Abstract
Subclinical levels of polysubstance use are a prevalent and understudied phenomenon. Alcohol is a substance commonly co‐used with other substances of other drug classes. These studies sought to determine the consumption effects of combining alcohol drinking and methamphetamine (MA) self‐administration. Male alcohol‐preferring P rats had continuous access to a two‐bottle alcohol drinking procedure in the home cage. Control rats remained alcohol naïve. Rats were also surgically implanted with intra‐jugular catheters and trained to self‐administer saline (control) or MA in daily 2‐hour sessions. We first measured the acquisition and maintenance of MA intake in alcohol‐consuming or control rats. MA intake was initially enhanced by alcohol consumption on a fixed ratio 1 schedule of reinforcement, but this effect did not prevail as the difficulty of the schedule (FR5 and progressive ratio) was increased. We next measured both alcohol consumption and preference before, during and after MA (or saline) self‐administration. MA self‐administration significantly reduced alcohol intake and preference ratios, a robust effect that persisted across several experimental variations. Interestingly, alcohol consumption rebounded following the cessation of MA self‐administration. The effects of MA self‐administration were specific to alcohol intake because it did not alter total fluid consumption or consumption of sucrose. MA self‐administration did not impact blood‐alcohol concentrations or alcohol‐induced loss of righting reflex suggesting no effect of MA intake on the alcohol metabolism or sensitivity. Together, the results suggest that MA intake disrupts alcohol consumption and preferences but not the reverse in alcohol‐preferring P rats.
Keywords: ethanol, polydrug use, psychostimulant
Introduction
Polysubstance use is the concurrent or sequential use of two or more psychoactive drugs and is an understudied global health issue. The overall prevalence of polysubstance use has been estimated between 15 and 60 percent depending on the drugs involved (Kedia et al. 2007; EMCDDA 2009). Alcohol is by far the most common substance that is co‐administered with illicit drugs (Kedia et al. 2007). Hospitals attribute an increasing number of admissions and deaths to polysubstance use with more than 75 percent of emergency room visits relating to alcohol combined with another substance (Coffin et al. 2003; SAMHSA 2014a). The prevalence of polysubstance use, especially when alcohol is one of those substances, is therefore high, yet there is little research investigating the combined effects of abused drugs.
Alcohol is frequently co‐used with psychostimulant drugs such as methamphetamine (MA) and cocaine (SAMHSA 2014b). An extensive body of research suggests that the co‐administration of alcohol and cocaine produces an active metabolite, cocaethylene that contributes to enhanced and prolonged subjective effects (McCance et al. 1995; Ikegami et al. 2002). Fewer studies have examined the effects of combined use of MA and alcohol, although these studies largely report a synergistic effect of combined MA and alcohol use. For example, heavy alcohol drinkers are more likely to report MA use than non‐drinkers, while primary MA users consistently use moderate levels of alcohol, and heavy MA use was associated with heavy alcohol use (Martin et al. 2006; O'Grady et al. 2008; Brecht et al. 2008). A recent report demonstrates that alcohol drinking predicts same day MA use further suggesting an interactive effect of the two drugs (Bujarski et al. 2014). The combined use of MA and alcohol results in enhanced euphoria and increased heart rate compared with either drug taken alone (Mendelson et al. 1995; Kirkpatrick et al. 2012), implying that the combined use of MA and alcohol produces synergistic drug effects. Despite data demonstrating the prevalence and significance of MA and alcohol co‐use, there are nearly no validated pre‐clinical models for studying polydrug use. The studies presented here sought to establish a pre‐clinical rat model aimed at examining patterns of co‐use of MA and alcohol using a drug self‐administration model and two‐bottle choice procedure, respectively.
Given the previous research showing synergistic effects of alcohol consumption on other psychostimulant drugs and epidemiological evidence of MA and alcohol co‐use, we hypothesized that the combined use of MA and alcohol would have synergistic effects on drug consumption with higher rates of intake when both substances are available. The present studies use the alcohol‐preferring P rats that have been selectively bred to consume alcohol to determine the combined effects of MA self‐administration and alcohol consumption. Our results suggest that MA self‐administration markedly decreases alcohol consumption but does not alter the subjective effects or metabolism of alcohol.
Materials and Methods
Animals
Male, selectively bred alcohol‐preferring P rats were bred at the Indiana University School of Medicine Animal Research Center (Indianapolis, IN, USA) and provided as part of the R24 Alcohol Research Resource Award grant (R24 AA015512) provided by NIAAA. Animals were shipped to the University of Colorado Boulder at approximately 30 days of age and underwent routine quarantine procedures for at least 2 weeks upon arrival at the University of Colorado Boulder. Prior to experimental procedures, rats were single‐housed for at least 1 week and procedures began when animals reached 300–325 g. All experimental procedures were conducted during the light period of a 12‐hour light/dark cycle and were completed in accordance with the guidelines established by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder.
Drugs
Methamphetamine hydrochloride was obtained from Sigma‐Aldrich (St. Louis, MO, USA). Alcohol (190 proof) from Decon Laboratories (King of Prussia, PA, USA) was diluted to 10–20 percent v/v with tap water for consumption.
Two‐bottle choice free alcohol consumption procedures
Animals had free choice between two bottles equipped with ball‐bearing sipper tubes in their home cage. One bottle contained tap water and the other contains Alcohol (10 or 20 percent, v/v). Some experiments included control animals that were given two water bottles. Bottles were available 5 days/week (Monday–Friday) with fresh solutions prepared at least once a week. Consumption from the bottles was measured daily in order to calculate percent preference of alcohol, grams of alcohol consumed and total fluid consumption. The duration of the two‐bottle procedure varied with each experiment and is detailed in the subsequent experimental protocols.
Methamphetamine self‐administration and extinction procedures
Self‐administration and extinction testing was performed in operant conditioning chambers (Med‐Associates, St. Albans, VT, USA) equipped with two response levers and an infusion pump. Rats were surgically implanted with an indwelling intra‐jugular catheter according to published procedures (Kavanagh et al. 2015). Rats recovered for at least 48 hours before self‐administration procedures began. Catheters were flushed daily with 0.1‐ml heparinized saline (20 U/ml heparin and 0.26 mg/ml gentamicin) to maintain catheter patency throughout the experiment. During self‐administration sessions, rats were allowed to self‐administer intravenous MA (0.1 mg/kg/100 µl infusion) or saline (100‐µl infusion) for 2 hours/day. MA/saline injections were delivered over a 5‐second period concurrent with illumination of a light cue placed directly over the drug‐paired lever. Each infusion was followed by a 15‐second timeout period during which the house light remained off and lever presses had no consequence. Responses on the inactive lever never had programmed consequences. The number of sessions and the schedule of reinforcement varied with each experiment and are detailed in each experimental protocol. In some experiments, animals underwent a brief (24 hour) withdrawal in the home cage followed by three daily 2‐hour extinction sessions. During each extinction session, lever responding was recorded, but the lever previously paired with MA resulted in no drug or cue delivery.
