregulated T‐cell response, in which the activity of pathogenic effector T‐cells is inadequately controlled by psoriatic regulatory T cells.12, 13, 14 However, the mechanisms underlying psoriatic Treg dysfunction have not been completely elucidated. In the current study, we examined the proportion of ectoenzymes CD39/CD73 and A2A R expression by peripheral blood Tregs in different types of psoriasis. Given the inhibitory function of pericellular adenosine against Teff, the change of co‐expression of CD73 and/or CD39 may participate in the attenuated suppression capacity of peripheral blood Tregs in psoriatic patients.
Figure 1.
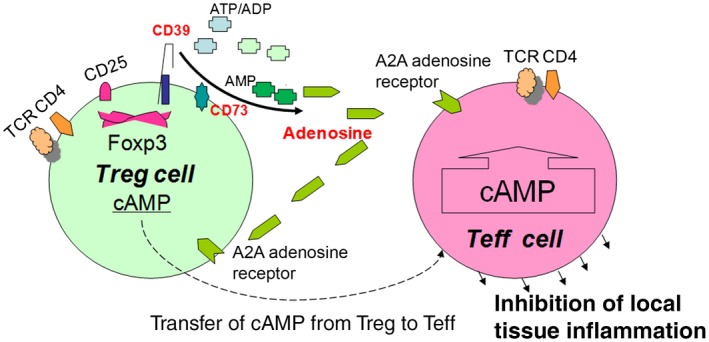
The role of CD39 and CD73 in Tregs. Tregs express CD39 and CD73. CD39 belongs to the ectonucleoside triphosphate diphosphohydrolase family, an ectoenzyme that hydrolyzes extracellular ATP/UTP, ADP/UDP to adenosine monophosphate (AMP). AMP is then degraded to nucleosides (adenosine) by CD73 (ecto‐5′‐nucleotidase), which in turn binds the adenosine receptor 2A (A2A R) expressed on T effector cells to elevate the intracellular cAMP which suppresses the proliferation of T effector cells.
Materials and Methods
Participants
A total of 30 patients with psoriasis (including 10 each with psoriasis vulgaris (PV), pustular psoriasis (PP) and erythrodermic psoriasis [PE]) and 10 age‐matched healthy volunteers were enrolled in this study (Table 1). The protocols involving human subjects were approved by the institutional review boards of Huashan Hospital, Fudan University, China and the University Hospitals of Cleveland, Case Western Reserve University, Ohio, USA. Informed consent was obtained from all participants. A medication‐free period of at least 1 month for oral drugs was required in all groups, and psoriatic patients refrained from using topical medications or phototherapy for at least 2 weeks before the blood collection procedures. The different types of psoriasis were defined mainly according to their clinical characteristics. The psoriasis area and severity index (PASI) was used to assess the severity of PV18 and the scores give a range from 0 to 72. For PP we used the method reported by Ohkawara,19 which evaluated the disease based on erythema, pustules, confluent pustules and enanthema.
Table 1.
