Abstract
Aims
Deciding if a diabetic foot ulcer is infected in a community setting is challenging without validated point‐of‐care tests. Four inflammatory biomarkers were investigated to develop a composite algorithm for mildly infected diabetic foot ulcers: venous white cell count, C‐reactive protein (CRP) and procalcitonin, and a novel wound exudate calprotectin assay. Calprotectin is a marker of neutrophilic inflammation.
Methods
In a prospective study, people with uninfected or mildly infected diabetic foot ulcers who had not received oral antibiotics in the preceding 2 weeks were recruited from community podiatry clinics for measurement of inflammatory biomarkers. Antibiotic prescribing decisions were based on clinicians’ baseline assessments and participants were reviewed 1 week later; ulcer infection was defined by clinicians’ overall impression from their two assessments.
Results
Some 363 potential participants were screened, of whom 67 were recruited, 29 with mildly infected diabetic foot ulcers and 38 with no infection. One participant withdrew early in each group. Ulcer area was 1.32 cm2 [interquartile range (IQR) 0.32–3.61 cm2] in infected ulcers and 0.22 cm2 (IQR 0.09–1.46 cm2) in uninfected ulcers. Baseline CRP for mild infection was 9.00 mg/ml and 6.00 mg/ml for uninfected ulcers; most procalcitonin levels were undetectable. Median calprotectin level in infected diabetic foot ulcers was 1437 ng/ml and 879 ng/ml in uninfected diabetic foot ulcers. Area under the receiver operating characteristic curve for a composite algorithm incorporating calprotectin, CRP, white cell count and ulcer area was 0.68 (95% confidence intervals 0.52–0.82), sensitivity 0.64, specificity 0.81.
Conclusions
A composite algorithm including CRP, calprotectin, white cell count and ulcer area may help to distinguish uninfected from mildly infected diabetic foot ulcers. Venous procalcitonin is unhelpful for mild diabetic foot ulcer infection.
What's new?
Distinguishing between mild infection and no infection in diabetic foot ulcers, a frequent position of equipoise in antibiotic prescribing, is challenging in the absence of objective evidence available at point of care.
We developed a novel wound exudate calprotectin assay which, when combined with venous C‐reactive protein, white cell count and ulcer area, provided a diagnostic algorithm for diabetic foot ulcer infection.
The combined algorithm has a specificity of 0.81 in distinguishing mild infection from no infection in a diabetic foot ulcer.
What's new?
Distinguishing between mild infection and no infection in diabetic foot ulcers, a frequent position of equipoise in antibiotic prescribing, is challenging in the absence of objective evidence available at point of care.
We developed a novel wound exudate calprotectin assay which, when combined with venous C‐reactive protein, white cell count and ulcer area, provided a diagnostic algorithm for diabetic foot ulcer infection.
The combined algorithm has a specificity of 0.81 in distinguishing mild infection from no infection in a diabetic foot ulcer.
Introduction
Diagnosis of diabetic foot ulcer infection continues to rely on symptoms, principally pain, and signs, including erythema, warmth, oedema and discharge. However, pain may be absent due to concomitant neuropathy and signs may be attenuated by vasculopathy 1. Failure to treat mild infection with antibiotics risks progression to severe infection and amputation. Conversely, unnecessary over‐prescription of antibiotics exposes the person to the risk of adverse effects, increases the risk of subsequent infection with resistant organisms, and contributes to increasing antimicrobial resistance in society, one of the highest current public health priorities 2. The financial burden of diabetic foot ulcers and associated amputations is large, approximately £650 million in England in 2011, exceeding 0.6% of the total health budget 3. There are still no objective biomarkers of diabetic foot ulcer infection available at the point of care, which is particularly relevant because most diabetic foot ulcer care occurs in the community 3.
