Abstract
Natural resources continue to be an invaluable source of new, novel chemical entities of therapeutic utility due to the vast structural diversity observed in them. The quest for new and better drugs has witnessed an upsurge in exploring and harnessing nature especially for discovery of antimicrobial, antidiabetic, and anticancer agents. Nature has historically provide us with potent anticancer agents which include vinca alkaloids [vincristine (VCR), vinblastine, vindesine, vinorelbine], taxanes [paclitaxel (PTX), docetaxel], podophyllotoxin and its derivatives [etoposide (ETP), teniposide], camptothecin (CPT) and its derivatives (topotecan, irinotecan), anthracyclines (doxorubicin, daunorubicin, epirubicin, idarubicin), and others. In fact, half of all the anti-cancer drugs approved internationally are either natural products or their derivatives and were developed on the basis of knowledge gained from small molecules or macromolecules that exist in nature. Three new anti-cancer drugs introduced in 2007, viz. trabectedin, epothilone derivative ixabepilone, and temsirolimus were obtained from microbial sources. Selective drug targeting is the need of the current therapeutic regimens for increased activity on cancer cells and reduced toxicity to normal cells. Nanotechnology driven modified drugs and drug delivery systems are being developed and introduced in the market for better cancer treatment and management with good results. The use of nanoparticulate drug carriers can resolve many challenges in drug delivery to the cancer cells that includes: improving drug solubility and stability, extending drug half-lives in the blood, reducing adverse effects in non-target organs, and concentrating drugs at the disease site. This review discusses the scientific ventures and explorations involving application of nanotechnology to some selected plant derived molecules. It presents a comprehensive review of formulation strategies of phytoconstituents in development of novel delivery systems like liposomes, functionalized nanoparticles (NPs), application of polymer conjugates, as illustrated in the graphical abstract along with their advantages over conventional drug delivery systems supported by enhanced biological activity in in vitro and in vivo anticancer assays.
Keywords: phytoconstituents, anti-cancer, nanotechnology, selective targeting, drug delivery systems
Graphical Abstract.
Versatile Drug delivery systems for anti-cancer Phytochemicals.
Introduction
Cancer is a major public health issue and one of the most common causes of morbidity and mortality worldwide and the second leading cause of death globally. It was responsible for 8.8 million deaths in 2015. Approximately 70% of deaths of cancer occur in low and middle-income countries. The annual number of new cases is projected to rise from 14.1 million in 2012 to 21.6 million in 2030. In addition, the economic impact of cancer is significant; in 2010, the total annual economic cost of cancer was estimated at approximately US$ 1.16 trillion, threatening economies at all income levels as well as causing financial catastrophe for individuals and families. One defining feature of cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries and which can then invade adjoining parts of the body and spread to other organs (metastases) which are a major cause of death from cancer (WHO Cancer Factsheet Feb, 2010).
Plants have been historically used in the alleviation of many diseases including cancer with over 60% of currently used anti-cancer drugs derived from natural sources. Nature is an attractive source of new therapeutic entities with plants, animals, marine organisms, and microorganisms all contributing to drugs with potential application as anti-cancer agents. Naturally occurring anticancer agents include vinca alkaloids, taxanes and its analogs, podophyllotoxin and its derivatives, camptothecin (CPT) and its derivatives, anthracyclines, and many others. In fact, half of all the anti-cancer drugs approved internationally are either natural products or their derivatives and were developed on the basis of knowledge gained from small molecules or macromolecules that exist in nature (Bhanot et al., 2011; Song et al., 2014).
Supplementary Table 1 represents the structural diversity of the anticancer agents obtained from natural resources.
Most of the clinically used anticancer drugs (formulated as conventional delivery systems) possess many significant limitations. These include their low solubility in water, unsuitability for oral administration, short half-life in the body, poor specificity associated with severe side effects (Cho et al., 2008). The therapeutic regimen is associated with severe systemic toxicity and development of multidrug resistance (MDR) primarily mediated by overexpression of proteins of the ATP binding cassette (ABC) transporter superfamily. These MDR proteins especially P-glycoprotein (Pgp) are responsible for energy dependent efflux of drugs, resulting in a reduced amount of chemotherapeutic agent present in cancer cells. Several Pgp inhibitors have been studied over the last few decades to overcome MDR in cancer. The first generation of ABC blockers [verapamil (VRP), cyclosporine A, and quinidine] are the most widely studied as these are already approved for other uses and can be repositioned and be clinically evaluated for new therapeutic use. Clinical studies on breast cancer have indicated that a combination of VCR and Pgp blocker VRP can enhance antitumor activity. This highlights the need to develop safe, effective, and novel drug delivery systems to target with MDR breast cancer (Taylor et al., 1997). Similar studies can be conducted on many anti-cancer phytochemicals in combination with Pgp blockers to improve their clinical efficacy in MDR cancer cell lines (Granja et al., 2016).
Another important factor is the tumor microenvironment that contributes to the development of MDR and affects a patient response to treatment. Nanocarriers offer promising delivery solutions for combination anticancer therapy that is required for treatment of MDR cancers. The benefits of nanocarriers include—their amenability to being engineered to achieve multiple effects using a single system, they improve the therapeutic index of drugs and can positively modulate their pharmacokinetic profile, they preferentially accumulate in the tumor microenvironment via EPR effect, their capacity to be conjugated to targeting moieties, and circumventing drug efflux by preferentially localizing agents in the perinuclear region of cell, away from membrane localized efflux pumps. These two drawbacks restrict the efficacy of these drugs and serve as the driving force for designing novel delivery systems to reduce the side effects and improve the clinical efficacy of existing drugs. It has also fuelled augmented research activity into development of new analogs of existing nature derived drugs and intensified search for new compounds from natural resources along with application of nanotechnology approaches to improve the safety and efficacy profiles of these drugs (Maeda, 2010; Thanki et al., 2013).
Nanotechnology has the potential to revolutionize cancer diagnosis and therapy. Often there is a defective, leaky vascular architecture associated with a tumor as a result of the poorly regulated nature of tumor angiogenesis. The interstitial fluid within a tumor is drained by a poorly formed lymphatic system. As a result submicron-sized particulate matter can easily extravasate into the tumor and be retained resulting in an “enhanced permeability and retention” (EPR) effect. This EPR effect is advantageous for a properly designed nanoparticulate system for passive targeting that allow nanocarriers loaded with cytotoxic agents to accumulate in the tumor tissues. Active targeting approaches acts by conjugating nanocarriers containing chemotherapeutics with molecules that bind to over-expressed antigens or receptors on the target cells. The biodistribution profile of nanocarriers can be fine-tuned by modifying their surface physico-chemical properties to target the tissue of interest, an outcome of recent advances in surface-engineering technology. This contributes to increased amount of drug reaching the targeted tumor sites with minimum systemic drug toxicity. Nanotechnologically modified drugs and drug delivery systems are increasing day by day in cancer treatment and some are used clinically with good results. Nanotechnology can be used for better cancer diagnosis, more efficient drug delivery to tumor cells, and molecular targeted cancer therapy that improving the therapeutic management of cancer patients (Mansoori et al., 2007; Schluep et al., 2009; Moorthi et al., 2011; Aliosmanoglu and Basaran, 2012).
The majority of current nanotechnology platforms for chemotherapy have involved repackaging of traditional anticancer agents into various forms of nanometer-sized delivery vehicles. Nanotechnology has provided a platform to improvise drug delivery using new concepts and carriers that conventional technologies have been unable to achieve. This review describes the application of nanotechnology in formulating drug delivery systems of some selected plant based anticancer agents [vincristine (VCR), paclitaxel (PTX), etoposide (ETP), curcumin (CUR), resveratrol, CPT, genistein, quercetin, and capsaicin (CAP) to name few of them] leading to an improved anticancer profile (Taratula et al., 2009).
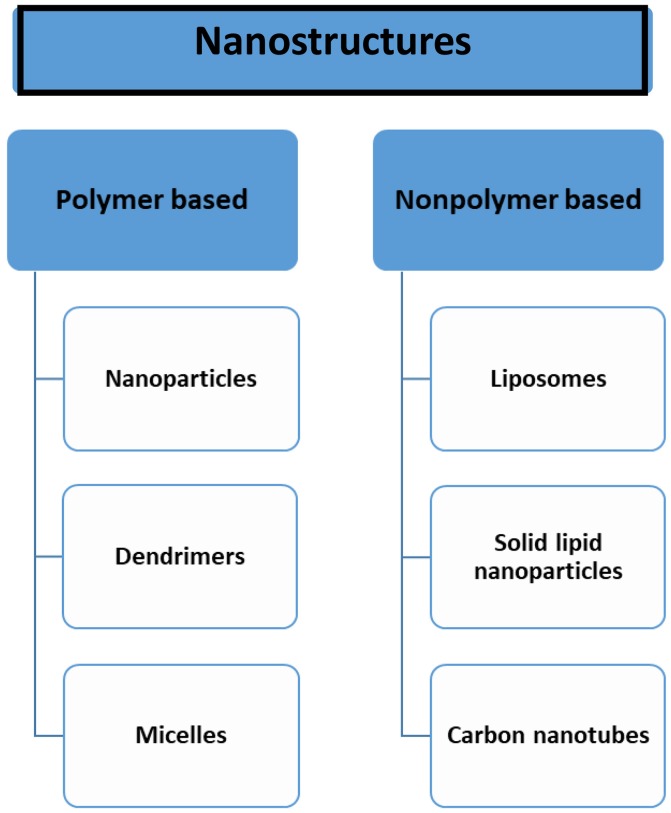
Figure 1 depicts the nano-drug delivery vehicles used in formulation development and Supplementary Table 2 gives an overview of the various methods of preparation of nanodrug delivery systems used in development.
