Abstract
Purpose
To evaluate the in‐scan and scan–rescan consistency of left ventricular (LV) in‐ and outflow assessment from 1) 2D planimetry; 2) 4D flow magnetic resonance imaging (MRI) with retrospective valve tracking, and 3) 4D flow MRI with particle tracing.
Materials and Methods
Ten healthy volunteers (age 27 ± 3 years) underwent multislice cine short‐axis planimetry and whole‐heart 4D flow MRI on a 3T MRI scanner twice with repositioning between the scans. LV in‐ and outflow was compared from 1) 2D planimetry; 2) 4D flow MRI with retrospective valve tracking over the mitral valve (MV) and aortic valve (AV), and 3) 4D flow MRI with particle tracing through forward and backward integration of velocity data.
Results
In‐scan consistency between MV and AV flow volumes is excellent for both 4D flow MRI methods with r ≥ 0.95 (P ≤ 0.001). In‐scan AV and MV flow by retrospective valve tracking shows good to excellent correlations versus AV and MV flow by particle tracing (r ≥ 0.81, P ≤ 0.004). Scan–rescan SV assessment by 2D planimetry shows excellent reproducibility (intraclass correlation [ICC] = 0.98, P < 0.001, coefficient of variation [CV] = 7%). Scan–rescan MV and AV flow volume assessment by retrospective valve tracking shows strong reproducibility (ICCs ≥ 0.89, P ≤ 0.05, CVs = 12%), as well as by forward and backward particle tracing (ICCs ≥ 0.90, P ≤ 0.001, CVs ≤ 11%). Multicomponent particle tracing shows good scan–rescan reproducibility (ICCs ≥ 0.81, P ≤ 0.007, CVs ≤ 16%).
Conclusion
LV in‐ and outflow assessment by 2D planimetry and 4D flow MRI with retrospective valve tracking and particle tracing show good in‐scan consistency and strong scan–rescan reproducibility, which indicates that both 4D flow MRI methods are reliable and can be used clinically.
Level of Evidence: 2
Technical Efficacy Stage: 2
J. Magn. Reson. Imaging 2018;47:511–522.
Keywords: 4D flow MRI, 2D planimetry, stroke volume, inflow, outflow
Accurate assessment of left ventricular (LV) in‐ and outflow volumes is crucial for the evaluation of cardiovascular disease and the distinction between health and disease. The most commonly used approach for assessing LV dimensions and stroke volume is multislice planimetry from cine short‐axis slices. However, this approach has important limitations for direct quantitation of in‐ and outflow volumes in the presence of (multivalve) regurgitation or intracardiac shunts. In such cases, a direct flow assessment at the LV inlet and outlet will resolve these limitations.1, 2 4D flow MRI (3D cine phase‐contrast [PC] magnetic resonance imaging [MRI] with three‐directional velocity‐encoding) represents all directions and spatial regions of blood flow velocity and has emerged as a suitable technique for comprehensive visualization and quantification of blood flow volumes over the valves.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 4D flow MRI with retrospective valve tracking enables accurate direct flow volume quantification through all four valves within a single acquisition, even in patients with valve regurgitation1, 2 and allows assessment of diastolic function in the presence of aortic or pulmonary valve regurgitation.7 4D flow MRI can also be used in combination with particle tracing to evaluate the intracardiac blood flow distribution and ventricular in‐ and outflow volumes.5, 8, 9, 10, 11 This approach allows unprecedented insight into normal intracardiac physiology and the way it changes due to congenital or acquired heart disease and may eventually lead to new parameters for early detection of cardiovascular disease.9, 10 Four functional flow components can be discriminated in a multicomponent particle tracing approach, as introduced by Bolger et al.8 This approach has been used to show ventricular in‐ and outflow in the healthy LV5 and right ventricle (RV),11 as well as for identification of changed ventricular flow in patients with various cardiovascular diseases.9, 10, 12 Validation of this method and comparison with other techniques for LV volumetry is currently lacking, as particle tracing was only compared to echocardiography and 2D phase‐contrast MRI5 and it was never tested against 2D planimetry or 4D flow MRI with retrospective valve tracking. Both retrospective valve tracking as well as particle tracing have shown good accuracy in LV in‐ and outflow assessment and low intra‐ and interobserver variation from repeated analysis.1, 2, 5 However, scan–rescan reproducibility of LV in‐ and outflow assessment by valve tracking and particle tracing remains unknown. Scan–rescan reproducibility is important in the clinical workflow since patients often undergo several MRI examinations during their life as follow‐up. Furthermore, scan–rescan reproducibility is essential to differentiate an abnormal stress response with prognostic importance from a normal response during a stress MRI‐examination.13 Therefore, the purpose of this study was to compare LV in‐ and outflow from 1) 2D planimetry, 2) 4D flow MRI with retrospective valve tracking, and 3) 4D flow MRI with particle tracing and to assess the scan–rescan reproducibility of these three methods.
Materials and Methods
Study Population
The study protocol was approved by the Medical Ethical Committee of the Leiden University Medical Center and informed consent was obtained from all participants. Ten healthy volunteers with no history of cardiac disease were included. All subjects underwent an MRI scan including whole‐heart 4D flow MRI between July 2015 and March 2016. The same scanning protocol was performed twice in the same session with a 10‐minute break between the scans and repositioning and replanning for every volunteer.