A pilot study of 45 individuals with diabetic foot ulcers reported that venous C‐reactive protein (CRP) levels combined with venous procalcitonin, another infection biomarker, may help in distinguishing between infected and non‐infected foot ulcers 4. For a CRP cut‐off value of 17 mg/L, negative predictive value was 0.91 and positive predictive value was 0.83. Validated point‐of‐care tests for CRP and other inflammatory biomarkers are now rapidly entering the market, providing the potential for testing in the community 5, 6. Calprotectin is a marker of neutrophilic inflammation and therefore may be useful as a marker of infection. It is currently largely used as a marker of inflammatory bowel disease through testing faecal samples, is available as a dipstick test, and is resistant to protease degradation 7. This makes it a candidate to directly assess wound exudate, whose relatively high protease levels prevent the use of several other candidate inflammatory biomarkers.
Diagnostic infection assays, including non‐specific testing of inflammatory markers, are likely to be most effective when combined with optimal clinical assessment and patient communication. For example, the use of a point‐of‐care test for CRP combined with an educational intervention more than halved antibiotic prescribing in primary care for lower respiratory tract infections without compromising patient safety 8.
The aim of our study was to determine the diagnostic accuracy of a combined inflammatory biomarker algorithm including venous white cell count, CRP and procalcitonin, and wound exudate calprotectin levels in predicting diabetic foot ulcer mild infection.
Patients and methods
Study design
The Updated List of Essential Items for Reporting Diagnostic Accuracy Studies (STARD) extension of the EQUATOR network has been followed to guide study reporting (Appendix S1) 9. A sequential recruitment observational design was selected to provide proof of principle and feasibility data, the intention being to use data from this study to inform subsequent evaluations of the potential use of point‐of‐care tests in future. Participants were recruited from six community podiatry clinics in South Wales, UK and the study received ethics approval from Wales Research Ethics Committee 6 (ref 14/WA/0085). Inclusion criteria were the presence of a full‐thickness skin defect located distal to the ankle that was assessed clinically as either uninfected or mildly infected according to the Infectious Disease Society of America International Working Group on the Diabetic Foot (IDSA‐IWGDF) classification system 10, in adults aged at least 18 years who met World Health Organization (WHO) diagnostic criteria for diabetes 11. Exclusion criteria were immunosuppression by medication or other medical conditions, recent antibiotic treatment, pregnancy or lactation, or IDSA‐IWGDF moderate or severe diabetic foot ulcer infection. During the first few weeks of recruitment it was noted that receipt of antibiotics within the preceding 6 weeks excluded a high proportion of potential participants and this criterion was reduced to 2 weeks, based on the dynamics of inflammatory biomarker production in response to infection 12.
It was anticipated that individuals with uninfected diabetic foot ulcers would be more prevalent and easier to recruit, and so it was prospectively decided to allow re‐recruitment of participants initially recruited with an uninfected diabetic foot ulcer, if they subsequently presented with a mildly infected diabetic foot ulcer. However, only data from their infected diabetic foot ulcer presentation would be used in the primary analysis of biomarker diagnostic accuracy, to avoid the same participant being counted twice in analyses.
Infection definition
Our infection definition was based on the clinician's assessment 1 week after initial recruitment incorporating any change from baseline, factoring in whether antibiotic therapy had been received in the subsequent week. The clinician obtained all the clinical information, including measurement of ulcer area, vasculopathy and neuropathy, while remaining blinded to assay results, including point‐of‐care test results.
Data collection and study procedures
At baseline, experienced podiatrists provided a clinical assessment of participants, including an overall assessment of appearing well or unwell, checking for peri‐ulcer erythema, tenderness, warmth, lymphangitis and osteomyelitis. Ankle brachial pressure indices (ABPIs) were recorded as a measure of vasculopathy, with a level less than 0.9 considered abnormal, and duplex scanning was undertaken in those with calcified vessels. Neuropathy was assessed by 10 g monofilament in the relevant foot. If the diabetic foot ulcer was judged to be infected at baseline on clinical grounds, oral antibiotics and antimicrobial dressings were prescribed based on local guidelines; oral antibiotics and antimicrobial dressings were not permitted in those whose diabetic foot ulcer was judged to be uninfected.
