Abstract
Objective
To compare the effectiveness of a hysteroscopic niche resection versus no treatment in women with postmenstrual spotting and a uterine caesarean scar defect.
Design
Multicentre randomised controlled trial.
Setting
Eleven hospitals collaborating in a consortium for women's health research in the Netherlands.
Population
Women reporting postmenstrual spotting after a caesarean section who had a niche with a residual myometrium of ≥3 mm, measured during sonohysterography.
Methods
Women were randomly allocated to hysteroscopic niche resection or expectant management for 6 months.
Main outcome measures
The primary outcome was the number of days of postmenstrual spotting 6 months after randomisation. Secondary outcomes were spotting at the end of menstruation, intermenstrual spotting, dysuria, sonographic niche measurements, surgical parameters, quality of life, women's satisfaction, sexual function, and additional therapy. Outcomes were measured at 3 months and, except for niche measurements, also at 6 months after randomisation.
Results
We randomised 52 women to hysteroscopic niche resection and 51 women to expectant management. The median number of days of postmenstrual spotting at baseline was 8 days in both groups. At 6 months after randomisation, the median number of days of postmenstrual spotting was 4 days (interquartile range, IQR 2–7 days) in the intervention group and 7 days (IQR 3–10 days) in the control group (P = 0.04); on a scale of 0–10, discomfort as a result of spotting had a median score of 2 (IQR 0–7) in the intervention group, compared with 7 (IQR 0–8) in the control group (P = 0.02).
Conclusions
In women with a niche with a residual myometrium of ≥3 mm, hysteroscopic niche resection reduced postmenstrual spotting and spotting‐related discomfort.
Tweetable abstract
A hysteroscopic niche resection is an effective treatment to reduce niche‐related spotting.
Keywords: Abnormal uterine bleeding, caesarean section, hysteroscopic resection, niche, postmenstrual spotting
Tweetable abstract
A hysteroscopic niche resection is an effective treatment to reduce niche‐related spotting.
Introduction
Long‐term complaints after caesarean section, such as postmenstrual spotting, dysmenorrhea, dyspareunia, or chronic pelvic pain, are frequently described in relation to the presence of a niche.1, 2, 3, 4, 5, 6, 7, 8
A post‐caesarean niche is defined as an indentation in the myometrium at the site of the uterine scar.9 Two independent prospective cohort studies reported that the presence of a niche after caesarean section increases the risk of postmenstrual spotting for more than 2 days from 15 to 30%.1, 3 Postmenstrual spotting may be caused by a mechanical outflow problem, with the retention of menstrual blood in a niche,4, 5, 7, 8 or by the accumulation of blood because of impaired uterine contractions at the site of the niche.5 Additionally, newly formed fragile vessels in the niche may play a role in the formation of blood or fluid in the niche and uterine cavity.
Sonohysterography is reported to measure the niche more accurately than sonography, with a better delineation of the niche.1, 3, 10 Sonohysterography allowed the observation of a niche in approximately 60% of women 2–12 months after a caesarean section.1, 3, 11, 12
Hysteroscopic, laparoscopic, or (laparoscopic‐assisted) vaginal niche resections have been developed.13 A hysteroscopic niche resection is the least invasive of these techniques, but requires a sufficient thick residual myometrium between the niche and the bladder to prevent bladder injury.13 A hysteroscopic niche resection can be performed in different ways: the lower rim (closest to the external cervical os) can be resected to facilitate menstrual outflow (Figure1);14, 15, 16, 17 both the lower and the upper part of the niche can be resected;18, 19, 20, 21 and this can be combined with coagulation of the vessels in the niche,16, 18, 19, 20, 21 or the entire niche surface.14, 15 Previous cohort studies reported a reduction of postmenstrual spotting in 80–90% of women, and a reduction in pain in 97% of women, in the absence of complications.13, 22, 23 The mean reduction in the number of days of spotting compared with baseline was reported in two studies, and varied between 2 and 4 days in 119 women.15,17 Apart from the flawed comparability and lack of randomisation, the studies did not use validated tools to measure the outcomes.13, 14, 15, 16, 17, 18, 19, 20, 21 We initiated a randomised controlled trial assessing the effect on postmenstrual spotting of a hysteroscopic niche resection versus no treatment.
Figure 1.
