ABSTRACT
BACKGROUND AND PURPOSE
In acute stroke, thromboembolism or spontaneous hemorrhage abruptly reduces blood flow to a part of the brain. To limit necrosis, rapid radiological identification of the pathological mechanism must be conducted to allow the initiation of targeted treatment. The aim of the Norwegian Acute Stroke Prehospital Project is to determine if anesthesiologists, trained in prehospital critical care, may accurately assess cerebral computed tomography (CT) scans in a mobile stroke unit (MSU).
METHODS
In this pilot study, 13 anesthesiologists assessed unselected acute stroke patients with a cerebral CT scan in an MSU. The scans were simultaneously available by teleradiology at the receiving hospital and the on‐call radiologist. CT scan interpretation was focused on the radiological diagnosis of acute stroke and contraindications for thrombolysis. The aim of this study was to find inter‐rater agreement between the pre‐ and in‐hospital radiological assessments. A neuroradiologist evaluated all CT scans retrospectively. Statistical analysis of inter‐rater agreement was analyzed with Cohen's kappa.
RESULTS
Fifty‐one cerebral CT scans from the MSU were included. Inter‐rater agreement between prehospital anesthesiologists and the in‐hospital on‐call radiologists was excellent in finding radiological selection for thrombolysis (kappa .87). Prehospital CT scans were conducted in median 10 minutes (7 and 14 minutes) in the MSU, and median 39 minutes (31 and 48 minutes) before arrival at the receiving hospital.
CONCLUSION
This pilot study shows that anesthesiologists trained in prehospital critical care may effectively assess cerebral CT scans in an MSU, and determine if there are radiological contraindications for thrombolysis.
Keywords: Cerebral CT, stroke, prehospital
Introduction
Revascularization of acute ischemic stroke is highly time dependent and the rapid diagnostic assessment in the acute phase is crucial.1 Different approaches to reduce symptom onset to treatment time (OTT) are constantly developing. These include streamlining in‐hospital logistics,2, 3, 4 public information campaigns,5, 6, 7 and developing prehospital prealert systems.8, 9 Neuroradiology is mandatory10 and cerebral CT or MRI examinations must be carried out to exclude intracerebral hemorrhage and large irreversible infarctions in all acute stroke patients.11, 12 Sensitivity in the detection of intracranial hemorrhage (within 6 hours of symptom onset) and availability of neuroradiological examinations makes cerebral CT the preferred diagnostic tool in the hyper acute setting.12
At present, recanalization therapy is only available to a small proportion of the acute ischemic stroke population.13 Time delay to treatment is associated with low efficacy combined with a higher risk of iatrogenic intracerebral hemorrhage.14 The use of prehospital cerebral CT scans in mobile stroke units (MSUs) is considered adequate to detect radiological contraindications for thrombolysis,10 and recent publications with in‐hospital specialists on board the ambulance or diagnostic work‐up via telemedicine15, 16, 17, 18 have reduced OTT. MSUs with telemedicine possibilities require a robust tele network coverage and the availability of in‐hospital specialists. Recent published studies report technical challenges16, 18, 19, 20, 21, 22, 23, 24 and none of these studies have assessed models in a rural health care system.
During the first preclinical part of the Norwegian Acute Stroke Prehospital Project (NASPP), we found that anesthesiologists trained in prehospital critical care from the national helicopter emergency medical service (HEMS) could interpret cerebral CT scans with excellent agreement when compared with the neuroradiologists.25 In this second part of the NASPP, we included prehospital advanced diagnostics in an MSU to develop an independent diagnostic platform of acute stroke patients in the Norwegian prehospital system and HEMS. The aim of this study was to assess the inter‐rater agreement between the pre‐ and in‐hospital interpretations of cerebral CT scans and selection for thrombolytic therapy.
Methods
The NASPP was approved by the Norwegian Regional Ethics Committee (2098/2013). Patients included completed informed written consent, after an initial verbal consent. The next of kin provided consent in situations where a written consent could not be completed by the patient. Study inclusion lasted 85 weekdays from 8 am to 8 pm between October 2014 and January 2016.