Wound swabs, without debridement, were collected for calprotectin measurement, as well as microscopy, culture and antibiotic sensitivity analysis. Venous blood was sampled for automated measurement of white cell count and CRP; serum was stored at –80 °C for analysis of procalcitonin levels. Quantification of procalcitonin was performed in duplicate by ELISA (Abcam, UK, ab100630). CRP was also measured from a pin prick of blood using a point‐of‐care instrument (QuikRead go CRP, Orion Diagnostica, Finland); levels were measured by a researcher and the result was not communicated to the clinician until after they had provided their overall assessment of ulcer infection status at the week 1 visit.
Participants were asked to return after 1 week for a repeat clinical examination and to repeat sampling of venous blood, wound exudate and point‐of‐care CRP testing. They also kept a diary during the initial first week and three subsequent weeks, recording ulcer symptoms, instigation of any antibiotics, adherence to treatment, other medications and dressings required, quality of life 13, functional impairment 14 and any ulcer complications, including hospitalization.
Calprotectin assay
Ulcer swabs were pre‐processed by vortexing the head of the swab in 3 ml of sterile saline for 5 s followed by sonication for 5 min; the head of the swab was then centrifuged at 100 g for 1 min. Duplicate supernatant samples were aliqoted and frozen at –80 °C for batched enzyme‐linked immunosorbent assay (ELISA) analysis within 1 year. A two‐site sandwich Calprotectin ELISA (MRP 8/14, S100A8/A9, DRG Diagnostics) at an initial dilution of 1 : 2 was used, absorbance being measured at 450 nm with a FLUOstar Optima microplate reader (BMG LABTECH Ltd, UK). Samples outside the upper limit of measurement were re‐run at dilutions of 1 : 20 or 1 : 200.
Sample size and statistical analysis
The largest previous study in the field recruited 23 participants with uninfected diabetic foot ulcers and 23 with infected diabetic foot ulcers, with a designed clinical prevalence for infection of 50% 4. Our intention was to exceed this study size, ideally recruiting 50 participants with uninfected diabetic foot ulcers and 50 with mildly infected diabetic foot ulcers, providing 95% confidence intervals (95% CI) of 0.77 to 0.96 around a positive predictive value (PPV) of 0.90 for the diagnostic algorithm. Inflammatory biomarker levels along with the gold standard dichotomous classification of diabetic foot ulcer infection formed the primary dataset. Cut‐off points that optimized sensitivity, specificity and the area under the receiver operating characteristic curve (AUROCC) were calculated using Youden's index and the Euclidean distance from the upper left hand corner to the co‐ordinates of the curve 15. Analyses to obtain AUROCC utilized bootstrapping in Stata/IC 13.1 with 5000 repetitions and bias‐corrected estimates are reported.
Results
During a 12‐month recruitment period, from October 2014 to September 2015, 363 potential participants were screened, of whom 67 were recruited. A breakdown of reasons for study non‐recruitment is given in Table 1, with recent antibiotic treatment being the most common cause. Some screened participants were excluded because their ulcer was not full thickness or was located proximal to the ankle, in the context that the clinics assessed a spectrum of patients with skin integrity problems. The primary dataset included 34 participants with uninfected diabetic foot ulcers and 27 with mildly infected diabetic foot ulcers (Fig. 1). Table 2 details the baseline characteristics of participants included in the primary dataset. Median ulcer area for the uninfected group was 0.22 cm2 [interquartile range (IQR) 0.09–1.46 cm2) compared with 1.32 cm2 for mildly infected diabetic foot ulcers (IQR 0.32–3.61 cm2).
Table 1.
Breakdown of reasons for study ineligibility in the 294 potential participants who were not recruited into the study. In some individuals more than one reason was identified
| Exclusion criterion | No. of participants | Percentage of ineligible individuals |
|---|---|---|
| Antibiotic treatment in last 2 weeks (post‐Nov 2014) | 82 | 28 |
| Ulcer not full thickness/below ankle | 74 | 25 |
| Moderate /severe infection | 48 | 16 |
| Not able to attend week 1 follow‐up | 37 | 13 |
| Antibiotic treatment in last 6 weeks (pre‐November 2014) | 27 | 9 |
| Not interested in participating | 26 | 9 |
| Immunosuppression | 20 | 7 |
| Unable to provide informed consent | 15 | 5 |
| People without diabetes | 11 | 4 |
Figure 1.