MRI Acquisition
Whole‐heart 4D flow MRI was obtained on a 3T MRI scanner (Ingenia, Philips Medical Systems, with Software Stream 4.1.3.0, Best, Netherlands) with maximal amplitude of 45 mT/m for each axis, slew rate of 200 T/m/sec, and a combination of FlexCoverage Posterior coil in the table top with a dStream Torso coil, providing up to 32 coil elements for signal reception. The orientation of the acquisition of 4D flow data was identical to the four‐chamber orientation (usually double‐oblique axial or coronal). Velocity‐encoding of 150 cm/s in all three directions was used in a standard four‐point encoding scheme, spatial resolution 3.0 × 3.0 × 3.0 mm3, flip angle 10°, echo time (TE) 3.7 msec, repetition time (TR) 10 msec, true temporal resolution 40 msec, sensitivity encoding (SENSE) factor 2 in the anterior–posterior direction and echo planar imaging (EPI) readout with a factor 5 for acceleration. No contrast agent was used. Concomitant gradient correction and local phase correction were performed from standard available scanner software and the heart was scanned in the isocenter of the magnet to minimize phase offset. For the 4D flow MRI acquisition, whole‐body specific absorption rate (SAR) was <0.3 W/kg and specific energy dose (SED) was <0.2 kJ/kg. Cine 2D left two‐chamber, four‐chamber, coronal, and sagittal aorta views and a cine multi‐2D short‐axis stack of slices were acquired, using steady‐state free‐precession sequences with TE/TR 1.5/3.0, 350 mm field‐of‐view, 45° flip angle, acquisition resolution 1.9 × 2.0 × 8.0 mm3. Retrospective gating was used with 30 phases reconstructed to represent one cardiac cycle. Free breathing was allowed without using motion suppression, three signal averages were taken to minimize effects of breathing motion. For the 2D cine acquisition, whole‐body SAR was <2.6 W/kg and SED was <0.5 kJ/kg.
MRI Analysis
Image analysis was performed by one observer with 2 years of experience in MRI (V.P.K.) and verified by one observer with over 15 years of experience in MRI (J.J.M.W.). Ventricular volume segmentation was done based on multislice 2D cine short‐axis images using in‐house developed MASS software (Leiden University Medical Center, Leiden, the Netherlands). The endocardial border was manually traced in all slices and phases and ventricular volume was calculated at the end‐diastolic (ED) and end‐systolic (ES) phases. Papillary muscles were disregarded and assumed to be included in the ventricular volume. SV was calculated as left ventricular end‐diastolic volume (LVEDV) – left ventricular end‐systolic volume (LVESV).
Phase wrapping artifacts in the 4D flow data were corrected in the source images.14 Retrospective valve tracking over the mitral valve (MV) and aortic valve (AV) was done using in‐house developed MASS software (Leiden University Medical Center) following previously published methods.1, 2 In short, streamlines of LV in‐ and outflow were visualized and multiplanar reformatting planes (MPR) were obtained perpendicular to these streamlines in all phases at the location of peak flow velocity. Through‐plane velocity‐encoded MPR images for all phases were reconstructed (typically in sets of five parallel slices with 5 mm interslice distance) on which borders of the MV or AV and myocardial wall (for through‐plane motion and velocity offset error correction, not necessarily in the same slice as the transvalvular flow velocity) were manually segmented. Correct positioning of the MV and AV and background contours were verified using the resulting velocity‐time curves. LV in‐ and outflow per heart beat were obtained as area under the curve from resulting flow rate‐time curves. For internal validation, the net LV inflow volume per heartbeat measured over the MV (MV flow) was compared with the net LV outflow volume per heartbeat measured over the AV (AV flow). Cardiac output (CO) was computed from the 4D flow data as LV outflow volume per heartbeat × heart rate (HR).
For segmenting the LV cavity in the 4D flow MRI data, the available segmentation of the 2D cine short‐axis acquisition was used. To correct for patient motion‐related misalignment between the two acquisitions, automated image‐based 3D rigid registration was performed using the phase with optimal depiction of the LV cavity in both scans with the Elastix image registration toolbox.15 In addition to the concomitant gradient correction as provided by the scanner software, prior to particle tracing the residual velocity offset errors were further minimized by subtracting the median velocity within the myocardial region at the moment of end‐systole for all voxels and at every time phase. The 4D velocity data was then used for the particle tracing algorithm, using fourth‐order Runge–Kutta numerical integration with a time‐step of one fifth of the actual temporal resolution (∼8 msec) to create pathlines. At end‐diastole, each voxel inside the LV was considered to represent one seed point (ie, one particle). Pathlines of the particles were calculated by integration of velocity data over time: backward tracing over the diastole and forward tracing over systole. In‐ and outflow was allowed over the MV and AV only; particles exiting the LV other than into the aorta (ie, aortic outflow) or the left atrium (ie, mitral regurgitation) were calculated and excluded from analysis. Using particle tracing, LV in‐ and outflow volumes (ie, flow volume over MV and AV, respectively) can be calculated in two ways. The first method is by performing a direct calculation by forward tracing to calculate AV flow and by backward tracing to calculate MV flow. The second method is by using the multicomponent particle tracing evaluation (ie, discriminating direct flow, delayed ejection flow, retained inflow, and residual volume) as introduced by Bolger et al8 and then summing direct flow and delayed ejection flow components to calculate the AV flow volume and summing direct flow and retained inflow components to calculate MV flow.
In‐Scan Comparison of MRI Methods
To evaluate in‐scan agreement (ie, measurements within one scan), SV by short‐axis 2D planimetry was compared to AV flow assessment from retrospective valve tracking and from particle tracing analysis. Furthermore, to evaluate agreement between the two 4D flow MRI methods, MV and AV flow values from retrospective valve tracking were compared to MV and AV flow values from particle tracing analysis. Figure 1 shows the three methods for LV in‐ and outflow assessment that were used.
Figure 1.
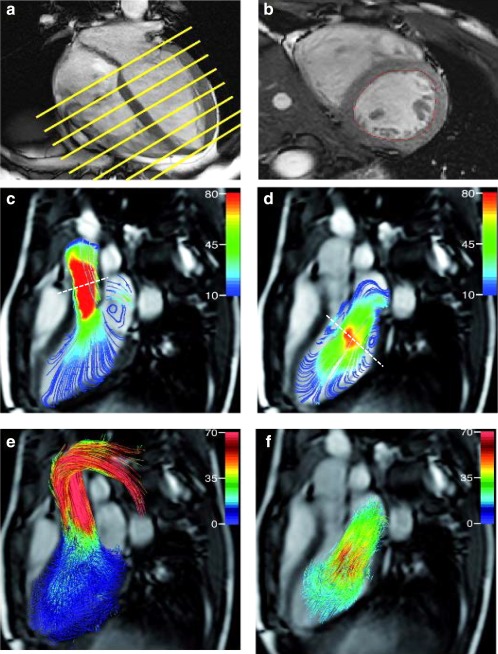
Three methods illustrating the assessment of LV in‐ and outflow volumes. Short‐axis planimetry (in a, planning for multislice 2D short‐axis is illustrated on four‐chamber view and in b, mid‐ventricular short‐axis slice is presented). Streamline representation of 4D flow MRI shows mid‐systolic aortic outflow (c) and early diastolic mitral (d) inflow volume with the positioning of the retrospective valve tracking indicated by a dashed line. (e) Outflow assessment over the aortic valve by forward particle tracing (e) and inflow assessment over the mitral valve by backward particle tracing (f).