Clinical and immunological profile of the studied patients
| Sex | Age, years | Disease duration, years | Score | CD39+/Treg (%) | CD73+/Treg (%) |
|---|---|---|---|---|---|
| Chronic plaque psoriasis vulgaris (PV, n = 10) | |||||
| M | 36 | 9 | 22.0 | 47.7 | 11.3 |
| M | 32 | 8 | 36.2 | 52.1 | 7.5 |
| F | 33 | 7 | 30.2 | 69.5 | 4.7 |
| M | 43 | 5 | 20.6 | 64.1 | 6 |
| F | 28 | 9 | 23.4 | 18.7 | 8.9 |
| F | 30 | 12 | 35.7 | 22.3 | 3.6 |
| M | 33 | 5 | 42.1 | 45.3 | 5.9 |
| F | 45 | 18 | 20.0 | 68.5 | 8.2 |
| M | 46 | 9 | 18.5 | 30.4 | 7.5 |
| M | 25 | 10 | 23.5 | 34 | 6.3 |
| Mean ± SD | 35.1 ± 4 | 9.2 ± 2 | 45.3 ± 10.7 | 7.0 ± 1.3 | |
| Erythrodermic psoriasis (PE, n = 10) | |||||
| M | 30 | 10 | – | 73.7 | 7.4 |
| F | 39 | 12 | – | 68.1 | 9.0 |
| F | 62 | 20 | – | 84.5 | 12.7 |
| F | 36 | 9 | – | 28.1 | 14.5 |
| M | 43 | 17 | – | 20.5 | 13.3 |
| M | 59 | 20 | – | 92.7 | 10.2 |
| F | 45 | 10 | – | 96.4 | 14.5 |
| M | 38 | 8 | – | 5.4 | 14.3 |
| M | 39 | 11 | – | 59.3 | 15.2 |
| F | 49 | 18 | – | 65.1 | 10.9 |
| Mean ± SD | 44 ± 5.8 | 13.5 ± 2.7 | – | 59.4 ± 18.1 | 12.2 ± 1.5 |
| Pustular psoriasis (PP, n = 10) | |||||
| F | 55 | 8 | 4 | 62.2 | 11.0 |
| F | 39 | 10 | 5 | 27.1 | 10.7 |
| M | 48 | 7 | 3 | 21.8 | 14.5 |
| F | 39 | 13 | 4 | 53.0 | 12.1 |
| F | 40 | 15 | 4 | 17.7 | 10.0 |
| F | 47 | 10 | 6 | 18.9 | 14.6 |
| M | 37 | 7 | 5 | 31.2 | 15.2 |
| M | 55 | 11 | 5 | 10.4 | 11.1 |
| F | 32 | 12 | 3 | 25.4 | 13.8 |
| F | 50 | 9 | 5 | 35.2 | 11.8 |
| Mean ± SD | 44.2 ± 4.6 | 10.2 ± 1.5 | 4.4 ± 0.6 | 30.3 ± 9.3 | 12.5 ± 1.1 |
| Controls (n = 10) | |||||
| Mean ± SD | 38 | 58.4 ± 14 | 13.2 ± 3.5 | ||
Cell isolation
Human PBMC was prepared from heparinised venous blood by Histopaque (Sigma‐Aldrich, St Louis, MO, USA) density gradient centrifugation according to the manufacturer's directions. CD4+ cells were separated from PBMC by negative selection on midiMACS columns (CD4+ T‐cell isolation kit; Miltenyi Biotec, Bergisch‐Gladbach, Germany) according to the manufacturer's instructions. The purity of the enriched CD4+ T‐cells exceeded 95% (Fig. 2).
Figure 2.
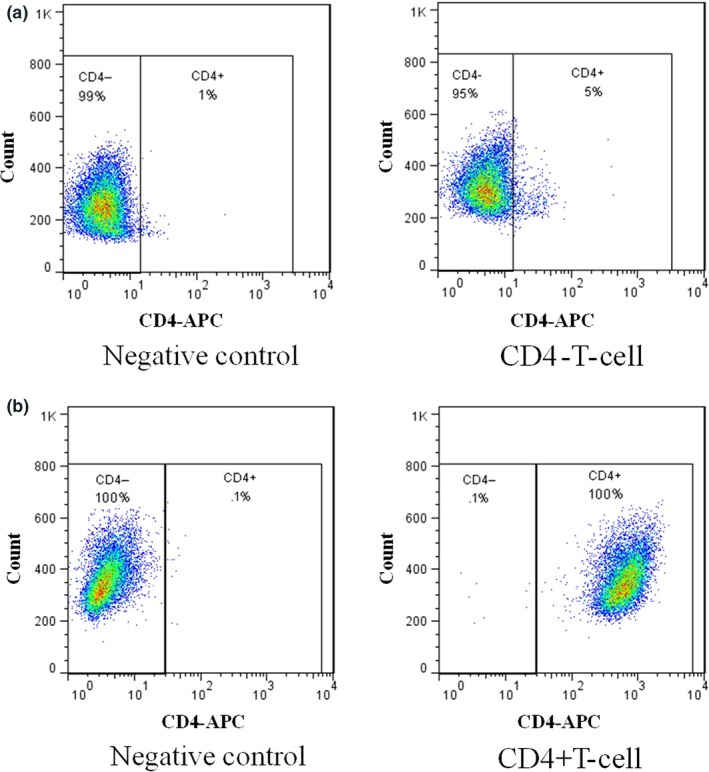
The purification of CD4+ T‐cell isolation. Flow cytometric analyses of purity of CD4+ T‐cells after negative selection. Control samples were stained with isotype‐matched control antibody. (a) The negative fraction contained less than 5% CD4 T‐cells. (b) The histogram shows that the purity of the CD4+ T‐cells was almost 100%.