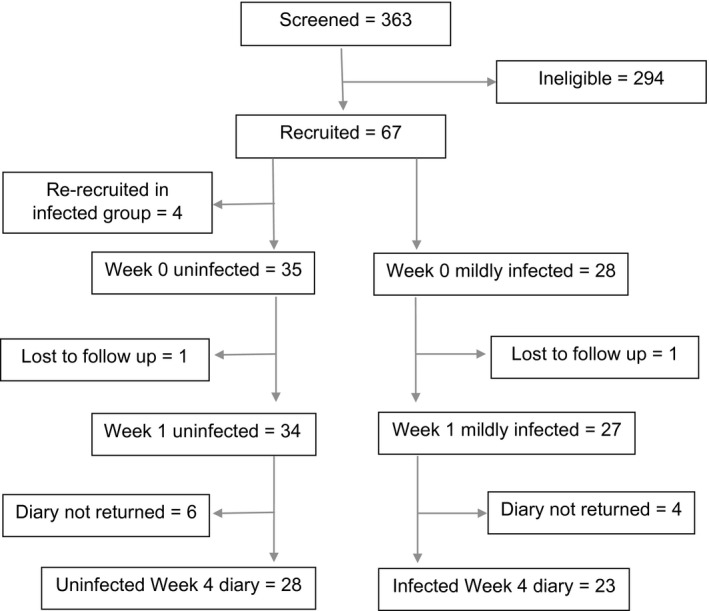
Participant flow diagram. A flow diagram summarizing participant recruitment into the INDUCE study. Uninfected or mildly infected status is defined by the clinician's overall clinical impression of the diabetic foot ulcer at week 1, incorporating response to antibiotics, if prescribed at baseline, while blinded to all test results. Four participants who were recruited with uninfected ulcers subsequently re‐presented with a mildly infected ulcer and were recruited again, but their data is only counted once, for the episode of infection.
Table 2.
Baseline characteristics of participants, subdivided by ulcer infection status
| Uninfected* (n = 34) | Infected* (n = 27) | |
|---|---|---|
| Age at recruitment (years) | 64.9 (11.0) | 66.4 (14.4) |
| Male† | 27 (79) | 19 (70) |
| Ethnicity White British/Welsh† | 32 (94) | 26 (96) |
| BMI (kg/m2) | 33.6 (5.5) | 28.3 (6.3) |
| Current smoker† | 2 (6) | 6 (22) |
| Ex‐smoker† | 18 (53) | 13 (48) |
| Years since diabetes diagnosis | 20.4 (9.9) | 16.6 (11.3) |
| HbA1c (mmol/mol)‡ | 64 (54–77) | 68 (54–110) |
| HbA1c (%)‡ | 8.0 (7.1–9.2) | 8.4 (7.1–12.2) |
| At least 1 previous ulcer in past year | 16 (47) | 11 (43) |
| Current ulcer area (cm2)‡ | 0.215 (0.090–1.463) | 1.320 (0.320–3.610) |
| Current ulcer duration (weeks)‡ | 16.0 (8.4–73.5) | 10.0 (1.1–66.0) |
| Previous antibiotic treatment of current ulcer | 23 (68) | 13 (48) |
| Ankle brachial pressure index abnormal in ipsilateral lower limb | 8 of 23 (35) | 8 of 15 (53) |
| Peripheral neuropathy in ulcerated foot | 33 (97.1) | 21 (77.8) |
Mean (sd) unless stated otherwise; †number (%) and ‡median (25th to 75th percentile).
