Abstract
Purpose
To demonstrate how reference data affect the quantification of the apparent diffusion coefficient (ADC) in long diffusion time measurements with diffusion‐weighted stimulated echo acquisition mode (DW‐STEAM) measurements, and to present a modification to avoid contribution from crusher gradients in DW‐STEAM.
Methods
For DW‐STEAM, reference measurements at long diffusion times have significant b 0 value, because b = 0 cannot be achieved in practice as a result of the need for signal spoiling. Two strategies for acquiring reference data over a range of diffusion times were considered: constant diffusion weighting (fixed‐b 0) and constant gradient area (fixed‐q 0). Fixed‐b 0 and fixed‐q 0 were compared using signal calculations for systems with one and two diffusion coefficients, and experimentally using data from postmortem human corpus callosum samples.
Results
Calculations of biexponential diffusion decay show that the ADC is underestimated for reference images with b > 0, which can induce an apparent time‐dependence for fixed‐q 0. Restricted systems were also found to be affected. Experimentally, the exaggeration of the diffusion time–dependent effect under fixed‐q 0 versus fixed‐b 0 was in a range predicted theoretically, accounting for 62% (longitudinal) and 35% (radial) of the time dependence observed in white matter.
Conclusions
Variation in the b‐value of reference measurements in DW‐STEAM can induce artificial diffusion time dependence in ADC, even in the absence of restriction. Magn Reson Med 79:952–959, 2018. © 2017 The Authors Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Keywords: stimulated echo, diffusion time, restriction, biexponential diffusion, apparent diffusion coefficient
INTRODUCTION
Dependence of the apparent diffusion coefficient (ADC) on diffusion time can reveal tissue properties with significance to neural health and disease. For instance, water molecules that are confined to a compartment exhibit reduced ADC with increased diffusion time. Long diffusion times can also probe membrane permeability. This dependence is often used in signal models that aim to improve biological specificity of diffusion MRI over nonspecific measures such as fractional anisotropy 1, 2, 3, 4, 5, 6, 7. To exploit these subtle changes in ADC, it is crucial that the measurements accurately reflect the diffusion propagator (ie, the displacement profiles over a range of diffusion times).
Diffusion time dependence has primarily been used to infer MRI measures of compartment size. Diffusion time dependence perpendicular to white matter tracts provides a marker of axon diameter, which has been proposed for improving tractography 8, 9, estimating conduction velocity 10, and monitoring of disease status 11. Long diffusion time signals can also characterize the mesoscopic organization of tissue 5, providing markers for axonal varicosities 12, undulations 13, and white matter fiber dispersion 14, 15. Finally, membrane permeability estimates based on diffusion time 6, 16 are a potential biomarker of apoptosis 17, tissue composition (eg, myelination 18), and tumor detection/classification 19, 20, 21.
Long diffusion time measurements face important technical challenges. Diffusion‐weighted spin‐echo (DW‐SE) measurements experience T2 signal loss that is intrinsically tied to long diffusion times and drastically reduces signal‐to‐noise ratio. Diffusion‐weighted stimulated echo acquisition mode (DW‐STEAM) 22 uncouples T2 signal loss from diffusion time and therefore may be more appropriate for long diffusion time measurements.
In this note, we demonstrate the importance of the sequence parameters for DW‐STEAM reference measurements, which are often referred to as b = 0, but in practice can have significant b‐value for long diffusion times. In particular, we show that these reference measurements can have a major effect on estimated ADCs for systems that exhibit non‐Gaussian diffusion, which is characteristic of all biological tissues. Crucially, including the b‐matrices in the ADC calculations is insufficient to draw valid conclusions about restrictions: The b‐values of both the diffusion‐weighted and reference measurements need to be kept constant over diffusion times.
THEORY
Diffusion Time Dependence of the ADC
In any diffusion‐weighted sequence, varying the diffusion time (Δ) between diffusion‐encoding gradients alters the diffusion‐weighting b if other parameters are kept constant:
| (1) |
Provided the displacement profile is Gaussian, diffusion measurements yield the same ADC, independent of the choice of b or Δ. However, with non‐Gaussian diffusion 23, 24, 25, 26, the choice of b is crucial for interpreting the diffusion time dependence of the ADC. For example, in biexponential diffusion—a simple departure from Gaussian diffusion—the ratio of a diffusion‐weighted signal, S(Δ), and a reference measurement, S0(Δ), is governed by
| (2) |
In this scenario, varying Δ with constant q (varying b) induces an apparent dependence of the ADC on diffusion time. This diffusion time dependence occurs for any protocol in which log(S/S0) is a nonlinear function of b, but would not be observed under constant b (varying q = γδG). Crucially, the need for constant b holds for both diffusion‐weighted and reference acquisitions with nonzero b‐value. This is particularly important for DW‐STEAM, in which reference measurements have significant b‐value as a result of the configuration of crusher gradients.
DW‐STEAM.
In DW‐SE, the range of diffusion times that can be probed is limited by T2 signal loss at long Δ. DW‐STEAM 22 is an attractive alternative that stores magnetization along the longitudinal axis during the mixing time (τm) between the second and third 90 ° pulse (Fig. 1). DW‐STEAM incurs a twofold signal reduction, but exhibits the much slower T1 relaxation during τm.
Figure 1.