Scan–Rescan Analysis
For the scan–rescan analysis, all data were presented blinded to the observer and all scans were analyzed in a random order. Scan–rescan analysis was performed to test the reproducibility of 1) SV assessment by 2D planimetry; 2) MV and AV flow volume assessment by 4D flow MRI with retrospective valve tracking; and 3) MV and AV flow volume assessment by 4D flow MRI with backward and forward particle tracing only and multicomponent particle tracing.
Statistical Analysis
Data analysis was performed using SPSS Statistics software (v. 23.0, IBM SPSS, Chicago, IL). Variables were tested for normal distribution using the Shapiro–Wilk test. Continuous data are expressed as mean ± standard deviation (SD) or as median with interquartile range (IQR), in case of non‐normality of the data. Mean differences were determined for in‐scan and scan–rescan comparison and for comparison of different methods. Significance was tested by a paired samples t‐test or, in case of non‐normality, the Wilcoxon signed‐rank test. Correlation between two methods or measurements done in repeated scans was tested by the Pearson correlation coefficient (r), or the Spearman correlation coefficient (ρ) in case of non‐normality of the data. The approach described by Bland and Altman16 was used to study systematic differences between the two scans. To detect a possible trend in bias in the Bland–Altman plots, linear regression was performed for the differences versus the means. Agreement between scans was assessed by determining the intraclass correlation (ICC) coefficient and coefficient of variation (CV). The CV was defined as the SD of the differences between two series of measurements divided by the mean of both measurements. Correlation and agreement were classified as follows for both r and ICC: >0.95: excellent, 0.95–0.85: strong, 0.85–0.70: good, 0.70–0.5: moderate, <0.5: poor. P < 0.05 was considered statistically significant.
Results
Volunteer characteristics are shown in Table 1. Fifty percent of the study group were male (5/10) and the mean age was 27 ± 3 years. HR was marginally significantly different between the two scans (scan 1: 62.3 ± 7.3; scan 2: 59.8 ± 7.6 bpm, P = 0.047). CO was not significantly different between the two scans (scan 1: 5.7 ± 0.8; scan 2: 5.7 ± 1.4 l/min, P = 0.88). 4D flow MRI data acquisition was successful in all volunteers. Mean acquisition time of the whole‐heart 4D flow MRI scan was 9.6 ± 1.4 minutes in scan 1 and 9.5 ± 1.7 minutes in scan 2. In two cases phase wrapping occurred. This was mathematically unwrapped in the source images of the respective velocity directions which presented the phase wrap.
Table 1.
Characteristics of the Volunteers
| Total population | ||||
|---|---|---|---|---|
| N | 10 | |||
| Male % | 50 (5) | |||
| Age (years) | 27 ± 3 | |||
| Height (cm) | 176 ± 7 | |||
| Weight (kg) | 69 ± 13 | |||
| BSA (m2) | 1.8 ± 0.2 | |||
| BMI (kg/m2) | 22 ± 3 |
| Scan 1 | Scan 2 | P‐value | ||
|---|---|---|---|---|
| HR (bpm) | 62.3 ± 7.3 | 59.8 ± 7.6 | 0.047 | |
| CO (l/min) | 5.7 ± 0.8 | 5.7 ± 1.4 | 0.88 |
Data are presented as mean ± standard deviation. BMI = body mass index, BSA = body surface area, HR = heart rate, bpm = beats per minute.
In‐Scan Comparison of MV Versus AV Flow for Both 4D Flow MRI Methods
Table 2 shows the in‐scan comparison of flow volume assessment through the MV and AV by retrospective valve tracking and by particle tracing. When using retrospective valve tracking, both scans show excellent correlation between MV and AV flow volumes (scan 1: r = 0.95, P < 0.001; scan 2: r = 0.98, P < 0.001). The mean difference between MV and AV flow was –2.7 ± 5.5 mL (P = 0.15) in scan 1 and –2.3 ± 5.7 mL (P = 0.23) in scan 2. The ICC was excellent (scan 1: 0.97, P < 0.001; scan 2: 0.98, P < 0.001) and the CV of MV and AV flow assessment by retrospective valve tracking was small in both scans (6%). Also, when using particle tracing analysis to determine LV in‐ and outflow, excellent in‐scan correlation between MV versus AV flow volumes was observed in both scans (scan 1: r = 0.95, P < 0.001; scan 2: r = 0.99, P < 0.001). The mean difference between MV and AV flow in scan 1 was –0.04 ± 11.3 mL (P = 0.99) and –5.9 ± 6.8 mL (P = 0.02) in scan 2. The ICC was strong (scan 1: 0.92, P = 0.001; scan 2: 0.93, P < 0.001). The CV of MV and AV flow assessment by particle tracing shows more variation (8–12%) than assessment by retrospective valve tracking.
Table 2.
