The Stranger and Danger Models
In 1989, Janeway (1) introduced a conceptual framework to understand how the innate immune system selectively responds to potentially threatening infections. Based on empirical observations, he proposed that the innate immune system must not only distinguish foreign cells from native cells, but also requires the presence of pathogenic costimulatory molecules to initiate inflammatory signaling cascades (1, 2). These signals, typified by gram-negative LPS and termed “pathogen-associated molecular patterns” (PAMPs), are molecularly distinct, foreign particles that serve as necessary immune adjuvants, causing local inflammation and tissue destruction (3). This theory was corroborated by the discovery of a new class of receptors known as PRRs (pattern-recognition receptors) (4, 5). This broad class of receptors, typified by the TLR (Toll-like receptor) family, bind structurally conserved moieties, such as microbial cell wall fragments and foreign DNA (Figure 1) (6). The activation of these receptors is responsible for local inflammation, most distinctly through the induction of multiple pathways, including the TNF (tumor necrosis factor)-α/NF-κB (nuclear factor-κB) signaling cascade as well as NLRP3 (nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing 3) inflammasome activation with production of inflammatory molecules, such as IL-1β and IL-18 (Figure 2). PRR activation also recruits and activates circulating leukocytes, such as macrophages and neutrophils, which enhance microbial killing through the release of reactive oxygen species, proteases, and IFNγ (7).
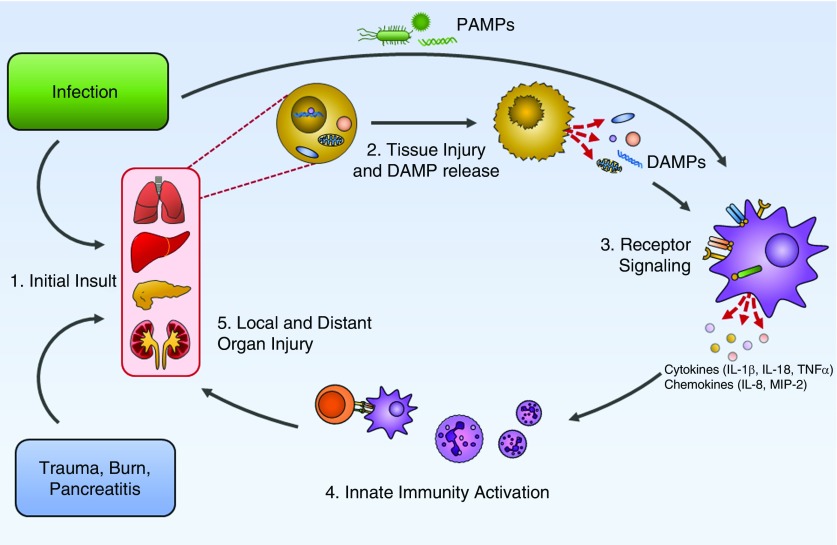
Figure 1.
Proposed schema for the pathogenesis of damage-associated molecular pattern (DAMP)- and pathogen-associated molecular pattern (PAMP)-mediated local and distal organ injury during critical illness. (1) Infection or sterile organ injury, such as trauma, burns, or pancreatitis, leads to direct initial insult. (2) Infection and local tissue injury releases PAMPs and DAMPs. (3) PAMPs and DAMPs bind Toll-like receptors and pathogen-recognition receptors, with subsequent cytokine and chemokine release. (4) Activation of the innate immune system with neutrophil recruitment and T-cell activation. (5) Resultant local and distant organ injury. MIP = macrophage inflammatory protein.
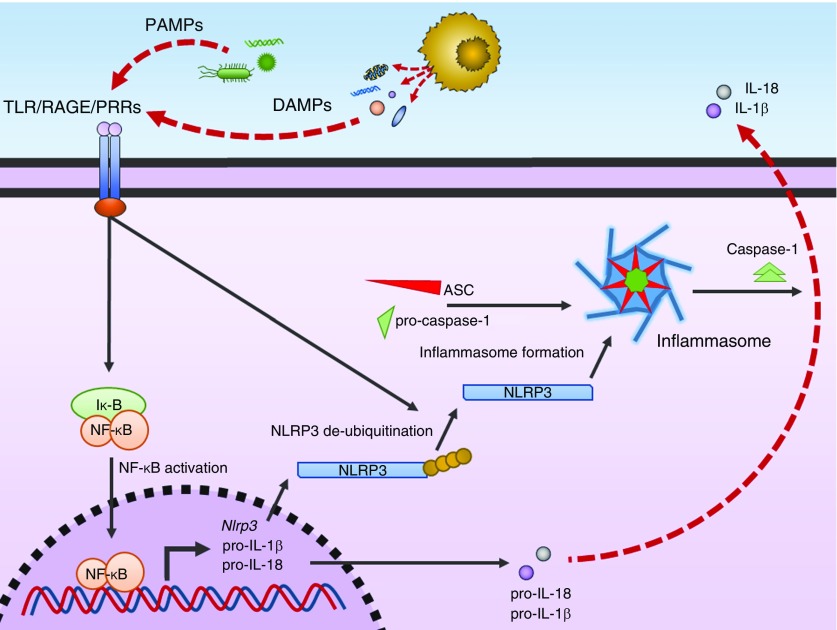
Figure 2.
Schema of damage-associated molecular pattern (DAMP)- and pathogen-associated molecular pattern (PAMP)-mediated activation of innate immunity and inflammation. PAMPs and DAMPs released from infection and tissue injury, respectively, bind cell surface TLRs (Toll-like receptors) and PRRs (pattern-recognition receptors), leading to activation of NF-κB (nuclear factor-κB). NF-κB translocates to the nucleus and promotes expression of pro–IL-18, pro–IL1β, and NLRP3 (nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing 3). NLRP3 is deubiquinated and combines with apoptosis-associated speck-like protein (ASC) and pro–caspase-1 to form the NLRP3 inflammasome. The activated NLRP3 inflammasome converts pro–caspase-1 to caspase-1. Caspase-1 subsequently then catalyzes the production of mature IL-18 and IL-1β. Iκ-B = inhibitor of κ-B; RAGE = receptor for advanced glycation end-products.
This framework, however, fails to explain the systemic inflammatory response observed during noninfectious critical illness, such as major trauma, burns, or cardiac arrest; nor does it explain the prolonged organ dysfunction in sepsis despite clearance of the original infection. In 1994, a complimentary model was proposed, coined the “danger” model (8). Based purely on theoretical grounds, the “danger” model hypothesized that unregulated, necrotic cell death must release endogenous molecules that trigger the innate immune system, leading to local “sterile” inflammation and tissue destruction. Definitive empirical evidence for this concept developed throughout the 1990s by observation of local cellular reaction to necrotic and apoptotic cell death. Several molecules were discovered in this period that can bind to PRRs and initiate local inflammatory responses (9–12). These activators of the innate immune system have been named alarmins, cell death-associated molecules, and damage-associated molecular patterns (DAMPs).