The Mobile Stroke Unit
This pilot study was carried out in a specially designed NASPP MSU (image 1) (Mercedes Sprinter, Germany) equipped with a cerebral CT scanner (CereTom Neurologica, Samsung, Korea), a point of care biochemical laboratory (pocH‐100i Automated Hematology Analyzer and HEMOCHRON Jr. Signature±, USA), and a telemedicine system (Meytech, Germany). The portable CereTom meets the American College of Radiology's recommended guidelines for Computed Tomography Dose Index.26 Images were of high quality with 8‐Slice, 17 lp/cm, and 1.25, 2.5, 5, and 10 mm thickness and with 3Hu @ 3 mm low contrast detectability and up to 512 × 512 image resolution. All CT scans were transferred with data‐transfer via CAMD, 3G, 4G (UMTS [HSDPA/HSUPA]). Audio and CCT data‐transfer using a DICOM format implements the pictures in hospital PACS. The Norwegian Governmental Radiation Protection Authority approved the CT scanner for prehospital use.
The Emergency Medical Communication Central (EMCC) used The Norwegian Index of Emergency Medicine as a guideline for dispatch.27 At patient location, the anesthesiologist on board the MSU completed a brief clinical examination and scored the patient according to the National Institute of Health Stroke Scale (NIHSS). A prehospital cerebral CT scan was carried out based on clinical and predefined inclusion criteria (Table 1).
Table 1.
Inclusion Criteria
| NASPP Inclusion Criteria |
|---|
|
|
|
|
NASPP = The Norwegian Acute Stroke Prehospital Project.
Cerebral CT Scan Assessment, Training, and Performance
In the NASPP MSU, all personnel wore a lead coated apron during the CT studies. The Norwegian Governmental Radiation Protection Authority controlled CT radiation exposure routinely. Anesthesiologists enrolled in NASPP were experienced prehospital critical care physicians, and a mandatory 2‐day course in cerebral CT scan assessment and web‐based certification in NIHSS by HealthCarePoint was carried out before participation in the study.28 The Stavanger Acute Medicine Foundation for Education and Research medical simulation center facilitated full‐scale simulations for all MSU personnel before the start of the study. Simulations involved acute stroke patient care aspects, technical implementation of the CT scanner, and laboratory skills.
Immediately after the MSU arrival at scene, a brief clinical examination, NIHSS, and blood tests were assessed before patients, eligible for a prehospital cerebral CT, were placed on the ambulance stretcher. The MSU anesthesiologist assessed the complete CT scan examination and the scans were simultaneously sent by teleradiology to the on‐call radiologist at the receiving hospital (Fig 1) The prehospital cerebral CT scan interpretation was for research purposes only, and blinded for the on‐call radiologists. All CT scans were retrospectivly interpreted by a neuroradiologist to set a “gold standard.” CT scans evaluated as of poor quality by the neuroradiologist, but considered adequate by the anesthesiologist and radiologist in the acute phase, are included in the analysis and interpreted as a “radiological contraindication.”
Figure 1.

The Norwegian Acute Stroke Prehospital Project (NASPP) Mobile Stroke Unit (MSU) staffed with critical care physican and paramedic.
Patients with CT scans with artifacts due to technical issues had a new scan taken on hospital admission. Information on age, gender, symptom onset, topography, brief history, and NIHSS was available for to the anesthesiologists, radiologists, and neuroradiologists. CT or MRI examinations from previous hospitalizations were blinded during the primary acute interpretation in the MSU to mimic the normally limited prehospital information.
The anesthesiologists and radiologists were asked to identify radiological contraindications for thrombolysis, and determine the radiological diagnosis defined as: (i) no significant pathology, (ii) intracranial hemorrhage, (iii) brain lesion, or (iv) hypodensity affecting more than 1/3 of the middle cerebral artery perfusion territory.
In‐hospital cerebral CT scan assessments were carried out by the on‐call radiology resident in the hyperacute phase to represent normal practice at the receiving hospital. They had no study‐specific training before the study. However, all residents had completed an educational program as part of their training which included cerebral CT evaluations in stroke patients. The residents had from 3 to 36 months experience working as a radiologist.
Statistical Methods
Data on the CT scans are presented as raw numbers. Agreement between pre‐ and in‐hospital assessments of the prehospital cerebral CT scans was calculated with Cohen's kappa for inter‐rater agreement. The kappa statistic uses values from 1 (indicating perfect agreement) through 0 (representing no agreement beyond what can be expected by chance), to –1 (indicating perfect disagreement). Inter‐rater agreement calculated by Cohen's kappa is considered a more robust measure than simple percent agreement, as it takes into account the possibility of the agreement occurring by chance.29 When interpreting results, kappa values < .2 represent negligible improvement over chance, .2‐.4 minimal agreement, .41‐.6 fair agreement, .61‐.8 good agreement, and >.81 excellent agreement.30 Robustness of the results was assessed using the nonparametric bootstrap.31 Population power analysis was not performed before the study due to the lack of literature published in this field. This study is therefore a pilot study. Time measurements had a skewed distribution, and were therefore summarized as median and quartiles.