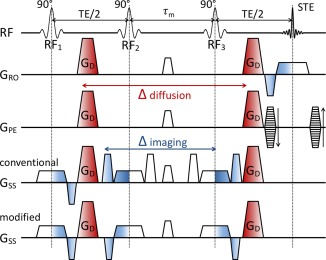
DW‐STEAM pulse sequence modification. The diffusion‐weighting effect of the diffusion‐encoding (red) and imaging gradients (blue) are indicated by color gradients. In the conventional scheme (fourth row), a pair of crushers is used on the slice‐select axis to remove unwanted signal from the third RF pulse. These crushers (and half of the slice‐select gradients) are separated by the mixing time τm, resulting in a substantial diffusion weighting at long diffusion times (see Supporting Fig. S1). This diffusion weighting—if not properly handled—results in considerable variation in b‐value with diffusion time, as well as directional bias in the diffusion gradient directions 27. Because in the DW‐STEAM sequence, the diffusion‐encoding gradient already provides sufficient dephasing of the signal from RF3, the crushers are superfluous and can be replaced by usual slice‐select refocussing. The modified scheme (bottom row) effectively removes the contribution of the imaging gradients that builds up during the mixing time by flipping the two crusher gradients to form pairs with the slice‐select gradients to refocus the diffusion encoding outside of the mixing time. Adapted from Kinchesh et al. 28.
However, in DW‐STEAM the diffusion weighting from non‐diffusion‐encoding gradients can become substantial at long τm as a result of crushers that remove signal created by the third radiofrequency (RF) pulse (Fig. 1). In diffusion‐weighted images, various strategies have been proposed to account for these gradients, including the use of the full b‐matrix in analysis 29 and adapting the diffusion gradients 27. We propose a simple modification to the DW‐STEAM sequence to reduce diffusion weighting from the crusher gradient (Fig. 1, bottom row). By negating the polarity of the crusher and slice‐select gradients, the net gradient area is minimized during the mixing time. In the remainder of this work, we consider the effect of the residual gradient area in reference images on ADC quantification.
Reference Images with Varying Diffusion Times
Calculating the ADC necessitates a reference measurement to divide out the signal contributions unrelated to the diffusion gradients. For DW‐STEAM, these contributions come from T1 and T2 decay. For measurements at multiple diffusion times, the variable T1 decay during the mixing times requires a separate reference image for each Δ.
Unlike DW‐SE, DW‐STEAM reference measurements accumulate significant b‐value at long diffusion times as a result of crusher gradients (Fig. 2a), which are smaller in amplitude than diffusion‐encoding pulses, but otherwise have similar properties, including separation by the mixing time.
Figure 2.
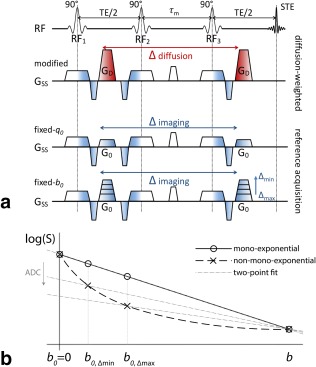
Reference measurements in the DW‐STEAM sequence. (a) DW‐STEAM reference measurements in which the gradient G 0 (fixed‐q 0; third row) or the b 0‐value are kept constant (fixed‐b 0; fourth row). In the DW‐STEAM sequence, the diffusion gradient (G d; second row) is used as an imaging gradient in the reference measurements (denoted G 0; applied over all three axes) to crush the signal from the third RF pulse. In the b 0 image, the G 0 is chosen according to the minimal q 0‐value that achieves this, which might result in a nonnegligible b 0‐value at long diffusion times. To obtain a constant b 0‐value over diffusion times, the G 0 needs to be increased for Δ < Δmax compared with the minimal value G0,Δmax. (b) ADC calculation of signals undergoing mono‐exponential and non‐mono‐exponential decay. For mono‐exponential decay (solid line), the behavior is linear in b, and any two‐point fit of (b, b 0) yields the same ADC. For the non‐mono‐exponential, the slope of the linear fit, and thus ADC, decreases if the b 0‐value increases over the measurements.
If the crusher gradient moment is kept constant over diffusion times (fixed‐q 0), the associated b‐value in the reference images (b 0) increases with diffusion time. An alternate design is to keep the b 0‐value constant over diffusion times (fixed‐b 0), which requires varying the crusher area, q 0. In this case, the minimum constant b 0‐value over diffusion times is determined by b 0 at the longest diffusion time b 0,Δmax.
It is instructive to compare the fixed‐b 0 and fixed‐q 0 approaches for mono‐ and biexponential decay, in which the latter is a representative deviation from Gaussian diffusion that is not driven by restriction. The ADC from a DW‐STEAM experiment can be calculated from a two‐point fit as (Fig. 2b)
| (3) |
For mono‐exponential decay, this calculation yields the same ADC independent of b 0. When the decay is biexponential (Fig. 2b, dashed line), however, a two‐point fit yields an ADC that decreases with increasing b 0 (Fig. 2b, dash‐dotted lines). Therefore, fixed‐q 0 designs yield an artificial time‐dependence of the ADC as a result of the variation in b 0‐value over diffusion times, which might be interpreted incorrectly as indicating restriction. Next we quantify this effect using simple simulations of unrestricted and restricted diffusion.
METHODS
ADC Calculations
The ADCs were compared between simulated measurements in which either b 0 or q 0 was held constant over diffusion times. For fixed‐b 0, the b 0,Δmax was chosen as [10,20,30]% of the b‐value of the diffusion‐weighted images to reflect the range found in literature 12, 19, 27, 30, 31. The b 0‐values are then
| (4) |
First, we evaluated the signal with and without the sequence modification (Supporting Fig. S1). Second, we investigated the influence of reference measurements on ADC estimates of a biexponential signal model and a biophysical tissue model. All calculations assume infinitely narrow pulses. Signal calculations were performed using the MISST toolbox 32, 33, 34 that uses the matrix formalism 35 to compute the signal for arbitrary pulse sequences.
The biexponential signal model is characterized by diffusion coefficients Ds (slow) and Df (fast) and volume fraction f s. Biexponential decay was simulated for four fast diffusion coefficients relative to Ds, given by Df = [1,3,5,7] × Ds. The first case (Df = Ds) represents mono‐exponential diffusion. The fast volume fraction f s was set to 0.33 in accordance with literature 36. Signal simulations were conducted to probe regimes of normalized diffusion time Δ/Δmax and contrast b ·Ds (b‐value in terms of signal contrast for the slow diffusion coefficient).