Flow cytometry analysis
CD4+ T‐cells were incubated with the appropriate monoclonal antibodies (mAb) first to identify cell surface markers, followed by fixation in fixation/permeabilisation buffer for intracellular marker identification. The samples were analyzed using a BD LSR flow cytometer (Becton Dickinson, San Jose, CA, USA). The following mAb were used: phycoerythrin (PE) antihuman CD73 (clone AD2; BD Biosciences), allophycocyanin (APC) antihuman CD25 (clone 2A3; BD Biosciences), fluorescein isothiocyanate (FITC) antihuman Foxp3 (clone PCH101 set; eBioscience, San Diego, CA, USA), phycoerythrin‐Cy7 antihuman CD39 (clone eBioA1; San Diego, CA, USA), human recombinant adenosine receptor 2A (Lifespan Biosciences, Seattle, WA, USA) and Pacific orange goat anti‐mouse immunoglobulin G antibody for second antibody (Invitrogen, San Diego, CA, USA).
For intracellular Foxp3 protein staining, CD4+ T‐cells were first stained with surface mAb (anti‐CD25‐APC, anti‐CD73‐PE, anti‐CD39‐PE‐Cy7 and A2AR mouse anti‐human mAb), and then were fixed and permeabilised using the Foxp3 Staining Buffer Set (eBioscience). Subsequently, the cells were stained with anti‐Foxp3‐FITC mAb, according to the manufacturer's instructions (eBioscience).
CD25high was identified based on the median fluorescence intensity (MFI) >120 for CD25 expression on CD4+ T‐cells.
Cells with a MFI < 120 were set as CD25mid. Flow cytometry data was analyzed using WinList (Verity Software House, Topsham ME, USA) software.
Statistical analysis
Data are expressed as mean ± SD, and P < 0.05 was considered statistically significant. Statistical significance between different types of psoriasis was determined by one‐way anova with a Bonferroni post hoc test. Student's t‐test was used for the comparison of two different samples. Correlations were calculated using the nonparametric Spearman's rank correlation test.
Results
CD39, CD73 expression levels on CD4+CD25highFoxp3+ Tregs from peripheral blood in healthy controls
On the basis of the CD25 expression level, human CD4+ T‐cells can be divided into three: CD25high, CD25mid and CD25− cells. Combined with Foxp3, flow cytometry analysis revealed that the CD25highFoxp3+ Tregs was highly enriched in CD39+ (58 ± 14%, P < 0.01, n = 10) relative to CD25midFoxp3− and CD25−Foxp3− cells (8 ± 2 and 7 ± 1%, respectively). CD73 surface expression was low in Tregs (13 ± 4%, P < 0.05). This is in contrast to murine Tregs, which are reported to express high levels of CD73.5 Double staining of CD25highFoxp3+ Treg subsets revealed that only a very small portion of cells were CD39+CD73+, whereas there was almost no expression on CD25midFoxp3− and CD25−Foxp3− (6 ± 1%, P < 0.01; Fig. 3a,b).
Figure 3.