DAMP release has been implicated in the development of a variety of both acute and chronic inflammatory conditions. In critically ill patients, DAMPs and activation of PRR-related pathways are involved in the pathogenesis of the sterile systemic inflammatory response syndrome and perpetuation of the multiorgan dysfunction syndrome (MODS) during sepsis (13, 14) and trauma (15). DAMPs have also been shown to be integral to the development of ventilator-induced acute lung injury (16) and drug-induced liver injury (17), and have been linked to outcomes after cardiac arrest (18).
To separate DAMPs from simple biomarkers or inflammatory cytokines, criteria have been proposed to formally characterize potential DAMPs (19, 20): 1) rapidly released in response to infection or tissue injury; 2) have effects on antigen-presenting cells that modulate immune activity; 3) active as a purified molecule at concentrations in pathophysiological situations; 4) selective elimination or inactivation should inhibit biological activity; and 5) have a separate biological role in noninflammatory states. Since the proposal of the initial “danger” hypothesis, numerous host-derived intracellular and extracellular molecules have been described in the literature as potential DAMPs, but few have met the above criteria (10, 21, 22). The most well-described and accepted DAMPs include nucleic acids, such as mitochondrial DNA (mtDNA) (22, 23) and nuclear DNA (24, 25), HMGB1 (high mobility group [HMG] box 1) (26), HSPs (heat shock proteins) (27), histones (28), and S100 proteins (29) (Table 1). Other potential DAMPs include intracellular uric acid (30), N-formyl peptides (31), defensins (32), cathelicidins (33), extracellular hyaluronic acid (34), and fibronectin (35).
Table 1.
Five Commonly Reported Damage-associated Molecular Patterns, Their Reported Receptors, Studies of Preclinical Models of Critical Illness, and Studies of Human Cohorts of Critical Illness
| DAMP | Receptor(s) | Nonhuman Studies | Human Studies |
|---|---|---|---|
| Mitochondrial DNA | TLR9 (39) and AIM2 (22) | Sepsis (22) | Sepsis (23, 56) |
| Trauma (52) | Trauma (51, 57–60) | ||
| ALI (52, 55) | Cardiac arrest (63) | ||
| Shock (153) | |||
| Burn (154) | |||
| Nuclear DNA | TLR9 (39) | Sepsis (138) | Sepsis (49, 50) |
| Trauma (52) | Trauma (53) | ||
| Cardiac arrest (61, 62) | |||
| Burn (155) | |||
| HMGB1 | TLR2 (67), TLR4 (67, 156), TLR9 (69), and RAGE (10) | Sepsis (66, 71) | Sepsis (10, 72, 73) |
| Trauma (74) | Trauma (74–77) | ||
| ALI (156, 157) | VILI (78) | ||
| Cardiac arrest (158) | Cardiac arrest (79, 160) | ||
| Burn (159) | Burns (161) | ||
| Heat shock proteins | TLR2 (162), TLR4 (162), SREC-1 (163), CD91 (164), and CD14 (165) | Sepsis (86–89) | Sepsis (91–93) |
| Trauma (166) | Trauma (96) | ||
| Shock (95) | Cardiac arrest (97) | ||
| ALI (167) | ALI (168) | ||
| S100 Proteins | TLR4 (100, 101) and RAGE (41) | Sepsis (107, 108) | Sepsis (109, 110) |
| Trauma (103, 104) | Trauma (111, 112) | ||
| ALI (169) | ALI (113–115) | ||
| Cardiac arrest (18, 116, 117) | |||
| Histones | TLR2 (120, 121), TLR4 (120, 121),TLR9 (119), and NLPR3 (122) | Sepsis (124) | Sepsis (125, 126) |
| Trauma (123) | Trauma (123) | ||
| ALI (123) | ALI (123) | ||
| Cardiac arrest (170) |
Definition of abbreviations: AIM2 = absent in melanoma 2; ALI = acute lung injury; DAMP = damage-associated molecular pattern; HMGB1 = high-mobility group box 1; NLPR3 = nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing 3; RAGE = receptor for advanced glycation end-products; SREC-1 = scavenger receptor expressed by endothelial cells I; TLR = Toll-like receptor; VILI = ventilator-induced lung injury.
Given the similarities between DAMPs and PAMPs, it should be no surprise that DAMPs also activate PRR pathways and lead to NF-κB and inflammasome activation. These cascades can, in turn, lead to local cellular necrosis, further release of DAMPs, and the propagation of dysregulated cell death (36). Specifically, DAMPs, such as HMGB1, mtDNA, S100 proteins, and histones, have been shown to activate TLRs, including TLR2 (37), TLR3 (38), TLR4 (37), and TLR9 (39). More recently, immunologically active receptors that recognize DAMPs, but not PAMPs, have been identified. For example, RAGE (the receptor for advanced glycation endproducts) is a plasma membrane receptor that has been shown to respond to a variety of DAMPs, including HMGB1 (40) and S100 proteins (41). RAGE was first discovered as a receptor for products of nonenzymatic glycation and oxidation of macromolecules (42). RAGE is found in high levels in lung tissue, but can also appear in innate immune cells, including macrophages and neutrophils. Once bound by its ligands, RAGE can activate NF-κB and mitogen-activated protein kinase pathways, leading to a proinflammatory state (43).
This review focuses on the best-described DAMPs and their role in the pathogenesis of critical illness, with a specific focus on sepsis, trauma, acute lung injury, and cardiac arrest. We also discuss current and future translational and therapeutic research in this field targeting DAMP-associated pathways in critical illness.
DAMPs and Their Role in Critical Illness
Nucleic Acids
Except for mature erythrocytes, all human cells contain nucleic acids in the form of nuclear DNA, RNA, and mtDNA. Microbial nucleic acids are well-known PAMPs (44–47). During times of cellular stress, the release of host nucleic acids into the cytosolic and extracellular space illicit an inflammatory response like that of microbial nucleic acids. In vitro stimulation with cell-free nuclear DNA can selectively stimulate the production of IL-6 by human monocytes (24). Similarly, mRNA has in vitro inflammatory activity through TLR3-dependent induction of the NK-κB pathway and IL-8 secretion (38). In human subjects, plasma nuclear DNA concentrations are higher in those undergoing treatment in a mixed ICU population compared with healthy control subjects and correlates with mortality and need for mechanical ventilation (25, 48). Compared with healthy control subjects, plasma nuclear DNA is higher in human subjects with sepsis or septic shock (49, 50). This correlation is also seen in murine models of hemorrhagic shock (51) and rate models of trauma (52). Plasma nuclear DNA concentrations are also associated with severity of trauma and the presence of acute lung injury (53).
Mitochondria are bacterial endosymbionts that have evolved to be vital organelles in cellular energy production. Mitochondria carry their own genetic code, such as mtDNA, and transcriptional machinery, allowing for production of mitochondria-specific proteins. mtDNA is also CpG enriched, like bacterial DNA. Cellular stress, such as stimulation with ATP and LPS, can release mtDNA into the cytosolic and extracellular space, leading to activation of the innate immune system through binding of TLR receptors, specifically TLR9 (22, 39). Human neutrophils treated with purified mtDNA demonstrate MMP (matrix metallopeptidase)-8 and MMP9 release. Mice injected with mitochondrial debris or purified mtDNA have higher levels of TNF-α, IL-6, and IL-1 in the plasma, higher levels of IL-6 and TNF-α in the liver, as well as increased markers of lung injury (54, 55).