Experiment 1: effect of 10 percent alcohol consumption on methamphetamine self‐administration
Experimental animals (10 percent alcohol) and controls (water) were exposed to the two‐bottle procedure throughout the course of the experiment. After 1 week of free choice consumption, rats were implanted with indwelling intra‐jugular catheters and trained to self‐administer MA (0.1 mg/kg/100 µl infusion) for 2 hours/day (Monday–Friday) while maintaining the two‐bottle procedure in the home cage. In order to assess the effects of alcohol consumption on acquisition and motivation to seek MA, rats were assessed on various reinforcement schedules. MA self‐administration was initiated on a Fixed Ratio 1 (FR1) schedule of reinforcement for 6 days, followed by 5 days on an FR5 schedule. Rats were then tested for 3 days on a progressive ratio schedule with the progression for response/injection ratios determined according to [5e(injection number x 0.2)]−5 (e.g. 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40 and 50). To measure the effect of alcohol consumption on extinction, a subset of animals that self‐administered MA on a FR1 and FR5 schedule went through a 24‐hour forced withdrawal in the home cage followed by daily 2‐hour extinction sessions for 3 days.
Experiment 2: effect of methamphetamine self‐administration on 10 and 20 percent alcohol consumption
The effect of MA self‐administration on alcohol intake and preference were evaluated using the two‐bottle procedure (10 or 20 percent alcohol) across three phases: (1) pre‐MA baseline, (2) MA co‐use and (3) post‐MA. After 1 week of free choice consumption, rats were implanted with indwelling intra‐jugular catheters and trained to self‐administer MA (0.1 mg/kg/100 µl infusion) or saline (control) for 2 hours/day (Monday–Friday) for 11 days on an FR1 schedule of reinforcement. An FR1 schedule was used to produce stable MA intake, while the effects of MA self‐administration were assessed on alcohol intake/preference. Following 24 hours of withdrawal from MA self‐administration in the home cage, animals underwent three daily 2‐hour extinction sessions. During both the self‐administration and extinction procedures, animals were maintained on the two‐bottle procedure in the home cage.
Experiment 3: effect of methamphetamine self‐administration on 20 percent alcohol consumption after prolonged alcohol consumption
Similar to Experiment 2, a three‐phase procedure was used to evaluated the effects of MA self‐administration on alcohol intake/preference in the two‐bottle procedure (5 days/week). However, Experiment 3 was aimed at evaluating whether more established alcohol drinking would still be susceptible to the effects of MA self‐administration. Thus, the pre‐MA alcohol consumption baseline period was prolonged to 24 days. During the pre‐MA baseline, alcohol drinking was facilitated with access to 10 percent alcohol for the initial 3 days prior to access to 20 percent alcohol for 21 days. After 24 days of the two‐bottle procedure, rats were implanted with indwelling intra‐jugular catheters and trained to self‐administer MA (0.1 mg/kg/100 µl infusion) or saline (control) for 2 hours/day (Monday–Friday) for 11 days on an FR1 schedule of reinforcement. Following 24 hour of withdrawal from MA self‐administration in the home cage, animals underwent three daily 2‐hour extinction sessions. During both the self‐administration and extinction procedures, animals were maintained on the two‐bottle 20 percent alcohol procedure in the home cage.
Experiment 4: effects of prior and concurrent methamphetamine self‐administration on initiation of alcohol consumption
Experiment 4 evaluated whether MA self‐administration would influence the initiation of alcohol consumption and subsequent effects on concurrent use. Thus, the two‐bottle procedure was implemented after the acquisition of MA self‐administration. Alcohol‐naïve animals were implanted with chronic intra‐jugular catheters and trained to self‐administer MA (0.1 mg/kg/100 µl infusion) or saline for 7 days. Following the seventh MA or saline self‐administration session, animals began the two‐bottle procedure with 10 percent alcohol. Animals continued daily MA/saline self‐administration sessions combined with the two‐bottle procedure in the home cage for 8 days. The two‐bottle procedure continued for an additional 3 days during which extinction training sessions were substituted for MA/saline self‐administration sessions.
Experiment 5: effects of prior methamphetamine self‐administration on subsequent initiation of alcohol consumption
Experiment 5 sought to identify whether MA self‐administration would impact alcohol intake/preference if it was not concurrent with the two‐bottle procedure. In this experiment, MA self‐administration was initiated and ceased prior to the implementation of the two‐bottle procedure. Alcohol‐naïve animals were trained to self‐administer MA (0.1 mg/kg/100 µl infusion) or saline for 10 days (Monday–Friday). Two days later, animals were exposed to the two‐bottle procedure (10 percent alcohol) for 13 days. Importantly, MA self‐administration and two‐bottle alcohol consumption were completely dissociated, and there was no overlap between MA and alcohol procedures.
Experiment 6: effects of methamphetamine self‐administration on blood alcohol levels
Blood alcohol analysis was conducted on one cohort of animals (n = 12) from Experiment 2. Blood was collected via a tail vein nick on day 14 of the alcohol drinking procedure (following 10 days of MA/saline self‐administration). Blood was collected 30 minutes following the administration of 1.0 g/kg alcohol via oral gavage. Blood plasma was isolated, and blood alcohol concentrations were measured using the Analox AM1 alcohol analyzer (Analox Instruments, UK).
Experiment 7: effects of methamphetamine self‐administration on alcohol‐induced loss of righting reflex
Experimental procedures were similar to those in Experiment 2 with all animals exposed to the two‐bottle procedure with 10 percent alcohol followed by 14 daily (Monday–Friday) 2‐hour self‐administration sessions (MA or saline). One hour following the final self‐administration session, all animals received an injection of alcohol (3.0 g/kg i.p.). Following the alcohol injection, animals were placed on their backs. The latency to lose and regain their righting reflex was recorded to indicate their sensitivity to alcohol. An animal that could right himself three times in 60 seconds was considered to have regained the righting reflex. Two animals from the saline self‐administration group failed to lose their righting reflex and were excluded from the final analysis. The dose and timing of alcohol injections and the criteria for regaining the righting reflex were based on previous studies (Ornelas et al. 2015).
Experiment 8: effects of methamphetamine self‐administration on sucrose consumption
In this experiment, animals were given a free choice between water and 0.1 percent sucrose to evaluate whether MA self‐administration altered the consumption and/or preference of a non‐alcoholic highly palatable sucrose solution. Animals were subjected to the two‐bottle procedure 5 days/week (Monday–Friday) for 13 days. After an initial 3 days of free choice access, animals were surgically implanted with intra‐jugular catheters and trained to self‐administer MA (0.1 mg/kg/100 µl infusion) or saline (control) for 2 hours/day (Monday–Friday). Animals continued daily self‐administration sessions combined with the two‐bottle procedure for 10 days.