Figure 1.
Nanotechnology based drug delivery vehicles.
Nanoparticles
The past two decades have witnessed many therapeutics based on nanoparticles (NPs) have been introduced in the market for therapeutic management of cancer. Advances in nanotechnology and an increased understanding of the importance of nanoparticle characteristics (size, shape, and surface properties) for biological interactions at the molecular level have created novel opportunities for development of NPs for versatile therapeutic applications.
Chen et al. prepared dual agent loaded—VCR and VRP poly(lactic-co-glycolic acid) (PLGA) NPs to reduce toxicity and enhance antitumor activity in multidrug resistant breast tumor Eenograft model. The PLGA NPs were prepared by emulsification/sonication method. They reported that the co-encapsulated NPs had lower toxicity (8.52 mg/kg) in comparison to VCR–VRP combination (4.93 mg/kg) and VCR–PLGANPs–VRP PLGANPs combination (7.85 mg/kg) in the acute toxicity study. The highest inhibition of tumor growth in the MCF-7/ADR (Adriamycin) human breast tumor xenograft was observed in the co-encapsulated NPs group (tumor mass = 0.32 g, inhibition efficiency = 64.04%) in comparison to VCR (tumor mass = 0.83 g, inhibition efficiency = 6.74%), VCR–VRP free combination (tumor mass = 0.62 g, inhibition efficiency = 30.34%), and VCR–PLGANPs plus VRP–PLGANPs combination (tumor mass = 0.47 g, inhibition efficiency = 47.19%). The in vitro and in vivo studies showcased that co-encapsulation of VCR and VRP into PLGA NPs at synergistic ratio exhibited good antitumor efficacy in MDR MCF-7/ADR human breast tumor xenograft models (Chen et al., 2014).
Das et al. loaded apigenin in PLGA nanoparticles (NAp) and studied its effect against ultraviolet B (UVB) and benzo(a)pyrene (BaP) induced skin tumor and mitochondrial dysfunction in mice. The results indicated superior effect for the Nap vs. apigenin alone attributed due to their smaller size, and faster mobility. The NAp reduced tissue damage and frequency of chromosomal aberrations, increased ROS accumulation to mediate mitochondrial apoptosis through modulation of several apoptotic markers and mitochondrial matrix swelling. The developed NAp showed ameliorative potential in combating skin cancer thereby showcasing better potential for use in therapeutic management of skin cancer (Das et al., 2013).
Wang et al. developed PTX and ETP co-loaded polymeric NPs and evaluated their cytotoxicity potential on MG63 and Saos-2 osteosarcoma cell lines. They used PLGA NPs to incorporate the two drugs by solvent evaporation method. The surface of these NPs was modified using polyethylene glycol (PEG) to prolong the blood circulation time. The co-encapsulated NPs exhibited a sustained release profile for PTX and ETP. In vitro cytotoxicity studies showed enhanced effect of combinational drug-loaded PLGA NPs (1.45 and 1.98 μg/ml) vs. the free drugs alone (PTX = 4.56 and 5.26 μg/ml; ETP = 6.12 and 7.15 μg/ml) and their combination (free ETP/PTX = 3.82 and 4.18 μg/ml) on MG63 and Saos-2 osteosarcoma cell lines owing to higher cellular uptake of NPs. The in vitro flow cytometry based cellular uptake studies indicated a time dependent cellular internalization of the NPs resulting in enhanced chemotherapeutic effect. The cell cycle progression studies indicated that the co-encapsulated PTX–ETP PLGA NPs arrested maximum number of cells at the same concentration as individual free drugs, the apoptosis fraction being almost double than the individual drugs in the early and late apoptosis phases of the cycle. These PTX–ETP PLGA NPs exhibited synergistic activity against osteosarcoma cancer cells (Wang et al., 2015).
Siddiqui et al. prepared PEG-PLA NPs of epigallocatechin-3-gallate (EGCG) recently and evaluated them on Mel 928 human melanoma cells (in vitro and in vivo studies). The results indicated an 8-fold increase in efficacy for the NPs vs. free EGCG. The in vivo studies indicated cell cycle phase arrest with modulation in the level of cyclins D1 and D3 protein expression for the EGCG NPs (Siddiqui et al., 2014).
Callewaert et al. developed surface modified NPs of ETP parenteral injectable solution (Teva®), loaded into PLGA or PLGA/P188-blended NPs using nanoprecipitation technique. The results of cytotoxicity studies conducted on murine C6 and F98 cell lines (glioblastoma) indicated that the use of PLGA and PLGA/P188 nanoencapsulation significantly enhanced the antitumor efficiency of ETP and gave a viable parenteral ETP formulation that circumvented the drawbacks of conventional injectable formulation (Callewaert et al., 2013).
Aygül et al. formulated PTX loaded PLGA NPs by emulsification solvent diffusion method. Cytotoxicity study of the PTX PLGA NPs was conducted on Caco-2 cells using the standard MTT assay. The results indicated that these NPs exhibited a significant cytotoxic effect in comparison to PTX alone—cell viability of 80% for PTX PLGA NPs vs. 90% for PTX alone (Aygül et al., 2013).
Bisht et al. developed polymeric NP-encapsulated curcumin (CUR) using micellar aggregates of cross-linked and random copolymers of N-isopropylacrylamide (NIPAAM), with N-vinyl-2-pyrrolidone (VP) and poly(ethyleneglycol)monoacrylate (PEG-A) that had a hydrophobic core and a hydrophilic shell. CUR has a major drawback of low oral bioavailability as demonstrated in many Phase I trials of CUR. Hence development nanotechnology based delivery systems that enable parenteral administration of CUR could potentially enhance the efficacy of CUR. This nanoCUR demonstrated comparable in vitro therapeutic efficacy to free CUR against human pancreatic cancer cell lines (BxPC3, AsPC1, MiaPaca, XPA-1, XPA-2, PL-11, PL-12, PL-18, PK-9, and Panc 2.03) as assessed by cell viability MTT assay. The results of cell uptake studies using fluorescence microscopy demonstrated enhanced uptake of nanoformulation of CUR by the pancreatic cell lines. This nanoformulation was found to enhance the solubility profile of CUR as well (Bisht et al., 2007).
Sebak et al. prepared NPs of noscapine with human serum albumin (HSA) for targeted delivery and evaluated them on SK-BR-3 breast cancer cells using pH coacervation method. The results indicated that the noscapine NPs were significantly better than free noscapine in reducing the cell viability of SK-BR-3 (Sebak et al., 2010).
Tang et al. developed PTX-loaded NPs of star-shaped cholic acid-core PLA-TPGS copolymer by a modified nanoprecipitation method and evaluated them in in vitro and in vivo studies on breast cancer MCF-7 cell lines. The results indicated that the CA-PLA-TPGS NPs had higher antitumor efficacy than the PLA-TPGS NPs and PLGA NPs (both in in vitro and in vivo studies) on MCF-7 cell lines demonstrating that these star-shaped cholic acid-core PLA-TPGS block copolymer could be considered as a potentially promising and effective strategy for breast cancer treatment (Tang et al., 2013).
Majumdar et al. encapsulated luteolin into a polymeric NPs comprising of PLA-PEG and evaluated them on H292 lung cancer cells. The results indicated the nanoluteolin exhibited a higher antiproliferative activity against H292 cells with lower IC50-values than free luteolin (Majumdar et al., 2014).
Sanna et al. formulated polymeric NPs encapsulating resveratrol (RSV) as novel prototypes for prostate cancer. Nanosystems, composed of a biocompatible blend of poly(epsilon-caprolactone) (PCL) and poly(D,L-lactic-co-glycolic acid)poly(ethylene glycol) conjugate (PLGA-PEG-COOH), were prepared by a nanoprecipitation technique. Cellular uptake of NPs was then evaluated in prostate cancer cell lines DU-145, PC-3, and LNCaP using confocal fluorescence microscopy and anti-proliferative efficacy was assessed using MTT assay. The results revealed that NPs were efficiently taken up by the PCa cell lines. These NPs significantly improved the cytotoxicity compared to that of free RSV (IC50 of NPs = 16 vs. 28.4 μM of free RSV) on DU-145 cell line (IC50 of NPs = 18 vs. 50.7 μM of free RSV) on LNCaP cell line, and (IC50 of NPs = 35.5 vs. 47.4 μM of free RSV) on PC-3 cell line (Sanna et al., 2013).