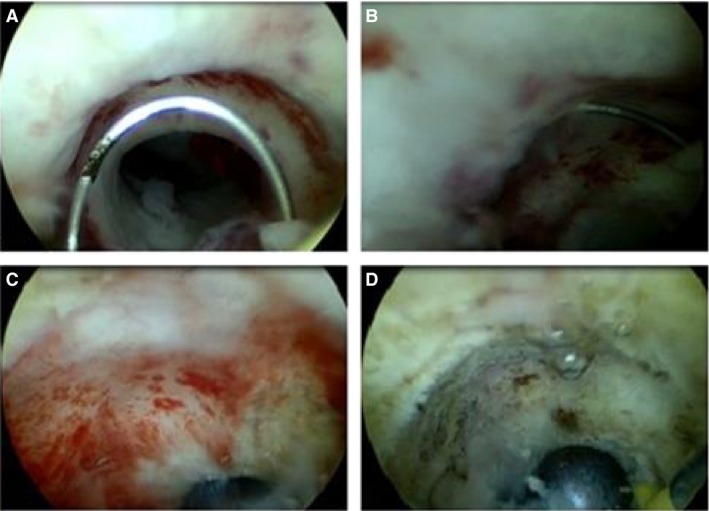
Hysteroscopic niche resection: (A) hysteroscopic view of the niche, with lower rim visible; (B) resection of the lower rim using a resectoscope; (C) coagulation of the niche surface using a rollerball; (D) hysteroscopic view on the site of the niche after the resection.
Methods
Trial design
We performed a multicentre randomised controlled trial in 11 hospitals that collaborate within the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology. The design and methods of this trial have been described in the published study protocol.24
Participants
Women with a previous caesarean section who presented with postmenstrual spotting, and in whom sonohysterography had shown a niche with a residual myometrium of at least 3 mm, were eligible. Postmenstrual spotting was defined as: (a) two or more days of intermenstrual spotting; or (b) two or more days of brownish discharge at the end of menstrual bleeding when the total period of menstrual bleeding exceeds 7 days. Postmenstrual spotting needed to be present for at least three consecutive months after the last caesarean section.
A niche was defined as an indentation in the uterine wall at the site of the caesarean scar with a depth of at least 2 mm, measured during sonohysterography according to a standardized protocol (Figure S1).24 Exclusion criteria included being under the age of 18 years, pregnancy, (suspected) malignancies, absence of cyclic bleeding periods caused by a levonorgestrel intrauterine device (IUD), continuous oral contraceptives or gonadotropin‐releasing hormone (GnRH) agonists, contraindications for spinal or general anaesthesia, atypical endometrial cells or cervical dysplasia in cervical cytology, uterine or cervical polyps, submucosal fibroids, cervical or pelvic infection in the cervical swab, a hydrosalpinx that communicates with the uterus, or an irregular cycle (>35 days or with an intercycle variation of 2 weeks or more). The absence of cyclic bleeding as a result of hormonal treatments was defined in the study protocol from the start of the study as an exclusion criterion, but was erroneously not reported in the published study protocol.24
Randomisation
After written informed consent was given, women were randomly allocated to either hysteroscopic niche resection within 1 month (intervention group) or to expectant management (control group). Randomisation was performed using the online randomisation program alea, using permuted blocks with a random block size of two or four women, and was stratified for treatment centre. The study was not blinded.
Training of participating gynaecologists
The gynaecologists who participated in the study were additionally trained and evaluated in their centre in the measurement of a niche and in performing a hysteroscopic niche resection by one of the experienced gynaecologists that performed niche resections in a previous pilot study (JHU, LVO, WHE, or HBR).24
Interventions
Hysteroscopic niche resection (intervention group)
The resection was performed under continuous sonographic evaluation. The lower rim of the niche, if prominently visible, was resected as described by Chang et al. and Fabres et al.14, 17 The niche surface was superficially coagulated with the use of a rollerball (Figures 1 and S2).24
Expectant management (control group)
Women in the control group were motivated to refrain from an additional intervention for 6 months after randomisation. They were encouraged to continue any hormonal medication during this period that they had used before randomisation. In case women wanted to undergo a hysteroscopic niche resection or to use other additional therapies before 6 months of follow‐up, we left the decision up to the gynaecologist and the participant, and participants remained included in the study.
Outcome measures
Women received digital questionnaires at baseline, and at 3 and 6 months after randomization, in which all outcomes (except for niche measurements) were assessed. Women were asked to fill out a 1‐month menstrual score chart at baseline, and again at 3 and 6 months after randomisation.25
The primary outcome was the self‐reported number of days with postmenstrual spotting during a menstrual cycle at 6 months after randomisation, which was also registered on a validated menstruation score chart.25
Secondary outcomes were: spotting at the end of the menstruation; intermenstrual spotting; menstrual‐related pain; discomfort experienced with spotting, as rated on a visual analogue scale (VAS) of 0–10; sonographic results (residual myometrium, depth of the niche, presence of intrauterine fluid); quality of life [Short Form 36 (SF‐36) and EuroQoL5D (EQ‐5D)];26, 27 women's satisfaction (five‐point Likert scale); sexual function [Female Sexual Function index (FSFI)],28 and pain during micturition at 3 and 6 months after randomisation. We also reported additional therapies at 6 months after randomisation.Women registered their VAS scores on a scale of 0–10 (i.e. on a line of 10 cm in length) for the various outcomes with 0 as ‘no discomfort or no pain at all’ and 10 as ‘the most discomfort or pain imaginable’.