Results for the inflammatory biomarker tests at baseline are in Table 3, subdivided by their infection status as determined by the study infection definition. The median wound exudate calprotectin level in samples from mildly infected diabetic foot ulcers was 1437 ng/ml (IQR 664–6420 ng/ml) compared with 879 ng/ml (IQR 586–2674 ng/ml) in uninfected ulcers. Venous procalcitonin results were limited by 41 of the 59 baseline samples having a level below the lower limit of assay detection, including 21 samples from those with infected ulcers.
Table 3.
Results of inflammatory biomarker tests, subdivided by ulcer infection status
| Test | Uninfected* | Infected* | AUROCC (95% CI) |
|---|---|---|---|
| White cell count (109 cells/L)† | 7.43 (1.50) N = 32 | 8.15 (1.95) N = 27 | 0.62 (0.47–0.76) |
| Laboratory venous C‐reactive protein (mg/L) | 4.5 (2.0–11.0) N = 32 | 7.0 (3.0–22.0) N = 27 | 0.62 (0.48–0.77) |
| Point‐of‐care venous C‐reactive protein (mg/L) | 6.0 (5.0–15.5) N = 34 | 9.0 (5.0–17.0) N = 27 | 0.54 (0.39–0.69) |
| Venous procalcitonin (pg/ml) | 21.8 (13.2–108) N = 13 (20 below limit) | 4.8 (1.96–14.1) N = 4 (21 below limit) | Unable to calculate |
| Wound exudate calprotectin (ng/ml) | 879 (586–2674) N = 33 | 1437 (664–6420) N = 25 | 0.56 (0.41–0.71) |
Values are median (25th to 75th centiles), except †mean (sd).
The inflammatory biomarker values were then combined in logistic regression models as predictors of diabetic foot ulcer infection, in each case creating a ‘likelihood score for infection’ (LSI) from which receiver operating characteristic curves could be plotted. Our original intention was to combine point‐of‐care test CRP, white cell count, procalcitonin and calprotectin values, however, the lack of measurable procalcitonin levels led to exclusion of this variable from the model. Participants lacking data for any of the required variables were not included, and so the LSI for the remaining three variables was based on 31 uninfected and 25 mildly infected diabetic foot ulcer participants. The AUROCC was 0.63 (95% CI 0.47–0.78), indicating no benefit for prediction of diabetic foot ulcer infection.
Having excluded procalcitonin from the combined algorithm, we decided to substitute a clinical parameter as the fourth variable. Ulcer area was chosen because results were available for all participants, there was a six‐fold difference in the mean value between infected and uninfected ulcers, and it is a straightforward measurement for clinicians to obtain. Combination of the three remaining biomarkers, point‐of‐care test CRP, white cell count and calprotectin with baseline ulcer area produced a composite algorithm demonstrating significant improvement in prediction of diabetic foot ulcer infection, with an AUROCC of 0.68 (95% CI 0.52–0.82). Using Youden's index and the Euclidean distance from the upper left hand corner provided a consistent cut‐off point for LSI of 1.77, giving a sensitivity of 0.64 and a specificity of 0.81, with a corresponding positive predictive value of 0.73 and negative predictive value of 0.75 (Appendix S2).
In terms of study safety, there were no serious adverse events during the 4 weeks covered by trial diaries. Five of the 28 participants (18%) with uninfected diabetic foot ulcers who returned their diary cards required oral antibiotics for diabetic foot ulcer infection in the subsequent 3 weeks after the week 1 assessment, compared with 15 of 23 with infected diabetic foot ulcers (65%) who required additional antibiotics during this period.