The same group developed novel cationic chitosan (CS)- and anionic sodium alginate (Alg)-coated PLGA NPs loaded with RSV by nanoprecipitation technique. Several studies have also been reported on the use of nanotechnology to vehicle RSV. In particular, RSV incorporation into mPEG-poly(ε-caprolactone)-based NPs resulted in significantly higher cytotoxicity against malignant glioma cells compared to an equivalent dose of free biomolecule. Solid-lipid NPs loaded with RSV also contributed to effectiveness of RSV on decreasing cell proliferation and demonstrated potential benefits for prevention of skin cancer. Moreover, other authors investigated the effect of RSV incorporated in liposome on the proliferation and UVB protection of cells, in order to enhance the efficacy in the prevention and treatment of human skin disorders caused by excessive exposure to UV radiation. Among the polymeric materials, PLGA is a widely used copolymer approved by the FDA for various medical and pharmaceutical applications, such as drug delivery. The combination of PLGA as a hydrophobic polymer and many natural hydrophilic biopolymers like gelatin or sodium alginate provides advantages for both hydrophilic and the hydrophobic nanoparticulate systems. CS and Alg are two major naturally occurring polysaccharides with hydrophilic characteristics that have gained increasing interest in the biomedical field particularly in the drug-delivery area. CS is a polycationic polymer that has one amino group and two hydroxylic functionalities in the repeating glycosidic residue. The amount of cationic or anionic polyelectrolytes employed as surface modifiers resulted in differences in size, surface charge, encapsulation capacity, and in vitro release behavior of RSV-loaded nanoprototypes. Stability studies revealed that encapsulation provides significant protection against light-exposure degradation, thereby contributing to increase the protection of the encapsulated RSV. The translational potential of these novel nanovehicles warrants biological evaluation in several experimental models, e.g., focusing on prostate and skin cancer as target diseases (Sanna et al., 2012).
Merlina et al. prepared PLGA NPs of ferulic acid by double emulsion method and evaluated them on NCI-H460 cells, non-small-cell lung carcinoma cell lines. The results depicted an increased anticancer effect for ferulic acid NPs vs. free ferulic acid. The ferulic acid NPs induced cytotoxicity reflected an increase in the level of reactive oxygen species, DNA damage, altered mitochondrial transmembrane potential, and apoptotic morphological changes demonstrating overall improved efficacy of the nanodrug delivery system (Merlina et al., 2012).
Gupta et al. prepared CUR NPs using silk fibroin polymer and a covalently bonded blend of silk fibroin and chitosan (SFCS) by capillary-microdot technique and evaluated them on MCF-7 and MDA-MB-453 breast cancer cell lines. The results indicated that the silk fibroin based CUR NPs displayed a higher cellular uptake and enhanced reduction in cell viability than the SFCS NPs in both breast cancer cell lines. Hence silk fibroin could prove to be a better nanodrug delivery vehicle and its application can be extended to development of NPs of many more phytochemicals (Gupta et al., 2009).
Magnetic nanoparticles
Magnetic nanoparticles (MNPs), in particular iron oxide (also called magnetite or Fe3O4) NPs and their multifunctionalized counterparts are an important class of nanoscale materials that have attracted great interest for their potential applications in drug delivery and disease diagnosis. Owing to the recent advances in synthesis and surface modification technologies, a variety of new potential applications have become feasible for this class of nanomaterials that may revolutionize current clinical diagnostic and therapeutic techniques. The well-developed surface chemistry of Fe3O4 MNPs allows loading of a wide range of functionalities, such as targeting ligands, imaging, and therapeutic features onto their surfaces. It is now possible to fine-tune the physical parameters of MNPs, such as size, shape, crystallinity, and magnetism. Furthermore, MNPs have the potential for replacement or modification of the coating materials post-synthesis allowing tailoring of the nanoparticle's surface charge, chemical groups, and overall size. Due to their unique physicochemical properties and ability to function at the cellular and molecular level of biological systems, MNPs are being actively investigated as the next generation of targeted drug delivery vehicle. The design of such drug delivery systems requires that the carriers be capable of selectively releasing their payloads at specific sites in the body and thereby treat disease deliberately without any harmful effect on the healthy tissues. In this regard, MNPs represent a promising option for selective drug targeting as they can be concentrated and held in position by means of an external magnetic field. This allows high dose drug-loads to be delivered to a desired target tissue while minimizing the exposure of healthy tissues to the side effects from highly toxic drugs, e.g., chemotherapeutic agents. In addition, preclinical and clinical studies have proven them to be safe and some formulations are now FDA approved for clinical imaging and drug delivery. Thus, fabrication of MNPs as drug conjugates has the potential to greatly benefit inflammatory disease and cancer treatments, and diagnostics (Verma et al., 2013; Ali et al., 2016).
Castillo et al. synthesized PEG coated CPT loaded iron oxide superparamagnetic NPs using an iron co-precipitation method under alkaline conditions and evaluated them on H460 lung cancer cell line. Superparamagnetic iron oxide NPs (SPION) are particularly promising as delivery systems due to their low toxicity and their ability to be used both in cancer diagnosis and therapy. CPT suffers from a reduced in vivo antitumor efficacy owing to its poor water-solubility and chemical instability. CPT derivatives with improved solubility and stability have been developed however their overall therapeutic impact is modest due to their lower activity when compared to CPT. They reported that surface modification of the NPs by PEG enabled higher payload of CPT. No significant difference in the cytotoxic activity was observed among the CPT loaded on either the PEGylated or bare magnetic NPs (% apoptotic cells 80% for both). However, a different behavior of PEGylated and non-PEGylated magnetic NPs can be expected due to longer circulation times of the PEGylated ones (Castillo et al., 2014).
Verma et al. developed magnetic core-shell NPs for aerosol delivery of quercetin (QUR) by nebulization and evaluated their cytotoxicity in lung cancer cell line A549. They coated the surface of the MNPs with PLGA to improve the dispersion of QUR (a poorly soluble drug) in aqueous medium, confer stability against oxidation and ensure biocompatibility of the delivery system. The biocompatibility of the MNPs was characterized by conducting in vitro and in vivo studies. The results indicated a significant reduction in the number of viable A549 cells for QUR-loaded PLGA-MNPs. This study provided proof of concept of the optimized nanoplatform technology of drug loaded nanoparticle delivery in lung cancer using aerosol formulation (Verma et al., 2013).
Liposomes
Liposomes are nanosized lipid carriers formed by the self-assembling phospholipid molecules in an aqueous environment. As liposomes are made up of lipids they are rapidly absorbed in liver and taken up by macrophages thus decreasing their efficacy. This can be avoided by coating liposome lipid surface with ligands such as monosialoganglioside or by incorporating cholesterol, polyvinylpyrrolidone polyacrylamide lipids, glucuronic acid lipids, or phospholipid distearoylphosphatidylcholine (DSPC) into liposomes that increases their circulating time in body. When the liposomes are coated with monosialoganglioside they are called stealth liposomes. The size of these liposomes is about 100 nm. The other type of liposomes i.e., non-stealth liposomes prepared from high phase transition temperature phospholipids helps in increasing circulation times and also accumulate within tumor tissue despite high levels of liver uptake. This surface modification of liposomes improves duration of drug release and also improves targeting of the drug to its site of action along with increased circulation time. Stability of the liposomes can be increased by incorporating cholesterol into it. The concentration of cholesterol is a crucial factor as it regulates the membrane properties. The advantages of using liposomal drugs as opposed to free drugs are well-documented in the literature and include the ability to selectively deliver liposomes to the desired site in the body (Chadha et al., 2008; Mehrabi et al., 2016).
Lu et al. prepared folic acid conjugated PEGylated liposomes of VCR (FA-PEG-LS/VCR) for multidrug resistant cancer therapy by thin-film hydration and extrusion method and evaluated their cytotoxicity on KBv200 cells (multidrug resistant variant), a human epidermoid nasopharyngeal carcinoma cell line using in vitro MTT assay and in vivo antitumor efficacy studies (tumor growth inhibition and apoptosis assessment studies by TUNEL). The results indicated that the IC50 of the PEGylated folic acid conjugated VCR liposomes was 23.99 nM vs. 1.10 μM for free VCR and 363.08 nM for PEG-LS/VCR. The results of in vivo experiments indicated that the folic acid conjugation significantly strengthened the antitumor efficacy of the PEGylated liposomes of VCR and also showed a higher apoptosis index in the TUNEL assay (24.1 vs. 14.4% and 11.8% for free VCR (Lu et al., 2013).
Lopes et al. formulated pH-sensitive liposomes of ursolic acid using lipid hydration method and evaluated them on MDA-MB-231 breast cancer cells. The results indicated that the liposomes had better anticancer activity than free ursolic acid indicating the improved anticancer activity of the nano-liposomes (Lopes de Araujo et al., 2013).