Three months after randomisation niche depth and residual myometrium were measured by transvaginal sonography according to a standardised method.24 Women were offered to undergo sonohysterography; however, this was omitted if women refused.
Sample size
At the time of study design, there was only one study reporting on the reduction in spotting days compared with baseline. The median reduction was 3.8 days, with an estimated standard deviation of 2.7 days.17 We expected a difference of 2 days in postmenstrual spotting to be clinically relevant. Given the lack of studies assessing the clinical relevance of a reduction in the number of spotting days we chose a conservative cut‐off value to reduce the risk of insufficient power. In order to achieve 90% power to detect a difference of 2 days of postmenstrual spotting between the intervention and control group after 6 months of follow‐up, with an estimated SD of 2.7 days, a two‐sided alpha of 0.05, and an anticipated drop‐out rate of 20%, we needed to include a total of 100 women.
Statistical analysis
All analyses were performed using spss 22. All tests were performed two sided and P < 0.05 was considered to indicate statistical significance.
Intention to treat (ITT) analysis
The difference between the two groups after 6 months of follow‐up regarding all continuous variables were analysed using the Mann–Withney U‐test for non‐parametric data (total number of postmenstrual spotting days during one menstrual cycle, days of spotting at the end of menstruation, days of intermenstrual spotting, dysmenorrhea, discomfort experienced as a result of spotting, SF‐36 domain scores, EuroQol scores, FSFI scores, niche depth, and residual myometrium). Baseline characteristics did not statistically differ between the two groups, and therefore we decided not to use the planned regression models to adjust for possible confounding factors.
Fisher's exact test was used for the binary data, such as the presence of (midcycle) intrauterine fluid and the presence of pain during micturition. Satisfaction with the randomised treatment was recoded into a binary outcome using ‘dissatisfied’ (combining dissatisfied, very dissatisfied, and neutral) or ‘satisfied’ (combining satisfied and very satisfied), and was analysed with the chi‐square test.
Predefined subgroup analyses
To identify a subgroup effect, we tested for an interaction for the following predefined subgroups: (1) number of postmenstrual spotting days (total days of spotting per cycle) at baseline (>25th and >75th percentile); (2) small versus larger niches at baseline (using a cut‐off of the residual myometrium of 3 mm, 5 mm, and <50% of total myometrial thickness); (3) one versus more previous caesarean sections.
Additional post hoc analyses
Given the large number of women who became amenorrheic during the first 6 months of follow‐up as a result of staring on continuous hormonal medication, or because of pregnancy, we executed an additional analysis excluding these women.
We also performed an analysis using the ‘last observation carried forward’ method for women who received an additional surgical intervention. We carried data from the last follow‐up period before this surgery forward to the 6‐month follow‐up.
Results
Participants
Between April 2012 and January 2015, 110 women were eligible and were asked to participate in the trial, of whom 103 women were randomised to the intervention (n = 52) or expectant management (n = 51) (Figure 2).
Figure 2.
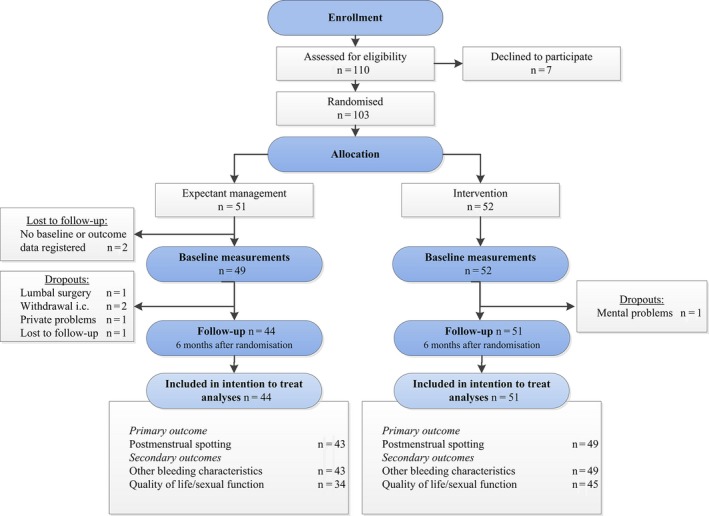
Flow chart.
ITT analysis
Outcomes were registered for 51 women in the intervention group and for 44 women in the control group after 6 months of follow‐up. The reasons for missing data were the withdrawal of a women's consent immediately after randomisation or for loss to follow‐up in the control group (Figure2). Six women became pregnant during the follow‐up period (three in each group). These women remained included in our ITT analysis.
Additional post hoc analyses
Women who became amenorrhoic during the first 6 months of follow‐up as a result of continuous hormonal therapy or a pregnancy were excluded from this analysis, resulting in 41 women in the intervention group and 35 women in the control group.
All 51 women of the intervention group and 44 women of the control group were included in the ‘last observation carried forward’ analysis.