Internal Consistency of Assessment of Flow Volumes Through the Mitral (MV) and Aortic Valve (AV) By Retrospective Valve Tracking and Particle Tracing Analysis
| MV flow (mL) | AV flow (mL) | Difference (AV‐MV) (mL)a | Pearson correlation coefficient | Intraclass correlation coefficient | ||
|---|---|---|---|---|---|---|
| Mean ± SD (min‐max) | Mean ± SD (min‐max) | Mean ± SD (min‐max) | ICC (95% C.I.) | Coefficient of variation (%) | ||
| Retrospective valve tracking scan 1 | 95.4 ± 17.0 (71.4‐121.2) | 92.7 ± 15.5 (68.4‐112.9) | −2.7 ± 5.5 (−11.2−7.1) | 0.95b | 0.97 (0.87‐0.99)b | 6 |
| Retrospective valve tracking scan 2 | 97.6 ± 24.0 (62.3‐135.0) | 95.3 ± 20.9 (66.0‐126.4) | ‐2.3 ± 5.7 (‐11.7‐6.8) | 0.98b | 0.98 (0.93‐1.00)b | 6 |
| Particle tracing scan 1 | 91.7 ± 23.6 (57.5‐133.1) | 91.6 ± 14.0 (71.5‐112.1) | ‐0.04 ± 11.3 (‐22.7‐14.0) | 0.95b | 0.92 (0.65‐0.98)c | 12 |
| Particle tracing scan 2 | 94.1 ± 20.0 (63.3‐121.8) | 88.1 ± 13.6 (68.2‐105.2) | ‐5.9 ± 6.8 (‐16.6‐5.0)c | 0.99b | 0.93 (0.56‐0.99)b | 8 |
Differences were calculated with the paired samples t‐test.
P < 0.001.
P < 0.05.
AV = aortic valve, MV = mitral valve.
In‐Scan Comparison of MV and AV Flow Between MRI Methods
Figure 2 shows the in‐scan comparison between SV assessment by short‐axis 2D planimetry versus AV flow assessment by retrospective valve tracking (Fig. 2a) and SV assessment by short‐axis 2D planimetry versus AV flow assessment by particle tracing analysis (Fig. 2b). AV flow assessment by retrospective valve tracking showed strong to excellent correlation with 2D planimetry (scan 1: r = 0.88, P = 0.001; scan 2: r = 0.95, P < 0.001). AV flow assessment by particle tracing showed excellent correlation with 2D planimetry (scan 1: r = 0.95, P < 0.001; scan 2: r = 0.95, P < 0.001). The Bland–Altman plots, also shown in Fig. 2, display small limits of agreements. However, SV by 2D planimetry versus AV flow assessment by retrospective valve tracking showed a significant trend in scan 1 (r = –0.64 P = 0.05), but not in scan 2 (r = –0.17 P = 0.64), as shown in Fig. 2a. This trend in scan 1 means that the differences between 2D planimetry and retrospective valve tracking increase with an increasing mean from both methods. A significant trend was also present in the measurement of SV by 2D planimetry versus AV flow assessment by particle tracing in both scans. The linear regression lines are plotted in Fig. 2b (scan 1: r = –0.85, P = 0.002; scan 2: r = –0.84 P = 0.002). This trend in both scans means that the differences between 2D planimetry and particle tracing increase with an increasing mean from both methods.
Figure 2.
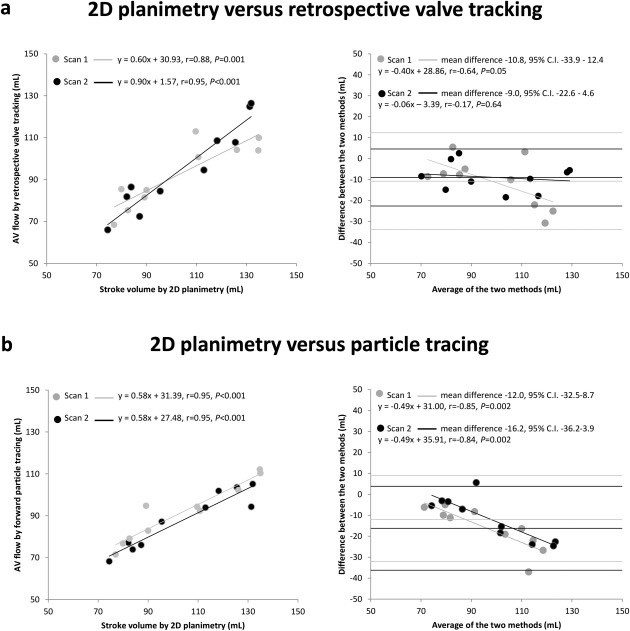
Scatterplots and Bland–Altman plots for comparison of left ventricular in‐ and outflow by 2D planimetry and 4D flow MRI with retrospective valve tracking and particle tracing (a) Left: scatterplot depicting the correlation between SV measured by 2D planimetry versus AV flow measured by retrospective valve tracking in scan 1 and scan 2; right: Bland–Altman plot depicting the agreement between SV measured by 2D planimetry versus AV flow measured by retrospective valve tracking in scan 1 and scan 2. (b) Left: scatterplot depicting the correlation between SV measured by 2D planimetry versus AV flow measured by forward particle tracing in scan 1 and scan 2; right: Bland–Altman plot depicting the agreement between SV measured by 2D planimetry versus AV flow measured by forward particle tracing in scan 1 and scan 2. The linear regression lines are plotted in the Bland–Altman plots.
Table 3 shows the in‐scan comparison of MV flow assessment by retrospective valve tracking versus MV flow assessment by particle tracing and the in‐scan comparison of AV flow assessment by retrospective valve tracking versus AV flow assessment by particle tracing. MV flow from retrospective valve tracking versus MV flow from particle tracing shows strong correlation in both scans (scan 1: r = 0.95, P < 0.001; scan 2: r = 0.88, P = 0.001). There was no significant difference between MV flow values from retrospective valve tracking and MV flow values from particle tracing (scan 1: 3.7 ± 9.1 mL, P = 0.23; scan 2: 3.6 ± 11.5 mL, P = 0.35). Also, AV flow from retrospective valve tracking versus AV flow from particle tracing shows good correlation in both scans (scan 1: r = 0.81, P = 0.004; scan 2: r = 0.87, P = 0.001) and no significant difference between AV flow values from retrospective valve tracking and AV flow values from particle tracing (scan 1: 1.1 ± 9.2 mL, P = 0.72; scan 2: and 7.2 ± 11.2 mL, P = 0.07).
Table 3.