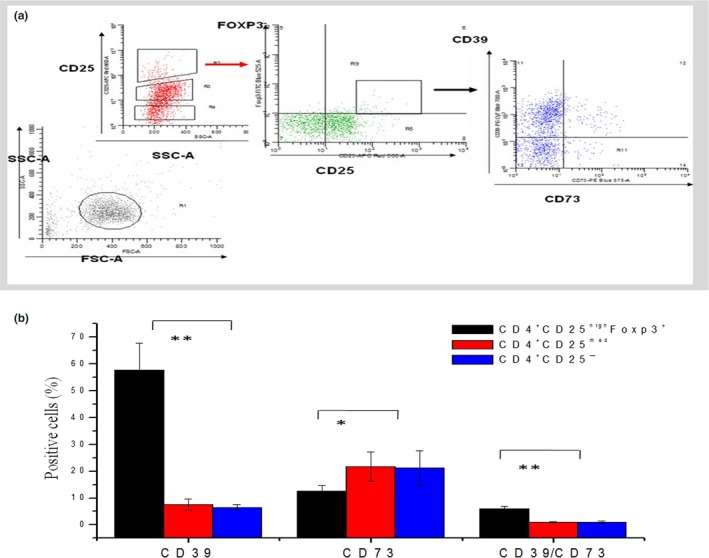
The proportion of CD39 and CD73 expressing CD25highFoxp3+Tregs in peripheral blood of healthy controls. Expression levels of CD39 and CD73 on normal CD25highFoxp3+ regulatory T‐cells (Tregs; n = 10). (a) Histogram depicts CD4+ (negative bead selection) cells defined by CD25 and Foxp3. CD25highFoxp3+ Tregs, CD25medFox3− and CD25−Foxp3−T‐cells were gated and further analyzed for expression of CD39 and CD73. (b) CD4+ CD25highFoxp3+ Tregs showed significantly higher expression levels of CD39 (P < 0.01) and lower proportion CD73 (P < 0.05) compared to CD25midFoxP3− and CD25− pool. (*P < 0.05, **P < 0.01, Student's t‐test).
CD39, CD73 expression levels on CD4+CD25highFoxp3+Tregs from peripheral blood in different types of psoriasis
The expression of CD73 was significant decreased on PV patient Tregs (7.0 ± 2%, n = 10) compared with healthy controls (13 ± 4%, n = 10), P < 0.01 (Fig. 4a,b). The double positive expression of CD39 and CD73 on Tregs was also decreased in PV (P < 0.05). The level of CD39 expressed by CD25highFoxp3+Tregs was significantly lower in PP patients than in normal controls (30 ± 9% vs 60 ± 14%, P < 0.01, Fig. 4b). There was no difference in expression between PE patients and controls. There was no statistically significant correlation between the percentage of CD73+Tregs and the severity of PV, and between CD39+Tregs and the severity of pustular psoriasis (Fig. 4c, r 2 = 0.247 and 0.03602, respectively).
Figure 4.
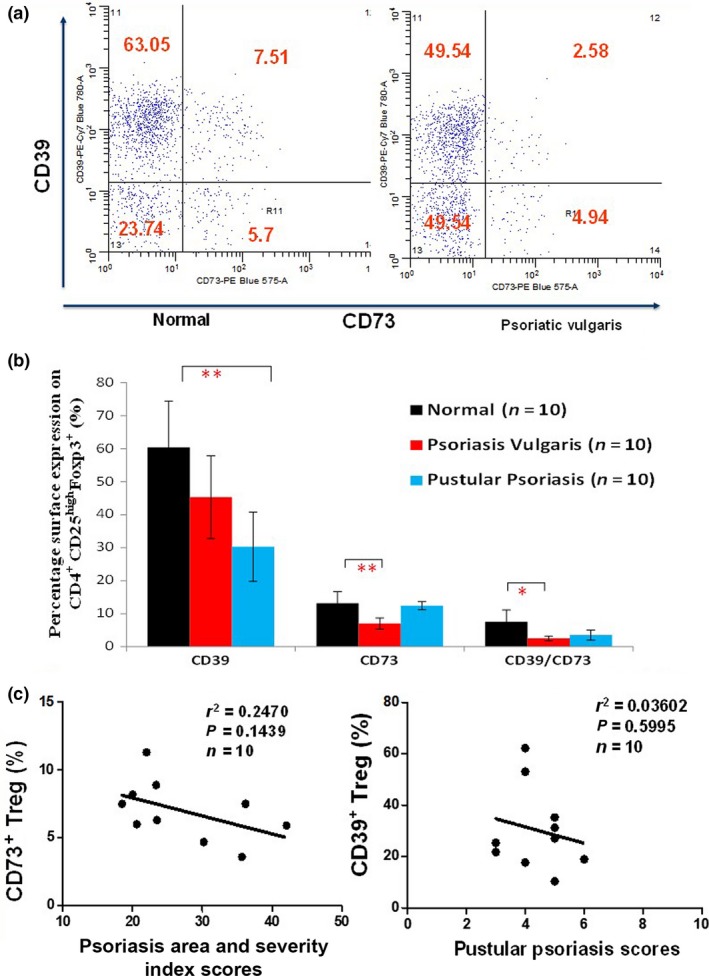