Discussion
Our results show that an algorithm including white cell count, CRP, wound exudate calprotectin and ulcer area has an AUROCC which is insufficient to recommend its use without further refinement. A sensitivity of 0.64 means that if 100 people with a mildly infected diabetic foot ulcer were tested, 64 would be correctly identified as having infection, and there would be 36 false negatives. A specificity of 0.81 means that if 100 people with an uninfected diabetic foot ulcer were tested, 81 would be correctly identified as having no infection and there would be 19 false positives indicating mild infection. Levels of the novel wound calprotectin biomarker were nearly doubled in mild diabetic foot ulcer infection, however, this result did not reach statistical significance. By contrast, venous procalcitonin levels were of no value in detecting mild diabetic foot ulcer infection. The parameters included in our algorithm are, or soon will be, available at point of care, which is an important consideration when most diabetic foot ulcer care occurs in the community and antibiotic decisions have to be made swiftly. We chose to restrict study participants to mild or no ulcer infection because this distinction represents the greatest challenge in diabetic foot ulcer antibiotic stewardship, in the context that moderate to severe infection is straightforward to diagnose from clinical parameters.
Venous procalcitonin or a combination of procalcitonin and CRP have been used as biomarkers of diabetic foot ulcer infection in several other studies. Jeandrot et al. calculated an AUROCC of 0.95 (sd 0.029) for procalcitonin combined with CRP 3, Uzun et al. demonstrated an AUROCC of 0.86 for procalcitonin alone 16, and Massara et al. found a 100‐fold increase in procalcitonin levels between infected and uninfected diabetic foot ulcers 17. In comparing our results with these other studies, it is possible that technical issues using different assays might account for some of the discrepancy, however participant selection criteria are the most likely reason. Most of the other studies recruited from hospitalized patients, with more severe diabetic foot ulcer infections. Massara et al. did not restrict recruitment to mild diabetic foot ulcer infection and 11 of the 15 patients in their infected group were pyrexial, with a temperature > 38.5 °C, graded as severe infection by the IDSA‐IWGDF classification system 17. The increased severity of infection is reflected by a mean CRP level of 121 mg/L, more than 10 times the level in our patients judged to have infected diabetic foot ulcers. Uzun et al. recruited participants who required admission to hospital; 28 of the 49 were receiving antibiotics at the time of admission and 7 of the 27 patients in the infected group had MRI evidence of osteomyelitis, indicating at least moderate infection 16. Jeandrot et al. did use the IDSA‐IWGDF classification system and compared people with mildly infected diabetic foot ulcers with those with uninfected diabetic foot ulcers 4. However, their study population was again recruited in a hospital setting, suggesting that more severe infection was likely, and people with other inflammatory conditions were excluded, reducing external validity.
We chose to screen and recruit sequential participants in a community setting because this is where most people with diabetic foot ulcers are treated, and where clinical equipoise occurs most frequently in considering possible systemic or topical antibiotic therapy for people with diabetic foot ulcers. Biomarkers available at point of care in a community setting have the greatest potential to avoid early infection being missed and to support clinicians in avoiding inappropriate empirical antibiotic therapy in the absence of objective evidence. In most people with moderate or severe infection requiring hospitalization, clinical parameters such as overt wound inflammation are sufficient to diagnose infection with confidence and biomarkers are relatively redundant. Inflammatory biomarkers, in particular erythrocyte sedimentation rate, may have a specific role to assist in the diagnosis of occult osteomyelitis, which can be difficult to diagnose clinically in the absence of immediate MRI resources 18.
In considering other strengths of our study, we selected an infection definition that is more robust than relying on a single clinical impression at one snap‐shot in time. Our definition was based on two separate clinical assessments, 1 week apart, allowing the clinician to integrate data regarding the natural history of the ulcer and incorporating response to antibiotic treatment, if given. All the clinicians involved in the study were podiatrists who were experienced in application of the IDSA‐IWGDF classification system and had completed an online educational tool to reinforce their clinical skills. Our definition has not, however, been subjected to validation studies to confirm that it performs better than a single assessment. We avoided incorporating microbiology results from ulcer swabs into our definition because all wounds are colonized with organisms and the method of sampling, comparing swabs with tissue samples, is known to produce inconsistent results 19.
In terms of study limitations, our definition for infection diagnosis has not been validated against the most robust gold standard of a peri‐ulcer tissue biopsy Gram stain and culture. We chose not to incorporate a tissue biopsy in our study because of the logistical challenge of performing biopsies in the community and ethical considerations in terms of enlarging the size of the diabetic foot ulcer in people with uninfected or only mildly infected ulcers. Difficulty in applying the gold standard for infection diagnosis in the field of diabetic foot ulcers persists, which led to our methodology of applying the IDSA‐IWGDF criteria on two separate occasions, one week apart, as a refinement when using clinical parameters.