Baseline data
Baseline characteristics did not differ statistically between the two groups (Table1). The median number of days of postmenstrual spotting at baseline was 8 days (interquartile range, IQR 5–12 days) in the intervention group, and 8 days (IQR 6–14 days) in the control group. The median level of discomfort with spotting was 7.8 (IQR 5.8–8.4) and 8.0 (IQR 6.6–9.0), respectively.
Table 1.
Baseline characteristics
| Characteristic | Intervention (n = 52) | Control (n = 49) |
|---|---|---|
| Age (years) | 36.6 ± 5.0 | 36.9 ± 4.9 |
| Body mass index (kg/m 2 ) | 24.4 ± 4.3 | 24.9 ± 4.2 |
| Smoking | 9 (17.3%) | 11 (22.9%) |
| Use of anticoagulants | 0 | 1 (2.1%) |
| Use of tranexamic acid | 0 | 1 (2.0%) |
| Parity | 2 (1–2) | 2 (1–3) |
| Number of caesarean sections | 1 (1–2) | 2 (1–3) |
| Time since last caesarean section (months) | 55.5 (27.8–80.3) | 39 (23–80) |
| History of uterine surgery | ||
| Curettage | 2 (3.8%) | 1 (2.0%) |
| Transcervical resection polyp | 1 (1.9%) | 1 (2.0%) |
| Transcervical resection fibroid | 0 | 0 |
| Fibroid enucleation | 1 (1.9%) | 0 |
| Wish to conceive | 18 (35.3%) | 12 (25%) |
| Fertility treatment after last caesarean section | 5 (9.6%) | 3 (6.3%) |
| Hormonal contraception | ||
| Oral contraception | 14 (26.9%) | 7 (14.3%) |
| Levonorgestrel intrauterine device | 1 (1.9%) | 1 (2.0%) |
| Nuvaring | 1 (1.9%) | 0 |
| Bleeding/micturition characteristics | ||
| Duration of bleeding complaints (months) | 36.5 (16–60) | 35 (14–56) |
| Total days of spottinga | 8 (5–12) | 8 (6–14) |
| Spotting end of menstruation | 4 (2–8) | 6 (3–11) |
| Intermenstrual spotting | 3 (0–5) | 2 (0–5) |
| Discomfort from spotting (0–10) | 7.8 (5.8–8.4) | 8.0 (6.6–9.0) |
| Dysmenorrhea (0–10) | 5.4 (0–7) | 7.0 (0–8.2) |
| Daily pain during micturition | 4 (7.7%) | 6 (12.2%) |
| Ultrasound findings | ||
| Residual myometrium (mm) | 4.0 (3.4–6.0) | 4.5 (3.6–6.6) |
| Depth niche (mm) | 6.0 (4.0–8.1) | 6.0 (4.2–7.4) |
| Intrauterine fluid | 11/51 (21.6%) | 4/45 (8.9%) |
| Quality of life and sexual function | ||
| SF‐36 physical component summary score | 53.9 (47.7–57.8) | 50.4 (39.7–56.9) |
| SF‐36 mental component summary score | 51.7 (43.7–55.6) | 49.4 (36.0–53.7) |
| EuroQol total score | 0.84 (0.78–1.0) | 0.83 (0.69–0.85) |
| FSFI total score | 18.3 (15.3–21.5) | 15.6 (13.4–20.5) |
Data are reported as mean ± standard deviation, as n (valid percentage), or as median (interquartile range, IQR).
No baseline characteristics differed statistically between the two groups.
Total days of spotting = the sum of the number of days spotting at the end of the menstruation and the number of days of intermenstrual spotting (postmenstrual spotting).
Surgical outcomes (intervention group)
In the intervention group, six women did not undergo the intervention. Two women had a strong preference for expectant management after randomisation, one feared the intervention, one feared anaesthesia, one became pregnant before the intervention, and in one woman the spotting complaints diminished. This resulted in 45 women receiving a hysteroscopic niche resection. The surgical outcomes are presented in Table S1.
Primary outcome
According to the ITT analysis, the median number of days of postmenstrual spotting at 6 months after randomisation was 4 days (IQR 2–7 days) in the intervention group versus 7 days (IQR 3–10 days) in the control group (P = 0.04; Table 2).
Table 2.