The proportion of CD39 and CD73 expressing CD25highFoxp3+ Tregs in peripheral blood of different types of psoriasis patients. (a) Representative diagrams showed CD39 and CD73 expressing regulatory T‐cells in peripheral blood of one healthy and one patient with psoriasis. The psoriasis vulgaris patient showed markedly reduced CD73 expression level in Tregs. (b) Cumulative data (n = 10) demonstrate a statistically significant lower single CD73 expression level of Tregs in psoriasis vulgaris patients compared to healthy controls (7 ± 2% vs 13 ± 4%, P < 0.01). Also, Tregs showed significant reduced double CD39/CD73 expression level (P < 0.05). While in pustular psoriasis patients, cumulative data (n = 10) demonstrate a 50% decrease in single CD39 expression in Tregs compared to healthy control subjects (30 ± 9% vs 60 ± 14%, P < 0.01). No significant expression differences were observed on single CD73 and double CD39/CD73 expression level (*P < 0.05, **P < 0.01, Student's t‐test). (c) Correlation between CD73+ Treg cells number, CD39+ Treg cells number and the psoriasis area and severity index score of psoriatic vulgaris patients (n = 10, r 2 = 0.247 and r 2 = 0.0045, respectively).
Furthermore, we compared the proportions of CD39+ and CD73+ Treg among different types of psoriasis. A significant reduction of CD39+ Tregs was observed in PP patients [PV (45 ± 10%), PP (30 ± 9%), PE (59 ± 18%)] (Bonferroni P < 0.0001), while the number of CD73+ Tregs was significantly lower in PV patients than in PP and PE patients [PV (7 ± 1%), PP (12 ± 2%), PE (12 ± 1%); Bonferroni P < 0.0001].
A2A R expression level In CD4+CD25−Teff cells in different types of psoriasis and healthy controls peripheral blood
Figure 5b shows the A2A R expression level in CD4+CD25‐Teff cells was reduced in PE and PP patients compared to PV patients and normal controls [PV (19 ± 4%), PP (3.95 ± 1%), PE (2.33 ± 1%); Bonferroni P < 0.0001]. No difference was observed in A2A R expression in CD4+CD25−Teff cells between PV patients and normal control, PE and PP patients.
Figure 5.