Among hospital nonsurvivors, mtDNA levels are higher at presentation to the emergency department and 72 hours after admission (23, 56). mtDNA levels are higher in patients who have undergone trauma admitted to the ICU compared with healthy control subjects, and correlates with severity of the inflammatory response and the development of MODS (51, 57–60). Similarly, plasma DNA levels correlate with mortality after out-of-hospital cardiac arrest (61, 62). In a separate cohort of 85 patients, cell-free mtDNA was superior to nuclear DNA in predicting survival at Day 3 after cardiac arrest (63).
HMGB1
HMGB1 is a member of the HMG family of proteins that are present in numerous eukaryotic organisms. HMG proteins reside within the nucleus and regulate transcription as a DNA chaperone (64). HMGB1 is released in response to cellular stress (10). Administration of purified HMGB1 activates the innate immune system (65), whereas inactivation of HMGB1 through antibodies or siRNA suppresses the inflammatory response (66). HMGB1 was first noted to be a potential late mediator of inflammation in 1999 by Wang and colleagues (10). Secretion of HMGB1 occurs 6–8 hours after LPS exposure, significantly later than TNF-α or IL-1. Release of HMGB1 extracellularly interacts with receptors, including TLR2, TLR4, TLR9, and RAGE (67–69), to release inflammatory cytokines, including TNF-α, IL-1, and IL-6 (10, 70).
HMGB1 was found to be elevated beginning 18 hours after cecal ligation and puncture (CLP) in mice, and remains elevated for up to 4 weeks (66, 71). Injections of anti-HMGB1 antibodies were able to ameliorate CLP-induced mortality (66). In humans, HMGB1 is elevated in patients with sepsis and septic shock, and is associated higher mortality (10, 72, 73). Compared with sepsis, traumatic injury is associated with a more rapid release of HMGB1 (74). In a murine model of hemorrhagic shock, treatment with anti-HMGB1 antibody improved survival at 24 hours after insult compared with control. In this same study, a human cohort of 25 patients admitted for trauma and hemorrhagic shock demonstrated peak plasma HMGB1 level within 6 hours of injury that remained elevated for at least 24 hours (74). In other studies, plasma HMGB1 is elevated within 30 minutes after trauma, and is associated with severity of injury and subsequent development of sepsis, MODS, and death (75–77). Elevated HMGB1 level has also been associated with patients requiring prolonged mechanical ventilation (78), as well as poor outcomes after cardiac arrest (79).
HSPs
HSPs are ubiquitous molecular chaperone proteins conserved across virtually all species. During normal cellular homeostasis, HSPs bind polypeptide chains to prevent protein aggregation and misfolding (80). The HSP70 family of at least 13 distinct human proteins is synthesized and released under a variety of environmental and pathological stresses, including infection (81), trauma (82), and hyperthermia (83). However, unlike HMGB1 or mtDNA, extracellular members of the HSP70 family can exhibit either inflammatory or antiinflammatory properties. T regulatory cells treated with purified HSP72, an endotoxin-inducible HSP70 family member, have decreased secretion of proinflammatory cytokines, such as IFNγ and TNF-α, and an increased secretion of antiinflammatory cytokines, such as IL-10 (84). In vitro treatment with HSP72 decreased activation of allogeneic T cells by immature dendritic cells (85).
Induction of several HSP70 family members through different methods was protective against animal models of sepsis (86–89). Aged transgenic hsp70−/− mice, which are deficient in the homolog for human HSP72, had higher mortality compared with wild-type mice subjected to CLP, Pseudomonas aeruginosa pneumonia, and Streptococcus pneumoniae pneumonia. In addition, these mice had higher levels of systemic inflammatory cytokines, including TNF-α, IL-6, IL-10, and IL-1β (90). In humans, HSPs are upregulated in adults (91, 92) and children (93) with sepsis. Polymorphisms in hsp70A1A, -A1B, and -A1L may also be correlated with increased morbidity after sepsis (94). In models of hemorrhage and resuscitation, mice have higher levels of HSP70 in the jejunum, lung, heart, kidney, and liver compared with sham controls (95). In a cohort of 67 patients admitted with trauma, HSP72 levels were significantly higher in those with injury severity scores greater than 16 compared with those with scores less than 16 and healthy control subjects. However, among patients admitted with severe trauma, higher HSP72 values at time of admission were associated with improved survival (96). Similarly, HSP72 is increased immediately after cardiac arrest and remains elevated over the following day (97). HSP72 levels are negatively correlated with proinflammatory cytokines, such as TNF-α and IL-6 (97).
S100 Proteins
S100 proteins are a family of more than 20 proteins seen exclusively within vertebrates. S100 proteins typically form homodimers that are capable of binding intracellular calcium and target proteins, serving as a calcium sensor for the regulation of effector proteins (98). Three specific S100 proteins (S100A8, S100A9, and S100A12) have been found to be specific to myeloid cells and highly regulated during the inflammatory process (29). S100A8, also known as myeloid-related protein 8 and S100A9, also known as myeloid-related protein 14, are present in circulatory granulocytes, but not tissue macrophages (29). During inflammation, the two proteins form a heterodimer and are released into the extracellular space (99). S100A8/S100A9 heterodimers regulate the inflammatory response through ICAM-1 (intercellular adhesion molecule 1)–mediated neutrophil chemotaxis and TLR4-mediated cytokine release (29, 100, 101). S100A12 is a more recently described S100 protein that activates the inflammatory axis through binding of either RAGE or TLR4 (41, 102). When released into the extracellular space, S100B can act as a ligand for RAGE-mediated signaling and NF-κB activation. S100B has been studied extensively in neurologic illness, and has been shown to be altered in numerous forms of brain injury, including trauma (103, 104), anoxia (105), and hemorrhage (106).
In sepsis models, S100A8/S100A9 is upregulated in the lung and plasma in mice during bacterial pneumonia. However, conflicting reports suggest that S100A8/S100A9 may be protective against bacterial dissemination and sepsis-associated mortality during infection with Klebsiella pneumoniae, but detrimental during infection with pneumococcal pneumonia (107, 108). Mice deficient in S100A9 are protected from endotoxin-induced lethal shock and Escherichia coli–induced abdominal sepsis (101). In humans, patients with septic shock who died had higher mRNA expression of S100A8 compared with survivors (109). S100A12 levels in the serum were higher in patients with sepsis from peritonitis, pneumonia, or urinary tract infection compared with healthy control subjects (110). After trauma, S100A8 and S100A12 are elevated, and reach their peak between 4 and 6 days after trauma (111). S100A8/S100A9 is also significantly higher in patients who have undergone trauma and who died on Day 1 of admission compared with survivors (112). S100A12 is elevated in the BAL fluid of subjects with acute respiratory distress syndrome compared with healthy control subjects (113–115). Significantly elevated levels of S100B after cardiac arrest have been associated with poor neurologic outcomes (18, 116) and lower rates of survival (117).