Data analysis
The effect of alcohol consumption on MA self‐administration was analyzed using MA infusions and lever responses as the dependent variables in separate two‐way mixed design ANOVA with session (within) and alcohol consumption (between) as factors. Consumption (g/kg/day) and preference (percent) were analyzed in separate two‐way mixed design ANOVAs with phase/day (within) and MA group (between) as factors. Preliminary analysis of the data using Mauchly's test indicated that the assumption of sphericity had been violated in many of the data sets. Greenhouse‐Geisser estimates of sphericity were therefore used to correct the degrees of freedom indicating sphericity. Significant interactions were followed with simple main effects analyses (one‐way ANOVA) and post hoc tests (Bonferonni's multiple comparisons). A between subjects t‐test was used to analyze the effects of MA self‐administration on blood alcohol concentrations (mg/dl) and time (minutes) to lose/regain righting reflex. Statistical significance was set at p < 0.05 for all tests.
Results
Alcohol consumption increases methamphetamine self‐administration during acquisition but fails to alter the motivation to seek methamphetamine
Experiment 1 tested the effect of alcohol consumption on the acquisition and motivation to seek MA using an operant MA self‐administration procedure (Fig. 1). Analysis of MA intake during the first 6 days of FR1 schedule of reinforcement revealed a significant main effect of days (F 3.26,225 = 2.97, p < 0.05) and a main effect of consumption group (F 1,45 = 4.52, p < 0.05). However, there was no significant interaction between days and consumption group during the FR1 phase (F 5,225 = 1.39, p = 0.23). During the FR5 and progressive ratio (PR) phases, there were no significant main effects of MA intake across days, consumption group and no significant interactions (Fig. 1b). Analysis of lever responding during the FR1, FR5 and PR schedules also revealed no effect of days, consumption group or interaction (Fig. 1c and d). There was a significant main effect of extinction responding across days (F 2,50 = 14.81, p < 0.05) but no main effect of consumption (F 2,50 = 2.34, p = 0.13) and no significant interaction (F 2,50 = 0.99, p = 0.38).
Figure 1.
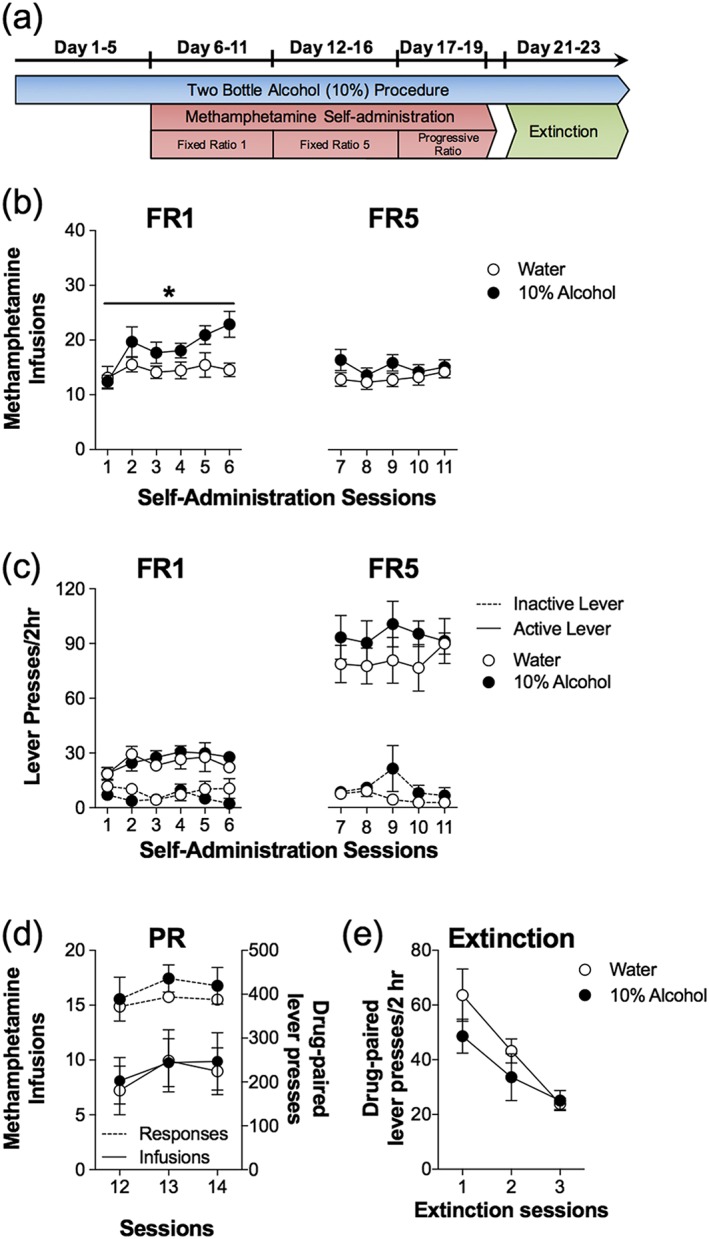
Alcohol consumption enhances methamphetamine (MA) self‐administration on a fixed ratio 1 (FR1) schedule of reinforcement. (a) Timeline illustrating the schedule of combining the 24‐hour two‐bottle choice procedure, daily 2‐hour self‐administration sessions and extinction sessions. (b) Average number of MA infusions (± SEM; 0.1 mg/kg/100 µl infusion) for each session of self‐administration under a FR1 and fixed ratio 5 (FR5) schedule of reinforcement. Alcohol consuming animals (n = 31) had significantly greater number of infusions during a FR1 schedule of reinforcement compared with water consuming animals (n = 16). However, this effect was not evident when MA self‐administration was conducted with an FR5 schedule of reinforcement. (c) Average number of drug‐paired and inactive lever responses (± SEM) for each session of self‐administration under a FR1 and FR5 schedule of reinforcement. Active, drug‐paired lever responses were greater than inactive lever responses, but no group effects were observed between the water and alcohol consumption groups. (d) Average number of MA infusions and total drug‐paired lever responses on the progressive ratio (PR) schedule of reinforcement. No significant group effects were observed over the three sessions of PR schedule testing. (e) There were no differences in the average number of active and inactive lever presses during extinction between alcohol and water consuming animals. *Significant from water‐drinking control (p < 0.05)
We also compared alcohol intake and preference in the two‐bottle procedure using an ABA design to assess changes in average alcohol intake and preference across the three experimental phases (pre‐MA baseline, MA co‐use and post‐MA extinction). Alcohol intake and preferences during these three phases is depicted in Fig. 2. A within‐subject ANOVA detected a significant decrease in average alcohol intake and preference during the MA co‐use phase that returned to baseline during the post‐MA extinction phase (F 2,26 = 11.41, p < 0.001 and F 2,26 = 9.72, p < 0.001, respectively). Total consumption, on the other hand, remained consistent throughout the experimental procedure (Fig. 2c and Table 1).