The biophysical model is a simple two‐compartment model with restricted intracellular diffusion in cylinders with radius r, diffusion coefficient Di and volume fraction f i, and hindered diffusion in the extracellular space with diffusion coefficient Dh. The evaluated ratios of the restricted and hindered diffusion coefficient were Dh = [1,0.75,0.50,0.25] × Di. Di was set to 2 μm2/ms and f i = 0.8, in keeping with the fraction found in white matter tissue 37. Experimental regimes investigated for this model were b = [0.0–8.5] ms/μm2 perpendicular to the cylinders and Δ = [10–500] ms.
MRI Data Acquisition
Postmortem human corpus callosum samples from two brains provided by the Thomas Willis Brain Collection in Oxford were scanned. Coronal slabs of 5‐mm thickness were cut at the level of the anterior commissure. From each slab, a 45 × 25 mm block was cut, including the medial corpus callosum and parts of the centrum semiovale, cingulate, and superior frontal gyri. The blocks were soaked in phosphate‐buffered saline for 72 h and then transferred to a syringe filled with Fluorinert (3M, St. Paul, MN) 24 h before imaging.
MRI was performed on a 9.4 Tesla (T) 160‐mm horizontal bore VNMRS preclinical scanner (Varian Inc, Corona, CA) with maximum gradient of 400 mT/m and a 26‐mm ID quadrature birdcage coil (Rapid Biomedical GmbH, Germany). DW‐STEAM using a single‐line readout was adapted to minimize the effect of imaging gradients on diffusion weighting 28. Measurements at eight diffusion times Δ = [70,100,150,200,250,300,350,400] ms were acquired using 30 directions distributed over the sphere. The repetition time (TR) was minimized for each diffusion time: TR = [2.4,2.4,2.4,2.4,2.4,2.6,3.6,4.1] s, with a minimum of 2.4 s to allow T1 recovery between excitations (T1 over our sample was estimated to be approximately 600 ms). The effective echo time was 16 ms. Five slices were acquired at 0.4 × 0.4 ×0.4 mm (matrix = 128 × 96; field of view = 51.2 × 38.4 mm). The complex data were resampled (matrix 64 × 48) and tapered with a Tukey window (α = 0.4) to boost SNR and reduce Gibbs ringing.
The diffusion‐weighted measurements used b = 3.5 ms·μm−2 with gradient duration δ = 2.22 ms and variable gradient strength |G| = 375–157 mT/m for Δ = 70–400 ms. For each Δ, two reference measurements were performed: one with constant q 0 = 0.023 μm−1 as the minimal value that adequately spoilt the signal generated from the third RF pulse (corresponding b 0 = 0.117–0.631 ms·μm−2) and one with constant b 0 = 0.631 ms·μm−2 as the b 0 value with q 0 at Δmax = 400 ms.
MR Data Analysis
Diffusion tensors were fitted to the DW‐STEAM data using FDT from FSL 38. The full b‐matrix (ie, accounting for imaging gradients) was used to calculate the b‐values and b‐vectors. Tensor fitting was performed for each diffusion time separately, and for the two reference data sets separately (fixed‐b 0 and fixed‐q 0), while using the same diffusion‐weighted measurements. Masks were created by thresholding two diffusion tensor metrics as follows: fractional anisotropy at > 0.20 for a white matter mask (which avoided including crossing‐fiber voxels, such as in the centrum semiovale that had a lower fractional anisotropy); and mean diffusivity > 0.4 μm2/ms for a gray matter mask. The diffusivities are presented for the longitudinal (the first eigenvalue) and radial (the average of the second and third eigenvalues) directions.
RESULTS
ADC Calculations
Figure 3a depicts mono‐exponential modeling of unrestricted, biexponential diffusion for the fixed‐b 0 and fixed‐q 0 schemes, with constant high b‐value across diffusion times and reference measurements with nonzero b 0‐value. For fixed‐b 0 (solid lines), the ADC is underestimated in comparison to a “true” b 0 = 0 ms/μm2 (bold black line). Higher values for b 0,Δmax result in larger underestimation of the ADC. Nevertheless, fixed‐b 0 does yield a constant ADC over diffusion times. The underestimation of the ADC also occurs for fixed‐q 0 (dashed lines), but even more importantly, fixed‐q 0 also induces an apparent diffusion time dependence in the absence of restriction, because the b 0‐value increases with diffusion time.
Figure 3.
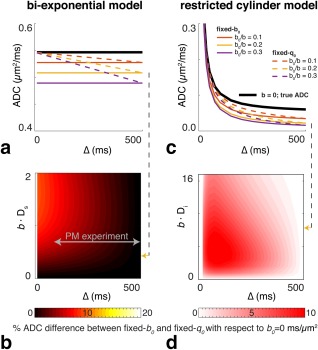
The effect of reference measurements on the ADC. The biexponential model is shown on the left (a, b), and the restricted cylinder model is shown on the right (c, d). (a, c) The line plots show specific examples for measurements with fixed‐q 0 (dashed lines) versus fixed‐b 0 (solid lines). Lines of different color show the behavior for different b 0,Δmax values, where b 0,Δmax = [0, 10, 20, 30]% of the b‐value. (b, d) The contour plots show differences in ADCs for the two reference measurement schemes; the color map represents the ADC difference between the fixed‐q 0 ADC and the fixed‐b 0 ADC as a percentage of the “true” ADC at b 0 = 0 ms/μm2. (a) ADC behavior in biexponential signal model (without restriction) at b × Ds = 0.5 (b = 2.5 ms·μm−2; Ds = 0.2 μm2·ms−1, with Df = 5 × Ds and f s = 0.3. b)% ADC differences between fixed‐b 0 and fixed‐q 0 for the biexponential model for Df = 5 × Ds and b 0/b = 0.2. (c) ADC behavior in the restricted cylinder model at b = 4.0 ms·μm−2; Di = Dh = 2.0 μm2·ms−1, with r = 5 μm and f i = 0.80. d)% ADC differences between fixed‐b 0 and fixed‐q 0 for the restricted cylinder model for Di = Dh and b 0/b = 0.2. Note that the actual deviation from truth (b 0 = 0 ms/μm2 measurement) is always maximal for fixed‐b 0, because the b 0‐value is determined by the longest diffusion time; however, our goal here is to quantify the difference between the two realistic measurement strategies: fixed‐b 0 and fixed‐q 0. The gray arrow indicates the regime of our postmortem measurements. All scales are linear. Supporting Figure S2 shows the effects of a different b 0/b ratios and different diffusion coefficient ratios. The effect of varying the intracellular volume fraction and the cylinder radius is provided in Supporting Figures S3 and S4, respectively.