Histones
Histones are intranuclear proteins that bind to DNA to enhance chromatin stability and allow for epigenetic regulation of DNA (28). Within the nucleus, histones have two major functions. Core histones compress DNA by wrapping 147 bp of DNA around an octameric core histone complex, forming the “nucleosome.” Linker histones then combine multiple nucleosomes together, forming chromatin (28). Both nucleosomes and free histones can be released into the extracellular space after unregulated necrotic cell death (118). When histones are released into the extracellular space, they target proinflammatory receptors, including TLR2, TLR4, and TLR9, as well as direct activation of the NLRP3 inflammasome (119–122). They also directly induce cell death through disruption of plasma membranes and subsequent calcium influx (123).
Administration of anti-histone antibodies reduced the mortality of mice in LPS, TNF-α, and CLP models of sepsis (124). Circulating histones are elevated in patients with sepsis compared with healthy control subjects (125, 126). In a cohort of 65 patients with sepsis, high histone levels were correlated with degree of organ failure, new-onset left ventricular dysfunction, and mortality (125). Administration of exogenous histones to mice produced similar levels of lung injury and coagulation abnormalities as those produced by trauma. Coadministration of anti-histone antibodies, or concurrent treatment with anti-histone antibodies 10 minutes before trauma, ameliorated these effects (123). Patients with trauma had elevated levels of circulating nucleosomes compared with healthy control subjects, and levels of circulating nucleosomes after trauma were correlated with development of post-traumatic respiratory failure (123). In another study of different ICU patients, extracellular plasma histone H4 (a core histone) concentration was most elevated in patients with multiple trauma as well as sepsis compared with ICU control subjects. Higher histone H4 concentration was also associated with need for renal replacement therapy and 90-day mortality (126).
Current Controversies and Unexplored Areas
Differential Expression of DAMPs during Critical Illness
Despite a robust body of preclinical studies demonstrating the effects of individual DAMPs on innate immunity and inflammation, no studies have elucidated precise stimuli or triggers that result in a differential expression of DAMPs. In a therapeutic study of intensive glycemic control using insulin in surgical ICU patients, HMGB1 and S100A12 were similarly elevated in both cohorts (127). In a cohort of patients with trauma in the emergency department, nuclear DNA and HSP70, but not mtDNA, were elevated compared with healthy control subjects (82). However, these findings contradict other studies of plasma mtDNA in patients with trauma, which show that mtDNA is elevated after trauma and is correlated to severity of injury and outcomes (51, 57–59). The lack of differential expression in the majority of published reports is not necessarily surprising. DAMPs are intracellular molecules that are typically passively released in the setting of cell stress and death, a feature common in many critical care states (128–130).
Mechanisms of Active DAMP Release
Although DAMPs are typically passively released in the setting of cell death, growing evidence suggests that certain DAMPs may also be actively secreted into the extracellular space. Monocytes and macrophages exposed to LPS or TNF-α can acetylate or phosphorylate the nuclear localization signals of HMGB1, leading to relocation of HMGB1 from the nucleus into the cytoplasmic space (131, 132). HMGB1 is then packaged into secretory lysosomes, where they can then be excreted into the extracellular space in response to triggers, such as lysophosphatidylcholine (133). HSPs have been shown to be trafficked to the cell surface via exosomes after stimulation with IFNγ or heat shock, a mechanism that is independent of the common protein-secretory pathway (134–136). Using time-lapse automated confocal imaging, Yousefi and colleagues (137) demonstrated that eosinophils released mtDNA, but not nuclear DNA, in a “catapult-like” manner after exposure to LPS, complement factor 5a, or eotaxin. The precise mechanism of this release is unclear, but appears to require activation of nicotinamide adenine dinucleotide phosphate reduced oxidase, but is independent of cell death (137). Whether these specific release mechanisms respond to all external environmental stressors or are trigger specific remains unknown. Further studies are also required to assess whether these lysosome- and exosome-dependent secretory pathways are shared among DAMPs or are specific for certain DAMPs.
Modulation of DAMP Expression or Release during Critical Illness
Preclinical studies exploring the modulation of DAMPs during critical illness have often focused on either the neutralization of DAMPs with antibodies or pharmacologic intervention before or after onset of critical illness. Use of anti-HMGB1 antibody has been shown to improve survival in murine models of sepsis and hemorrhagic shock (66, 74). The same group has also reported that (−)-epigallocatechin-3-gallate (EGCG), an ingredient in green tea, suppressed LPS-induced release of HMGB1 from macrophages, and rescued mice from lethal sepsis after CLP. EGCG also inhibited HMGB1-mediated release of inflammatory cytokines by blocking aggregation of exogenous HMGB1 on the surface of macrophages. No other DAMPs were evaluated, so it is unclear whether the beneficial effects of EGCG are specific to HMGB1 or applicable to other DAMPs.
Mice that are treated with DNase 4 or 6 hours after CLP have reduced cell-free DNA, suppressed organ damage, and reduced bacterial dissemination (138). Similarly, intratracheal DNase administration to rat lungs before or after intratracheal infection with P. aeruginosa mitigated endothelial injury and mtDNA in the BAL (139). Thus far, no human studies have directly inhibited DAMP release or binding. Interestingly, some of the antiinflammatory effects of HSP72 may be related its modulation of HMGB1 release. In macrophages, release of HSP72 with mild heat shock or overexpression via gene transfection leads to inhibition of HMGB1 cytoplasmic translocation and release after oxidative stress, LPS, and TNF-α treatment (140, 141).
Modulation of DAMP–Receptor Interaction during Critical Illness
Investigators have also explored the possibility of modulating DAMP signaling through the blocking of common DAMP receptors, including TLR4 and RAGE. TAK-242 (resatorvid) is a small-molecule–specific inhibitor of TLR4 signaling through binding of the intracellular domain of TLR4. In mice, administration of TAK-242 before or after LPS challenge suppressed expression of inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Mice treated with TAK-242 before or after LPS challenge also demonstrated lower markers of organ injury, such as alanine aminotransferase and total bilirubin (142). However, a multicenter, randomized, double-blind, placebo-controlled trial of TAK-242 in 274 patients with severe sepsis and shock or respiratory failure was stopped early due to lack of efficacy in suppressing serum IL-6 levels (143).
E5564 (eritoran) is a synthetic LPS derived from the endotoxin of Rhodobacter sphaeroides. Eritoran blocks LPS-mediated cytokine release through the binding of an internal pocket of MD2, a protein necessary to facilitate the binding of LPS to TL4 (144). Phase I clinical studies demonstrated reductions in vital sign changes as well as decreased expression of TNF-α and IL-6 after treatment with eritoran in healthy subjects challenged with LPS (145). However, follow-up phase II and phase III trials failed to demonstrate a difference in 28-day mortality after treatment with eritoran among patients with severe sepsis and organ dysfunction (146, 147).
van Zoelen and colleagues (148) have previously demonstrated that RAGE-deficient mice show improved survival after intranasal inoculation with S. pneumoniae compared with wild-type mice. Similarly, the use of an anti-RAGE monoclonal antibody was protective in murine sepsis with both CLP and S. pneumoniae inoculation (149, 150). RAGE signaling has been associated with increased lethality in mice exposed to CLP or S. pneumoniae inoculation (148, 150). However, more recently van Zoelen and colleagues have also shown that RAGE is protective during K. pneumoniae–induced pneumonia. Thus, current work on RAGE has yielded inconsistent results and remains preclinical. These studies highlight the complexity of treating human disease when effective therapies observed in preclinical disease models sometimes (oftentimes) are not replicated in human diseases.