Figure 2.
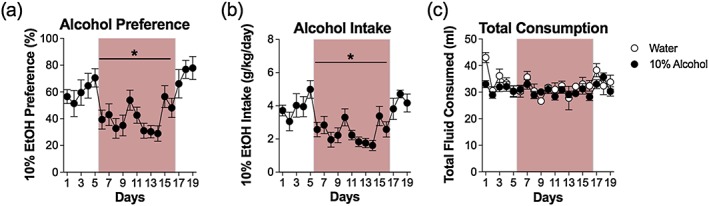
Concurrent methamphetamine (MA) self‐administration decreases alcohol preference and consumption but not total consumption. (a) Average daily alcohol preference during pre‐MA baseline, MA co‐use and post‐MA extinction phases are shown for the 10 percent ethanol group in Experiment 1. Average preference during the MA co‐use phase (40.8 ± 2.2 percent; indicated by rose shading) was significantly reduced compared with both pre‐MA baseline (60.5 ± 3.7 percent) and post‐MA extinction (70.1 ± 5.3 percent). (b) Average daily alcohol intake during pre‐MA baseline, MA co‐use and post‐MA extinction phases are shown for the 10 percent ethanol group in Experiment 1. Average intake during the MA co‐use phase (2.36 ± 0.1 g/kg/day; indicated by rose shading) was significantly reduced compared with both pre‐MA baseline (3.94 ± 0.3 g/kg/day) and post‐MA extinction (4.00 ± 0.3 g/kg/day). (c) Total fluid consumption during the three phases was not altered. * Significant from pre‐MA baseline and post‐MA extinction (p < 0.001)
Table 1.
Statistical tests of total fluid consumption revealed no effect of methamphetamine self‐administration.
| Days | Self‐administration Group | Days × Self‐administration | |
|---|---|---|---|
| Exp. 1 | F 2.38,150 = 2.01, p < 0.05* | F 1,10 = 0.40, p = 0.54 | F 15,150 = 1.61, p = 0.08 |
| Exp. 2a | F 16,176 = 1.56, p = 0.08 | F 1,11 = 4.13, p = 0.07 | F 16,176 = 0.78, p = 0.71 |
| Exp. 2b | F 5.47,240 = 4.12, p < 0.05* | F 1,15 = 0.20, p = 0.66 | F 16,240 = 1.06, p = 0.39 |
| Exp. 3 | F 4.63,660 = 8.35, p < 0.05* | F 1,20 = 0.06, p = 0.80 | F 33,660 = 8.35, p = 0.88 |
| Exp. 4 | F 5.25,187 = 2.59, p < 0.05* | F 1,17 = 1.62, p = 0.22 | F 11,187 = 0.77, p = 0.66 |
| Exp. 5 | F 1.55,090 = 2.13, p < 0.05* | F 1,19 = 3.29, p = 0.09 | F 11,209 = 0.89, p = 0.55 |
| Exp. 8 | F 3.35,144 = 12.00, p < 0.05* | F 1,16 = 0.57, p = 0.46 | F 9,144 = 0.66, p = 0.75 |
Significant change in total consumption across days in both self‐administration groups.
Methamphetamine self‐administration decreases consumption and preference for 10 and 20 percent alcohol
Experiment 2 examined the effect of MA self‐administration on alcohol consumption in the home cage. There were significant main effects of alcohol intake across days (10 percent: F 5.44,320 = 3.99, p < 0.05; 20 percent: F 5.20,224 = 5.91, p < 0.05) and preference across days (10 percent: F 5.06,400 = 5.81, p < 0.05; 20 percent: F 4.76,240 = 5.61, p < 0.05). There was a main effect of self‐administration on alcohol intake (10 percent: F 1,20 = 13.53, p < 0.05; 20 percent: F 1,14 = 7.21, p < 0.05) but no overall main effect of self‐administration on alcohol preference (10 percent: F 1,25 = 1.98, p = 0.17; 20 percent: F 1,15 = 4.94, p = 0.063). There were also significant interactions between days and self‐administration (Intake: 10 percent: F 5.44,320 = 2.87, p < 0.05; 20 percent: F 5.20,224 = 4.94, p < 0.05; Preference: 10 percent: F 5.06,400 = 4.39, p < 0.05; 20 percent: F 4.76,240 = 1.96, p < 0.05). Post hoc analyses revealed that alcohol consumption was decreased after initiation of MA self‐administration and during subsequent extinction sessions but not during the baseline phase as illustrated in Fig. 3. There were no differences in total consumption of water and alcohol between MA and saline groups (Table 1). Similarly, average MA infusions during the self‐administration procedure was similar between the 10 and 20 percent alcohol consumption groups (18.0 ± 0.8 and 18.1 ± 0.5 infusions, respectively), and no changes were observed on extinction responding (data not shown).
Figure 3.
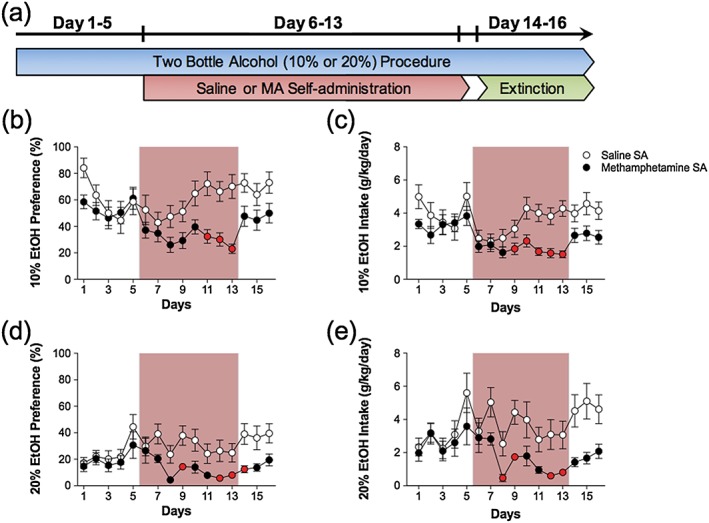
Methamphetamine (MA) self‐administration reduces 10 and 20 percent alcohol consumption and preference. (a) Timeline illustrating the schedule of combining the two‐bottle choice procedure with daily 2‐hour self‐administration sessions and extinction sessions. The preference for (b) 10 percent and (d) 20 percent alcohol was significantly reduced during MA self‐administration (rose shading). Similarly, intake of (c) 10 percent and (e) 20 percent alcohol was also significantly reduced during MA self‐administration (rose shading). The red symbols indicate significance between MA and saline self‐administration groups on that day (p < 0.05); n = 9–21/group
Methamphetamine self‐administration decreases consumption and preference for alcohol after prolonged alcohol consumption
Experiment 3 assessed whether MA self‐administration would disrupt alcohol consumption following prolonged alcohol consumption. There was a main effect of day for both intake and preference (Intake: F 4.88,660 = 27.08, p < 0.05; Preference: F 4.68,660 = 22.04, p < 0.05). The main effects of self‐administration for alcohol intake and preference did not reach statistical significance (Intake: F 1,20 = 4.03, p = 0.058; Preference: F 1,20 = 4.28, p = 0.052). However, there was a significant interaction between days and self‐administration for both intake and preference (Intake: F 4.88,660 = 7.50, p < 0.05: Preference: F 4.68,660 = 5.96, p < 0.05). Post hoc analyses revealed that alcohol consumption was only decreased after initiation of MA self‐administration and during subsequent extinction sessions (Fig. 4). Animals in the MA self‐administration group averaged 21.2 ± 1.83 infusions, and there were no differences in total consumption of water and alcohol between MA and saline groups, as detailed in Table 1.