Bleeding characteristics and quality of life after 6 months of follow‐up, by intention‐to‐treat analysis
| Outcomes | Intervention n = 51 | Control n = 44 | P |
|---|---|---|---|
| Bleeding/micturition characteristics | |||
| Total days of spottinga | 4 (2–7) | 7 (3–10) | 0.04 |
| Spotting at the end of the menstruation | 3 (2–5) | 5 (2–8) | 0.13 |
| Intermenstrual spotting | 0 (0–0) | 0 (0–3) | 0.15 |
| Discomfort from spotting (0–10) | 2.0 (0–6.8) | 6.9 (0.4–8.0) | 0.02 |
| Dysmenorrhea (0–10) | 3.0 (0–6.2) | 4.3 (0–7.3) | 0.37 |
| Daily pain during micturition | 2 (4.7%) | 3 (7.9%) | 0.67 |
| Quality of life and sexual function | |||
| SF‐36 physical component summary score | 53.1 (45.4–58.7) | 52.1 (46.6–57.7) | 0.67 |
| SF‐36 mental component summary score | 52.6 (47.0–56.9) | 50.0 (44.5–54.2) | 0.05 |
| EuroQol total score | 0.84 (0.81–1.0) | 0.83 (0.72–1.0) | 0.33 |
| FSFI total score | 13.5 (9.8–21.6) | 15.1 (10.0–21.3) | 0.61 |
Data are reported as n (valid percentage) or as median (IQR). Analyses are by intention to treat.
Total days of spotting = the sum of the number of days spotting at the end of the menstruation and the number of days of intermenstrual spotting (postmenstrual spotting).
The post hoc analyses excluding women with amenorrhoea during follow‐up as a result of continuous hormonal therapy or pregnancy, and the ‘last observation carried forward’ analysis, showed consistent findings with the ITT analysis (Table S2).
Given the small sample sizes in the various subgroups we did not execute the intended subgroup analyses.
Secondary outcomes
During the 6 months of follow‐up, 13 additional surgical interventions were performed in the control group versus none in the intervention group (P < 0.01). Among these 13 women, nine underwent hysteroscopic niche resection, two underwent endometrial ablation, one underwent transcervical resection of a fibroid (that was missed at baseline), and one underwent laparoscopic hysterectomy because of persisting symptoms. The number of additional medical hormonal therapies did not differ between the two groups (Table S3).
At 6 months after randomisation, the median discomfort with spotting was 2.0 (IQR 0–6.8) versus 6.9 (IQR 0.4–8.0) (on a VAS of 0–10) in the intervention and control groups, respectively (P = 0.02). Other menstrual characteristics and number of women with pain during micturition did not differ statistically between the two groups (Table2). Postmenstrual spotting did not differ after 3 months of follow‐up, with 6 days (IQR 2–9 days) reported in the intervention group and 7 days (IQR 2–10 days) reported in the control group (P = 0.54).
After 6 months of follow‐up, quality of life measured with EuroQol and the total physical component score of the SF‐36 did not differ statistically between the two groups. The total mental component score of the SF‐36 was slightly higher in the intervention group (52.6, IQR 47.0–56.9) than in the control group (50.0, IQR 44.5–54.2) (P = 0.05; Table2). More women in the intervention group were (very) satisfied with the randomised treatment (71.1%) in comparison with the control group (37.5%); (RR 2.2; 95% CI 1.23–3.80).
Transvaginal sonography 3 months after randomisation was performed in 36 out of 51 women (70.6%) in the intervention group, and in 23 out of 44 women (52.3%) in the control group. The depth of the niche and the thickness of the residual myometrium did not differ statistically between the two groups after 3 months of follow‐up, and within the two groups in comparison with baseline (Table S4). Niche depth and residual myometrium were evaluated with sonohysterography in only 24 women (47.1%) of the intervention group and 13 women (29.5%) of the control group, mainly because women were not motivated to undergo a second sonohysterography.
Complications
No complications occurred during the niche resection. One woman developed fever and lower abdominal pain after the intervention, which was diagnosed as pelvic inflammatory disease and treated with antibiotics. No complications occurred in the control group.
Discussion
Main findings
Hysteroscopic niche resection reduced the median number of days of postmenstrual spotting by 3 days after 6 months of follow‐up compared with expectant management, and by 4 days compared with baseline. Discomfort related to spotting (on a VAS of 0–10) was five points lower in the intervention group in comparison with the control group after 6 months of follow‐up. After the intervention, the median number of days of discomfort related to spotting reduced from eight at baseline to two after 6 months of follow‐up. More women were (very) satisfied in the intervention group compared with the control group, and more surgical additional interventions were performed in the control group. The residual myometrium using transvaginal ultrasonography at 3 months did not change in comparison with baseline or with the control group.
Strengths and limitations
Strengths
This trial is the first randomised controlled trial to evaluate the effectiveness of a hysteroscopic niche resection versus expectant management in women with niche‐related postmenstrual spotting. Randomisation was performed with allocation concealment through a web‐based randomisation program, which reduced the chance for selection bias. The surgeons were trained and assessed in their execution of this new intervention in order to perform it in a standardised manner. All questionnaires were completed without interference of care‐providers, thereby reducing the risk of socially acceptable answers. We used validated questionnaires and standardised methods for the measurement of niche characteristics.29
Limitations
The study could not be blinded for the participant or for the surgeon, and therefore the Hawthorne effect cannot be excluded;30 however, knowing the allocation of the intervention is part of real life, and its contribution to the (perceived) effectiveness of the intervention could be taken into account.