The contour plot (Fig. 3b) quantifies, for an example regime b 0/b = 0.2 and Df = 5 × Ds, the difference between the ADCs calculated with fixed‐q 0 and fixed‐b 0 measurements expressed as a percentage of the “true” b 0 = 0 ms/μm2 (ie, ( )/ADCb = 0 × 100%). At low b‐values, the induced diffusion time dependence is small. However, a commonly used regime in experiments at long diffusion time, ie, b × Ds≈[0.2–1.0] induces a large diffusion time dependence. The minimum b 0‐value at the longest diffusion time and increasing difference in the biexponential signal components exacerbate the induced time dependence of the ADC (Supporting Fig. 2a). Conventional interpretations would incorrectly attribute the variation with diffusion time to restriction, despite this being a fundamentally unrestricted system.
In the case of genuine restriction (Figs. 3c and 3d), ADC decreases are observed that are similar in magnitude to the biexponential model for the ratio's Dh/Di that are common in these restriction models (Dh = [0.5–1] × Di). The differences between fixed‐q 0 and fixed‐b 0 decrease as the displacement profiles in the two compartments become more similar (eg, as Dh approaches the intracellular perpendicular ADC, as well as at short Δ where it even becomes negative as the diffusion in the intracellular space becomes free (Di > Dh)) (Supporting Fig. 2b). For any nonzero b 0‐value (both fixed‐b 0 and fixed‐q 0), the signal attenuation curves differ from the “true” b 0 = 0 ms/μm2, ultimately affecting the estimation of the model parameters.
Postmortem MRI Data
Figure 4 depicts the diffusivities measured in two postmortem samples. Although the diffusivities differ between the samples, most likely because of differences in tissue preparation 39, 40, they follow similar trends. Diffusivity decreases are observed with increasing diffusion time for both longitudinal and radial directions, as well as in different tissue types (white and gray matter). This diffusion time dependence is exaggerated when the signal is normalized by the fixed‐q 0 measurement compared with the fixed‐b 0 measurement: The slope for fixed‐q 0 is larger than for fixed‐b 0. Differences in diffusion time dependence between fixed‐b 0 and fixed‐q 0 are quantified in Table 1.
Figure 4.
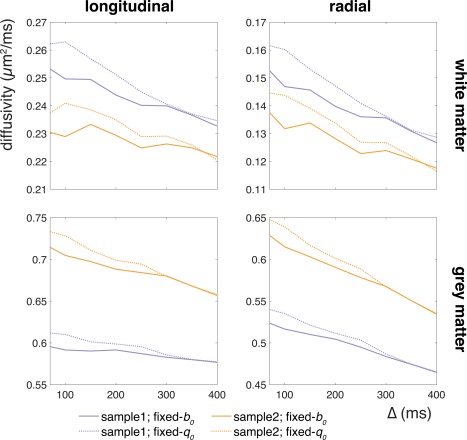
Differences in the diffusion time dependence of the ADC with two normalization strategies (fixed‐q 0 and fixed‐b 0). Panels show the parallel (left column) and perpendicular (right column) diffusivities averaged over voxels in a white matter (top row) and gray matter (bottom row) mask. The two samples are plotted in different colors. The diffusivity decreases with diffusion time for all cases, but in each case is exaggerated when fixed‐q 0 normalization is used. Note that the diffusivities for both reference image sets converge at the longest diffusion time where the b 0‐values for both reference measurement strategies are equal (however, these correspond to separate acquisitions, and thus the measurements are not identical).
Table 1.
ADC Differences between Fixed‐q 0 and Fixed‐b 0 Strategies
| ΔADC (%) | Fixed‐q 0 | Fixed‐b 0 | Fixed‐q 0 effect |
|---|---|---|---|
| White matter longitudinal | 7–11 | 4–8 | 44–80 |
| White matter radial | 19–20 | 15–17 | 35–35 |
| Gray matter longitudinal | 6–10 | 3–8 | 32–86 |
| Gray matter radial | 14–17 | 11–15 | 20–28 |
Note: The values in columns 1 and 2 indicate the difference in ADC between the short diffusion time (Δ = 70 ms) and the long diffusion time measurement (Δ = 400 ms), expressed as a percentage of the short diffusion time measurement. The third column shows the percentage of the ADC dependence as observed under fixed‐b 0, which is added by dependence induced by fixed‐q 0 measurements. The ranges indicate values for the two samples.
DISCUSSION
We demonstrate that the choice of acquisition parameters for the reference b 0‐image is essential for interpreting ADC measurements using DW‐STEAM. In a system that can be characterized by multiple diffusion coefficients, reference measurements with nonzero b‐value lead to underestimation of the “composite” ADC. As this underestimation increases with b 0‐value, the common approach of using a constant crusher gradient strength for the reference measurement at each diffusion time induces an artificial diffusion time dependence. Therefore, in experiments that rely on subtle variations of the ADC over a long interval of diffusion times, incorrect normalization could lead to misinterpretation of the results.