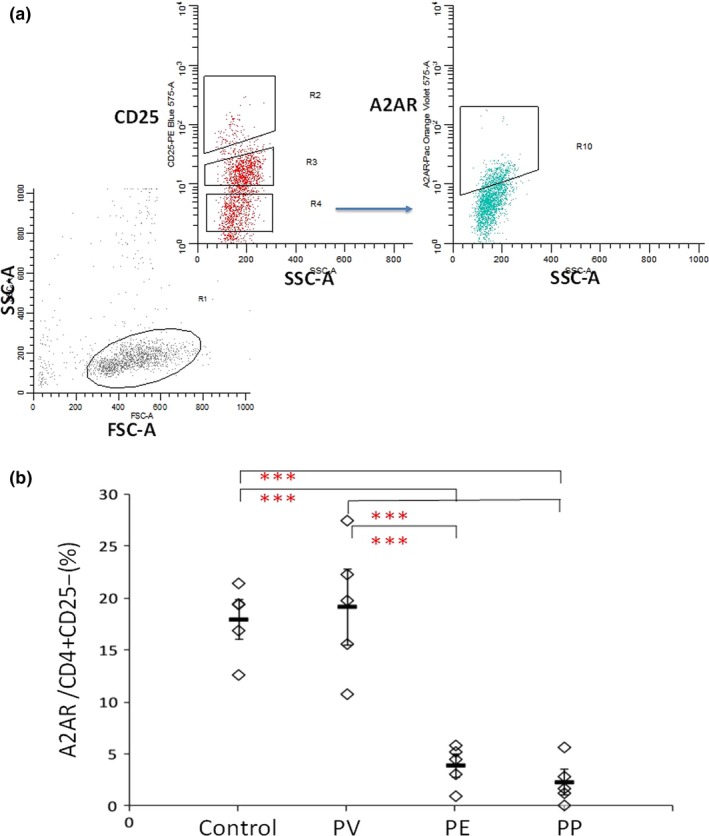
Proportion of adenosine receptor 2A (A2A R>) in Teff cells among different types of psoriasis patients. (a) CD4+ cells were identified based on their characteristic properties shown in the forward scatter (FSC) and sideward scatter (SSC). CD4+ CD25− T‐cells were gated and further analysed for expression of A2A R. (b) Data shown are A2A R +/CD4+ CD25− ratios in peripheral blood of normal controls, psoriasis vulgaris (PV), pustular psoriasis, and psoriatic erythroderma patients, representing a summary of 10 (normal), 10 (PV), 10 (PE) and 10 pustular psoriasis with PV (PP) independent experiments. Data are mean ± SD. (***P < 0.0001, Bonferroni correction).
Discussion
Treg‐mediated immune suppression involves many molecular mechanisms. Ectonucleotidases CD39 and CD73 have been found to be expressed on the surface of murine and human Treg.20, 21 Extracellular adenosine is a hydrolysis product of adenosine triphosphate cleaved in tandem by CD39 and CD73. In conjunction with the receptor expressed on Teff cells (A2A R), adenosine leads to the elevation of cyclic adenosine monophosphate (cAMP) levels, which plays a crucial role in immunosuppression (Fig. 1).5 Psoriatic CD4+CD25high Tregs are functionally deficient in suppressing effector T‐cell proliferation.12, 13, 14 We have just published our findings on skin‐infiltrating CD39+Foxp3+ and CD73+Foxp3+ in different psoriatic lesions. The relative reduced expressions of CD39 and CD73 in Foxp3+ Tregs may imply there is a different mechanism for Tregs function impairment.22 In the present study, we sought to assess the expression levels of CD39/CD73 specifically on peripheral blood Tregs involved in psoriatic pathology.
There is no single surface marker that has been identified for Treg cells. Both Foxp3 and CD25 expression on Treg cell, have limitations. We therefore combined these two molecular markers to define Treg cells more accurately.23, 24
Our data on the phenotypical analysis of ectoenzymes in Tregs in healthy controls show that nearly 60% of CD25highFoxp3+ human Tregs were positive for CD39, which is consistent with other research findings.8, 24 The surface expression of CD73, described as a characteristic surface marker of murine Treg,5 was observed in only a small proportion of human Treg (almost 13%). Since CD73 has a synergistic function with CD39, the unbalanced expression of these two molecules suggests that CD73 is readily shed from the surface of human lymphocytes.25
Flow cytometric analyses revealed that the proportion of CD73+ cells among CD25highFoxp3+ Tregs in the blood of PV patients was markedly reduced (Fig. 3). However, we could not detect other changes in CD39+ Tregs or A2A R expression on effector T‐cells in PV patients. Taken together, our data show that the production of extracellular adenosine monophosphate (AMP) adenosine is mediated by normal CD39 levels in Tregs, but the striking reduction in the number of CD73+ Tregs may ultimately affect extracellular adenosine generation. This, in turn, leds to a reduction in intracellular cAMP accumulation, which provides a possible mechanistic explanation for the dysfunction of the suppressive capacity of psoriatic Treg cells.