Results
During NASPP study inclusion period, 68 patients completed a prehospital cerebral CT scan in the MSU and 51 scans were included for analysis (Fig 2). The study population had more females (59%) than men (41%) and the mean age was 68 years.
Figure 2.
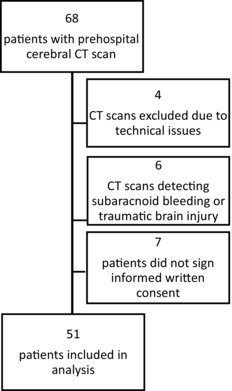
Patient flow chart.
Inter‐rater agreement on finding radiological contraindications had a kappa of .87 representing excellent agreement between the MSU anesthesiologists and the on‐call radiologists (Fig 3). The neuroradiologists and the on‐call radiologists had a good inter‐rater agreement with a kappa of .68. MSU anesthesiologists and the neuroradiologists had a kappa of .81.
Figure 3.
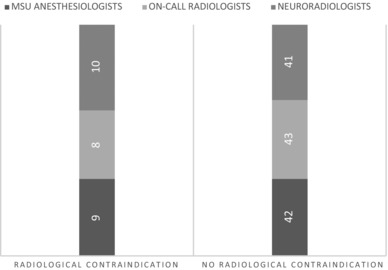
Detection of radiological contraindications for thrombolysis in prehospital cerebral CT scan assessments (n = 51). MSU = mobile stroke unit.
Classifying prehospital cerebral CT scans into four predefined diagnostic categories had a kappa value of .87 between the MSU anesthesiologists and the on‐call radiologists (Fig 4). Kappa between the on‐call radiologists and neuroradiologists was .40, and between the anesthesiologists and neuroradiologists kappa was .41.
Figure 4.
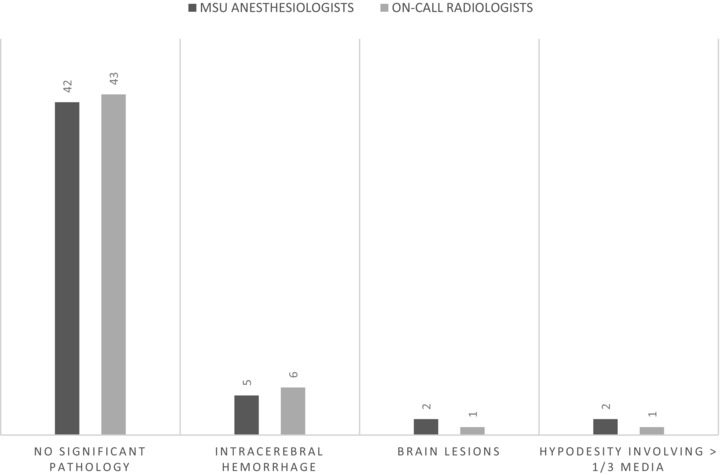
Radiological diagnosis in prehospital cerebral CT scan assessment (n = 51). MSU = mobile stroke unit.
The radiologists classified six cerebral CT scans as intercranial hemorrhage, and the anesthesiologists identified five scans with radiological signs of bleeding (Fig 3). One scan was misclassified as a brain lesion by the anesthesiologists, and identified as a hemorrhagic intracerebral hemorrhage by the radiologists and neuroradiologists. This CT scan was defined as a contraindication for thrombolysis by all the physicians. The neuroradiologists identified 10 CT scans as having radiological contraindications for thrombolysis. One of these was not found by the anesthesiologists. This patient's cerebral CT scan had a few slices with artifacts and was therefore classified as a contraindication by the neuroradiologists. The anesthesiologists decided that the patient was not eligible for thrombolysis, due to a combination of comorbidity, medication use, and age.
The median prehospital diagnostic decision time, defined as time from first patient contact to a completed prehospital cerebral CT scan (including interpretation), was 10 minutes (7 and 14 minutes). Median time from symptom onset to cerebral CT in the MSU was 1 hour and 17 minutes (59 minutes and 2 hour 20 minutes). Median transportation time to hospital was 39 minutes (31 and 48 minutes).