Bomana et al. prepared liposomes of VCR for achieving liposome circulation longevity, drug retention characteristics and in vivo antitumor activity. They encapsulated VCR inside EPC/cholesterol and DSPC/cholesterol vesicles by pH-gradient driven drug uptake processes. Encapsulation of VCR in DSPC/cholesterol liposomes resulted in a 1.7- to 2.1-fold increase in the LD50-values compared to free VCR when administered intravenously in DBA-2J and CD-1 mice models. Administration of free and liposomal VCR at doses in the range between 0.5 and 3.0 mg/kg resulted in a significant increase in the mean survival times and in the percent increase in life span (% ILS)-values of DBN-2J mice bearing either P388 or L1210 peritoneal tumors. The activity of VCR against tumors in DBA/2J mice appeared to be closely correlated to the VCR circulation longevity in the formulation indicating the benefits of liposomes as drug delivery systems that improve the toxicity profile of VCR and help increase the accumulation of drug at tumor sites (Bomana et al., 1995).
Odeh et al. prepared thymoquinone liposomes and evaluated them on MCF-7 cancer cells and fibroblast cells. The results indicated that the liposomes suppressed the proliferation of MCF-7 cells and exerted very low toxicity on normal periodontal ligament fibroblasts (Odeh et al., 2012).
Many research groups formulated berberine liposomes using different lipid compositions and evaluated them on selected cancer cell lines. The results indicated enhanced cytotoxicity in comparison to free berberine (Wen et al., 2011; Lin et al., 2014; Sailor et al., 2015).
Carbon nanotubes
Carbon nanotubes are long, thin cylinders made up of carbon. These are synthetic rods that are only half the width of DNA. These are large macromolecules that are unique for their size, shape, and remarkable physical properties. The carbon nanotubes are derived from Graphene. As in grapheme carbon atoms are arranged in sp2 bonded structure they form honeycomb like patterns. They are of two types single-wall carbon nanotubes (SWCNTs) that have single layer of graphene and multi-wall carbon nanotubes (MWCNTs) that have more than one well of graphene. MWCNTs consist of concentric cylinders with the regular periodic interlayer spacing with a hollow center. This central core has a spacing of around 0.34–0.39 nm. This inner diameter differs depending on the number of layers. The outer diameter of these nanotubes ranges from 20 to 30 nm. The tips of MWCNTs are usually closed and their ends are capped. A property of carbon nanotubes is that they absorb near-infrared light waves and pass harmlessly through cells. However, when a beam of near-infrared light falls on carbon nanotubes, the excitation of electrons in the nanotubes occurs as a result the excess energy is produced in the form of heat that leads to the thermal destruction of cancer cells in-vivo. The surface of cancer cells contains numerous of receptors for vitamins known as folate, thus the nanotubes coated with the folate molecules would be attracted to folate receptors of diseased cells. This treatment induces coagulative necrosis, a form of cell death that involves protein denaturation and membrane lysis. Use of MWCNTs enables ablation of tumors with low laser power (3 W/cm2) and very short treatment times with minimal local toxicity and no evidence of systemic toxicity (Popov, 2014).
Tian et al. synthesized and functionalized multi-walled carbon nanotubes (MWNTs) as anticancer drug carriers for loading PTX using folic acid as the targeting ligand. These functionalized MWNTs exhibited good aqueous solubility, biocompatibility, and high targeting ability as indicated by in vitro cytotoxicity studies performed on HeLa cell line using MTT assay. The results showed significant enhancement in the cytotoxic capability thus improving the antitumor activity of the drug. The f-MWNTs-PTX complexes exhibited efficient targeting intracellular delivery and enhanced antitumor activity. These studies reinforce the application of functionalized carbon nanotubes-based complexes as promising drug delivery platform for improved cancer therapy (Tian et al., 2011).
Dendrimers
Dendrimers are spherical macromolecules having highly branched structure of large number of peripheral groups that aid in encapsulation of hydrophobic drug compounds. They consist of a central core, branching units and terminal functional groups. The environment of the nanocavities and solubilizing properties of these cavities depend on the central core. Liquid crystals show the combined properties of both liquid and solid states. They can be made to form different size and shapes, with alternative polar and non-polar layers which includes aqueous drug solutions. Because of their unique physical properties (like monodispersity, water solubility and encapsulation ability, these macromolecules are very helpful in production of drug delivery vehicles. The properties of dendrimers are dominated by the functional groups on the molecular surface; however, there are examples of dendrimers with internal functionality. Dendrimers have a well-defined nanoscale architecture and large internal volume make them an attractive option for drug delivery and other biomedical applications. Their systematic structural architecture. The unique properties of dendrimers, as compared to linear polymers, render them of interest for intracellular drug delivery system for cancer therapy. Dendritic encapsulation of functional molecules allows for the isolation of the active site, a structure which mimics that of active sites in biomaterials. Also, it is possible to make water-soluble dendrimers, unlike most polymers, by functionalizing their outer shell with charged species or other hydrophilic groups (Abdel-Rahman and Al-Abd, 2013).
Malar et al. prepared dendrosomal CAP nanoformulation by esterification process and evaluated them for in vitro anticancer activity on VERO, Hep 2, and MCF-7 cell lines. The results indicated that these dendrimers showed a significant cytotoxicity on VERO cell line with an IC50 of 1.25 μg/mL and on MCF-7 and HEp2 cell lines with an IC50 of 0.62 μg/mL (Malar and Bavanilathamuthiah, 2015).
Sharma et al. formulated dendrimers of gallic acid with polyamidoamine (PAMAM) using Tomalia's divergent growth method and evaluated them on MCF-7 breast cancer cells. These dendrimers provided a high degree of surface functionality and versatility for drug loading. The IC50-values indicated that the gallic acid-loaded PAMAM dendrimers were significantly better than the free gallic acid proving the benefit of dendrimers as a suitable nanotechnology platform enhanced cytotoxicity on MCF-7 breast cancer cells (Sharma et al., 2011).
Micelles
Micelles are collection of amphiphilic surfactant molecule that spontaneously aggregate and forms a spherical vesicle in water (size range of several tens of nanometers). The inner core of micelle is hydrophobic, thus can help in incorporation of hydrophobic drugs which are then released by some drug delivery mechanism. Conventional micelles consist of hydrophilic head and a hydrophobic tail made of small molecules consisting of the hydrocarbon portion of long fatty acids. They are most of times used as carriers for hydrophobic drugs and can be administered directly into the circulation. The molecular weight of polymer micelles are often high thus enabling maximum storage in the tissue of solid cancers. Micelles enter the tumor tissue easily as compared to other tissues. The concentration of micelles is often one order higher than in the surrounding area. The drug can be dissolved in the hydrophobic micelle core, or are bound chemically on the biodegradable polymer carrier. The preparation of polymeric micelles is simple but controlling the rate of drug release from these polymeric micelles is a tedious job. So the surface modified micelles were prepared, the chemical bond on the surface helps to control drug release. The activation of the micelles occurs in the tumor tissue environment thus preventing the drug release in the blood while circulation thus decreasing the toxicity of drug to normal cells (Lu et al., 2013; Lu and Park, 2013).
Qiu et al. developed luteolin polymeric micelles with monomethoxy poly(ethylene glycol)-poly(3 caprolactone) (MPEG-PCL) by self-assembly method and evaluated them on C-26 colon carcinoma cells. They studied the pharmacokinetics of free luteolin and the luteolin MPEG-PCL micelles in rats, the results indicating a higher bioavailable concentration of luteolin for the luteolin MPEG-PCL micelles. The in vitro cytotoxicity studies reflected a lower IC50 for the MPEG-PCL luteolin micelles suggesting that encapsulation of luteolin into MPEG-PCL micelles can potentially enhance the bioavailability and cytotoxicity (Qiu et al., 2013).
Blanco et al. prepared PEG-PLGA polymeric micelles of β-lapachone using film sonication method and evaluated them in mice with subcutaneous A549 lung tumor. The results of biodistribution studies of in mice indicated prolonged blood circulation and higher concentration of β-lapachone. In addition, the in vitro administration of the micelles to LLC tumors led to DNA damage and PARP-1 hyperactivation (Blanco et al., 2010).
Kumari et al. synthesized of block copolymeric micelles, methoxy-poly(ethylene glycol)-poly(D/L-lactide) (mPEG-PLA) to encapsulate CUR and evaluated their cytotoxicity in murine cancer cells, B16F10 (melanoma) and human breast cancer, MDA-MB-231 cell lines. The results of cellular uptake studies (flow cytometry studies) indicated that the cellular uptake of CUR in CUR-mPEG-PLA formulation was higher than that of free CUR in both the cell lines. The cytotoxicity of CUR was higher in mPEG-PLA micelles indicating mPEG-PLA polymeric micelles to be an efficient nanocarrier for CUR (Kumari et al., 2016).
Dong et al. prepared self-assembled biodegradable star-shaped polymeric micelles of honokiol using monomethoxy poly(ethylene glycol) (MPEG) and poly(3-caprolactone) (PCL) by direct dissolution ultrasonication method and evaluated them on CT26 murine colon carcinoma cells. The results indicated the polymeric micelles had a significantly enhanced dose related cytotoxicity on the CT26 cells (Dong et al., 2010).