Regarding other study limitations, it would have been ideal to measure toe pressures rather than ABPIs in our study population, but ABPI measurement remained standard practice when our study was performed. In addition, it should be noted that the dynamics of biomarker responses to infection caused us to exclude people who had received oral antibiotics within the preceding 2 weeks, resulting in 28% of screened individuals being ineligible for the study. It follows that our results are not generalizable to those who have received oral antibiotics in the previous fortnight.
Building on the results from our calprotectin wound swab assay, it may be that the focus of biomarker development for diabetic foot ulcer infection should be directed towards further wound fluid assays. It is perhaps unlikely that circulating venous biomarkers are substantially raised by mild infection in a foot ulcer, particularly when the vascular supply to the foot is compromised. In addition, interference from other comorbid inflammatory conditions is less likely to affect the results. Other wound exudate biomarkers under investigation include levels of lactate 20, interleukin‐6 21, human neutrophil elastase and cathepsin G 22. These could be combined with calprotectin to produce a composite wound exudate assay. Subsequent clinician education is important to encourage behaviour change and to prevent over‐reliance on test results. Ultimately, an RCT is needed utilizing a composite point‐of‐care test and accompanied by an educational resource, to determine whether antibiotic prescribing can be safely targeted for people with infected diabetic foot ulcers, avoiding inappropriate antibiotic use in people without diabetic foot ulcer infection.
In summary, our study demonstrated that a novel wound exudate calprotectin biomarker shows promise in assisting with the diagnosis of mild diabetic foot ulcer infection, a scenario in which clinical equipoise is frequently encountered regarding whether to offer antibiotic therapy. Combination of calprotectin with venous white cell count and CRP, and ulcer area improved diagnostic accuracy but the AUROCC of 0.68 remains insufficient to recommend the composite algorithm without further refinement in a primary care setting. Nevertheless, our algorithm's specificity of 0.81 provides some much‐needed objective evidence to help clinicians avoid mass antibiotic prescribing in non‐infected diabetic foot ulcers, contributing to prevention of antimicrobial resistance.
Funding sources
Funding to undertake the study was provided by a Research for Patient and Public Benefit grant (no. 1018) from Health and Care Research Wales (HCRW). The funder had no involvement in study design, data collection, data analysis, preparation of the manuscript or decision to submit for publication. The views expressed in this publication are those of the authors and not necessarily those of HCRW. Oxford Biosystems Ltd provided point‐of‐care instruments and assay reagents to measure C‐reactive protein free of charge.
Competing interests
None declared.
Supporting information
Appendix S1. Updated List of Essential Items for Reporting Diagnostic Accuracy Studies (STARD).
Appendix S2. Receiver operating characteristic curve for ‘likelihood score for infection’ and infection status.
Acknowledgements
We wish to thank the members of the trial steering committee (TSC), the chairman Prof. Colin Dayan, Cardiff University, statistician Dr Jonathan Gillard, Cardiff University, and patient representatives Dai Williams and Geraldine Pittman. Amanda Iles, Janine Bates and Judith Evans of the Centre for Trials Research, Cardiff University kindly provided administrative support. Miguel Cossio, also from the Centre for Trials Research, Cardiff University, provided study database expertise. Dr Bastiaan Hoogendoorn and Dr Catherine Naseriyan, both from Cardiff University School of Medicine, assisted with the biochemical assays. We thank Jane Lewis, Cardiff Metropolitan University, for providing podiatry advice and Dr Robin Howe of Public Health Wales for microbiology advice. Podiatrists Angela Jones and Helen Richards were invaluable in recruiting participants and we also wish to thank Health and Care Research Wales for supporting study recruitment.