The size of this induced diffusion time dependence depends on the tissue's diffusion properties and the sequence parameters. In simulations, we demonstrated that stronger deviation from mono‐exponential decay and a larger b 0‐value for the reference measurement induces a larger diffusion time dependence. For typical acquisition parameters, the artificial effect can be on the order of the diffusion time dependence as a result of restrictions (Table 1, column 3). Data acquired from postmortem corpus callosum samples indicated that a considerable portion of the diffusion time dependence observed using fixed‐q 0 references is consistent with multiple diffusion coefficients in the system. Nonetheless, the ADC still exhibited decreases with diffusion time under fixed‐b 0 references, both perpendicular and parallel to the bundle, suggesting true restriction effects.
Although most studies investigating diffusion time dependence of ADC measures report a fixed b‐value, reporting of the b 0‐values in DW‐STEAM is often omitted or inaccurate (ie, reported as b = 0 ms/μm2 or “non‐diffusion weighted”). The present investigation demonstrates that using the b‐matrix is only sufficient to account for this effect when the underlying diffusion process is well characterized by a single diffusion coefficient. Both b and b 0 must be kept constant to draw conclusions about restriction. As a case in point, the use of fixed‐b 0 measurements has changed our original interpretations of the data in Figure 4, which aimed to investigate diffusion time dependence along white matter fibers. There is not very strong evidence for a diffusion time dependence of the ADC in the longitudinal direction when a fixed‐b 0 is used, whereas the fixed‐q 0 data appear to suggest a small but consistent dependence.
In our simulations, a biexponential signal was generated to evaluate the effects of DW‐STEAM reference measurements in the presence of non‐Gaussian diffusion, which is well established in brain tissue 16. However, the choice of a biexponential system in our simulations is motivated by its simplicity and not to imply that this is the most appropriate tissue model. Our results extend to any form of non‐mono‐exponential signal decay.
The introduction of diffusion time dependence through variation in the b‐values is limited to situations in which two‐point fits are used to calculate a summary ADC from measurements with varying diffusion time. A model fit (eg, kurtosis (44), biexponential (45), CHARMED (46), AxCaliber (47)) to the nonnormalized data could be used to extract accurate and informative quantities from DW‐STEAM measurements, although these models would have to be adapted to account for T1 decay during the mixing time.
For models that rely on accurate estimation of ADC values (12,48), it becomes important to carefully consider the measurement regime, as not all regimes are equally affected. The exact sampling locations of the reference and diffusion‐weighted measurements along the decay curve determine the extent of the induced diffusion time dependence. In the low‐contrast regime, the effect is small: The linear extrapolation to the b = 0 ms/μm2 intercept is reasonably accurate. Higher b 0‐values for the reference measurement at the longest diffusion time spread the sampled points for fixed‐q 0 design over a larger range of the decay curve, and therefore result in a larger induced diffusion time dependence (Fig. 2).
Although these effects were demonstrated in postmortem tissue, the results presented here should apply to in vivo data. Postmortem tissue has been shown to exhibit similar diffusion time dependence to in vivo tissue (49). Our measurements had a slight residual variation in the b‐value with Δ and gradient direction (b min = 3.39 ms·μm−2 at Δ = 70 ms quadratically increasing to b max = 3.45 ms·μm−2 at Δ = 400 ms along the slice‐select axis). Considering the relatively small size of the b‐value variation in comparison to the b 0‐variation and the use of the same diffusion‐weighted data in both analyses, this does not invalidate our fixed‐b 0 versus fixed‐q 0 comparison and conclusions. Another remaining issue is the directional bias 27 caused by the particular direction of the diffusion weighting in the b 0‐image, with respect to all directions in the shell. One solution for this would be to acquire a reference image for each direction and diffusion time at the cost of significant increase in scan time.
CONCLUSIONS
Future experiments with the DW‐STEAM sequence using variable diffusion times should be designed to avoid variable b‐values in the reference measurements to draw valid conclusions about quantities related to restriction, such as length scales and permeability. Incorporating the full b‐matrix is insufficient: To avoid confounding the effects of restriction and possible multiple diffusion coefficients in the system under study, it is important to keep the b‐value as well as the b 0‐value constant over diffusion times. Unlike reference measurements with fixed‐q 0, use of a constant b 0‐value does not induce an apparent diffusion time dependence in the absence of restrictions. Fixed‐b 0 is therefore preferable in experiments that draw conclusions from the relation between the ADC and diffusion time.
Supporting information
Fig. S1. DW‐STEAM sequence modification efficacy. Resultant b‐values for conventional and modified DW‐STEAM sequence according to the postmortem protocol are shown in the top row. All gradients were applied in the slice‐select direction with the b‐value from the diffusion gradient set to b diff = 3.5, b diff = 0.7 (fixed‐b 0) and b diff=0.0 ms·μm−2 over all diffusion times, as well as one in which the gradient was kept constant over diffusion times (fixed‐q 0). As measured in the (worst‐case) direction along the slice‐select axis, the conventional implementation of the DW‐STEAM sequence results in a large range of b‐values over the diffusion times in our postmortem protocol. The b‐value accumulated from the imaging gradients alone is given by the blue line. The bottom row shows the ADC for conventional and modified DW‐STEAM. For these calculations, we used values for the parameters of the biexponential signal model close to those found in our postmortem samples (ie, Ds = 0.2, Df = 1.0; fs = 0.33), as well as q‐values for crusher and slice‐select gradients representative for our postmortem MR protocol (ie, q c = q s = 0.046 μm−1). For the conventional sequence implementation, the undesired b‐value variation implies considerable variation of the calculated ADC in the absence of restriction. The sequence modification, however, prevents the variation in b‐value and results in a flat ADC over diffusion times if the b‐value and b 0‐value are constant. Next to demonstrating that the sequence modification is adequate, this indicates the importance of keeping the b‐value and b 0‐value of the diffusion‐weighted images constant with diffusion time.