Future Directions
As highlighted in this review, systemic and tissue-specific levels of various DAMPs are associated with progression of key critical illnesses. From the basic and early translational research performed in recent decades, the importance of these cellular breakdown products in inflammatory signal transduction has become clear. In the near term, several best-studied DAMPs, such as nucleic acids, may play a role in prognostication in the ICU. However, the focus on DAMPs research should not focus on its prognostic significance in human cohorts, but more on the precise mechanisms of DAMP-induced inflammation and cell injury. In many conditions encountered in the ICU, multiple DAMPs may be crucial to the development of a specific response. Prior work has focused on individual markers and interaction with specific PRRs. In most clinical situations, there are complex, and likely redundant, interactions between different DAMP–PRR pathways. Moreover, differential levels and patterns of DAMP kinetics may play an important role in the diagnosis of syndromic disease states, such as sterile systemic inflammatory response syndrome and ongoing occult infection. There is much work to be done to understand stimulus-specific differential DAMP responses. Likely, there is a complex interplay between host genomic and metabolic parameters and DAMP-specific response to certain stimuli that will vary in different patient populations, and this needs further elucidation. Further understanding of the feedback loops that can propagate DAMP release and ongoing cell death will be important in designing clinical interventions in these conditions. Recent work has highlighted the importance of necroptotic cell death in the immunologically active release of various cellular components, many of which are DAMPs (151). Truly understanding when and where in the host these pathways are appropriately activated versus dysregulated will be key to appropriately targeting inhibitors.
Conclusions
Given the past failures of antiinflammatory therapy, it is necessary to move beyond animal and cell culture models of DAMPs. Unfortunately, there is no “time zero” in many ICU diseases, and the time course of the immune response to insult is often not known. Humans are more complex than most laboratory animals, carrying immune histories and comorbidities over a long and varied life span. Importantly, robust real-world translational data are needed regarding DAMP, PRR, and pathway propagation, such as the important contribution of Nakahira and colleagues (22). Deep understanding of subphenotypes and differential individual time-dependent phases of classic syndromic disease states, such as sepsis and trauma, which characterize the innate immune system response, will be integral (152). Taking these data, and understanding the dysregulated cell death pathway activation through a combination of clinical and molecular methods, will be important to designing adaptive, personalized clinical trials with a high likelihood of finding targeted therapies.
Footnotes
Supported by NIH grants P01 HL108801, R01 HL079904, R01 HL055330, and R01 HL060234 (A.M.K.C.); and KL2TR000458-10 (E.J.S.).
Author Contributions: K.C.M., A.M.K.C., and E.J.S. contributed to the conception and design of the study; K.C.M., E.J.S., and M.A.P. contributed to the drafting of the manuscript; K.C.M., A.M.K.C., and E.J.S. contributed to the editing of the manuscript; K.C.M. contributed to the creation of the figures; A.M.K.C. and E.J.S. contributed to the editing of the figures; all authors contributed to the final approval of all submitted contents.
Originally Published in Press as DOI: 10.1164/rccm.201612-2460PP on October 4, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Dresser DW. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961;191:1169–1171. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 6.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 7.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 9.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 11.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg B, Urnovitz HB, Stricker RB. Beyond danger: unmethylated CpG dinucleotides and the immunopathogenesis of disease. Immunol Lett. 2000;73:13–18. doi: 10.1016/s0165-2478(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 13.Denk S, Perl M, Huber-Lang M. Damage- and pathogen-associated molecular patterns and alarmins: keys to sepsis? Eur Surg Res. 2012;48:171–179. doi: 10.1159/000338194. [DOI] [PubMed] [Google Scholar]
- 14.Kang J-W, Kim S-J, Cho H-I, Lee S-M. DAMPs activating innate immune responses in sepsis. Ageing Res Rev. 2015;24:54–65. doi: 10.1016/j.arr.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Hirsiger S, Simmen H-P, Werner CML, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm. 2012;2012:315941. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuipers MT, van der Poll T, Schultz MJ, Wieland CW. Bench-to-bedside review: damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care. 2011;15:235. doi: 10.1186/cc10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BHF, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 18.Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Abe R, et al. S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care. 2009;13:R121. doi: 10.1186/cc7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 20.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Nakahira K, Hisata S, Choi AMK. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. 2015;23:1329–1350. doi: 10.1089/ars.2015.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakahira K, Haspel JA, Rathinam VAK, Lee S-J, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahira K, Kyung S-Y, Rogers AJ, Gazourian L, Youn S, Massaro AF, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577. doi: 10.1371/journal.pmed.1001577. [Discussion, p. e1001577.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atamaniuk J, Kopecky C, Skoupy S, Säemann MD, Weichhart T. Apoptotic cell-free DNA promotes inflammation in haemodialysis patients. Nephrol Dial Transplant. 2012;27:902–905. doi: 10.1093/ndt/gfr695. [DOI] [PubMed] [Google Scholar]
- 25.Wijeratne S, Butt A, Burns S, Sherwood K, Boyd O, Swaminathan R. Cell-free plasma DNA as a prognostic marker in intensive treatment unit patients. Ann N Y Acad Sci. 2004;1022:232–238. doi: 10.1196/annals.1318.036. [DOI] [PubMed] [Google Scholar]
- 26.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 27.Wallin RPA, Lundqvist A, Moré SH, von Bonin A, Kiessling R, Ljunggren H-G. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–135. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 28.Silk E, Zhao H, Weng H, Ma D. The role of extracellular histone in organ injury. Cell Death Dis. 2017;8:e2812. doi: 10.1038/cddis.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 30.Kono H, Chen C-J, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazeldine J, Hampson P, Opoku FA, Foster M, Lord JM. N-Formyl peptides drive mitochondrial damage associated molecular pattern induced neutrophil activation through ERK1/2 and P38 MAP kinase signalling pathways. Injury. 2015;46:975–984. doi: 10.1016/j.injury.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 32.Xie G-H, Chen Q-X, Cheng B-L, Fang X-M. Defensins and sepsis. Biomed Res Int. 2014;2014:180109. doi: 10.1155/2014/180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J, et al. Cathelicidins: family of antimicrobial peptides: a review. Mol Biol Rep. 2012;39:10957–10970. doi: 10.1007/s11033-012-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobili V, Alisi A, Torre G, De Vito R, Pietrobattista A, Morino G, et al. Hyaluronic acid predicts hepatic fibrosis in children with nonalcoholic fatty liver disease. Transl Res. 2010;156:229–234. doi: 10.1016/j.trsl.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz Martín G, Prieto Prieto J, Veiga de Cabo J, Gomez Lus L, Barberán J, González Landa JM, et al. Plasma fibronectin as a marker of sepsis. Int J Infect Dis. 2004;8:236–243. doi: 10.1016/j.ijid.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, et al. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 38.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 39.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian J, Avalos AM, Mao S-Y, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 42.van Zoelen MA, Achouiti A, van der Poll T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit Care. 2011;15:208. doi: 10.1186/cc9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: a new therapeutic target? Curr Opin Investig Drugs. 2000;2006:985–991. [PubMed] [Google Scholar]