Wei et al. formulated honokiol micelles using poly(3-caprolactone)-poly(ethylene glycol)-poly(3-caprolactone) copolymer (PCEC) and evaluated them on A549 human lung adenocarcinoma cells. The results indicated comparable anti-proliferative effect of free honokiol and honokiol-copolymeric micelles on A549 cells and were comparable showcasing their application in treatment of lung cancer (Wei et al., 2009).
Many phytodrug-loaded polymeric micelles for anticancer therapy are under investigation in preclinical studies to improve drug efficacy. Some of these are in various phases of clinical trials for e.g., a block copolymer of PEG and polyglutamate (PGlu) conjugated with 7-ethyl-10-hydroxy-campothecin for breast cancer reemphasizing the existing gap for novel delivery systems and scope for new therapeutic modalities for cancer management (Oerlemans et al., 2010).
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNPs) are widely used as a nanocarrier system for many drugs. These particles have size ranging from 50 to 1,000 nm and are made up of lipids which is stable at room temperature and body temperature. The lipids used in preparation of SLNPs include lipid acids, mono-, di-, or triglycerides, glyceride mixtures, or waxes that are stabilized using biocompatible surfactants. Over other drug deliveries the SLNPs have advantages of physical stability, protection of labile drugs from degradation, controlled release and ease of preparation. Production of SLNPs is relatively cost efficient and amenable to large scale production. The storage and drug leakage problems are very less in SLNPs than in liposomes (Ekambaram et al., 2012).
The SLNPs prepared using biodegradable polymeric materials showed significant decrease in toxicity and acidity. Most of lipophilic compounds can be efficiently incorporated into SLNPs thus the encapsulation of cytotoxic compounds in SLNPs can prove to be a best for oral administration of the drugs. A number of SLNPs or SLN-based systems have been developed for delivery of cytotoxic drugs such as doxorubicin, idarubicin, PTX, camptothecan, 7-ethyl-10-hydroxy-20(S)-CPT, ETP, flurodooxyuridine (FudR) and retinoic acid, and cholesterylbutyrate (Wei et al., 2015).
Serpe et al. prepared PTX loaded SLNPs that showed almost similar cytotoxic compared to the equivalent amount of drug in free solution. The in vivo efficacy of PTX loaded SLNPs was compared with free drug formulation using murine breast cancer mice model. It was found that the group of animal treated with PTX loaded SLNPs had significantly smaller tumor size and lower percent inhibition (Serpe et al., 2004).
Xu et al. prepared SLNPs of silibinin containing TPGS and phosphatidylcholine by using thin film hydration method and evaluated on MDA-MB-231 breast cancer cells. The results demonstrated enhanced cellular uptake of SLNPs almost twice that of free silibinin. These were corroborated by similar results in cell viability, invasion and migration assays. An interesting observation made by them was the suppression of the invasive and migratory capabilities of MDA-MB-231 cells at a concentration of 20 mg/mL of SLNPs via downregulation of the MMP-9 and Snail pathways (Xu et al., 2013).
Shen et al. coated SLNPs with hyaluronic acid so as to enhance its anti-tumor activity. The PTX was used as anti-cancer agent. The PTX-SLNPs were prepared by film-ultrasonic method. These SLNPs were coated with hyaluronic acid as cancer stem cells shows presences of CD44 that binds specially to hyaluronic acid. The in vitro and in vivo cytotoxicity evaluation was performed on B16F10 melanoma cells and in mouse xenograft model, respectively. The results showed the efficient intracellular delivery of PTX and induced substantial apoptosis in CD44+ cells in vitro. These PTX loaded HA-SLNPs targeted the tumor bearing tissues and subsequently exhibited significant antitumor effect with a low dose of PTX as compared to the pure drug (Shen et al., 2015).
An exhaustive review of the nanotech platforms for delivering some phytoconstituents as liposomes, nanoemulsions, micelles, SLNPs, and nanolipid carriers (NLCs) has been discussed with remarkable increase in their anticancer activity, an outcome of nanotechnology advantages (Chuan et al., 2015). A detailed summary of the application of nanotechnology for delivery of combination chemotherapy with many advantages over conventional combination therapy is presented and they have described many phytodrug combinations with synthetic and nature derived anticancer drugs (PCT and ETP; TPT and VCR; VCR and QUR; PCT and DOX; CUR and DOX; PCT and CUR to mention some) that have been developed as NPs, liposomes, lipid-polymer hybrid NPs and micelles delivering enhanced therapeutic efficacy for the combination drugs (Chen et al., 2017). Table 1 gives the comparative summary of the various nanotech delivery systems.
Table 1.
Comparison of different nanotech delivery carriers for selected phytochemicals.
| Type | Nanoparticles | Solid Lipid Nanoparticles | Liposomes | Carbon Nanotubes | Micelles | Dendrimers |
|---|---|---|---|---|---|---|
| Advantages |
|
|
|
|
|
|
| Limitations |
|
|
|
|
|
|
| Phytochemicals incorporated | VCR, ETP, PTX, CUR, RSV, CPT, QUR, EGCG | CUR, PTX, CPT, ETP | VCR, BER, QUR, thymoquinone, ursolic acid, ETP, CUR, RSV | PTX, QUR | CUR, PTX | Capsaicin |
| References | Gu et al., 2007 Cho et al., 2008 Mishra et al., 2010 Lim et al., 2011 Zu et al., 2011 Pimple et al., 2012, Siu et al., 2012 Tang et al., 2013, Mallamma et al., 2014, Sundar et al., 2014, Siddiqui et al., 2015, Suryani and Ismail, 2015 Granja et al., 2016 Han et al., 2016 Sajan et al., 2016 Salar and Kumar, 2016, Thadakapally et al., 2016 Yang et al., 2016 Zhou et al., 2016 |
Wong et al., 2007 Ekambaram et al., 2012 Yassin et al., 2013 Abd-Allah et al., 2014 |
Chadha et al., 2008 Narayanan et al., 2009 Ramanaa et al., 2012 Venegas et al., 2012 Shah et al., 2014 Mehrabi et al., 2016 |
Li et al., 2011 Tian et al., 2011 Popov, 2014 |
Nakanishi et al., 2001 Husseini and Pitt, 2008 Maeda et al., 2009 Mourya et al., 2011 Lu et al., 2013 Powar and Sharma, 2016 |
Naha et al., 2010 Abdel-Rahman and Al-Abd, 2013 Baig et al., 2015 Malar and Bavanilathamuthiah, 2015 Yang et al., 2015 |
Zhang et al. prepared co-encapsulated micelles of β-lapachone and PTX using PEG-PLA copolymer and evaluated them on A549 lung cancer cells. These micelles showcased enhanced antiproliferative effect vs. the individual drug micelles (IC50 = 0.16 mM for the co-encapsulated micelles, IC50 = 4.5 mM for β-lapachone micelles and 0.32 mM for PTX micelles). A very useful synergistic effect was observed for the co-encapsulated two agents against lung cancer (Zhang et al., 2015).
Conclusion
About 65% of anticancer drugs introduced over the last 25 years have been derived from the natural sources. Chemical synthesis (either partial or total) has played an important role in supplying these nature derived compounds in large quantities and in preparing their novel analogs. Many of these compounds suffer from low solubility and poor bioavailability; Nanotechnology offers the advantages of increased bioavailability, prolongation of drug circulation time, multiple drug loading, all contributing to improved efficacy, and decreased toxicity. These nanotech based therapeutic delivery systems have many advantages such as water solubility, lower toxicity, biocompatibility, and amenability of their surface to further modifications for related applications. There is immense scope for the nature derived molecules to be formulated into nanotechnology based drug delivery system targeting the tumor microenvironment to combat MDR as nanotechnology based combination drug formulations. There is an existing gap and research initiatives to synthesize more tumor targeted nanotherapeutic delivery systems with high quality and yield of cytotoxic agents obtained from natural resources could prove effective in the overall management of cancer. Novel nanoformulations containing a synergistic combination of plant based drugs along with synthetic drugs could prolong drug circulation times, provide coordinated drug release, a better efficacy to toxicity ratio that could lead them into clinical trials and eventually to the bedside. Efficient formulation targeting strategies and evaluation of the targeting efficiency of NPs, and conforming to international standards for their toxicology and biocompatibility could pave the way for clinically viable phytochemical based anticancer therapies.