Fig. S2. Effect of reference measurements on the ADC. (a) Biexponential signal model (without restriction) with f s = 0.33. (b) Restricted cylinder model with Di = 2 μm2/ms, f i = 0.80, and r = 5 μm. In each panel, the columns show the results of different ratios of the diffusion coefficients in the respective models. The line plots in the top row of each panel show specific examples for measurements with fixed‐q 0 (dashed lines) and fixed‐b 0 (solid lines). Lines of different color show the behavior for different b 0,Δmax values, in which b 0,Δmax = [0, 10, 20, 30] % of the b‐value. The three rows of contour plots show differences in ADCs for the two reference measurement schemes, where each row shows the results for a different b 0/b ratio. The color map represents the ADC difference between the fixed‐q 0 ADC and the fixed‐b 0 ADC as a percentage of the “true” ADC at b 0 = 0 ms/μm2. The top row in (a) plots the ADC versus Δ curves for b × Ds = 0.5 (b = 2.5 ms·μm−2; Ds = 0.2 μm2·ms−1, with Df = 5 × Ds and f s = 0.33) (ie, representative of our postmortem experiment). A larger difference between diffusion coefficients leads to increased underestimation of the ADC (with respect to the “true” b 0 = 0 ms/μm2), as does a larger b 0/b ratio. This is further quantified in the contour plots, where the effect of contrast parameter b × Ds can be examined. In (b) the top row shows the ADC versus Δ curves for b = 4.0 ms·μm−2, with r = 5 μm and f i = 0.80. Here, the difference between the fixed‐b 0 and fixed‐q 0 decreases with increasing difference between the compartment diffusion coefficients, because the intracellular compartment is highly restricted and the ADCi is low. As Dh decreases, the compartmental ADCs are more similar and the differences between fixed‐b 0 and fixed‐q 0 diminish. For very short Δ, in which diffusion approaches free diffusion in the intracellular compartment, the % ADC difference measure becomes negative when Di > Dh. Note that the actual deviation from truth (b 0 = 0 ms/μm2 measurement) is always maximal for fixed‐b 0, because the b 0‐value is determined by the longest diffusion time; however, our goal here is to quantify the difference between the two realistic measurement strategies: fixed‐b 0 and fixed‐q 0. Different columns show the effect of a larger difference between the diffusion coeffiecients. The gray arrow indicates the regime of our postmortem measurements. All scales are linear. The effect of varying the intracellular volume fraction and the cylinder radius is provided in Supporting Figures S3 and S4, respectively.
Fig. S3. The effect of varying volume fraction on the ADC in the presence of multiple diffusion coefficients. (a) The ADC versus Δ curves (top row) and % ADC differences between fixed‐b 0 and fixed‐q 0 for the biexponential model with Df = 5 × Ds. The biexponential model exhibits high sensitivity to volume fraction variation, because it severely alters the ADC differences between the two pools (fast and slow). (b) ADC versus Δ curves (top row) and % ADC differences between fixed‐b 0 and fixed‐q 0 for the restricted cylinder model with Dh = Di and r = 5 μm. Although the volume fraction changes the ADC, the relative difference between fixed‐b 0 and fixed‐q 0 are found to be very similar.
Fig. S4. The effect of cylinder radius on the ADC in the presence of multiple diffusion coefficients in the restricted cylinder model.
These authors contributed equally to this work.
REFERENCES
- 1. Stanisz GJ, Szafer A, Wright GA, Henkelman RM. An analytical model of restricted diffusion in bovine optic nerve. Magn Reson Med 1997;37:103–111. [DOI] [PubMed] [Google Scholar]
- 2. Assaf Y, Blumenfeld‐Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med 2008;59:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJM, Dyrby TB. Orientationally invariant indices of axon diameter and density from diffusion MRI. NeuroImage 2010;52:1374–1389. [DOI] [PubMed] [Google Scholar]
- 4. Lam WW, Jbabdi S, Miller KL. A model for extra‐axonal diffusion spectra with frequency‐dependent restriction. Magn Reson Med 2015;73:2306–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proc Natl Acad Sci U S A 2014;111:5088–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Latour LL, Svoboda K, Mitra PP, Sotak CH. Time‐dependent diffusion of water in a biological model system. Proc Natl Acad Sci U S A 1994;91:1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lasič S, Nilsson M, Lätt J, Ståhlberg F, Topgaard D. Apparent exchange rate mapping with diffusion MRI. Magn Reson Med 2011;66:356–365. [DOI] [PubMed] [Google Scholar]
- 8. Sherbondy AJ, Rowe MC, Alexander DC. MicroTrack: an algorithm for concurrent projectome and microstructure estimation. Med Image Comput Comput Assist Interv 2010;13:183–190. [DOI] [PubMed] [Google Scholar]
- 9. Girard G, Rutger Fick, Descoteaux M, Deriche R, Wassermann D. AxTract: microstructure‐driven tractography based on the Ensemble Average Propagator. Inf Process Med Imaging 2015;24:675–686. [DOI] [PubMed] [Google Scholar]
- 10. Horowitz A, Barazany D, Tavor I, Bernstein M, Yovel G, Assaf Y. In vivo correlation between axon diameter and conduction velocity in the human brain. Brain Struct Funct 2015;220:1777–1788. [DOI] [PubMed] [Google Scholar]
- 11. Horowitz A, Tavor I, Hoffmann C, Miron S, Achiron A, Assaf Y. The in‐vivo dynamics of axon diameter in multiple sclerosis In Proceedings of the Annual Meeting of the Organization for Human Brain Mapping, Geneva, Switzerland; 2016. p 1232. [Google Scholar]
- 12. Fieremans E, Burcaw LM, Lee H‐H, Lemberskiy G, Veraart J, Novikov DS. In vivo observation and biophysical interpretation of time‐dependent diffusion in human white matter. NeuroImage 2016;129:414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nilsson M, Lätt J, Ståhlberg F, van Westen D, Hagslätt H. The importance of axonal undulation in diffusion MR measurements: a Monte Carlo simulation study. NMR Biomed 2012;25:795–805. [DOI] [PubMed] [Google Scholar]
- 14. Mollink J, Kleinnijenhuis M, Sotiropoulos SN, Ansorge O, Jbabdi S, Miller KL. Diffusion restriction along fibres: How coherent is the corpus callosum? In Proceedings of the 23rd Annual Meeting of ISMRM, Toronto, Canada; 2015. p 2783.