Figure 4.
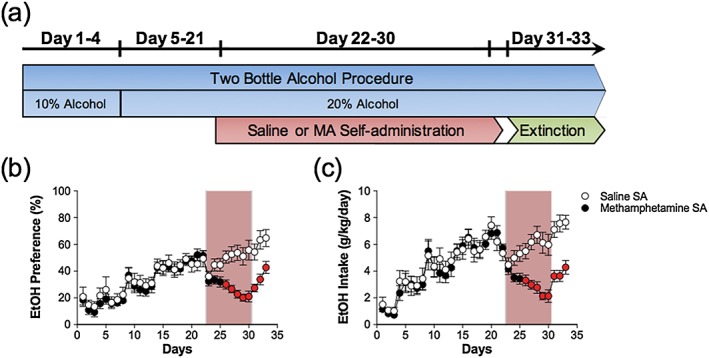
Methamphetamine (MA) self‐administration reduces alcohol consumption and preference after prolonged alcohol consumption. (a) Timeline illustrating schedule of combining the 24‐hour two‐bottle choice procedure and daily 2‐hour self‐administration sessions and extinction sessions. (b) Preference and (c) intake of alcohol after prolonged alcohol consumption were both significantly reduced during MA self‐administration (rose shading). The red symbols indicate significance between MA and saline self‐administration groups on that day (p < 0.05); n = 10–14/group
Methamphetamine self‐administration reduces subsequent initiation of alcohol consumption
Experiment 4 tested whether MA self‐administration would alter subsequent alcohol consumption. There was a main effect of day for both alcohol intake and preference (Intake: F 3.56,154 = 14.10, p < 0.05; Preference: F 4.40,154 = 4.28, p < 0.05), as well as significant interactions of intake and preference between days and self‐administration (Intake: F 3.56,154 = 2.80, p < 0.05; Preference: F 4.40,154 = 3.81, p < 0.05). There was no main effect of self‐administration for either alcohol intake or preference (Intake: F 1,14 = 4.52, p = 0.052; Preference: F 1,14 = 4.50, p < 0.052). Post hoc analyses revealed that alcohol consumption was decreased on days with concurrent MA use, as illustrated in Fig. 5. There were no differences in total consumption of water and alcohol between MA and saline groups (Table 1).
Figure 5.
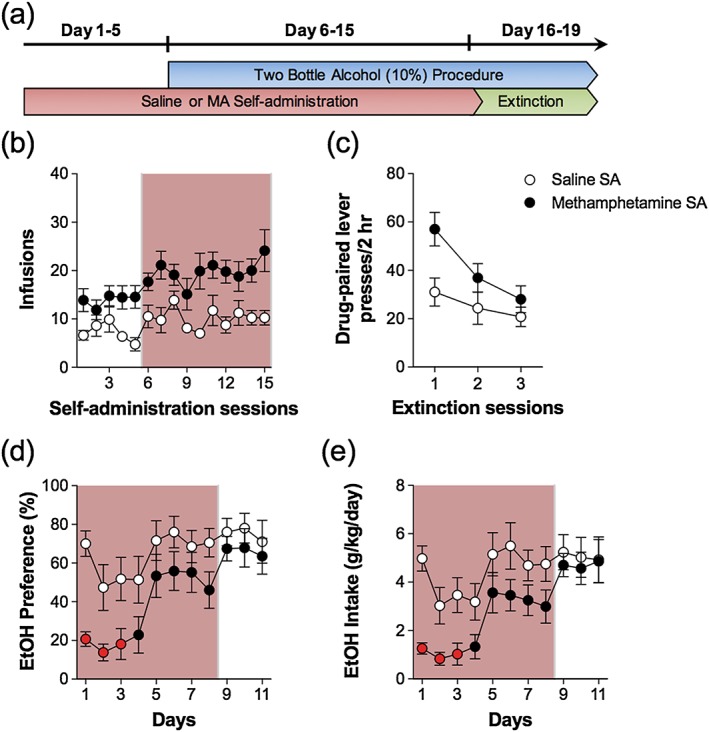
Initiating methamphetamine self‐administration prior to concurrent alcohol consumption procedures reduces initial alcohol consumption and preference. (a) Timeline illustrating the schedule of combining the 24‐hour two‐bottle choice procedure and daily 2‐hour self‐administration sessions and extinction sessions. (b) Average number of MA infusions (± SEM; 0.1 mg/kg/100 µl infusion) during each 2‐hour session over the 5 days prior to alcohol consumption and the 10 days concurrent with alcohol consumption (rose shading). (c) Average number of drug‐paired lever presses for each 2‐hour session during the three‐day extinction procedure. Prior self‐administration of methamphetamine reduces (d) preference and (e) intake of 10 percent alcohol during the beginning of the co‐use phase (rose shading) but not during the later days of the co‐use phase. The red symbols indicate significance between MA and saline self‐administration groups on that day (p < 0.05); n = 9–11/group
Methamphetamine self‐administration has no lasting effects on future alcohol consumption
Experiment 5 evaluated whether previous MA self‐administration would have lasting effects on future alcohol consumption. Figure 6 illustrates that MA self‐administration has no lasting effects on subsequent alcohol intake or preference. The main effect of day for both alcohol intake and preference was significant (Intake: F 4.26,228 = 17.65, p < 0.05; Preference: F 4.37,209 = 12.88, p < 0.05); however, the main effect of self‐administration group was not significant (Intake: F 1,19 = 1.55, p = 0.23; Preference: F 1,19 = 2.50, p < 0.13). There was also no statistically significant interaction between days and self‐administration group for intake or preference (Intake: F 4.26,228 = 0.60, p = 0.84; Preference: F 4.37,209 = 0.35, p = 0.97). There were no differences in total consumption of water and alcohol between MA and saline groups (Table 1).
Figure 6.