Author contributions
TK has conceived the idea, edited, and built the manuscript. PG has conducted literature review and collated the information and prepared the draft review article.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge and appreciate the help and support rendered by Mr. Chintan Bhavsar for designing the graphics and figures of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2017.01002/full#supplementary-material
References
- Abd-Allah F. I., Dawaba H. M., Samy A. M., Nutan M. T. (2014). Application of solvent injection method to develop stable, sustained release solid lipid nanoparticles of curcumin. Int. J. Dev. Res. 4, 2734–2742. [Google Scholar]
- Abdel-Rahman M. A., Al-Abd A. M. (2013). Thermoresponsive dendrimers based on oligoethylene glycols: design, synthesis and cytotoxic activity against MCF-7 breast cancer cells. Eur. J. Med. Chem. 69, 848–854. 10.1016/j.ejmech.2013.09.019 [DOI] [PubMed] [Google Scholar]
- Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S. W., Zarghami N., Hanifehpour Y., et al. (2013). Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 8:102. 10.1186/1556-276X-8-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Zafar H., Zia M., Haq I., Phull A. R., Ali J. S., et al. (2016). Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 9, 49–67. 10.2147/NSA.S99986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliosmanoglu A., Basaran I. (2012). Nanotechnology in cancer treatment. J. Nanomed. Biotherapeut. Discov. 2, 1–3. 10.4172/2155-983X.1000107 [DOI] [Google Scholar]
- Aygül G., Yerlikaya F., Caban S., Vural I., Çapan Y. (2013). Formulation and in vitro evaluation of paclitaxel loaded nanoparticles. Hacettepe Univ. J. Faculty Pharm. 33, 25–40. [Google Scholar]
- Badini G. A., Vega V., Ebing A., Mishra D., Szary P., Prida V. M., et al. (2011). Template-assisted self-assembly of individual and clusters of magnetic nanoparticles. Nanotechnology 22:285608 10.1088/0957-4484/22/28/285608 [DOI] [PubMed] [Google Scholar]
- Baig T., Nayak J., Dwivedi V., Singh A., Srivastava A., Tripathi P. K. (2015). A review about dendrimers: synthesis, types, characterization and applications. Int. J. Adv. Pharmacy Biol. Chem. 4, 44–59. [Google Scholar]
- Bhanot A., Sharma R., Noolvi M. N. (2011). Natural sources as potential anti-cancer agents: a review. Int. J. Phytomed. 3, 9–26. [Google Scholar]
- Bisht S., Feldmann G., Soni S., Ravi R., Karikar C., Maitra A., et al. (2007). Polymeric nanoparticle-encapsulated curcumin (“Nanocurcumin”): a novel strategy for human cancer therapy. J. Nanobiotechnol. 5, 1–18. 10.1186/1477-3155-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco E., Bey E. A., Khemtong C., Yang S. G., Setti-Guthi J., Chen H., et al. (2010). Beta-lapachone micellar nanotherapeutics for non-small cell lung cancer therapy. Cancer Res. 70, 3896–3904. 10.1158/0008-5472.CAN-09-3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomana N. L., Bally M. B., Cullis P. R., Mayer L. D., Web M. S. (1995). Encapsulation of vincristine in liposomes reduces its toxicity and improves its anti-tumor efficacy. J. Liposome Res. 5, 523–541. 10.3109/08982109509010240 [DOI] [Google Scholar]
- Cal C., Garban H., Jazirehi A., Yeh C., Mizutani Y., Bonavida B. (2003). Resveratrol and cancer: chemoprevention, apoptosis, and chemo-immunosensitizing activities. Curr. Med. Chem. Anti Cancer Agents. 3, 77–93. 10.2174/1568011033353443 [DOI] [PubMed] [Google Scholar]
- Callewaert M., Dukic S., Gulick L. V., Vittier M., Gafa V., Andry M. C., et al. (2013). Etoposide encapsulation in surface-modified poly(lactide-co-glycolide) nanoparticles strongly enhances glioma antitumor efficiency. J. Biomed. Mater. Res. A 101, 1319–1327. 10.1002/jbm.a.34442 [DOI] [PubMed] [Google Scholar]
- Cao S., Chen H., Xiang S., Hong J., Weng L., Zhu H., Liu Q. (2015). Anti-cancer effects and mechanisms of capsaicin in chili peppers. Am. J. Plant Sci. 6, 3075–3081. 10.4236/ajps.2015.619300 [DOI] [Google Scholar]
- Castillo P. M., Mata M., Casula M. F., Sánchez-Alcázar J. A., Zaderenko A. P. (2014). PEGylated versus non-PEGylated magnetic nanoparticles as camptothecin delivery system. Beilstein J. Nanotechnol. 5, 1312–1319. 10.3762/bjnano.5.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha R., Kapoor V. K., Thakur D., Kaur R., Arora P., Jain D. V. S. (2008). Drug carrier systems for anticancer agents: a review. J. Sci. Ind. Res. 185–197. [Google Scholar]
- Chaturvedi S. P., Kumar V. (2012). Production techniques of lipid nanoparticles: a review. Res. J. Pharm. Biol. Chem. Sci. 3, 525–541. [Google Scholar]
- Chen D., Xie F., Sun D., Yin C., Gao J., Zhong Y. (2017). Nanomedicine-mediated combination drug therapy in tumor. Open Pharm Sci. J. 4, 1–10. 10.2174/1874844901704010001 [DOI] [Google Scholar]
- Chen Y., Zheng X. L., Fang D. L., Yang Y., Zhang J. K., Li H. L., et al. (2014). Dual agent loaded PLGA nanoparticles enhanced antitumor activity in a multidrug-resistant breast tumor eenograft model. Int. J. Mol. Sci. 15, 2761–2772. 10.3390/ijms15022761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. J., Wang X., Nie S. M., Chen Z., Shin D. M. (2008). Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 14, 1310–1316. 10.1158/1078-0432.CCR-07-1441 [DOI] [PubMed] [Google Scholar]
- Chuan L., Zhang J., Yu-Jiao Z., Shu-Fang N., Jun C., Wang Q., et al. (2015). Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 13, 641–652. 10.1016/S1875-5364(15)30061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G. M., Newman D. J. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Das S., Das J., Samadder A., Paul A., Khudabukhsh A. R. (2013). Efficacy of PLGA loaded apigenin nanoparticles in benzo[a]pyrene and ultraviolet induced skin cancer of mice: mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 62, 670–680. 10.1016/j.fct.2013.09.037 [DOI] [PubMed] [Google Scholar]
- Dhanikula A. B., Panchagnula R. (1999). Localized paclitaxel delivery. Int. J. Pharm. 183, 85–100. 10.1016/S0378-5173(99)00087-3 [DOI] [PubMed] [Google Scholar]
- Dong P. W, Wang X. H., Gu Y. C., Wang Y. J., Wang Y. J., Gong C. Y., et al. (2010). Self-assembled biodegradable micelles based on star-shaped PCL-b-PEG copolymers for chemotherapeutic drug delivery. Colloids Surf. A 358, 128–134. 10.1016/j.colsurfa.2010.01.037 [DOI] [Google Scholar]
- Ekambaram P., Sathali A. A. H., Priyanka K. (2012). Solid lipid nanoparticles: a review. Sci. Revs. Chem. Commun. 2, 80–102. [Google Scholar]
- Elkholi I. E., Hazem N. M., ElKashef W. F., Sobh M. A., Shaalan D., Sobh M., et al. (2014). Evaluation of anti-cancer potential of capsaicin-loaded trimethyl chitosan-based nanoparticles in HepG2 hepatocarcinoma cells. J. Nanomed. Nanotechnol. 5, 1–8. 10.4172/2157-7439.1000240 [DOI] [Google Scholar]
- Ferraz da Costa D. C., Fialho E., Silva J. L. (2017). Cancer chemoprevention by resveratrol: the p53 tumor suppressor protein as a promising molecular target. Molecules 22, 1–24. 10.3390/molecules22061014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja A., Pinheiro M., Reis S. (2016). Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 8:E307. 10.3390/nu8050307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F. X., Karnik R., Wang A. Z., Alexis F., Levy-Nissenbaum E., Hong S., et al. (2007). Targeted nanoparticles for cancer therapy. Nano Today 2, 14–21. 10.1016/S1748-0132(07)70083-X [DOI] [Google Scholar]
- Gupta V., Aseh A., Ríos C. N., Aggarwal B. B., Mathur A. B. (2009). Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 4, 115–122. 10.2147/IJN.S5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F. Y., Thurecht K. J., Whittaker A. K., Smith M. T. (2016). Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 7:185. 10.3389/fphar.2016.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseini G. A., Pitt W. G. (2008). Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 60, 1137–1152. 10.1016/j.addr.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juillerat-Jeanneret L. (2008). The targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug nanoparticles. Drug Discov. Today 13, 1099–1106. 10.1016/j.drudis.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Kabanov A. V., Batrakova E. V., Alakhov V. Y. (2002). Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control Release 82, 189–212. 10.1016/S0168-3659(02)00009-3 [DOI] [PubMed] [Google Scholar]
- Kumari P., Omkara S. M., Sravan K. N., Srividya M., Balaram Ghosh B., Swati B. (2016). Curcumin delivery by poly(lactide)-based co-polymeric micelles: an in vitro anticancer study. Pharm Res. 33, 826–841. 10.1007/s11095-015-1830-z [DOI] [PubMed] [Google Scholar]