- 44.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 45.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 46.Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 48.Saukkonen K, Lakkisto P, Varpula M, Varpula T, Voipio-Pulkki L-M, Pettilä V, et al. Association of cell-free plasma DNA with hospital mortality and organ dysfunction in intensive care unit patients. Intensive Care Med. 2007;33:1624–1627. doi: 10.1007/s00134-007-0686-z. [DOI] [PubMed] [Google Scholar]
- 49.Rhodes A, Wort SJ, Thomas H, Collinson P, Bennett ED. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit Care. 2006;10:R60. doi: 10.1186/cc4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saukkonen K, Lakkisto P, Pettilä V, Varpula M, Karlsson S, Ruokonen E, et al. Finnsepsis Study Group. Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin Chem. 2008;54:1000–1007. doi: 10.1373/clinchem.2007.101030. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gan L, Chen X, Sun T, Li Q, Zhang R, Zhang J, et al. Significance of serum mtDNA concentration in lung injury induced by hip fracture. Shock. 2015;44:52–57. doi: 10.1097/SHK.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 53.Lo YM, Rainer TH, Chan LY, Hjelm NM, Cocks RA. Plasma DNA as a prognostic marker in trauma patients. Clin Chem. 2000;46:319–323. [PubMed] [Google Scholar]
- 54.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J-Z, Liu Z, Liu J, Ren J-X, Sun T-S. Mitochondrial DNA induces inflammation and increases TLR9/NF-κB expression in lung tissue. Int J Mol Med. 2014;33:817–824. doi: 10.3892/ijmm.2014.1650. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Kung C-T, Hsiao S-Y, Tsai T-C, Su C-M, Chang W-N, Huang C-R, et al. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J Transl Med. 2012;10:130. doi: 10.1186/1479-5876-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu X, Yao Y, Wu G, Lv T, Luo L, Song Y. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PLoS One. 2013;8:e72834. doi: 10.1371/journal.pone.0072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simmons JD, Lee Y-L, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258:591–596. doi: 10.1097/SLA.0b013e3182a4ea46. [Discussion, pp. 596–598.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care. 2013;28:1027–1031. doi: 10.1016/j.jcrc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One. 2015;10:e0120549. doi: 10.1371/journal.pone.0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnalich F, Menéndez M, Lagos V, Ciria E, Quesada A, Codoceo R, et al. Prognostic value of cell-free plasma DNA in patients with cardiac arrest outside the hospital: an observational cohort study. Crit Care. 2010;14:R47. doi: 10.1186/cc8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gornik I, Wagner J, Gašparović V, Miličić D, Degoricija V, Skorić B, et al. Prognostic value of cell-free DNA in plasma of out-of-hospital cardiac arrest survivors at ICU admission and 24h post-admission. Resuscitation. 2014;85:233–237. doi: 10.1016/j.resuscitation.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Arnalich F, Codoceo R, López-Collazo E, Montiel C. Circulating cell-free mitochondrial DNA: a better early prognostic marker in patients with out-of-hospital cardiac arrest. Resuscitation. 2012;83:e162–e163. doi: 10.1016/j.resuscitation.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 64.Fink MP. Bench-to-bedside review: high-mobility group box 1 and critical illness. Crit Care. 2007;11:229. doi: 10.1186/cc6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson U, Wang H, Palmblad K, Aveberger A-C, Bloom O, Erlandsson-Harris H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park JS, Svetkauskaite D, He Q, Kim J-Y, Strassheim D, Ishizaka A, et al. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 68.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin: mediation of neurite outgrowth and co-expression of RAGE and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 69.Valdés-Ferrer SI, Rosas-Ballina M, Olofsson PS, Lu B, Dancho ME, Li J, et al. High-mobility group box 1 mediates persistent splenocyte priming in sepsis survivors: evidence from a murine model. Shock. 2013;40:492–495. doi: 10.1097/SHK.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, et al. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–392. [PubMed] [Google Scholar]
- 71.Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, et al. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 2007;33:1347–1353. doi: 10.1007/s00134-007-0691-2. [DOI] [PubMed] [Google Scholar]
- 73.Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 74.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, et al. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X-W, Karki A, Zhao X-J, Xiang X-Y, Lu Z-Q. High plasma levels of high mobility group box 1 is associated with the risk of sepsis in severe blunt chest trauma patients: a prospective cohort study. J Cardiothorac Surg. 2014;9:133. doi: 10.1186/s13019-014-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Zoelen MAD, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–445. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- 79.Omura T, Kushimoto S, Yamanouchi S, Kudo D, Miyagawa N. High-mobility group box 1 is associated with neurological outcome in patients with post-cardiac arrest syndrome after out-of-hospital cardiac arrest. J Intensive Care. 2016;4:37. doi: 10.1186/s40560-016-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 81.Delogu G, Lo Bosco L, Marandola M, Famularo G, Lenti L, Ippoliti F, et al. Heat shock protein (HSP70) expression in septic patients. J Crit Care. 1997;12:188–192. doi: 10.1016/s0883-9441(97)90031-9. [DOI] [PubMed] [Google Scholar]
- 82.Timmermans K, Kox M, Vaneker M, van den Berg M, John A, van Laarhoven A, et al. Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med. 2016;42:551–561. doi: 10.1007/s00134-015-4205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang RC, Wang CI, Chen HW, Chou FP, Lue SI, Hwang KP. Heat shock treatment decreases the mortality of sepsis in rats. Kaohsiung J Med Sci. 1998;14:664–672. [PubMed] [Google Scholar]
- 84.Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenschuh S, et al. HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(−) T cells. PLoS One. 2012;7:e51747. doi: 10.1371/journal.pone.0051747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stocki P, Wang XN, Dickinson AM. Inducible heat shock protein 70 reduces T cell responses and stimulatory capacity of monocyte-derived dendritic cells. J Biol Chem. 2012;287:12387–12394. doi: 10.1074/jbc.M111.307579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hauser GJ, Dayao EK, Wasserloos K, Pitt BR, Wong HR. HSP induction inhibits iNOS mRNA expression and attenuates hypotension in endotoxin-challenged rats. Am J Physiol. 1996;271:H2529–H2535. doi: 10.1152/ajpheart.1996.271.6.H2529. [DOI] [PubMed] [Google Scholar]
- 87.Chu EK, Ribeiro SP, Slutsky AS. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25:1727–1732. doi: 10.1097/00003246-199710000-00025. [DOI] [PubMed] [Google Scholar]