Discussion
The results from NASPP show that anesthesiologists trained in prehospital critical care in an MSU model can, after limited training, effectively detect radiological contraindications for thrombolysis, with high level of agreement compared to on‐call radiologists at the receiving hospital. The results suggest that advanced diagnostics of acute stroke may be carried out closer in time to symptom onset and independent of in‐hospital stroke specialists and telemedicine.
According to the American Heart Association Guidelines, cerebral CT scans may be interpreted by any specialty if the physician has acquired expertise in the field.32 The level of expertise is not defined, however, and kappa levels > .8 are recommended as the minimal accepted agreement when implementing new models in health care.33 In our study, MSU anesthesiologists and on‐call radiologists had excellent agreement (kappa .87), and the prehospital interpretation was completed in median 39 minutes before arriving at the in‐hospital CT facilities of the receiving hospital.
To set a gold standard for the interpretation, a consultant neuroradiologist assessed the same cerebral CT scans retrospectively. Kappa values between the anesthesiologists, radiologists, and neuroradiologists showed a good agreement. Similar results have been published when testing the level of agreement between in‐hospital stroke physicians and radiologists.34 Comparing classification of radiological pathology in predefined categories had fair agreement with the neuroradiologists. However, the interpretation in the hyperacute setting by anesthesiologists and radiologists compared to the retrospective evaluation by the neuroradiologists may have biased the results. The anesthesiologists were trained to be time efficient and leave detailed diagnostic evaluations for later where the neuroradiologists may have identified more subtle changes and spent more time on each scan.35 This may be different if the anesthesiologists had more training in neuroradiology of stroke,36 and should be considered when planning future studies.
The crucial time factor in acute stroke with the number needed to treat rapidly increasing after the first 60–90 minutes (“golden hour”)29 strongly suggests that optimal prehospital models may make it possible to initiate treatment within the best possible time window.37 The limited access to specialists in rural areas suggests a model run independently by the emergency medical services rather than in‐hospital neurologists and neuroradiologists via tele network interpretations.38 The NASPP MSU conducted a prehospital cerebral CT scan and interpretation in median 10 minutes, before transportation to the in‐hospital cerebral CT facilities. In rural areas, transportation time alone may limit the number of patients eligible for thrombolytic treatment.39 The NASPP MSU provides an independent diagnostic platform and may be directly implemented and run by the already existing prehospital system. A JAMA editorial from 2015 suggested that the MSU concept should evolve into a neurological emergency unit,40 and highlighted the need to serve a broader patient population. In a recent publication from NASPP, the time efficiency of prehospital cerebral CT diagnostics was illustrated by a case series of prehospital triage to neurosurgical care.41 Prehospital cerebral CT diagnostics in close relation to patient location correctly assessed by the prehospital system may open for initiation of time crucial treatment and direct triage to comprehensive centers in acute stroke as well as other neurological emergencies. This may save both time and brain in rural areas.
Study Strengths and Limitations
The patient population in NASPP was enrolled from a real‐time prehospital setting in a rural area. Patient characteristics were also representative for the stroke population in Norway,42 apart from a slightly lower mean age and higher proportion of women. The NASPP study collected data prospectively in online forms during assessment of the enrolled patients and this allowed for analysis of real‐time ratings.
This study has some limitations. First, the number of patients included is rather low. Patients included in the analysis were limited to those meeting the predefined study inclusion criteria (Table 1). The aim of this study was focused on the identification of radiological contraindications to thrombolysis in patients identified as suspected acute stroke by the EMCC. In future studies, inclusion criteria may include all patients with symptom of acute cerebrovascular disease, such as acute intense headache which may be due to a warning leak from a cerebral aneurysm. Second, 4 of the 68 patients (5% and 8%) were excluded due to lack of image quality or artifacts on their CT scans.
Conclusion
This NASPP pilot study shows for the first time that anesthesiologists trained in prehospital critical care may effectively assess prehospital cerebral CT scans to select patients for thrombolysis, in acute stroke. The NASPP MSU is a time efficient and accurate acute stroke assessment model, and may therefore be implemented as part of the prehospital system, not as an additional specialized medical unit. The results suggest that further studies should be carried out in the prehospital setting which may eventually lead to the introduction of an independent prehospital diagnostic platform.
Acknowledgments and Disclosures: The Norwegian Air Ambulance Foundation for funding this study. Ann Kristin Wiik, Head of Research Department, The Norwegian Air Ambulance Foundation for administrative support throughout the study. Professor Hans Morten Lossius, General Secretary of The Norwegian Air Ambulance Foundation for pilot work and making the Norwegian MSU a reality.