Diabet. Med. 35, 255–261 (2018)
References
- 1. Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous manifestations of diabetes mellitus: a review. Am J Clin Dermatol 2017; 18: 541–553. [DOI] [PubMed] [Google Scholar]
- 2. Gulland A. Antimicrobial resistance is now widespread, warns WHO. BMJ 2014; 348: g3062. [DOI] [PubMed] [Google Scholar]
- 3. Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med 2014; 31: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 4. Jeandrot A, Richard JL, Combescure C, Jourdan N, Finge S, Rodier M et al Serum procalcitonin and C‐reactive protein concentrations to distinguish mildly infected from non‐infected diabetic foot ulcers: a pilot study. Diabetologia 2008; 51: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pfäfflin A, Schleicher E. Inflammation markers in point‐of‐care testing (POCT). Anal Bioanal Chem 2009; 393: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 6. Seamark DA, Backhouse SN, Powell R. Field‐testing and validation in a primary care setting of a point‐of‐care test for C‐reactive protein. Ann Clin Biochem 2003; 40: 178–180. [DOI] [PubMed] [Google Scholar]
- 7. Chen CC, Huang JL, Chang CJ, Kong MS. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J Pediatr Gastroenterol Nutr 2012; 55: 541–547. [DOI] [PubMed] [Google Scholar]
- 8. Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ 2009; 338: b1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L et al STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351: h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG et al 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54: 132–173. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization (WHO) . Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated report of a WHO Consultation. Geneva: WHO, 2011. [PubMed]
- 12. Schmit X, Vincent JL. The time course of blood C‐reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection 2008; 36: 213–219. [DOI] [PubMed] [Google Scholar]
- 13. Price PE, Harding KG. Cardiff Wound Impact Schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004; 1: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. EuroQol Group . EuroQol – a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 15. Kelly MJ, Dunstan FD, Lloyd K, Fone DL. Evaluating cutpoints for the MHI‐5 and MCS using the GHQ‐12: a comparison of five different methods. BMC Psychiat 2008; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uzun G, Solmazgul E, Curuksulu H, Turhan V, Ardic N, Top C et al Procalcitonin as a diagnostic aid in diabetic foot infections. Tohoku J Exp Med 2007; 213: 305–312. [DOI] [PubMed] [Google Scholar]
- 17. Massara M, De Caridi G, Serra R, Barillà D, Cutrupi A, Volpe A et al The role of procalcitonin as a marker of diabetic foot ulcer infection. Int Wound J 2017; 14: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Victoria van Asten SA, Geradus Peters EJ, Xi Y, Lavery LA. The role of biomarkers to diagnose diabetic foot osteomyelitis. a meta‐analysis. Curr Diabetes Rev 2016; 12: 396–402. [DOI] [PubMed] [Google Scholar]
- 19. Nelson EA, Wright‐Hughes A, Brown S, Lipsky BA, Backhouse M, Bhogal M et al Concordance in diabetic foot ulceration: a cross‐sectional study of agreement between wound swabbing and tissue sampling in infected ulcers. Health Technol Assess 2016; 20: 1–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Löffler M, Zieker D, Weinreich J, Löb S, Königsrainer I, Symons S et al Wound fluid lactate concentration: a helpful marker for diagnosing soft‐tissue infection in diabetic foot ulcers? Preliminary findings. Diabet Med 2011; 28: 175–178. [DOI] [PubMed] [Google Scholar]
- 21. Ambrosch A, Lobmann R, Pott A, Preissler J. Interleukin‐6 concentrations in wound fluids rather than serological markers are useful in assessing bacterial triggers of ulcer inflammation. Int Wound J 2008; 5: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasmann A, Gewessler U, Hulla E, Schneider KP, Binder B, Francesko A et al Sensor materials for the detection of human neutrophil elastase and cathepsin G activity in wound fluid. Exp Dermatol 2011; 20: 508–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Updated List of Essential Items for Reporting Diagnostic Accuracy Studies (STARD).
Appendix S2. Receiver operating characteristic curve for ‘likelihood score for infection’ and infection status.