Comparison Between Retrospective Valve Tracking and Particle Tracing for MV and AV Flow Quantification
| Statistics | MV retrospective valve tracking versus MV particle tracing | AV retrospective valve tracking versus AV particle tracing |
|---|---|---|
| Scan 1 | ||
| Mean difference (mL) | 3.7 ± 9.1 | 1.1 ± 9.2 |
| P‐value* | 0.23 | 0.72 |
| Pearson correlation coefficient | 0.95 | 0.81 |
| P‐value | <0.001 | 0.004 |
| Scan 2 | ||
| Mean difference (mL) | 3.6 ± 11.5 | 7.2 ± 11.2 |
| P‐value* | 0.35 | 0.07 |
| Pearson correlation coefficient | 0.88 | 0.87 |
| P‐value | 0.001 | 0.001 |
| *P‐values were calculated with the paired samples t‐test | ||
AV = aortic valve, MV = mitral valve.
Scan–Rescan Analysis of MRI Methods
Results of the scan–rescan analysis for flow assessment by all three methods (2D planimetry, 4D flow MRI with retrospective valve tracking, and particle tracing) are presented in Table 4. Scan–rescan assessment of SV by 2D planimetry showed excellent correlation between both scans (r = 0.95, P < 0.001) with an excellent ICC (0.98, P < 0.001) and low CV (7%), indicating excellent reproducibility. Also, scan–rescan assessment of MV and AV flow volumes by retrospective valve tracking from 4D flow MRI showed good reproducibility with good to strong Pearson correlation coefficients (MV: r = 0.90, P < 0.001; AV: r = 0.83, P = 0.003) and a strong ICC (MV: 0.92, P < 0.001; AV: 0.89, P = 0.002). CVs for both MV and AV flow assessment by retrospective valve tracking are low (MV: 12%; AV: 12%). MV and AV flow assessment by particle tracing from backward (MV) and forward (AV) particle tracing only shows good to strong correlation between both scans (MV: r = 0.89, P = 0.001; AV: r = 0.83, P = 0.003), nonsignificant differences (MV: 2.4 ± 10.7 mL, P = 0.50; AV: –3.5 ± 8.2 mL, P = 0.21), and strong ICCs (MV: 0.94, P < 0.001; AV: 0.90, P = 0.001). CVs for both MV and AV flow assessment by backward and forward particle tracing only are low (MV: 11%; AV: 9%). MV and AV flow assessment by particle tracing using the multicomponent analysis shows good correlation between the values from both scans (MV: r = 0.76, P = 0.01; AV: r = 0.72, P = 0.02), a nonsignificant difference (MV: 0.8 ± 13.6 mL; AV:, –5.8 ± 10.7 mL) and good to strong ICCs (MV: 0.88, P = 0.003; AV: 0.81, P = 0.007). CVs for both MV and AV flow assessment by multicomponent particle tracing are acceptable (MV: 16%; AV: 12%). Scatterplots and Bland–Altman plots for flow assessment by 2D planimetry and 4D flow MRI with retrospective valve tracking and particle tracing are shown in Fig. 3. No trend is present in the Bland–Altman plot for the assessment of SV in scan 1 versus SV in scan 2 (r = –0.10, P = 0.80), as shown in Fig. 3a. The Bland–Altman plot for the assessment of AV and MV flow from retrospective valve tracking (Fig. 3b) shows a significant trend for the scan–rescan assessment of MV flow, but not for AV flow (MV: r = 0.63, P = 0.05; AV: r = 0.48, P = 0.16). This trend in MV flow assessment by retrospective valve tracking means that the differences between MV flow assessment by retrospective valve tracking in scan 1 and scan 2 increase with an increasing mean from both scans. The Bland–Altman plot for the assessment of AV and MV flow from particle tracing (Fig. 3c) shows no significant trend for the scan–rescan assessment of MV and AV flow (MV: r = –0.34, P = 0.34; AV: r = –0.05, P = 0.89).
Table 4.
Scan‐Rescan Comparison of 2D Stroke Volume (SV) and Flow Through the Mitral (MV) and Aortic Valve (AV)
| Scan 1 | Scan 2 | Difference (mL)a | Pearson correlation coefficient | Intraclass correlation coefficient | Coefficient of variation (%) | |
|---|---|---|---|---|---|---|
| Mean ± SD (min ‐max) | Mean ± SD (min ‐max) | Mean ± SD (min‐max) | ICC (95% C.I.) | |||
| 2D planimetry | ||||||
| SV (mL) | 103.5 ± 22.8 (77.0‐135) | 104.3 ± 22.1 (74.4‐131.9) | 0.8 ± 7.0 (‐9.2‐12.9) | 0.95b | 0.98 (0.91‐0.99)b | 7 |
| Retrospective valve tracking | ||||||
| MV flow (mL) | 95.4 ± 17.0 (71.4‐121.2) | 97.6 ± 24.0 (62.3‐135.0) | 2.2 ± 11.3 (‐16.7‐17.6) | 0.90b | 0.92 (0.70‐0.98)b | 12 |
| AV flow (mL) | 92.7 ± 15.5 (68.4‐112.9) | 95.3 ± 20.9 (66.0‐95.3) | 2.6 ± 11.7 (‐18.4‐20.7) | 0.83c | 0.89 (0.58‐0.97)c | 12 |
| Backward and forward particle tracing for LV in‐ and outflow | ||||||
| MV flow (Backward only) (mL) | 91.7 ± 23.6 (57.5‐133.1) | 94.1 ± 20.0 (63.3‐121.8) | 2.4 ± 10.7 (‐11.3‐18.2) | 0.89c | 0.94 (0.77‐0.99)b | 11 |
| AV flow (Forward only) (mL) | 91.6 ± 14.0 (71.5‐112.1) | 88.1 ± 13.6 (68.2‐105.2) | ‐3.5 ± 8.2 (‐18.7‐9.5) | 0.83c | 0.90 (0.61‐0.97)c | 9 |
| Multicomponent particle tracing for LV in‐ and outflow | ||||||
| MV flow (Direct flow + retained inflow) (mL) | 82.9 ± 20.1 (56.0‐113.5) | 83.7 ± 19.2 (57.1‐109.6) | 0.8 ± 13.6 (‐12.7‐26.5) | 0.76c | 0.88 (0.48‐0.97)c | 16 |
| AV flow (Direct flow + delayed ejection flow) (mL) | 89.9 ± 13.8 (71.2‐109.5) | 84.1 ± 14.5 (59.2‐103.3) | ‐5.8 ± 10.7 (‐22.7‐13.3) | 0.72c | 0.81 (0.29‐0.95)c | 12 |
Differences were calculated with the paired samples t‐test.