The authors have no conflict of interest.
References
- 1. Saver JL. Time is brain—quantified. Stroke 2006;37:263‐6. [DOI] [PubMed] [Google Scholar]
- 2. Monks T, Pitt M, Stein K, et al. Maximizing the population benefit from thrombolysis in acute ischemic stroke: a modeling study of in‐hospital delays. Stroke 2012;43:2706‐11. [DOI] [PubMed] [Google Scholar]
- 3. Meretoja A, Strbian D, Mustanoja S, et al. Reducing in‐hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012;79:306‐13. [DOI] [PubMed] [Google Scholar]
- 4. Kendall J, Dutta D, Brown E. Reducing delay to stroke thrombolysis—lessons learnt from the stroke 90 project. Emerg Med J 2015;32:100‐4. [DOI] [PubMed] [Google Scholar]
- 5. Wolters FJ, Paul NL, Li L, et al. Sustained impact of UK FAST‐test public education on response to stroke: a population‐based time‐series study. Int J Stroke 2015;10:1108‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishijima H, Kon T, Ueno T, et al. Effect of educational television commercial on pre‐hospital delay in patients with ischemic stroke. Neurol Sci 2016;37:105‐9. [DOI] [PubMed] [Google Scholar]
- 7. Mellon L, Hickey A, Doyle F, et al. Can a media campaign change health service use in a population with stroke symptoms? Examination of the first Irish stroke awareness campaign. Emerg Med J 2014;31:536‐40. [DOI] [PubMed] [Google Scholar]
- 8. Faiz KW, Sundseth A, Thommessen B, et al. Prehospital delay in acute stroke and TIA. Emerg Med J 2013;30:669‐74. [DOI] [PubMed] [Google Scholar]
- 9. Kim SK, Lee SY, Bae HJ, et al. Pre‐hospital notification reduced the door‐to‐needle time for iv t‐PA in acute ischaemic stroke. Eur J Neurol 2009;16:1331‐5. [DOI] [PubMed] [Google Scholar]
- 10. John S, Stock S, Cerejo R, et al. Brain imaging using mobile CT: current status and future prospects. J Neuroimaging 2015;26:5‐15. [DOI] [PubMed] [Google Scholar]
- 11. Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: a joint statement by the American Society of Neuroradiology, the American College of Radiology and the Society of Neurointerventional Surgery. J Am Coll Radiol 2013;10:828‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vachha BA, Schaefer PW. Imaging patterns and management algorithms in acute stroke: an update for the emergency radiologist. Radiol Clin North Am 2015;53:801‐26. [DOI] [PubMed] [Google Scholar]
- 13. Adeoye O, Sucharew H, Khoury J, et al. Recombinant tissue‐type plasminogen activator plus eptifibatide versus recombinant tissue‐type plasminogen activator alone in acute ischemic stroke: propensity score‐matched post hoc analysis. Stroke 2015;46:461‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minnerup J, Wersching H, Teuber A, et al. Outcome after thrombectomy and intravenous thrombolysis in patients with acute ischemic stroke: a prospective observational study. Stroke 2016;47:1584‐92. [DOI] [PubMed] [Google Scholar]
- 15. Walter S, Kostopoulos P, Haass A, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol 2012;11:397‐404. [DOI] [PubMed] [Google Scholar]
- 16. Ebinger M, Winter B, Wendt M, et al. Effect of the use of ambulance‐based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA 2014;311:1622‐31. [DOI] [PubMed] [Google Scholar]
- 17. Cerejo R, John S, Buletko AB, et al. A mobile stroke treatment unit for field triage of patients for intraarterial revascularization therapy. J Neuroimaging 2015;25:940‐5. [DOI] [PubMed] [Google Scholar]
- 18. Bowry R, Parker S, Rajan SS, et al. Benefits of stroke treatment using a mobile stroke unit compared with standard management: the BEST‐MSU study run‐in phase. Stroke 2015;46:3370‐4. [DOI] [PubMed] [Google Scholar]
- 19. Barrett KM, Pizzi MA, Kesari V, et al. Ambulance‐based assessment of NIH Stroke Scale with telemedicine: a feasibility pilot study. J Telemed Telecare 2016;23:476‐83. [DOI] [PubMed] [Google Scholar]
- 20. Lippman JM, Smith SN, McMurry TL, et al. Mobile telestroke during ambulance transport is feasible in a rural EMS setting: the iTREAT study. Telemed J E Health 2015;22:507‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liman TG, Winter B, Waldschmidt C, et al. Telestroke ambulances in prehospital stroke management: concept and pilot feasibility study. Stroke 2012;43:2086‐90. [DOI] [PubMed] [Google Scholar]