- Li R., Wu R., Zhao L., Hu Z., Guo S., Pan X., et al. (2011). Folate and iron difunctionalized multiwall carbon nanotubes as dual-targeted drug nanocarrier to cancer cells. Carbon 49, 1797–1805. 10.1016/j.carbon.2011.01.003 [DOI] [Google Scholar]
- Lim K. J., Bisht S., Bar E. E., Maitra A., Eberhart C. G. (2011). A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol. Ther. 11, 1–10. 10.4161/cbt.11.5.14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Li L., Thanh X. N., Ahmed M. E. A., Mario G., Guang Y. (2014). Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B. 41, 7149–7159. 10.1039/C4TB00876F [DOI] [PubMed] [Google Scholar]
- Lopes de Araujo S. C., Novais M. V. M., Teixeira C. S., Honorato-Sampaio K., Pereira M. T., Ferreira L. A. M., et al. (2013). Preparation, physicochemical characterization, and cell viability evaluation of long-circulating and pH-sensitive liposomes containing ursolic acid. Bio Med. Res. Int. 2013:467147 10.1155/2013/467147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Wang C., Feng L., Yang X., Wang F. (2013). Folic acid-conjugated liposomal vincristine for multidrug resistant cancer therapy. Asian J. Pharm. Sci. 8, 118–127. 10.1016/j.ajps.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Park K. (2013). Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 453, 198–214. 10.1016/j.ijpharm.2012.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. (2010). Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate Chem. 21, 797–802. 10.1021/bc100070g [DOI] [PubMed] [Google Scholar]
- Maeda H., Bharate G. Y., Daruwalla J. (2009). Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 71, 409–419. 10.1016/j.ejpb.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Majumdar D., Jung K. H., Zhang H. Z., Nannapaneni S., Wang X., Amin R. M., et al. (2014). Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity. Cancer Prev. Res. 7, 65–73. 10.1158/1940-6207.CAPR-13-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malar C., Bavanilathamuthiah G. (2015). Dendrosomal capsaicin nanoformulation for the in vitro anticancer effect on hep 2 and MCF-7 cell lines. Int. J. Appl. Bioeng. 9, 30–35. 10.18000/ijabeg.10133 [DOI] [Google Scholar]
- Mallamma T., Bharathi D. R., Lakshmi R. G., Vyjayanthimala T., Nagasubbareddy J., Naveen R. (2014). Etoposide-loaded nanoparticles made from poly-e-caprolactone (PCL): formulation, characterization, in vitro drug release for controlled drug delivery system. Int. J. Biopharm. 5, 5–12. [Google Scholar]
- Mansoori G. A., Mohazzabi P., McCormack P. (2007). Nanotechnology in cancer prevention, detection and treatment: bright future lies ahead. World Rev. Sci. Tech. Sust. Dev. 2, 226–257. 10.1504/WRSTSD.2007.013584 [DOI] [Google Scholar]
- Mehrabi M., Esmaeilpour P., Akbarzadeh A., Saffari Z., Farahnak M., Farhangi A., et al. (2016). Efficacy of PEGylated liposomal etoposide nanoparticles on breast cancer cell lines. Turk. J. Med. Sci. 46, 567–571 10.3906/Sag-1412-67 [DOI] [PubMed] [Google Scholar]
- Merlina J. J. P. N., Prasada R. N., Shibli S. M. A., Sebeel M. (2012). Ferulic acid loaded poly-D,L-lactide-co-glycolide nanoparticles: systematic study of particle size, drug encapsulation efficiency and anticancer effect in non-small cell lung carcinoma cell line in vitro. Biomed. Prev. Nutr. 2, 69–76. 10.1016/j.bionut.2011.12.007 [DOI] [Google Scholar]
- Mishra B., Patel B. B., Tiwari S. (2010). Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 6, 9–24. 10.1016/j.nano.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Moorthi C., Manavalan R., Kathiresan K. (2011). Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharmaceut. Sci. 14, 67–77. 10.18433/J30C7D [DOI] [PubMed] [Google Scholar]
- Mourya V. K., Inamdar N., Nawale R. B., Kulthe S. S. (2011). Polymeric micelles: general considerations and their applications. Ind. J. Pharm. Edu. Res. 45, 128–138. [Google Scholar]
- Naha P. C., Davoren M., Lyng F. M., Byrne H. J. (2010). Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A1 cells. Toxicol. Appl. Pharmacol. 246, 91–99. 10.1016/j.taap.2010.04.014 [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Fukushima S., Okamoto K., Suzuki M., Matsumura Y., Yokoyama M., et al. (2001). Development of the polymer micelle carrier system for doxorubicin. J. Control Release 74, 295–302. 10.1016/S0168-3659(01)00341-8 [DOI] [PubMed] [Google Scholar]
- Narayanan N. K., Nargi D., Randolph C., Narayanan B. A. (2009). Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 125, 1–8. 10.1002/ijc.24336 [DOI] [PubMed] [Google Scholar]
- Odeh F., Ismail S. I., Abu-Dahab R., Mahmoud I. S., Bawab A. (2012). Thymoquinone in liposomes: a study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 19, 371–377. 10.3109/10717544.2012.727500 [DOI] [PubMed] [Google Scholar]
- Oerlemans C., Bult W., Bos M., Storm G., Nijsen J. F. W., Hennink W. E. (2010). Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm. Res. 27, 2569–2589. 10.1007/s11095-010-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimple S., Manjappa A. S., Ukawala M., Murthy R. S. R. (2012). PLGA nanoparticles loaded with etoposide and quercetin dihydrate individually: in vitro cell line study to ensure advantage of combination therapy. Cancer Nano. 3, 25–36. 10.1007/s12645-012-0027-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkowski K., Mazurek A. P. (2000). Biological properties of genistein: a review of in vitro and in vivo data. Acta Pol. Pharm. 57, 135–155. [PubMed] [Google Scholar]
- Popov V. N. (2014). Carbon nanotubes: properties and application. Mater. Sci. Eng. 43, 61–102. 10.1016/j.mser.2003.10.001 [DOI] [Google Scholar]
- Powar P. V., Sharma P. H. (2016). Polymeric micelle as a multifunctional therapeutics. J. Innov. Appl. Pharm. Sci. 1, 24–33. [Google Scholar]
- Qiu J. F., Gao X., Wang B. L., Wei X. W., Gou M. L., Men K., et al. (2013). Preparation and characterization of monomethoxy poly(ethylene glycol)-poly(Îμ-caprolactone) micelles for the solubilization and in vivo delivery of luteolin. Int. J. Nanomed. 8, 3061–3069. 10.2147/IJN.S45062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique M. M. A., Iqbal J. (2011). Production of carbon nanotubes by different routes - a review. J. Encap. Adsorp. Sci. 1, 29–34. 10.4236/jeas.2011.12004 [DOI] [Google Scholar]
- Ramanaa L. N., Sharmab S., Sethuramana S., Rangab U., Krishnana U. M. (2012). Investigation on the stability of saquinavir loaded liposomes: Implication on stealth, release characteristics and cytotoxicity. Int. J. Pharm. 431, 120–129. 10.1016/j.ijpharm.2012.04.054 [DOI] [PubMed] [Google Scholar]
- Reis C. P., Neufeld R. J., Ribeiro A. J., Francisco Veiga F. (2006). Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine 2, 8–21. 10.1016/j.nano.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Ruiz-Torres V., Encinar J. A., Herranz-López M., Pérez-Sánchez A., Galiano V., Barrajón-Catalán E., et al. (2017). An updated review on marine anticancer compounds: the use of virtual screening for the discovery of small-molecule cancer drugs. Molecules 22:1037. 10.3390/molecules22071037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailor G., Seth A. K., Parmar G., Chauhan S., Javia A. (2015). Formulation and in vitro evaluation of berberine containing liposome optimized by 32 full factorial designs. J. Appl. Pharm. Sci. 5, 23–28. 10.7324/JAPS.2015.50704 [DOI] [Google Scholar]
- Sajan J., Rosmy S., Cinu T. A., Jyoti H., Aleykutty N. A. (2016). Ligand conjugated tumor targeted nanoparticle drug delivery system of vincristine: 32 full factorial design and in vitro evaluation. Pharm. Lett. 8, 25–30. [Google Scholar]
- Salar R. K., Kumar N. (2016). Synthesis and characterization of vincristine loaded folic acid–chitosan conjugated nanoparticles. Res. Efficient Technol. 2, 199–214. 10.1016/j.reffit.2016.10.006 [DOI] [Google Scholar]