- 15. Kleinnijenhuis M, Mollink J, Kinchesh P, Lam WW, Galinsky VL, Frank LR, Smart SC, Jbabdi S, Miller KL. Monte Carlo diffusion simulations disambiguate the biophysical mechanisms of diffusion hinderance along tracts. In Proceedings of the 23rd Annual Meeting of the ISMRM, Toronto, Ontario, Canada; 2015. p 2756.
- 16. Van Zijl PC, Moonen CT, Faustino P, Pekar J, Kaplan O, Cohen JS. Complete separation of intracellular and extracellular information in NMR spectra of perfused cells by diffusion‐weighted spectroscopy. Proc Natl Acad Sci U S A 1991;88:3228–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bailey C, Giles A, Czarnota GJ, Stanisz GJ. Detection of apoptotic cell death in vitro in the presence of Gd‐DTPA‐BMA. Magn Reson Med 2009;62:46–55. [DOI] [PubMed] [Google Scholar]
- 18. Nilsson M, Lätt J, van Westen D, Brockstedt S, Lasič S, Ståhlberg F, Topgaard D. Noninvasive mapping of water diffusional exchange in the human brain using filter‐exchange imaging. Magn Reson Med 2013;69:1573–1581. [DOI] [PubMed] [Google Scholar]
- 19. Lampinen B, Szczepankiewicz F, van Westen D, Englund E, C Sundgren P, Lätt J, Ståhlberg F, Nilsson M. Optimal experimental design for filter exchange imaging: apparent exchange rate measurements in the healthy brain and in intracranial tumors. Magn Reson Med 2017;77:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lasič S, Oredsson S, Partridge SC, Saal LH, Topgaard D, Nilsson M, Bryskhe K.Apparent exchange rate for breast cancer characterization. NMR Biomed 2016;29:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teruel JR, Cho GY, Moccaldi RTM, Goa PE, Bathen TF, Feiweier T, Kim SG, Moy L, Sigmund EE. Stimulated echo diffusion tensor imaging (STEAM‐DTI) with varying diffusion times as a probe of breast tissue. J Magn Reson Imaging 2017;45:84–93. [DOI] [PubMed] [Google Scholar]
- 22. Merboldt KD, Hänicke W, Frahm J. Diffusion imaging using stimulated echoes. Magn Reson Med 1991;19:233–239. [DOI] [PubMed] [Google Scholar]
- 23. Niendorf T, Dijkhuizen RM, Norris DG, van Lookeren Campagne M, Nicolay K. Biexponential diffusion attenuation in various states of brain tissue: implications for diffusion‐weighted imaging. Magn Reson Med 1996;36:847–857. [DOI] [PubMed] [Google Scholar]
- 24. Assaf Y, Mayk A, Cohen Y. Displacement imaging of spinal cord using q‐space diffusion‐weighted MRI. Magn Reson Med 2000;44:713–722. [DOI] [PubMed] [Google Scholar]
- 25. Alexander DC, Barker GJ, Arridge SR. Detection and modeling of non‐Gaussian apparent diffusion coefficient profiles in human brain data. Magn Reson Med 2002;48:331–340. [DOI] [PubMed] [Google Scholar]
- 26. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non‐gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005;53:1432–1440. [DOI] [PubMed] [Google Scholar]
- 27. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundell H, Alexander DC, Dyrby TB. High angular resolution diffusion imaging with stimulated echoes: compensation and correction in experiment design and analysis. NMR Biomed 2014;27:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kinchesh P, Kleinnijenhuis M, Miller KL, Smart S. Minimizing diffusion encoding of slice selection in stimulated echo imaging. In Proceedings of the 23rd Annual Meeting of ISMRM. Toronto, Canada; 2015. p 2791.
- 30. Clark CA, Le Bihan D. Water diffusion compartmentation and anisotropy at high b values in the human brain. Magn Reson Med 2000;44:852–859. [DOI] [PubMed] [Google Scholar]
- 31. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 32. D’Arceuil H, de Crespigny A. The effects of brain tissue decomposition on diffusion tensor imaging and tractography. NeuroImage 2007;36:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller KL, Stagg CJ, Douaud G, et al. Diffusion imaging of whole, post‐mortem human brains on a clinical MRI scanner. NeuroImage 2011;57:167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mollink J, Kleinnijenhuis M, Jbabdi S, Sotiropoulos SN, Ansorge O, Miller KL. Fiber dispersion in the corpus callosum revealed with postmortem diffusion weighted imaging and PLI. In Proceedings of the Annual Meeting of the Organization for Human Brain Mapping, Honolulu, HI; 2015. p 4103.
- 35. Kleinnijenhuis M, Mollink J, Kinchesh P, et al. Monte Carlo simulations disambiguate the biophysical mechanisms of diffusion hindrance along tracts. In Proceedings of the Annual Meeting of the Organization for Human Brain Mapping, Honolulu, HI; 2015. p 1769.
- 36. Lam WW, Miller KL, Kleinnijenhuis M, Jbabdi S. Longitudinally hindered diffusion of in vivo human white matter at long diffusion time. In Proceedings of the 23rd Annual Meeting of ISMRM. Toronto, Canada; 2015. p 2776.