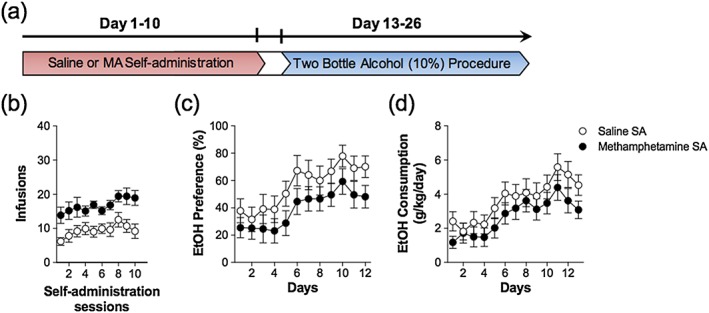
Temporally dissociating prior methamphetamine (MA) self‐administration from two‐bottle alcohol procedure has no lasting impact on subsequent alcohol consumption or preference. (a) Timeline illustrating the schedule of combining the 24‐hour two‐bottle choice procedure and daily 2‐hour self‐administration sessions. (a) Prior MA self‐administration did not significantly alter alcohol (b) preference or (c) intake when MA and alcohol were not administered concurrently; n = 10–11/group
Methamphetamine self‐administration does not alter blood alcohol concentrations
Experiment 6 utilized tail vein blood collected from animals in Experiment 2 during the self‐administration phase of the experiment to determine differences in blood alcohol concentrations 30 minutes following 1.0 g/kg alcohol administered via oral gavage. Figure 7 illustrates that there was no significant difference in blood alcohol concentrations following MA or saline self‐administration (t 10 = 0.87, p = 0.40).
Figure 7.
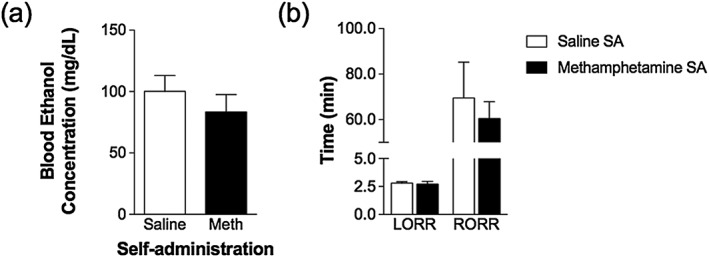
Methamphetamine (MA) self‐administration does not alter blood alcohol concentrations or alcohol intoxication. Blood was collected 30 minutes following oral administration of 1.0 g/kg alcohol in animals that self‐administered MA (n = 7) or saline (n = 7) for 14 days in daily 2‐hour self‐administration sessions. (a) No significant differences were detected between the two self‐administration groups. Similarly, the loss and regain of the righting reflex was measured following administration 3.0 mg/kg alcohol (i.p.) in animals that self‐administered MA (n = 7) or saline (n = 5) for 14 days in daily 2‐hour self‐administration sessions. (b) No significant differences in either measure were observed between the self‐administration groups
Methamphetamine self‐administration does not alter the sensitivity to alcohol‐induced sedation
Experiment 7 tested sensitivity to alcohol intoxication using a righting reflex test. We measured the time for animals to lose and regain their righting reflex after an injection of 3.0 g/kg alcohol. Figure 7 illustrates no significant differences between MA and saline self‐administration groups in the time to lose righting reflex (t 10 = 0.28, p = 0.79) or regain righting reflex (t 10 = 0.57, p = 0.58).
Methamphetamine self‐administration has no effect on sucrose consumption
Experiment 8 examined whether MA self‐administration would affect consumption of a 0.1 percent sucrose solution to evaluate MA effects on consumption of a highly palatable, non‐drug reward. Figure 8 illustrates that the sucrose solution was highly preferred and consumed in both groups. There were main effects of sucrose intake and preference across days (Intake: F 3.49,144 = 13.70, p < 0.05; Preference: F 1.17,144 = 10.97, p < 0.05) but no differences between the self‐administration groups (Intake: F 1,6 = 0.71, p = 0.41; Preference: F 1,16 = 0.76, p = 0.40). There were also no significant interactions between days and self‐administration for intake or preference (Intake: F 4.26,228 = 0.99, p = 0.45; Preference: F 4.37,209 = 0.61, p = 0.79). There were no differences in total consumption of water and sucrose between MA and saline groups, as detailed in Table 1.
Figure 8.
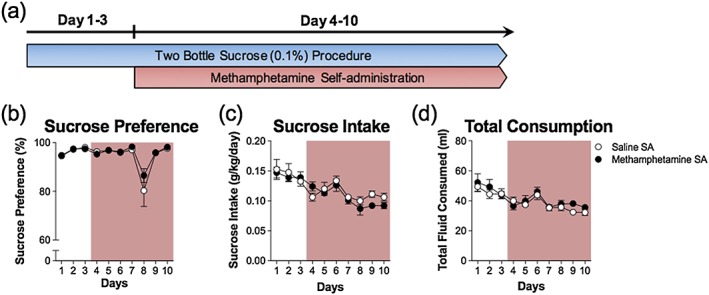
Methamphetamine (MA) self‐administration has no effect on sucrose consumption or preference. (a) Timeline illustrating the schedule of combining the 24‐hour two‐bottle choice procedure and daily 2‐hour self‐administration sessions. MA self‐administration did not alter sucrose preference (b) or intake (c). (d) Total fluid consumption during the three phases was not altered. The rose colored area indicates concurrent MA self‐administration and two‐bottle sucrose procedures; n = 10/group
Discussion
This study is the first to implement a co‐use model of MA self‐administration and free choice alcohol consumption to identify the interactive effects on volitional consumption behaviors. Alcohol consumption enhanced MA self‐administration during the acquisition phase when rats were trained on an FR1 schedule of reinforcement. This effect did not persist when rats were advanced to an FR5 and progressive ratio schedule of reinforcement suggesting that alcohol consumption may enhance the acquisition of MA taking but does not alter the motivation for MA seeking under a progressive ratio schedule of reinforcement. We also showed that alcohol consumption did not alter MA seeking during extinction conditions. Surprisingly, we observed robust decreases in alcohol intake during MA self‐administration. These effects did not seem to be related to MA‐induced changes in alcohol metabolism or sensitivity.
The observed MA‐induced decrease in alcohol intake was surprising because MA is frequently co‐administered with alcohol in humans, and dexamphetamine use, an active metabolite of MA, has been shown to facilitate heavy drinking (Green & Moore 2009; Bujarski et al. 2014). Co‐use of MA and alcohol produces enhanced subjective effects in humans suggesting a synergistic effect (Mendelson et al. 1995; Kirkpatrick et al. 2012). Combining MA with alcohol is not known to produce an active metabolic such as cocaethylene, a metabolite produced with co‐administration of alcohol and cocaine that results in enhanced and prolonged drug effects (McCance et al. 1995; Ikegami et al. 2002). However, MA and cocaine both enhance extracellular dopamine through DAT reversal and inhibition, respectively. Therefore, it is surprising that we observe decreased rather than enhanced alcohol intake and preference during MA co‐use. It is possible that these observations were confounded by the use of alcohol‐preferring P rats. P rats were derived from a Wistar stock of rats through bidirectional selective breeding (Li et al. 1987). It is possible that the selective breeding resulted in idiosyncratic neurobiological or behavioral changes that correspond with unique drug interactive effects that are unlike non‐selected outbred rats, potentially limiting the extrapolation of these finding. Future studies should consider exploring similar interactions in additional rat lines, including outbred rats having high and low alcohol intake/preferences.