- Sanna V., Roggio A. M., Siliani S., Piccinini M., Marceddu S., Mariani A., et al. (2012). Development of novel cationic chitosan- and anionic alginate-coated poly(D,L-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int. J. Nanomed. 7, 5501–5516. 10.2147/IJN.S36684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna V., Siddiqui I. A., Sechi M., Mukhtar H. (2013). Resveratrol-loaded nanoparticles based on poly(epsiloncaprolactone) and poly(D,L-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol. Pharm. 10, 3871–3881. 10.1021/mp400342f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluep T., Hwang J., Hildebrandt I. J., Czernin J., Choi C. H. J., Alabi C. A., et al. (2009). Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET imaging and tumor histological measurements. Proc. Natl. Acad. Sci. U.S.A. 106, 11394–11399. 10.1073/pnas.0905487106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebak S., Mirzaei M., Malhotra M., Kulamarva A., Prakash S. (2010). Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysis. Int. J. Nanomed. 20, 525–532. 10.2147/IJN.S10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe L., Canaparoa R., Gascoc M. R., Eandia M., Zaraa G. P. (2004). Cytotoxicity of anticancer drugs incorporated in solid lipid nanoparticles on HT-29 colorectal cancer cell line. Eur. J. Pharm. Biopharm. 58, 673–680. 10.1016/j.ejpb.2004.03.026 [DOI] [PubMed] [Google Scholar]
- Shah S. M., Goel P. N., Jain A. S., Pathak P. O., Padhye S. G., Govindarajan S., et al. (2014). Liposomes for targeting hepatocellular carcinoma: use of conjugated arabinogalactan as targeting ligand. Int. J. Pharm. 477, 128–139. 10.1016/j.ijpharm.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Shanmugam M. K., Rane G., Kanchi M. M., Arfuso F., Chinnathambi A., Zayed M. E., et al. (2015). The multifaceted role of curcumin in cancer prevention and treatment. Molecules 20, 2728–2769. 10.3390/molecules20022728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Gautam S. P., Gupta A. K. (2011). Surface modified dendrimers. Bioorg. Med. Chem. 19, 3341–3346. 10.1016/j.bmc.2011.04.046 [DOI] [PubMed] [Google Scholar]
- Shen H., Shi S., Zhang Z., Gong T., Sun X. (2015). Coating solid lipid nanoparticles with hyaluronic acid enhances antitumor activity against melanoma stem-like cells. Theranostics 5, 755–771. 10.7150/thno.10804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng-Nan S., Chao W., Zan-Zan Z., Yang-Long H., Venkatramana S. S., Zhi-Chuan X. (2014). Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B. 23:037503 10.1088/1674-1056/23/3/037503 [DOI] [Google Scholar]
- Siddiqui I. A., Bharali D. J., Nihal M., Adhami V. M., Khan N., Chamcheu J. C., et al. (2014). Excellent anti-proliferative and pro-apoptotic effects of epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine 10, 1619–1626. 10.1016/j.nano.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Siddiqui I. A., Sanna V., Ahmad N., Sechi M., Mukhtar H. (2015). Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 1384, 20–31. 10.1111/nyas.12811 [DOI] [PubMed] [Google Scholar]
- Sisodiya P. S. (2013). Plant derived anticancer agents: a review. Int. J. Res. Dev. Pharm. L. Sci. 2, 293–308. [Google Scholar]
- Siu Y. S., Li L., Leung M. F., Lee K. L. D., Li P. (2012). Polyethylenimine-based amphiphilic core-shell nanoparticles: study of gene delivery and intracellular trafficking. Biointerphases 7:16. 10.1007/s13758-011-0016-4 [DOI] [PubMed] [Google Scholar]
- Song Y. H., Sun H., Zhang A., Yan G., Han Y., Wang X. (2014). Plant-derived natural products as leads to anti-cancer drugs. J. Med. Plant Herb. Ther. Res. 2, 6–15. [Google Scholar]
- Sundar V. D., Dhanaraju M. D., Sathyamoorthy N. (2014). Fabrication and characterization of etoposide loaded magnetic polymeric microparticles. Int. J. Drug Deliv. 6, 24–35. [Google Scholar]
- Suryani, Martien R., Ismail H. (2015). Preparation of curcumin nanoparticles and cellular uptake study on HeLa cells, in International Conference on Latest Trends in Food, Biological & Ecological Sciences (Dubai: ). [Google Scholar]
- Tang X., Cai S., Zhang R., Liu P., Chen H., Zheng Y., et al. (2013). Paclitaxel-loaded nanoparticles of star-shaped cholic acid-core PLA-TPGS copolymer for breast cancer treatment. Nanoscale Res. Lett. 8:420. 10.1186/1556-276X-8-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taratula O., Garbuzenko O. B., Kirkpatrick P., Pandya I., Savla R., Pozharov V. P., et al. (2009). Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J. Control. Release. 140, 284–293. 10.1016/j.jconrel.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Dalton W. S., Mosley K., Dorr R. T., Salmon S.E. (1997). Combination chemotherapy with cyclophosphamide, vincristine, adriamycin, and dexamethasone plus oral quinine and verapamil in patients with advanced breast cancer. Breast Cancer Res. Treat. 42, 7–14. 10.1023/A:1005716214718 [DOI] [PubMed] [Google Scholar]
- Thadakapally R., Aafreen A., Aukunuru J., Habibuddin M., Jogala S. (2016). Preparation and characterization of PEG-albumin-curcumin nanoparticles intended to treat breast cancer. Indian J. Pharm. Sci. 78, 65–72. 10.4103/0250-474X.180250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanki K., Rahul P., Gangwal R. P., Abhay T., Sangamwar A. T., Jain S. (2013). Oral delivery of anticancer drugs: challenges and opportunities. J. Control. Release. 170, 15–40. 10.1016/j.jconrel.2013.04.020 [DOI] [PubMed] [Google Scholar]
- Tian Z., Shi Y., Yin M., Shen H., Jia N. (2011). Functionalized multiwalled carbon nanotubes anticancer drug carriers: synthesis, targeting ability and antitumor activity. Nano Biomed. Eng. 3, 157–162. 10.5101/nbe.v3i3.p157-162 [DOI] [Google Scholar]
- Venegas B., Zhu W., Haloupek N. B., Lee J., Zellhart E., Suga I. P., et al. (2012). Cholesterol superlattice modulates ca4p release from liposomes and ca4p cytotoxicity on mammary cancer cells. Biophys. J. 10, 2086–2094. 10.1016/j.bpj.2012.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Crosbie-Staunton K., Satti A., Gallagher S., Ryan K. B., Doody T., et al. (2013). Magnetic core-shell nanoparticles for drug delivery by nebulisation. J Nanobiotechnol. 11, 1–12. 10.1186/1477-3155-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Yu X. C., Xu S. F., Xu M. (2015). Paclitaxel and etoposide co-loaded polymeric nanoparticles for the effective combination therapy against human osteosarcoma. J. Nanobiotechnol. 13:22 10.1186/s12951-015-0086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Chen C., Liu J., Liu C., Posocco P., Liu X., et al. (2015). Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. U.S.A. 112, 2978–2983. 10.1073/pnas.1418494112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. W., Chang Y. G., Shia S., Fua S. Z., Menc K., Zeng S., et al. (2009). Self-assembled honokiol-loaded micelles based on poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) copolymer. Int. J. Pharm. 369, 170–175. 10.1016/j.ijpharm.2008.10.027 [DOI] [PubMed] [Google Scholar]
- Wen W., Qian H., Xu T., Hanquiang L., Junqi T., Ying Y., et al. (2011). Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 102, 1287–1292. 10.1111/j.1349-7006.2011.01933.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. L., Bendayan R., Rauth A. M., Li Y., Wu X. Y. (2007). Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 59, 491–504. 10.1016/j.addr.2007.04.008 [DOI] [PubMed] [Google Scholar]
- WHO Cancer Factsheet Feb (2010). NMH.
- Xu P., Yin Q., Shen J., Chen L., Yu H., Zhang Z., et al. (2013). Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int. J. Pharm. 454, 21–30. 10.1016/j.ijpharm.2013.06.053 [DOI] [PubMed] [Google Scholar]
- Yang A., Liu Z., Yan B., Zhou M., Xiong X. (2016). Preparation of camptothecin-loaded targeting nanoparticles and their antitumor effects on hepatocellular carcinoma cell line H22. Drug Deliv. 23, 1699–1706. 10.3109/10717544.2014.950767 [DOI] [PubMed] [Google Scholar]
- Yang Q., Yang Y., Li L., Sun W., Zhu X., Huang Y. (2015). Polymeric nanomedicine for tumor-targeted combination therapy to elicit synergistic genotoxicity against prostate cancer. ACS Appl. Mater. Interf. 7, 6661–6673. 10.1021/am509204u [DOI] [PubMed] [Google Scholar]
- Yassin A. E. B., Albekairy A., Alkatheri A., Sharma R. K. (2013). Anticancerloaded solid lipid nanoparticles: high potential advancement in chemotherapy. Dig. J. Nanomater. Biostruct. 8, 905–916. [Google Scholar]
- Zhang L., Chen Z., Yang K., Liu C., Gao J., Qian F. (2015). β-lapachone and paclitaxel combination micelles with improved drug encapsulation and therapeutic synergy as novel nanotherapeutics for NQO1-targeted cancer therapy. Mol. Pharmaceutics. 12, 3999–4010. 10.1021/acs.molpharmaceut.5b00448 [DOI] [PubMed] [Google Scholar]
- Zhou H., Liu X., Wu F., Zhang J., Wu Z., Yin H., et al. (2016). Preparation, characterization, and antitumor evaluation of electrospun resveratrol loaded nanofibers. J. Nanomater. 2016:5918462 10.1155/2016/5918462 [DOI] [Google Scholar]
- Zu Y., Wang D., Zhao X., Jiang R., Zhang Q., Zhao D., et al. (2011). A novel preparation method for camptothecin (CPT) loaded folic acid conjugated dextran tumor-targeted nanoparticles. Int. J. Mol. Sci. 12, 4237–4249. 10.3390/ijms12074237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.