P < 0.001.
P < 0.05.
AV = aortic valve, MV = mitral valve, SV = stroke volume.
Figure 3.
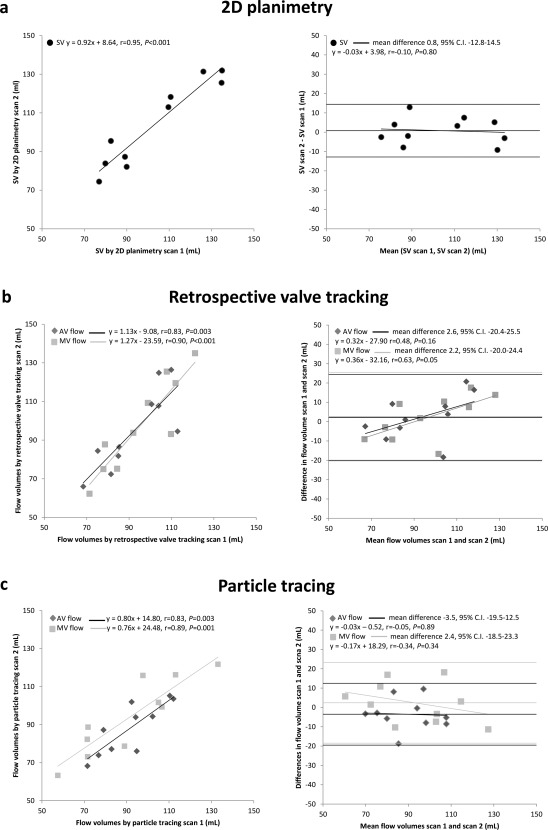
Scatterplots and Bland–Altman plots for comparison of left ventricular in‐ and outflow by 2D planimetry and 4D flow MRI with retrospective valve tracking and 4D flow MRI with backward and forward particle tracing. (a) Left: scatterplot depicting the correlation between SV measured by 2D planimetry in scan 1 and scan 2; right: Bland–Altman plot depicting the agreement between SV measured by 2D planimetry in scan 1 and scan 2. (b) Left: scatterplot depicting the correlation between MV flow measured by retrospective valve tracking with 4D flow MRI in scan 1 and scan 2 and the correlation between AV flow measured by retrospective valve tracking with 4D flow MRI in scan 1 and scan 2; right: Bland–Altman plot depicting the agreement between MV flow measured by retrospective valve tracking with 4D flow MRI in scan 1 and scan 2 and the agreement between AV flow measured by retrospective valve tracking with 4D flow MRI in scan 1 and scan 2. (c) Left: scatterplot depicting the correlation between MV flow measured by 4D flow MRI with backward particle tracing in scan 1 and scan 2 and the correlation between AV flow measured by 4D flow MRI with backward particle tracing in scan 1 and scan 2; right: Bland–Altman plot depicting the agreement between MV flow measured by 4D flow MRI with backward particle tracing in scan 1 and scan 2 and the agreement between AV flow measured by 4D flow MRI with backward particle tracing in scan 1 and scan 2. The linear regression lines are plotted in the Bland–Altman plots.
Percentages of the multicomponent particle tracing analysis are: 40.1 [36.6–41.9]% direct flow, 20.4 ± 4.5% delayed ejection flow, 14.8 ± 4.3% retained inflow and 24.1 ± 4.6% residual volume in scan 1 and 40.4 ± 5.2% direct flow, 18.4 ± 3.5% delayed ejection flow, 17.6 ± 4.1% retained inflow and 23.6 ± 3.3% residual volume in scan 2. In scan 1, 13 ± 5% of the particles was excluded, as this portion of the total particles entered or exited the LV other than through the MV or AV, and in scan 2, 15 ± 5% of the particles was excluded. Scan–rescan analysis of the four components of 4D flow MRI with multicomponent particle tracing analysis shows nonsignificant mean differences between the components of less than 3% (–0.1 [–5.9–5.7]% (P = 0.80) for direct flow, –2.0 ± 5.8% (P = 0.30) for delayed ejection flow, 2.8 ± 4.3% (P = 0.07) for retained inflow and –0.5 ± 4.8% (P = 0.75) for residual volume).
Discussion
4D flow MRI allows unprecedented assessment of intracardiac flow volumes and provides new insights into normal physiology and the way this is altered by congenital or acquired heart disease. Furthermore, 4D flow MRI allows improved quantification of LV in‐ and outflow1, 2, 3, 5 and may provide new parameters so assess cardiac dysfunction.9, 10
In the current study, LV in‐ and outflow was assessed by 4D flow MRI with retrospective valve tracking and by particle tracing and both compared to 2D planimetry. Scan–rescan reproducibility of all these methods was tested. The main findings of the study are: 1) In‐scan consistency between flow volumes over the MV and the AV is excellent for both 4D flow MRI methods; 2) In‐scan AV flow assessment by both retrospective valve tracking and particle tracing correlates strongly with 2D planimetry SV assessment; 3) In‐scan comparison between MV and AV flow assessed by retrospective valve tracking and MV and AV flow assessed by particle tracing shows good to strong correlations with no significant differences; 4) Scan–rescan reproducibility of SV assessment by 2D planimetry is excellent and reproducibility of MV and AV flow by both retrospective valve tracking and particle tracing analysis is good to strong; however, retrospective valve tracking and particle tracing analysis show a higher coefficient of variation than 2D planimetry.