We observed strikingly reduced CD39 expression in the Tregs population and A2A R levels on T effector cells of pustular psoriasis patients (Fig. 3b, 5b). Jenabian and colleagues26 reported that CD39+ Tregs suppress the interleukin‐2 expression of activated CD4+ T‐cells more efficiently than CD39− Tregs, and the CD39+ Tregs appeared to be bona fide Tregs. Chemotaxis of neutrophils is the main reason for the formation of pustules, while inadequate activity of the CD39/CD73 axis has been associated with the uncontrolled activation of neutrophil chemotaxis.27 Significant impaired CD39+Treg and A2A R +Teff levels in pustular patients (compared to normal controls, PV and PE patients) may be one of the reasons for the difference between pustular psoriasis lesions and other types of psoriasis.
Until now, there have been no published studies on peripheral blood Treg cells in pustular psoriasis. Further studies should address the Tregs function in pustular psoriasis and whether ectoenzyme CD39 is involved in the function's abnormalities.
Borsellino and colleagues8 were the first to report that patients with the remitting/relapsing form of multiple sclerosis had strikingly reduced numbers of CD39+ Treg cells in the blood, while there were no changes in the CD39+ Treg cell number after the treatment of immunomodulators or in a clinical remission state. Increased levels of CD39+ Treg were also correlated with poor prognosis in sepsis patients,28 which suggests that CD39+ Treg expression could be used as a prognostic biomarker. However, we could not detect a correlation between the severity of psoriasis patients (including PV and pustular psoriasis) and CD39/CD73 expression in Tregs.
Our research compared CD39 and CD73 expression levels in peripheral blood Tregs among three different types of psoriasis. A decreased CD39+ Tregs level was found in pustular psoriasis compared with PV and PE patients, which is consistent with the significant reduction of CD39+ Treg cells in pustular psoriasis lesions found in our previous study.21
Future research requires an expansion in the number of study participants, further stratified according to their disease course (early stage, remitting/relapsing form and advanced stage) and functional assays of the capacity of these enzymes, which will widen our understanding of the role of ectoenzymes CD39 and CD73 and A2A R function in psoriatic Treg cells.
Acknowledgements
This study was supported by funding from National Natural Science Foundation of China, Grant no. 81502715 and by funding from the Science and Technology Commission of Shanghai Municipality, Grant no. 13JC1402302.
Ling Han, MD. Hideaki Sugiyama, MD. Qi Zhang, PhD. Kexiang Yan, PhD. Xu Fang, MD. Thomas S McCormick, MD. Kevin D Cooper, MD. Qiong Huang, MD.
Conflict of interest: none.
