Complications from preterm birth, especially due to lung disease, are the leading cause of neonatal mortality and an important contributor to child and adult morbidities. Improved neonatal care has shifted the survival of premature infants from 32 weeks gestational age (GA) in the presurfactant era to the current lower limit of 23 weeks GA; however, extreme prematurity (23–29 weeks GA) has an increased respiratory morbidity that persists into childhood (1, 2). The complex origins of adult respiratory disease can often be traced back to the fetal and early childhood period when the lung is undergoing rapid development.
In addition to airways and parenchymal disease, preterm infants often have early pulmonary vascular disease (PVD), as defined by abnormal vascular growth and function (3). Strong laboratory and clinical data show that early disruption of pulmonary vascular growth (angiogenesis) impairs alveolarization, leading to a reduced surface area and a high risk for PVD (4, 5). Our recent clinical studies showed that abnormal echo findings consistent with PVD at postnatal Day 7 were strongly associated with the subsequent diagnosis and severity of bronchopulmonary dysplasia (BPD), the need for supplemental oxygen therapy, and later signs of PVD at 36 weeks corrected age (5).
Moreover, we do not fully understand why some preterm infants develop respiratory problems while others do not. The structural development of the human lung is dependent upon numerous growth factors and complex pathways, including angiogenic signaling. A better understanding of the underlying proteomic profiles may help to explain the heterogeneity across infants and could serve as biomarkers to identify those at highest risk for adverse events. An aptamer-based proteomic platform was shown to be useful in several recent studies of other diseases (6–9). This new technology has the ability to measure proteins (including low-abundance proteins) over a large dynamic range and provides a unique opportunity to study a large number of proteins (n = 1,121) using a small amount of blood (only 50 μl of plasma), which is essential in studies of preterm infants.
We performed a prospective study of preterm infants with circulating blood samples collected at ∼7 days of age. A machine learning approach was applied to protein measurements obtained using a novel aptamer-based proteomics technology. This preliminary evaluation identified proteins associated with echocardiographic evaluations of early PVD.
Methods
All data were prospectively obtained as part of an observational research study that included subjects who were enrolled between July 2006 and March 2012 at hospitals affiliated with two academic institutions (University of Colorado School of Medicine and Indiana University School of Medicine). The protocol was approved by the institutional review board of each participating site, and written informed consent was received from the parents or guardians of all participants. Research echocardiograms were standardized at both sites before initiation of the study and were performed in subjects at 7 days of age. All echocardiograms were interpreted by a single cardiologist who remained masked to the subjects’ clinical status to ensure consistent evaluations of the findings. The criteria for enrollment and PVD determination have been published previously (5).
We prospectively collected blood samples at Day 7 (± 48 h) from 100 infants during their first week after birth. Plasma samples were collected using an ethylenediaminetetraacetic acid plasma tube and centrifuged after phlebotomy. The supernatant was removed, aliquoted, and placed in a freezer at −80°C. At the conclusion of the study, a proteomic analysis was conducted on an aliquot using the SOMAscan assay at the laboratories of SomaLogic, Inc. The SOMAscan proteomic assay is described in detail elsewhere (10).
Random forests of 5,000 classification trees were made using the randomForest package in RStudio (RStudio Team 2015, RStudio, Inc.; http://www.rstudio.com/) to assess relationships between proteins and PVD. Area-under-the-curve random forests (AUCRF) were made using the AUCRF package to iteratively eliminate the lowest-ranked proteins from a random forest and calculate the area under the forest’s receiver operating characteristic curve to determine the number of proteins that created the most efficient classifier. The subset of only the 18 top-ranked proteins (as determined by AUCRF) was then used to create the final random forest.
Results
The cohort was comprised of 100 preterm infants, 44 of whom were diagnosed with PVD at Day 7. Across the PVD and non-PVD groups, there was no difference in GA (mean, 26 weeks for both groups), sex (females 43% vs. 57%), or birthweight z-score (mean, −0.3 for both groups). Clinical status varied with regard to BPD at 36 weeks, and cesarean section delivery also did not differ between groups.