The hysteroscopic resection was executed later than planned in the protocol: 55% of women in the intervention group received it in the second or third month after randomisation. For this reason we considered the data after 3 months of follow‐up as not reliable, because this moment of follow‐up would be still in the healing phase after the surgical procedure.
Experience with performing the intervention and the number of included women differed between the various centres, and therefore the effect of a learning curve cannot be excluded. To reduce the effect of a learning curve on outcomes between the study groups, women were stratified per centre.
Quality of life was only measured using generic questionnaires; these may be not responsive enough to measure differences in discomfort as a result of spotting. Disease‐specific validated questionnaires have not yet been developed.
The number of women who withdrew immediately after randomisation or who were lost to follow‐up was particularly high in the control group. This should be taken into account interpreting the results of the ITT analysis. According to our sample size calculation, however, we maintained sufficient power despite these losses.
Many additional medical therapies were applied during the 6 months of follow‐up in both groups, and many additional surgical interventions were used in the control group, which might have biased our primary outcome in the ITT analysis. For this reason we performed an additional analysis excluding all women with amenorrhea, which showed consistent findings with the ITT analysis; however, the required number for adequate power was not reached in this analysis. Median spotting at baseline was particularly high (10 days) in controls who underwent additional surgical therapy. This may explain the reduction in spotting in the entire control group at follow‐up, compared with baseline. This may have resulted in an underestimation of the effect in our study. This is underlined by the fact that the effect of the intervention became more pronounced in the additional analysis using the last observation carried forward.
Interpretation
The reduction in the number of postmenstrual spotting days after 6 months of follow‐up is in line with previous publications on hysteroscopic niche resection. The mean reported reduction in spotting in one prospective and one retrospective cohort study varied between 2 and 4 days.15, 17 Two recent non‐comparative studies in 144 women reported a resolution of spotting in 80% of women; however, a reduction in the number of days of spotting was not reported.22, 23
Although our trial reported a modest reduction in postmenstrual spotting, the reduction in discomfort related to spotting was substantial. This suggests that even a modest reduction of some days is relevant for women with these symptoms, although it did not result in a difference in generic quality of life.
Proximal resection of the niche may in theory cause cervical incompetence in a subsequent pregnancy. In addition, we expected the niche resection to increase the size of the niche and wanted to prevent any unneeded enlargement in volume. Although we did not find a reduction in the residual myometrium in our study, we need to interpret these results with caution, because not all women showed up for the sonographic measurements at follow‐up. Additionally, we did not measure the length and volume of the niche, which in theory may enlarge after resection (of the lower rim) of the niche.
A hysteroscopic niche resection should only be performed if the residual myometrium between the niche and the bladder is sufficiently thick to prevent bladder injury. The cut‐off value of the residual myometrium in various studies varies between 2.5 and 4.0 mm, as measured using sonohysterography.13, 22, 23
We have only included women with relatively small niches (≥3 mm), and thus the outcomes should not be extrapolated to women with large niches. In women with a large niche (with residual myometrium of <3 mm) with severe symptoms and desiring to conceive, a laparoscopic niche resection may be considered. Given the limited studies evaluating this method and the lack of randomised trials this intervention should only be offered in a research setting.
It is important to realise that not all niches cause symptoms. Treatment should only be performed in order to reduce symptoms, and thus niches without symptoms should not be treated. Although oral contraceptives or levonorgestrel IUD are less invasive therapeutic options, and might therefore be offered first, their effectiveness for this indication has not yet been proven. In women with an actual desire to conceive or with contraindications for hormone treatment, this is not an option. Given the reported reduction in the number of spotting days and discomfort in favour of the hysteroscopic niche resection in our trial, it may be considered in symptomatic women with a niche with sufficient residual myometrium (≥3 mm).
Every woman must tradeoff the limited reduction of spotting against the burden of the procedure. In addition, we need to inform women about the uncertainty of whether the reduction in spotting will persist in the longer term, and it is not expected to affect their generic quality of life. The same accounts for the unknown effect of a hysteroscopic niche resection on the risk for scar rupture in labour, pregnancy implantation involving the scar, and related morbidly adherent placenta or cervical incompetence in future pregnancies.
Future perspectives
Long‐term follow‐up is needed to evaluate the sustainability of a hysteroscopic niche resection and the cost‐effectiveness. Larger studies are needed to evaluate the effect of a hysteroscopic niche resection on reproductive outcomes of subsequent pregnancies, including possible cervical incompetence.
Future preferably randomised studies are needed to evaluate the effectiveness of hormones compared with hysteroscopic niche resection.