- 88.Jing L, Wu Q, Wang F. Glutamine induces heat-shock protein and protects against Escherichia coli lipopolysaccharide–induced vascular hyporeactivity in rats. Crit Care. 2007;11:R34. doi: 10.1186/cc5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol (1985) 2001;90:2403–2410. doi: 10.1152/jappl.2001.90.6.2403. [DOI] [PubMed] [Google Scholar]
- 90.McConnell KW, Fox AC, Clark AT, Chang N-YN, Dominguez JA, Farris AB, et al. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J Immunol. 2011;186:3718–3725. doi: 10.4049/jimmunol.1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hashiguchi N, Ogura H, Tanaka H, Koh T, Nakamori Y, Noborio M, et al. Enhanced expression of heat shock proteins in activated polymorphonuclear leukocytes in patients with sepsis. J Trauma. 2001;51:1104–1109. doi: 10.1097/00005373-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 92.Zhang R, Wan X, Zhang X, Kang Q, Bian J, Yu G, et al. Plasma HSPA12B is a potential predictor for poor outcome in severe sepsis. PLoS One. 2014;9:e101215. doi: 10.1371/journal.pone.0101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wheeler DS, Fisher LE, Jr, Catravas JD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005;6:308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- 94.Ramakrishna K, Pugazhendhi S, Kabeerdoss J, Peter JV. Association between heat shock protein 70 gene polymorphisms and clinical outcomes in intensive care unit patients with sepsis. Indian J Crit Care Med. 2014;18:205–211. doi: 10.4103/0972-5229.130571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiang JG, Bowman PD, Wu BW, Hampton N, Kiang AG, Zhao B, et al. Geldanamycin treatment inhibits hemorrhage-induced increases in KLF6 and iNOS expression in unresuscitated mouse organs: role of inducible HSP70. J Appl Physiol (1985) 2004;97:564–569. doi: 10.1152/japplphysiol.00194.2004. [DOI] [PubMed] [Google Scholar]
- 96.Pittet J-F, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–617. doi: 10.1097/00005373-200204000-00001. [Discussion, p. 617.] [DOI] [PubMed] [Google Scholar]
- 97.Timmermans K, Kox M, Gerretsen J, Peters E, Scheffer GJ, van der Hoeven JG, et al. The involvement of danger-associated molecular patterns in the development of immunoparalysis in cardiac arrest patients. Crit Care Med. 2015;43:2332–2338. doi: 10.1097/CCM.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 98.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 99.Zwadlo G, Brüggen J, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988;72:510–515. [PMC free article] [PubMed] [Google Scholar]
- 100.Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 101.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MAD, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 102.Foell D, Wittkowski H, Kessel C, Lüken A, Weinhage T, Varga G, et al. Proinflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am J Respir Crit Care Med. 2013;187:1324–1334. doi: 10.1164/rccm.201209-1602OC. [DOI] [PubMed] [Google Scholar]
- 103.Raabe A, Grolms C, Sorge O, Zimmermann M, Seifert V. Serum S-100B protein in severe head injury. Neurosurgery. 1999;45:477–483. doi: 10.1097/00006123-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 104.Goyal A, Failla MD, Niyonkuru C, Amin K, Fabio A, Berger RP, et al. S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J Neurotrauma. 2013;30:946–957. doi: 10.1089/neu.2012.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kecskes Z, Dunster KR, Colditz PB. NSE and S100 after hypoxia in the newborn pig. Pediatr Res. 2005;58:953–957. doi: 10.1203/01.PDR.0000182591.46087.7D. [DOI] [PubMed] [Google Scholar]
- 106.Lai PMR, Du R. Association between S100B levels and long-term outcome after aneurysmal subarachnoid hemorrhage: systematic review and pooled analysis. PLoS One. 2016;11:e0151853. doi: 10.1371/journal.pone.0151853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Achouiti A, Vogl T, Endeman H, Mortensen BL, Laterre P-F, Wittebole X, et al. Myeloid-related protein-8/14 facilitates bacterial growth during pneumococcal pneumonia. Thorax. 2014;69:1034–1042. doi: 10.1136/thoraxjnl-2014-205668. [DOI] [PubMed] [Google Scholar]
- 108.Achouiti A, Vogl T, Van der Meer AJ, Stroo I, Florquin S, de Boer OJ, et al. Myeloid-related protein-14 deficiency promotes inflammation in staphylococcal pneumonia. Eur Respir J. 2015;46:464–473. doi: 10.1183/09031936.00183814. [DOI] [PubMed] [Google Scholar]
- 109.Payen D, Lukaszewicz A-C, Belikova I, Faivre V, Gelin C, Russwurm S, et al. Gene profiling in human blood leucocytes during recovery from septic shock. Intensive Care Med. 2008;34:1371–1376. doi: 10.1007/s00134-008-1048-1. [DOI] [PubMed] [Google Scholar]
- 110.Achouiti A, Föll D, Vogl T, van Till JWO, Laterre P-F, Dugernier T, et al. S100A12 and soluble receptor for advanced glycation end products levels during human severe sepsis. Shock. 2013;40:188–194. doi: 10.1097/SHK.0b013e31829fbc38. [DOI] [PubMed] [Google Scholar]
- 111.Uhle F, Lichtenstern C, Brenner T, Fleming T, Koch C, Hecker A, et al. Role of the RAGE axis during the immune response after severe trauma: a prospective pilot study. Mediators Inflamm. 2015;2015:1691491. doi: 10.1155/2015/691491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Austermann J, Friesenhagen J, Fassl SK, Petersen B, Ortkras T, Burgmann J, et al. Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflammatory conditions Cell Rep 201492112–2123.[Published erratum appears in Cell Rep 11:849.] [DOI] [PubMed] [Google Scholar]
- 113.Wittkowski H, Sturrock A, van Zoelen MAD, Viemann D, van der Poll T, Hoidal JR, et al. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit Care Med. 2007;35:1369–1375. doi: 10.1097/01.CCM.0000262386.32287.29. [DOI] [PubMed] [Google Scholar]
- 114.Lorenz E, Muhlebach MS, Tessier PA, Alexis NE, Duncan Hite R, Seeds MC, et al. Different expression ratio of S100A8/A9 and S100A12 in acute and chronic lung diseases. Respir Med. 2008;102:567–573. doi: 10.1016/j.rmed.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jabaudon M, Blondonnet R, Roszyk L, Pereira B, Guérin R, Perbet S, et al. Soluble forms and ligands of the receptor for advanced glycation end-products in patients with acute respiratory distress syndrome: an observational prospective study. PLoS One. 2015;10:e0135857. doi: 10.1371/journal.pone.0135857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shinozaki K, Oda S, Sadahiro T, Nakamura M, Abe R, Nakada T-A. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation. 2009;80:870–875. doi: 10.1016/j.resuscitation.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 117.Calderon LM, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC Post Cardiac Arrest Service. Combining NSE and S100B with clinical examination findings to predict survival after resuscitation from cardiac arrest. Resuscitation. 2014;85:1025–1029. doi: 10.1016/j.resuscitation.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holdenrieder S, Stieber P, Bodenmüller H, Busch M, Von Pawel J, Schalhorn A, et al. Circulating nucleosomes in serum. Ann N Y Acad Sci. 2001;945:93–102. doi: 10.1111/j.1749-6632.2001.tb03869.x. [DOI] [PubMed] [Google Scholar]