References
- 1. Sakaguchi S, Sakaguchi N, Asano M et al Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25): breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J. Immunol. 1995; 155: 1151–64. [PubMed] [Google Scholar]
- 2. Bach J‐F. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 2003; 3: 189–98. [DOI] [PubMed] [Google Scholar]
- 3. Sakaguchi S. Naturally arising Foxp3‐expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non‐self. Nat. Immunol. 2005; 6: 345–52. [DOI] [PubMed] [Google Scholar]
- 4. Su H, Longhi MS, Wang P et al Human CD4+CD25 (high) CD127 (low/neg) regulatory T cells. Methods Mol. Biol. 2012; 806: 287–99. [DOI] [PubMed] [Google Scholar]
- 5. Deaglio S, Dwyer KM, Gao W et al Adenosine generation catalyzed by CD39 and C D73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007; 204: 1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sitkovsky M, Lukashev D, Deaglio S et al Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br. J. Pharmacol. 2008; 153 (Suppl. 1): S457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004; 25: 33–9. [DOI] [PubMed] [Google Scholar]
- 8. Borsellino G, Kleinewietfeld M, Di Mitri D et al Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007; 110: 1225–32. [DOI] [PubMed] [Google Scholar]
- 9. Mandapathil M, Szczepanski MJ, Szajnik M et al Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin. Cancer Res. 2009; 15: 6348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rissiek A, Baumann I, Cuapio A et al The expression of CD39 on regulatory T cells is genetically driven and further upregulated at sites of inflammation. J. Autoimmun. 2015; 58: 12–20. [DOI] [PubMed] [Google Scholar]
- 11. Schon MP, Boehncke WH. Psoriasis. N. Engl. J. Med. 2005; 352: 1899–912. [DOI] [PubMed] [Google Scholar]
- 12. Sugiyama H, Gyulai R, Toichi E et al Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005; 174: 164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodman WA, Levine AD, Massari JV et al IL‐6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009; 183: 3170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang Q, Han L, Fang X et al A study of the function of CD4+CD25+ regulatory T cells in peripheral blood of patients with psoriasis vulgaris. Zhonghua Pi Fu Bing Xue Za Zhi 2010; 43: 251–5 (Chinese). [Google Scholar]
- 15. Yamasaki K, Nakagawa H, Kubo Y et al Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52‐week, open‐label study. Br. J. Dermatol. 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Elston DM. Seborrheic dermatitis, psoriasis, recalcitrant palmoplantar eruptions, pustular dermatitis, and erythroderma In: James WD, Berger TG, Elston DM. (eds). Andrews’ Diseases of the Skin: Clinical Dermatology, 10th edn New York, NY: Elsevier Health Sciences, 2006; 10: 194–5. [Google Scholar]
- 17. Saikaly SK, Mattes M. Biologics and pediatric generalized pustular psoriasis: an emerging therapeutic trend. Cureus 2016; 6: e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician's global assessment. J. Am. Acad. Dermatol. 2004; 51: 563–9. [DOI] [PubMed] [Google Scholar]
- 19. Ohkawara A. To Propose the Diagnostic Criteria for Severity Rating of Pustular Psoriasis. A Report of the MHW Investigation and Research Team in Specific and Rare Refractory Skin Diseases in 1997. Tokyo: Ministry of Health, Labor and Welfare, 1998; 44–5. [Google Scholar]
- 20. Dwyer K, Deaglio S, Crikis S. Salutary roles of CD39 in transplantation. Transplant. Rev. 2007; 21: 54–63. [Google Scholar]
- 21. Mandapathil M, Lang S, Gorelik E et al Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J. Immunol. Methods 2009; 346: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang HY, Yan KX, Huang Q et al Target tissue ectoenzyme CD39/CD73‐expressing Foxp3+ regulatory T cells in patients with psoriasis. Clin. Exp. Dermatol. 2015; 40: 182–91. [DOI] [PubMed] [Google Scholar]
- 23. Sugiyama H, Matsue H, Nagasaka A et al CD4+CD25high regulatory T cells are markedly decreased in blood of patients with pemphigus vulgaris. Dermatology 2007; 214: 210–20. [DOI] [PubMed] [Google Scholar]
- 24. Mandapathil M, Hilldorfer B, Szczepanski MJ et al Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 2010; 285: 7176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Airas L, Niemelä J, Salmi M et al Differential regulation and function of CD73, a glycosyl‐phosphatidylinositol‐linked 70‐kD adhesion molecule, on lymphocytes and endothelial cells. J. Cell Biol. 1997; 136: 421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jenabian MA, Seddiki N, Yatim A et al Regulatory T cells negatively affect IL‐2 production of effector T cells through CD39/adenosine pathway in HIV infection. PLoS Pathog. 2013; 9: e1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antonioli L, Pacher P, Sylvester Vizi E et al CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013; 19: 355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang H, Xu R, Lin F et al High circulating CD39(+) regulatory T cells predict poor survival for sepsis patients. Int. J. Infect. Dis. 2015; 30: 57–63. [DOI] [PubMed] [Google Scholar]