Conclusion
In conclusion, a hysteroscopic niche resection reduces postmenstrual spotting, and the discomfort from spotting, compared with expectant management after 6 months of follow‐up in women with a niche with a residual myometrium of at least 3 mm.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Contribution to authorship
AVE, LVO, WHE, HBR, and JHU made substantial contributions to the design and drafting of this article. AVE, KOU, SZW, and JHU contributed to the analysis and interpretation of the data. AVE, LVO, WHE, ATH, PKE, HQU, WKU, MBO, PGE, LVL, MHO, HVL, SVE, WRE, KOU, SZW, HBR, BMO, and JHU critically revised and approved this final version for publication.
Details of ethics approval
The study was approved by the National Central Committee on Research Involving Human Subjects (CCMO – NL38397.029.11) on 4 April 2012, by the ethics committee of the VU University Medical Centre Amsterdam on 2 February 2012 (ref. no. 2011/397), and by the boards of directors of all participating hospitals. Netherlands Trial Register NTR3269 (http://www.trialregister.nl), registered 1 February 2012. The protocol of this trial has been published in Biomed Central Women's health (https://bmcwomenshealth.biomedcentral.com/articles/10.1186/s12905-015-0260-8/open-peer-review).
Funding
This study is funded by ZonMw, a Dutch organization for Health Research and Development (project number 80‐82305‐97‐12030) and was co‐funded by the VU University Medical Centre Amsterdam. These sponsors had no role in the study design, data collection, data analysis, data interpretation or writing of this article.
Supporting information
Figure S1. Niche during sonohysterography before the intervention.
Figure S2. Resection of the lower part of the niche.
Table S1. Surgical outcomes.
Table S2. Post hoc analysis.
Table S3. Additional therapies, by intention‐to‐treat analysis.
Table S4. Ultrasound findings after 3 months of follow‐up.■
Acknowledgements
We thank the women who participated in the study and all other persons that were involved in the study (in participating hospitals and within the Dutch Consortium for Studies in Women's Health and Reproduction).
Vervoort AJMW, van der Voet LF, Hehenkamp WJK, Thurkow AL, van Kesteren PJM, Quartero H, Kuchenbecker W, Bongers M, Geomini P, de Vleeschouwer LHM, van Hooff MHA, van Vliet H, Veersema S, Renes WB, Oude Rengerink K, Zwolsman SE, Brölmann HAM, Mol BWJ, Huirne JAF. Hysteroscopic resection of a uterine caesarean scar defect (niche) in women with postmenstrual spotting: a randomised controlled trial. BJOG 2018; 125:326–334.
Linked article This article is commented on by T El‐Toukhy p. 335 in this issue. To view this artcle visit https://doi.org/10.1111/1471-0528.14823.
References
- 1. Bij de Vaate AJ, Brolmann HA, van der Voet LF, van der Slikke JW, Veersema S, Huirne JA. Ultrasound evaluation of the Cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol 2011;37:93–9. [DOI] [PubMed] [Google Scholar]
- 2. Bij de Vaate AJ, van der Voet LF, Naji O, Witmer M, Veersema S, Brolmann HA, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol 2014;43:372–82. [DOI] [PubMed] [Google Scholar]
- 3. van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HA, Huirne JA. Long‐term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG 2014;121:236–44. [DOI] [PubMed] [Google Scholar]
- 4. Fabres C, Aviles G, De La Jara C, Escalona J, Munoz JF, Mackenna A, et al. The cesarean delivery scar pouch: clinical implications and diagnostic correlation between transvaginal sonography and hysteroscopy. J Ultrasound Med 2003;22:695–700. [DOI] [PubMed] [Google Scholar]
- 5. Thurmond AS, Harvey WJ, Smith SA. Cesarean section scar as a cause of abnormal vaginal bleeding: diagnosis by sonohysterography. J Ultrasound Med 1999;18:13–16. [DOI] [PubMed] [Google Scholar]
- 6. Wang CB, Chiu WW, Lee CY, Sun YL, Lin YH, Tseng CJ. Cesarean scar defect: correlation between Cesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet Gynecol 2009;34:85–9. [DOI] [PubMed] [Google Scholar]
- 7. Erickson SS, Van Voorhis BJ. Intermenstrual bleeding secondary to cesarean scar diverticuli: report of three cases. Obstet Gynecol 1999;93:802–5. [DOI] [PubMed] [Google Scholar]
- 8. Van HA, Temmerman M, Dhont M. Cesarean scar dehiscence and irregular uterine bleeding. Obstet Gynecol 2003;102:1137–9. [PubMed] [Google Scholar]
- 9. Monteagudo A, Carreno C, Timor‐Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med 2001;20:1105–15. [DOI] [PubMed] [Google Scholar]