- 37. Marschar AM, Kuder TA, Stieltjes B, Nagel AM, Bachert P, Laun FB. In vivo imaging of the time‐dependent apparent diffusional kurtosis in the human calf muscle. J Magn Reson Imaging 2015;41:1581–1590. [DOI] [PubMed] [Google Scholar]
- 38. Nilsson M, Lätt J, Nordh E, Wirestam R, Ståhlberg F, Brockstedt S. On the effects of a varied diffusion time in vivo: Is the diffusion in white matter restricted? Magn Reson Imaging 2009;27:176–187. [DOI] [PubMed] [Google Scholar]
- 39. Assaf Y, Cohen Y. Assignment of the water slow‐diffusing component in the central nervous system using q‐space diffusion MRS: implications for fiber tract imaging. Magn Reson Med 2000;43:191–199. [DOI] [PubMed] [Google Scholar]
- 40. De Santis S, Jones DK, Roebroeck A. Including diffusion time dependence in the extra‐axonal space improves in vivo estimates of axonal diameter and density in human white matter. NeuroImage 2016;130:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burcaw LM, Fieremans E, Novikov DS. Mesoscopic structure of neuronal tracts from time‐dependent diffusion. NeuroImage 2015;114:18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richardson S, Siow B, Panagiotaki E, Schneider T, Lythgoe MF, Alexander DC. Viable and fixed white matter: diffusion magnetic resonance comparisons and contrasts at physiological temperature. Magn Reson Med 2014;72:1151–1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. DW‐STEAM sequence modification efficacy. Resultant b‐values for conventional and modified DW‐STEAM sequence according to the postmortem protocol are shown in the top row. All gradients were applied in the slice‐select direction with the b‐value from the diffusion gradient set to b diff = 3.5, b diff = 0.7 (fixed‐b 0) and b diff=0.0 ms·μm−2 over all diffusion times, as well as one in which the gradient was kept constant over diffusion times (fixed‐q 0). As measured in the (worst‐case) direction along the slice‐select axis, the conventional implementation of the DW‐STEAM sequence results in a large range of b‐values over the diffusion times in our postmortem protocol. The b‐value accumulated from the imaging gradients alone is given by the blue line. The bottom row shows the ADC for conventional and modified DW‐STEAM. For these calculations, we used values for the parameters of the biexponential signal model close to those found in our postmortem samples (ie, Ds = 0.2, Df = 1.0; fs = 0.33), as well as q‐values for crusher and slice‐select gradients representative for our postmortem MR protocol (ie, q c = q s = 0.046 μm−1). For the conventional sequence implementation, the undesired b‐value variation implies considerable variation of the calculated ADC in the absence of restriction. The sequence modification, however, prevents the variation in b‐value and results in a flat ADC over diffusion times if the b‐value and b 0‐value are constant. Next to demonstrating that the sequence modification is adequate, this indicates the importance of keeping the b‐value and b 0‐value of the diffusion‐weighted images constant with diffusion time.
Fig. S2. Effect of reference measurements on the ADC. (a) Biexponential signal model (without restriction) with f s = 0.33. (b) Restricted cylinder model with Di = 2 μm2/ms, f i = 0.80, and r = 5 μm. In each panel, the columns show the results of different ratios of the diffusion coefficients in the respective models. The line plots in the top row of each panel show specific examples for measurements with fixed‐q 0 (dashed lines) and fixed‐b 0 (solid lines). Lines of different color show the behavior for different b 0,Δmax values, in which b 0,Δmax = [0, 10, 20, 30] % of the b‐value. The three rows of contour plots show differences in ADCs for the two reference measurement schemes, where each row shows the results for a different b 0/b ratio. The color map represents the ADC difference between the fixed‐q 0 ADC and the fixed‐b 0 ADC as a percentage of the “true” ADC at b 0 = 0 ms/μm2. The top row in (a) plots the ADC versus Δ curves for b × Ds = 0.5 (b = 2.5 ms·μm−2; Ds = 0.2 μm2·ms−1, with Df = 5 × Ds and f s = 0.33) (ie, representative of our postmortem experiment). A larger difference between diffusion coefficients leads to increased underestimation of the ADC (with respect to the “true” b 0 = 0 ms/μm2), as does a larger b 0/b ratio. This is further quantified in the contour plots, where the effect of contrast parameter b × Ds can be examined. In (b) the top row shows the ADC versus Δ curves for b = 4.0 ms·μm−2, with r = 5 μm and f i = 0.80. Here, the difference between the fixed‐b 0 and fixed‐q 0 decreases with increasing difference between the compartment diffusion coefficients, because the intracellular compartment is highly restricted and the ADCi is low. As Dh decreases, the compartmental ADCs are more similar and the differences between fixed‐b 0 and fixed‐q 0 diminish. For very short Δ, in which diffusion approaches free diffusion in the intracellular compartment, the % ADC difference measure becomes negative when Di > Dh. Note that the actual deviation from truth (b 0 = 0 ms/μm2 measurement) is always maximal for fixed‐b 0, because the b 0‐value is determined by the longest diffusion time; however, our goal here is to quantify the difference between the two realistic measurement strategies: fixed‐b 0 and fixed‐q 0. Different columns show the effect of a larger difference between the diffusion coeffiecients. The gray arrow indicates the regime of our postmortem measurements. All scales are linear. The effect of varying the intracellular volume fraction and the cylinder radius is provided in Supporting Figures S3 and S4, respectively.
Fig. S3. The effect of varying volume fraction on the ADC in the presence of multiple diffusion coefficients. (a) The ADC versus Δ curves (top row) and % ADC differences between fixed‐b 0 and fixed‐q 0 for the biexponential model with Df = 5 × Ds. The biexponential model exhibits high sensitivity to volume fraction variation, because it severely alters the ADC differences between the two pools (fast and slow). (b) ADC versus Δ curves (top row) and % ADC differences between fixed‐b 0 and fixed‐q 0 for the restricted cylinder model with Dh = Di and r = 5 μm. Although the volume fraction changes the ADC, the relative difference between fixed‐b 0 and fixed‐q 0 are found to be very similar.
Fig. S4. The effect of cylinder radius on the ADC in the presence of multiple diffusion coefficients in the restricted cylinder model.