Our studies indicate that decreases in alcohol consumption do not appear to result from MA‐induced alterations in alcohol metabolism or alcohol‐induced intoxication. We show that blood‐alcohol concentrations and alcohol‐induced sedative effects are not altered in MA self‐administrating animals. It is important to note that our studies did not examine the dose‐responsivity of alcohol‐induced sedation or other alcohol effects that are often observed at lower doses of alcohol. Thus, it remains possible that MA self‐administration produces a leftward shift in alcohol sensitivity, whereby aversive effects are observed at alcohol concentrations that are typically preferred (e.g. 10 percent alcohol). Future studies would benefit from a more extensive characterization of lower alcohol doses and concentrations.
It is also important to consider the effect of MA on general consumption behavior as an alternative explanation for the reduction in alcohol intake. To this end, we observed no changes in total fluid consumption during the self‐administration phases and no effect of MA on sucrose consumption suggesting that there were no overt alterations in overall consumption behaviors. At low doses, MA produces a mild aversive taste reactivity to sucrose, but this is not observed at higher MA doses (Parker 1993; Cagniard & Murphy 2009). It is therefore unlikely that the reduction in alcohol consumption is due to non‐specific MA‐induced changes in alcohol sensitivity, generalized effects on consumption or a MA‐induced taste aversion.
Our findings are the first to examine the effects of MA self‐administration on alcohol intake and reveal that MA decreases alcohol consumption and preference. There are, however, several studies reporting the effects of amphetamine on alcohol consumption. The results of these studies are largely inconsistent and provide mixed support for our findings. For example, some studies report amphetamine‐induced decreases in alcohol consumption (Halladay et al. 1999; Pohorecky & Sweeny 2012), while others show amphetamine‐induced increases or no effect on alcohol intake (Levy & Ellison 1985; Hubbell et al. 1991; Samson et al. 1993; Fahlke et al. 1994). A notable procedural difference between our study and much of the published work is the use of experimenter‐delivered amphetamine, compared with our paradigm that utilizes MA self‐administration. There is a growing body of research revealing biological differences in animals that self‐administer MA or cocaine compared with those who receive passively administered drugs (Stefanski et al. 1999; Galici et al. 2000; Twining et al. 2009). It is possible that neurobiological and motivational changes resulting from volitional administration of MA affects alcohol consumption differently than passive administration. The majority of these studies also use sweetened alcohol that may increase the incentive to consume alcohol, perhaps masking the ability to observe decreased alcohol consumption and preference. Lastly, amphetamine has lower potency than MA, and it is possible that amphetamine is not sufficient to consistently reduce alcohol intake (Hall et al. 2008; Goodwin et al. 2009). Further studies are needed to fully understand the interactive effects of MA and alcohol that we observed.
It is well established that drugs of abuse produce their subjective effects through potent interactions in the mesocorticolimbic dopamine (DA) system that is comprised of neuronal projections originating in the ventral tegmental area and terminating in forebrain regions, such as the nucleus accumbens. Both alcohol and MA increase extracellular DA in the mesolimbic system, although the mechanisms are quite distinct. Alcohol is thought to modulate the activity of ventral tegmental area gamma‐Aminobutyric acid interneurons to increase vesicular DA release in terminal areas (Weiss et al. 1993; Morikawa & Morrisett 2010; Theile et al. 2011). MA, on the other hand, blocks vesicular monoamine transporters resulting in a concentration differential across the plasma membrane that reverses the plasmalemmal transporter (Volz et al. 2007; Vergo et al. 2007). It is therefore surprising that MA self‐administration diminishes alcohol drinking given the powerful effects that both drugs have on the mesocorticolimbic dopamine system.
It is possible that MA self‐administration reduces alcohol intake by producing lingering extracellular dopamine levels that interfere with the rewarding effects of alcohol. Our studies show that alcohol consumption is only reduced during concurrent MA use, suggesting a transient effect that could be explained by an excess of extracellular dopamine. Previous studies utilizing dopamine agonists to enhance dopamine transmission provide support for this notion. Acute treatments of non‐selective dopamine receptor agonists, as well as selective D1 and D2 receptor agonists, decrease alcohol consumption in alcohol‐preferring rats (Weiss et al. 1990; Dyr et al. 1993; McMillen et al. 2013). Further support comes from a recent study where the catechol‐o‐methyltransferase inhibitor, talcapone, reduced alcohol consumption in a cued‐access paradigm (McCane et al. 2014). Together, these studies suggest that increasing dopamine neurotransmission by inhibiting dopamine breakdown or enhancing dopamine receptor signaling may decrease alcohol intake.
An important caveat to our studies was that consumption of MA and alcohol was assessed in separate sessions dissociated temporally. That is, rats did not have access to both MA and alcohol simultaneously in the same session, prohibiting us from measuring the direct interactive effects between the drugs. Despite this limitation, we show that a 2‐hour MA self‐administration session impacts alcohol consumption over the following 24‐hour period. It is important to note that the MA effects on alcohol consumption were not observed after 24‐hour withdrawal and generally had no lasting impact when tested during MA abstinence. The results of our findings necessitate further studies using additional alcohol consumption models to fully characterize the impact of MA on alcohol consumption that would enable the determination of possible mechanisms.
In conclusion, our results show that self‐administered MA reduces home‐cage alcohol consumption and preference in alcohol‐preferring P rats. Alcohol consumption had minimal effects on MA intake and did not appear to alter MA reinforcement or seeking. MA had a near immediate and short‐lived impact on alcohol consumption that was only maintained, while both drugs were consumed within the same 24‐hour period. MA did not have a lasting impact on alcohol consumption during MA abstinence. MA did not alter the metabolism or sensitivity of alcohol. Future studies should be aimed at determining a mechanism for this effect of MA on alcohol consumption and preference.
Acknowledgements
This work was supported by United States Public Health Service grant DA033358 Supplement.
Authors Contribution
MCW and RKB were responsible for the study concept and design. MCW and JS performed the surgical and self‐administration procedures. EMG, JS and MCW were responsible for measuring fluid consumption. MCW and RKB performed the injections for experimenter‐delivered alcohol. MCW collected tail vein blood and performed blood‐alcohol analysis. MCW and RKB conducted data analysis. MCW, EMG and RKB drafted the manuscript. All authors critically reviewed content and approved the final version for publication.