- 10. Osser OV, Jokubkiene L, Valentin L. Cesarean section scar defects: agreement between transvaginal sonographic findings with and without saline contrast enhancement. Ultrasound Obstet Gynecol 2010;35:75–83. [DOI] [PubMed] [Google Scholar]
- 11. Valenzano MM, Mistrangelo E, Lijoi D, Fortunato T, Lantieri PB, Risso D, et al. Transvaginal sonohysterographic evaluation of uterine malformations. Eur J Obstet Gynecol Reprod Biol 2006;124:246–9. [DOI] [PubMed] [Google Scholar]
- 12. Osser OV, Jokubkiene L, Valentin L. High prevalence of defects in Cesarean section scars at transvaginal ultrasound examination. Ultrasound Obstet Gynecol 2009;34:90–7. [DOI] [PubMed] [Google Scholar]
- 13. van der Voet LF, Vervoort AJ, Veersema S, BijdeVaate AJ, Brolmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: a systematic review. BJOG 2014;121:145–56. [DOI] [PubMed] [Google Scholar]
- 14. Fabres C, Arriagada P, Fernandez C, Mackenna A, Zegers F, Fernandez E. Surgical treatment and follow‐up of women with intermenstrual bleeding due to cesarean section scar defect. J Minim Invasive Gynecol 2005;12:25–8. [DOI] [PubMed] [Google Scholar]
- 15. Feng YL, Li MX, Liang XQ, Li XM. Hysteroscopic treatment of postcesarean scar defect. J Minim Invasive Gynecol 2012;19:498–502. [DOI] [PubMed] [Google Scholar]
- 16. Wang CJ, Huang HJ, Chao A, Lin YP, Pan YJ, Horng SG. Challenges in the transvaginal management of abnormal uterine bleeding secondary to cesarean section scar defect. Eur J Obstet Gynecol Reprod Biol 2011;154:218–22. [DOI] [PubMed] [Google Scholar]
- 17. Chang Y, Tsai EM, Long CY, Lee CL, Kay N. Resectoscopic treatment combined with sonohysterographic evaluation of women with postmenstrual bleeding as a result of previous cesarean delivery scar defects. Am J Obstet Gynecol 2009;200:370–4. [DOI] [PubMed] [Google Scholar]
- 18. Florio P, Gubbini G, Marra E, Dores D, Nascetti D, Bruni L, et al. A retrospective case‐control study comparing hysteroscopic resection versus hormonal modulation in treating menstrual disorders due to isthmocele. Gynecol Endocrinol 2011;27:434–8. [DOI] [PubMed] [Google Scholar]
- 19. Gubbini G, Casadio P, Marra E. Resectoscopic correction of the “isthmocele” in women with postmenstrual abnormal uterine bleeding and secondary infertility. J Minim Invasive Gynecol 2008;15:172–5. [DOI] [PubMed] [Google Scholar]
- 20. Gubbini G, Centini G, Nascetti D, Marra E, Moncini I, Bruni L, et al. Surgical hysteroscopic treatment of cesarean‐induced isthmocele in restoring fertility: prospective study. J Minim Invasive Gynecol 2011;18:234–7. [DOI] [PubMed] [Google Scholar]
- 21. Marra E. Resectoscopic treatment of “Isthmocele”: “Isthmoplasty”. In: P C, F A, D DA, M B, MA R, editors.: Gynaecol Surg; 2009. p. S108‐S9.
- 22. Li C, Guo Y, Liu Y, Cheng J, Zhang W. Hysteroscopic and laparoscopic management of uterine defects on previous cesarean delivery scars. J Perinat Med 2014;42:363–70. [DOI] [PubMed] [Google Scholar]
- 23. Raimondo G, Grifone G, Raimondo D, Seracchioli R, Scambia G, Masciullo V. Hysteroscopic treatment of symptomatic cesarean‐induced isthmocele: a prospective study. J Minim Invasive Gynecol 2015;22:297–301. [DOI] [PubMed] [Google Scholar]
- 24. Vervoort AJ, van der Voet LF, Witmer M, Thurkow AL, Radder CM, van Kesteren PJ, et al. The HysNiche trial: hysteroscopic resection of uterine caesarean scar defect (niche) in patients with abnormal bleeding, a randomised controlled trial. BMC Womens Health 2015;15:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maarse M, BijdeVaate AJM, Huirne JAF, Brölmann HAM. De maandkalender; een hulpmiddel voor een efficiënte menstruatieanamnese. NTOG 2012;124:231–6. [Google Scholar]
- 26. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 27. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108. [DOI] [PubMed] [Google Scholar]
- 28. Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self‐report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 29. Naji O, Abdallah Y, Bij de Vaate AJ, Smith A, Pexsters A, Stalder C, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol 2012;39:252–9. [DOI] [PubMed] [Google Scholar]
- 30. Berthelot JM, Le GB, Maugars Y. The Hawthorne effect: stronger than the placebo effect? Joint Bone Spine 2011;78:335–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Niche during sonohysterography before the intervention.
Figure S2. Resection of the lower part of the niche.
Table S1. Surgical outcomes.
Table S2. Post hoc analysis.
Table S3. Additional therapies, by intention‐to‐treat analysis.
Table S4. Ultrasound findings after 3 months of follow‐up.■