- 119.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J, et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23:1375–1388. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang H, Chen H-W, Evankovich J, Yan W, Rosborough BR, Nace GW, et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol. 1950;2013:2665–2679. doi: 10.4049/jimmunol.1202733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alhamdi Y, Abrams ST, Cheng Z, Jing S, Su D, Liu Z, et al. Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit Care Med. 2015;43:2094–2103. doi: 10.1097/CCM.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 126.Ekaney ML, Otto GP, Sossdorf M, Sponholz C, Boehringer M, Loesche W, et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care. 2014;18:543. doi: 10.1186/s13054-014-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ingels C, Derese I, Wouters PJ, Van den Berghe G, Vanhorebeek I. Soluble RAGE and the RAGE ligands HMGB1 and S100A12 in critical illness: impact of glycemic control with insulin and relation with clinical outcome. Shock. 2015;43:109–116. doi: 10.1097/SHK.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 128.Hofer S, Brenner T, Bopp C, Steppan J, Lichtenstern C, Weitz J, et al. Cell death serum biomarkers are early predictors for survival in severe septic patients with hepatic dysfunction. Crit Care. 2009;13:R93. doi: 10.1186/cc7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 130.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 131.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Youn JH, Shin J-S. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 133.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 135.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 136.Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-γ enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 138.Mai SHC, Khan M, Dwivedi DJ, Ross CA, Zhou J, Gould TJ, et al. Canadian Critical Care Translational Biology Group. Delayed but not early treatment with DNase reduces organ damage and improves outcome in a murine model of sepsis. Shock. 2015;44:166–172. doi: 10.1097/SHK.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 139.Simmons JD, Freno DR, Muscat CA, Obiako B, Lee Y-LL, Pastukh VM, et al. Mitochondrial DNA damage associated molecular patterns in ventilator-associated pneumonia: Prevention and reversal by intratracheal DNase I. J Trauma Acute Care Surg. 2017;82:120–125. doi: 10.1097/TA.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007;179:1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, et al. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)–induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007;178:7376–7384. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, Iizawa Y. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 143.Rice TW, Wheeler AP, Bernard GR, Vincent J-L, Angus DC, Aikawa N, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 144.Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4–directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304:1093–1102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 145.Lynn M, Rossignol DP, Wheeler JL, Kao RJ, Perdomo CA, Noveck R, et al. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis. 2003;187:631–639. doi: 10.1086/367990. [DOI] [PubMed] [Google Scholar]
- 146.Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, et al. Eritoran Sepsis Study Group Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis Crit Care Med 20103872–83.[Published erratum appears in Crit Care Med 38:1925.] [DOI] [PubMed] [Google Scholar]
- 147.Opal SM, Laterre P-F, Francois B, LaRosa SP, Angus DC, Mira J-P, et al. ACCESS Study Group. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 148.van Zoelen MAD, Schouten M, de Vos AF, Florquin S, Meijers JCM, Nawroth PP, et al. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol. 2009;182:4349–4356. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 149.Lutterloh EC, Opal SM, Pittman DD, Keith JC, Jr, Tan X-Y, Clancy BM, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007;11:R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Christaki E, Opal SM, Keith JC, Jr, Kessimian N, Palardy JE, Parejo NA, et al. A monoclonal antibody against RAGE alters gene expression and is protective in experimental models of sepsis and pneumococcal pneumonia. Shock. 2011;35:492–498. doi: 10.1097/SHK.0b013e31820b2e1c. [DOI] [PubMed] [Google Scholar]
- 151.Moreno-Gonzalez G, Vandenabeele P, Krysko DV. Necroptosis: a novel cell death modality and its potential relevance for critical care medicine. Am J Respir Crit Care Med. 2016;194:415–428. doi: 10.1164/rccm.201510-2106CI. [DOI] [PubMed] [Google Scholar]
- 152.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu Q, Wood CR, Cimen S, Venkatachalam AB, Alwayn IPJ. Mitochondrial damage-associated molecular patterns (MTDs) are released during hepatic ischemia reperfusion and induce inflammatory responses. PLoS One. 2015;10:e0140105. doi: 10.1371/journal.pone.0140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szczesny B, Brunyánszki A, Ahmad A, Oláh G, Porter C, Toliver-Kinsky T, et al. Time-dependent and organ-specific changes in mitochondrial function, mitochondrial DNA integrity, oxidative stress and mononuclear cell infiltration in a mouse model of burn injury. PLoS One. 2015;10:e0143730. doi: 10.1371/journal.pone.0143730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chiu TW, Young R, Chan LYS, Burd A, Lo DYM. Plasma cell-free DNA as an indicator of severity of injury in burn patients. Clin Chem Lab Med. 2006;44:13–17. doi: 10.1515/CCLM.2006.003. [DOI] [PubMed] [Google Scholar]
- 156.Deng Y, Yang Z, Gao Y, Xu H, Zheng B, Jiang M, et al. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS One. 2013;8:e64375. doi: 10.1371/journal.pone.0064375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–L965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 158.Xu M, Zhou G-M, Wang L-H, Zhu L, Liu J-M, Wang X-D, et al. Inhibiting high-mobility group box 1 (HMGB1) attenuates inflammatory cytokine expression and neurological deficit in ischemic brain injury following cardiac arrest in rats. Inflammation. 2016;39:1594–1602. doi: 10.1007/s10753-016-0395-2. [DOI] [PubMed] [Google Scholar]
- 159.Huang L-F, Yao Y-M, Zhang L-T, Dong N, Yu Y, Sheng Z-Y. The effect of high-mobility group box 1 protein on activity of regulatory T cells after thermal injury in rats. Shock. 2009;31:322–329. doi: 10.1097/SHK.0b013e3181834070. [DOI] [PubMed] [Google Scholar]
- 160.Oda Y, Tsuruta R, Fujita M, Kaneda K, Kawamura Y, Izumi T, et al. Prediction of the neurological outcome with intrathecal high mobility group box 1 and S100B in cardiac arrest victims: a pilot study. Resuscitation. 2012;83:1006–1012. doi: 10.1016/j.resuscitation.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 161.Lantos J, Földi V, Roth E, Wéber G, Bogár L, Csontos C. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010;33:562–567. doi: 10.1097/SHK.0b013e3181cd8c88. [DOI] [PubMed] [Google Scholar]
- 162.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Häcker H, et al. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 163.Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A, et al. T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J Immunol. 1950;2009:3092–3098. doi: 10.4049/jimmunol.0901235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 166.Baker TA, Romero J, Bach HH, IV, Strom JA, Gamelli RL, Majetschak M. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage*. Crit Care Med. 2012;40:876–885. doi: 10.1097/CCM.0b013e318232e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Villar J, Cabrera N, Casula M, Flores C, Valladares F, Muros M, et al. Mechanical ventilation modulates Toll-like receptor signaling pathway in a sepsis-induced lung injury model. Intensive Care Med. 2010;36:1049–1057. doi: 10.1007/s00134-010-1799-3. [DOI] [PubMed] [Google Scholar]
- 168.Ganter MT, Ware LB, Howard M, Roux J, Gartland B, Matthay MA, et al. Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L354–L361. doi: 10.1152/ajplung.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hiroshima Y, Hsu K, Tedla N, Wong SW, Chow S, Kawaguchi N, et al. S100A8/A9 and S100A9 reduce acute lung injury. Immunol Cell Biol. 2017;95:461–472. doi: 10.1038/icb.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jeppesen AN, Hvas A-M, Grejs AM, Duez CHV, Sorensen BS, Kirkegaard H. Post-cardiac arrest level of free-plasma DNA and DNA-histone complexes. Acta Anaesthesiol Scand. 2017;61:523–531. doi: 10.1111/aas.12882. [DOI] [PubMed] [Google Scholar]