Excellent reproducibility of SV assessment by 2D planimetry has already been shown in an earlier study by Grothues et al.17 The current study, with an updated scan protocol, shows similar results with respect to scan–rescan reproducibility of SV assessment by 2D planimetry. However, in the case of multiple valve lesions or intracardiac shunting, SV assessment from 2D planimetry can be insufficient. In such cases 4D flow MRI with retrospective valve tracking will be beneficial, as it will provide a direct assessment of flow at the inlet and outlet of the LV (MV and AV) and valve regurgitation can be directly quantified.1, 2 Currently, the most common clinical MRI flow assessment technique is 2D cine PC MRI with a static imaging plane and velocity encoding in a single (ie, through‐plane) direction.18 However, several studies have already shown that 4D flow MRI with retrospective valve tracking is more accurate than 2D cine PC MRI for transvalvular flow volume assessment.1, 3, 6 4D flow MRI has multiple advantages over 2D cine PC MRI. First of all, 4D flow MRI represents all directions and spatial regions of flow.19 Second, when using 4D flow MRI with retrospective valve tracking, positioning of measurement planes can be changed dynamically in every time frame and adjusted to the orientation of the flow direction, while with 2D cine PC MRI, the measurement plane cannot adapt to the motion of the heart and the flow direction during the cardiac cycle.1, 2, 4, 6 Furthermore, with 4D flow MRI multiple measurement planes can be defined from a single acquisition, while 2D cine PC MRI requires a repeated acquisition when assessing flow over multiple valves, which increases the chance of inconsistencies because of heart rate variation between the acquisitions. Also, planning of a 3D volume acquisition is more straightforward than planning 2D acquisition planes. A further clinical benefit of 4D flow MRI with retrospective valve tracking is that diastolic function can be assessed, especially in the presence of aortic or pulmonary valve regurgitation leading to two sources of diastolic ventricular inflow.7
Roes et al1 showed excellent intra‐ and interobserver agreement for flow volume measurements by retrospective valve tracking over all four valves. The current study confirms the excellent in‐scan consistency for MV and AV flow. Also, in the current study AV flow assessment by 4D flow MRI with retrospective valve tracking is compared to SV assessment using 2D planimetry, which shows strong agreement. Furthermore, our study extends these findings by demonstrating excellent scan–rescan reproducibility with good to strong correlations (MV flow r = 0.90, P < 0.001, AV flow r = 0.83, P = 0.003) and strong ICCs. The CV is small, but shows more variation than SV assessment by 2D planimetry (CV 12% vs. 7%). These results indicate that 4D flow MRI with retrospective valve tracking is a reliable method that can be used clinically to assess flow volumes, also over repeated examinations.
LV in‐ and outflow can also be assessed by using particle tracking derived from 4D flow MRI. Bolger et al8 introduced the use of multicomponent particle tracing to quantify four different functional components of blood flow distribution in the left ventricle (direct flow, retained inflow, delayed ejection, residual volume). This comprehensive assessment of intracardiac flow provided unique insights into normal intracardiac flow and how this is affected by congenital and acquired heart disease. Although the clinical usefulness needs to be established, potentially new parameters for early detection of cardiac dysfunction can be derived from this approach.9, 10 Furthermore, several studies5, 8 have used particle tracing in the evaluation of LV in‐ and outflow. However, a validation of in‐scan and scan–rescan consistency and reproducibility and comparison with other techniques for LV volumetry is currently lacking. The current study presents this necessary validation, which is necessary for a full interpretation of quantified differences in LV stroke volume in a follow‐up study or in a rest‐stress evaluation. Eriksson et al5 introduced a semiautomated analysis approach based on the method by Bolger et al8 in order to reduce user‐dependency and enhance reproducibility. They have shown that multicomponent particle tracing can be used to evaluate ventricular flow patterns in healthy subjects as well as patients with dilated cardiomyopathy.10 In their study, in‐scan comparison of MV flow (direct flow + retained inflow) and AV flow (direct flow + delayed ejection flow) assessed with particle tracing did not show significant differences.5 Furthermore, low intra‐ and interobserver variability was shown for the assessment of the flow volumes by particle tracing.5 A recent 3T MRI study by Calkoen et al,9 using multicomponent particle tracing analysis to assess transvalvular flow in 32 patients with corrected atrioventricular septal defect, showed a statistically significant in‐scan difference between MV and AV flow volumes assessed with multicomponent particle tracing. In the current study, MV flow and AV flow were not only calculated from the multicomponent analysis over the full cardiac cycle, as was done in the previous studies. Here, also forward particle tracing over systole for AV flow and backward particle tracing over diastole for MV flow were performed. Using this last approach, in‐scan as well as scan–rescan consistency was strong. The possible explanation for this may be that for multicomponent particle tracing, numerical integration over the full cardiac cycle is required, while forward and backward particle tracing only requires integration over systole and diastole, respectively. Adding more time steps in the integration procedure will accumulate more error over the full cardiac cycle.
Based on the Bland–Altman analyses, some significant trends in bias were seen when the differences between measurements were correlated with the mean of the measurements. This should be taken into account when comparing the different methods for SV assessment. However, in the evaluation of serial scans (during follow‐up or in case of a rest‐stress protocol), generally one specific method will be used to detect changes in SV. In that perspective, CV and ICC are of more importance for reproducibility of the method of choice.
A limitation of the current study is the low number of samples (ie, 10 volunteers). Nevertheless, our findings show good in‐scan and scan–rescan reproducibility of both 4D flow MRI‐derived measurements of LV in‐ and outflow. Additionally, no patient data were used in this study, as it would be unethical to repeat the long 4D flow MRI acquisition in a scan/rescan study in a routine clinical evaluation of patients. Still, scan–rescan analysis of transvalvular flow assessment should also be tested in the presence of valve lesions, shunt flow, and decreased cardiac function. Another important limitation is the lack of a gold standard. We compared LV outflow to 2D planimetry. This method is currently considered the reference method for LV volume assessment when image analysis is performed in a standardized manner.20 However, planimetry from multislice short‐axis acquisitions is also subject to variability, as images are acquired using multiple breath‐holds. In‐scan consistency, however, which was used in the current study to compare flow volumes through MV and AV, may be considered as an internal reference standard. 3D cine data might perform better than multislice 2D cine. However, this is a novel approach that is not available on all MRI platforms, and was not available for our study. Also, image segmentation for such approach also requires a 3D algorithm instead of the current algorithm that was used for 2D planimetry. Such a 3D approach first requires validation in order to use it in a comparison study, as was presented here. Another limitation is the fact that retrospective valve tracking is semiautomatic, as in previous studies, since manual interaction was required to position measurement planes. However, retrospective valve tracking can be performed with low intra‐ and interobserver variation, as has been previously published.1, 2 Compared to 2D cine PC MRI with velocity encoding in a single direction, the adjustment of measurements planes requires more processing time (1–2 min per valve). Therefore, an automatic valve tracking procedure could further improve reproducibility and reduce analysis time.