- 22. Fong WC, Ismail M, Lo JW, et al. Telephone and teleradiology‐guided thrombolysis can achieve similar outcome as thrombolysis by neurologist on‐site. J Stroke Cerebrovasc Dis 2015;24:1223‐8. [DOI] [PubMed] [Google Scholar]
- 23. Itrat A, Taqui A, Cerejo R, et al. Telemedicine in prehospital stroke evaluation and thrombolysis: taking stroke treatment to the doorstep. JAMA Neurol 2016;73:162‐8. [DOI] [PubMed] [Google Scholar]
- 24. Wu TC, Nguyen C, Ankrom C, et al. Prehospital utility of rapid stroke evaluation using in‐ambulance telemedicine: a pilot feasibility study. Stroke 2014;45:2342‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hov MR, Nome T, Zakariassen E, et al. Assessment of acute stroke cerebral CT examinations by anaesthesiologists. Acta Anaesthesiol Scand 2015;59:1179‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American College of Radiology's guidelines for Computed Tomography Dose Index (CTDI) . 2008. American College of Radiology website. Available at: https://www.acr.org/Quality-Safety/Radiology-Safety/Radiation-Safety. Accessed June 14, 2017.
- 27. Norwegian Medical Association . 2015. Norwegian index for medical emergency assistance. Available at: http://traumeplan.no/wp-content/uploads/2015/03/Norsk-indeks-for-medisinsk-n%C3%B8dhjelp.pdf. Accessed June 14, 2017.
- 28. NIH Stroke Scale Training Campus . 1998. Available at: https://secure.trainingcampus.net/uas/modules/trees/windex.aspx?rx=nihss-english.trainingcampus.net. Accessed June 14, 2017.
- 29. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307‐10. [PubMed] [Google Scholar]
- 30. Katz DJ, Wild D, Elmore JG, et al. Epidemiology, Biostatistics and Preventive Medicine. Philadelphia, PA: Saunders Elsevier; 2007:105‐19. [Google Scholar]
- 31. Efron B. An Introduction to the Bootstrap. Boca Raton, FL: Chapmall and Hall/CRC; 1993. [Google Scholar]
- 32. Adams HP Jr., del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;115:478‐534. [DOI] [PubMed] [Google Scholar]
- 33. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012;22:276‐82. [PMC free article] [PubMed] [Google Scholar]
- 34. Spokoyny I, Raman R, Ernstrom K, et al. Pooled assessment of computed tomography interpretation by vascular neurologists in the STRoKE DOC telestroke network. J Stroke Cerebrovasc Dis 2014;23:511‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demaerschalk BM, Bobrow BJ, Raman R, et al. CT interpretation in a telestroke network: agreement among a spoke radiologist, hub vascular neurologist, and hub neuroradiologist. Stroke 2012;43:3095‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wardlaw JM, Farrall AJ, Perry D, et al. Factors influencing the detection of early CT signs of cerebral ischemia: an internet‐based, international multiobserver study. Stroke 2007;38:1250‐6. [DOI] [PubMed] [Google Scholar]
- 37. von Kummer R. Effect of training in reading CT scans on patient selection for ECASS II. Neurology 1998;51:S50‐2. [DOI] [PubMed] [Google Scholar]
- 38. Audebert HJ, Saver JL, Starkman S, et al. Prehospital stroke care: new prospects for treatment and clinical research. Neurology 2013;81:501‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simonsen SA, Andresen M, Michelsen L, et al. Evaluation of pre‐hospital transport time of stroke patients to thrombolytic treatment. Scand J Trauma Resusc Emerg Med 2014;22:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warach S. Prehospital thrombolysis for stroke: an idea whose golden hour has arrived. JAMA Neurol 2015;72:9‐10. [DOI] [PubMed] [Google Scholar]
- 41. Hov MR, Ryen A, Finsnes K, et al. Pre‐hospital CT diagnosis of subarachnoid hemorrhage. Scand J Trauma Resusc Emerg Med 2017;25:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Norwegian Stroke Registry. Available at: https://www.kvalitetsregistre.no/registers/norsk-hjerneslagregister. Accessed June 10, 2017.