Winkler, M. C. , Greager, E. M. , Stafford, J. , and Bachtell, R. K. (2018) Methamphetamine self‐administration reduces alcohol consumption and preference in alcohol‐preferring P rats. Addiction Biology, 23: 90–101. doi: 10.1111/adb.12476.
References
- Brecht ML, Huang D, Evans E, Hser YI (2008) Polydrug use and implications for longitudinal research: ten‐year trajectories for heroin, cocaine, and methamphetamine users. Drug Alcohol Depend 96:193–201. DOI: 10.1016/j.drugalcdep.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Roche DJO, Lunny K, et al (2014) The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug Alcohol Depend 142:127–132. DOI: 10.1016/j.drugalcdep.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Murphy NP (2009) Taste reactivity and its modulation by morphine and methamphetamine in C57BL/6 and DBA/2 mice. Physiol Behav 96:412–420. DOI: 10.1016/j.physbeh.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Galea S, Ahern J, et al (2003) Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–98. Addict Abingdon Engl 98:739–747. [DOI] [PubMed] [Google Scholar]
- Dyr W, McBride WJ, Lumeng L, et al (1993) Effects of D1 and D2 dopamine receptor agents on ethanol consumption in the high‐alcohol‐drinking (HAD) line of rats. Alcohol 10:207–212. [DOI] [PubMed] [Google Scholar]
- EMCDDA (2009) Annual Report 2009: The State of the Drug Problem in Europe.
- Fahlke C, Hansen S, Engel JA, Hård E (1994) Effects of ventral striatal 6‐OHDA lesions or amphetamine sensitization on ethanol consumption in the rat. Pharmacol Biochem Behav 47:345–349. [DOI] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP (2000) Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol 387:59–62. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, et al (2009) Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem 284:2978–2989. DOI: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Moore D (2009) “Kiddie drugs” and controlled pleasure: recreational use of dexamphetamine in a social network of young Australians. Int J Drug Policy 20:402–408. DOI: 10.1016/j.drugpo.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Wagner GC, Hsu T, et al (1999) Differential effects of monoaminergic agonists on alcohol intake in rats fed a tryptophan‐enhanced diet. Alcohol 18:55–64. [DOI] [PubMed] [Google Scholar]
- Hall DA, Stanis JJ, Avila HM, Gulley JM (2008) A comparison of amphetamine‐ and methamphetamine‐induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology (Berl) 195:469–478. DOI: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell CL, Marglin SH, Spitalnic SJ, et al (1991) Opioidergic, serotonergic, and dopaminergic manipulations and rats' intake of a sweetened alcoholic beverage. Alcohol 8:355–367. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Olsen CM, Fleming SM, et al (2002) Intravenous ethanol/cocaine self‐administration initiates high intake of intravenous ethanol alone. Pharmacol Biochem Behav 72:787–794. [DOI] [PubMed] [Google Scholar]
- Kavanagh KA, Schreiner DC, Levis SC, et al (2015) Role of adenosine receptor subtypes in methamphetamine reward and reinforcement. Neuropharmacology 89:265–273. DOI: 10.1016/j.neuropharm.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia S, Sell MA, Relyea G (2007) Mono‐ versus polydrug abuse patterns among publicly funded clients. Subst Abuse Treat Prev Policy 2:33 DOI: 10.1186/1747-597X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, et al (2012) Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology (Berl) 219:191–204. DOI: 10.1007/s00213-011-2390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy AD, Ellison G (1985) Amphetamine‐induced enhancement of ethanol consumption: role of central catecholamines. Psychopharmacology (Berl) 86:233–236. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM (1987) Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol 1:91–6. [PubMed] [Google Scholar]
- Martin I, Lampinen TM, McGhee D (2006) Methamphetamine use among marginalized youth in British Columbia. Can J Public Health Rev Can Sante Publique 97:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance EF, Price LH, Kosten TR, Jatlow PI (1995) Cocaethylene: pharmacology, physiology and behavioral effects in humans. J Pharmacol Exp Ther 274:215–223. [PubMed] [Google Scholar]
- McCane AM, Czachowski CL, Lapish CC (2014) Tolcapone suppresses ethanol intake in alcohol‐preferring rats performing a novel cued access protocol. Alcohol Clin Exp Res 38:2468–2478. DOI: 10.1111/acer.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen BA, Lommatzsch CL, Sayonh MJ, Williams HL (2013) Interactions of a dopamine D1 receptor agonist with glutamate NMDA receptor antagonists on the volitional consumption of ethanol by the mHEP rat. Pharm Basel Switz 6:469–479. DOI: 10.3390/ph6040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Upton R, Jacob P 3rd (1995) Methamphetamine and ethanol interactions in humans. Clin Pharmacol Ther 57:559–68. DOI: 10.1016/0009-9236(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA (2010) Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol 91:235–88. DOI: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady KE, Arria AM, Fitzelle DM, Wish ED (2008) Heavy drinking and polydrug use among college students. J Drug Issues 38:445–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas LC, Novier A, Van Skike CE, et al (2015) The effects of acute alcohol on motor impairments in adolescent, adult, and aged rats. Alcohol 49:121–126. DOI: 10.1016/j.alcohol.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Parker LA (1993) Taste reactivity responses elicited by cocaine‐, phencyclidine‐, and methamphetamine‐paired sucrose solutions. Behav Neurosci 107:118–129. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Sweeny A (2012) Amphetamine modifies ethanol intake of psychosocially stressed male rats. Pharmacol Biochem Behav 101:417–426. DOI: 10.1016/j.pbb.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (2014a) The DAWN Report: Alcohol and Drug Combinations are More Likely to Have a Serious Outcome than Alcohol Alone in Emergency Department Visits Involving Underage Drinking.
- SAMHSA (2014b) The DAWN Report: Emergency Department Visits Involving Methamphetamine: 2007–2011. [PubMed]
- Samson HH, Hodge CW, Tolliver GA, Haraguchi M (1993) Effect of dopamine agonists and antagonists on ethanol‐reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull 30:133–141. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, et al (1999) Neuroadaptations in the dopaminergic system after active self‐administration but not after passive administration of methamphetamine. Eur J Pharmacol 371:123–35. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA (2011) GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience 172:94–103. DOI: 10.1016/j.neuroscience.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS (2009) Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci 123:913–925. DOI: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M, Lotharius J (2007) Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res 1185:18–32. DOI: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, Fleckenstein AE (2007) The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine‐induced dopaminergic deficits. J Neurochem 101:883–8. DOI: 10.1111/j.1471-4159.2006.04419.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF (1993) Oral alcohol self‐administratoin stimulates dopamine release in the rat nucleus accumbens: genetic and motivation determinants. J Pharmacol Exper Ther 267:250–258. [PubMed] [Google Scholar]
- Weiss F, Mitchiner M, Koob GF (1990) Free‐choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharm 101:178–186. [DOI] [PubMed] [Google Scholar]