In conclusion, SV assessment by 2D planimetry, and MV and AV flow assessment by 4D flow MRI with retrospective valve tracking and particle tracing, show strong to excellent in‐scan consistency and good to strong scan–rescan reproducibility and correlation, which indicates that 4D flow MRI with retrospective valve tracking and 4D flow MRI with particle tracing analysis are reliable methods to assess LV in‐ and outflow to be used clinically.
Acknowledgment
Contract grant sponsor: Dutch Heart Foundation; contract grant number: 2013T091 and 2014T087 (to V.P.K. and R.L.F.v.d.P., respectively); Contract grant sponsor: ZonMw; contract grant number: 104003001 (to J.J.M.W.)
The authors thank Pieter J. van den Boogaard, BSc for help in providing the MRI data.
References
- 1. Roes SD, Hammer S, van der Geest RJ, et al. Flow assessment through four heart valves simultaneously using 3‐dimensional 3‐directional velocity‐encoded magnetic resonance imaging with retrospective valve tracking in healthy volunteers and patients with valvular regurgitation. Invest Radiol 2009;44:669–675. [DOI] [PubMed] [Google Scholar]
- 2. Westenberg JJ, Roes SD, Ajmone Marsan N, et al. Mitral valve and tricuspid valve blood flow: accurate quantification with 3D velocity‐encoded MR imaging with retrospective valve tracking. Radiology 2008;249:792–800. [DOI] [PubMed] [Google Scholar]
- 3. Calkoen EE, Roest AA, Kroft LJ, et al. Characterization and improved quantification of left ventricular inflow using streamline visualization with 4DFlow MRI in healthy controls and patients after atrioventricular septal defect correction. J Magn Reson Imaging 2015;41:1512–1520. [DOI] [PubMed] [Google Scholar]
- 4. Calkoen EE, Westenberg JJ, Kroft LJ, et al. Characterization and quantification of dynamic eccentric regurgitation of the left atrioventricular valve after atrioventricular septal defect correction with 4D flow cardiovascular magnetic resonance and retrospective valve tracking. J Cardiovasc Magn Resonance 2015;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eriksson J, Carlhall CJ, Dyverfeldt P, Engvall J, Bolger AF, Ebbers T. Semi‐automatic quantification of 4D left ventricular blood flow. J Cardiovasc Magn Reson 2010;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. She HL, Roest AA, Calkoen EE, et al. Comparative evaluation of flow quantification across the atrioventricular valve in patients with functional univentricular heart after Fontan's surgery and healthy controls: measurement by 4D flow magnetic resonance imaging and streamline visualization. Congenit Heart Dis 2017;12:40–48. [DOI] [PubMed] [Google Scholar]
- 7. van der Hulst AE, Westenberg JJ, Kroft LJ, et al. Tetralogy of Fallot: 3D velocity‐encoded MR imaging for evaluation of right ventricular valve flow and diastolic function in patients after correction. Radiology 2010;256:724–734. [DOI] [PubMed] [Google Scholar]
- 8. Bolger AF, Heiberg E, Karlsson M, et al. Transit of blood flow through the human left ventricle mapped by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2007;9:741–747. [DOI] [PubMed] [Google Scholar]
- 9. Calkoen EE, de Koning PJ, Blom NA, et al. Disturbed intracardiac flow organization after atrioventricular septal defect correction as assessed with 4D flow magnetic resonance imaging and quantitative particle tracing. Invest Radiol 2015;50:850–857. [DOI] [PubMed] [Google Scholar]
- 10. Eriksson J, Bolger AF, Ebbers T, Carlhall CJ. Four‐dimensional blood flow‐specific markers of LV dysfunction in dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2013;14:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fredriksson AG, Zajac J, Eriksson J, et al. 4‐D blood flow in the human right ventricle. Am J Physiol Heart Circ Physiol 2011;301:H2344–2350. [DOI] [PubMed] [Google Scholar]
- 12. Fredriksson AG, Svalbring E, Eriksson J, et al. 4D flow MRI can detect subtle right ventricular dysfunction in primary left ventricular disease. J Magn Reson Imaging 2016;43:558–565. [DOI] [PubMed] [Google Scholar]
- 13. Luijnenburg SE, Mekic S, van den Berg J, et al. Ventricular response to dobutamine stress relates to the change in peak oxygen uptake during the 5‐year follow‐up in young patients with repaired tetralogy of Fallot. Eur Heart J Cardiovasc Imaging 2014;15:189–194. [DOI] [PubMed] [Google Scholar]
- 14. Lotz J, Meier C, Leppert A, Galanski M. Cardiovascular flow measurement with phase‐contrast MR imaging: basic facts and implementation. Radiographics 2002;22:651–671. [DOI] [PubMed] [Google Scholar]
- 15. Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. Elastix: a toolbox for intensity‐based medical image registration. IEEE Trans Med Imaging 2010;29:196–205. [DOI] [PubMed] [Google Scholar]
- 16. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 17. Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two‐dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29–34. [DOI] [PubMed] [Google Scholar]
- 18. Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging. Magn Reson Q 1991;7:229–254. [PubMed] [Google Scholar]
- 19. Markl M, Chan FP, Alley MT, et al. Time‐resolved three‐dimensional phase‐contrast MRI. J Magn Reson Imaging 2003;17:499–506. [DOI] [PubMed] [Google Scholar]
- 20. Schulz‐Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]