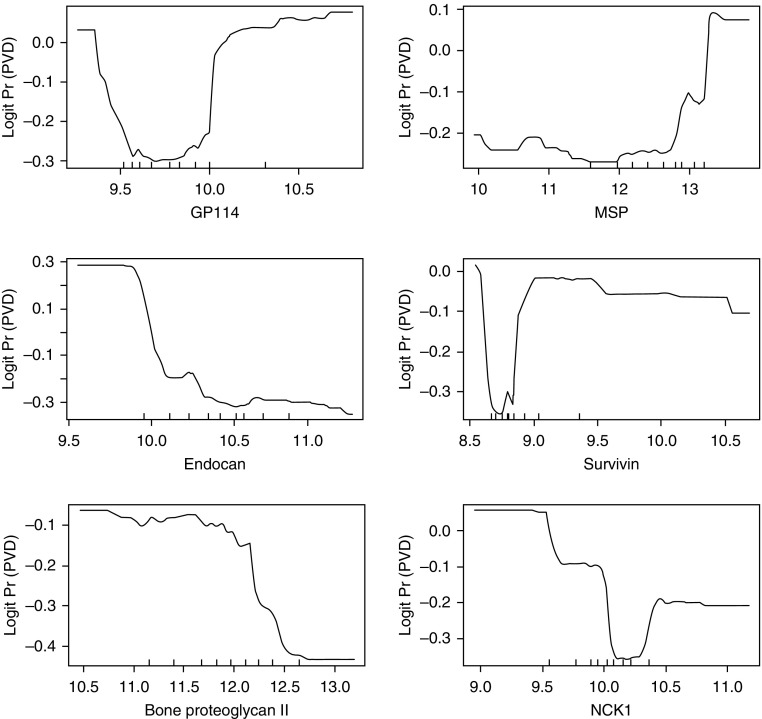
The protein selection process, based on the out-of-bag area under the curve (c-index), resulted in the selection of 18 proteins with the highest area under the curve of 0.82. These 18 proteins had a classification error rate of 24% and discriminated well between those who developed and those who did not develop PVD. A brief description of these 18 proteins is provided in Table 1, and for a subset of proteins, the association with PVD is graphically displayed in Figure 1.
Table 1.
Description of 18 Selected Proteins
| Protein | Uniprot # | Gene Ontology Biological Process | Pathway |
|---|---|---|---|
| ON (SPARC) | P09486 | Cellular response to growth factor stimulus, ECM organization, heart development, lung development, negative regulation of angiogenesis | ECM proteoglycans |
| STK16 | O75716 | Cellular response to transforming growth factor β stimulus | |
| SCGF-α | Q9Y240 | Positive regulation of cell proliferation | |
| Endocan | Q9NQ30 | Angiogenesis, positive regulation of cell proliferation | |
| NCK1 (cytoplasmic protein) | P16333 | Translation regulation | VEGFA–VEGFR2 pathway |
| Survivin | O15392 | Apoptosis, transcription regulation | Mitotic prometaphase |
| PDGF-AA | P04085 | Developmental protein, growth factor | Non-integrin membrane–ECM interactions |
| PDGF-BB | P01127 | Developmental protein, growth factor | Non-integrin membrane–ECM interactions |
| MMP7 | P09237 | Collagen degradation | Degradation of the ECM, activation of matrix metalloproteinases |
| PDE5A | O76074 | Negative regulation of cardiac muscle contraction, positive regulation of cardiac muscle hypertrophy, relaxation of cardiac muscle | cGMP effects |
| APP | P05067 | Heparin binding, protease inhibitor, serine protease inhibitor | ECM proteoglycans |
| PF-4 | P02776 | Cytokine, heparin binding | Cell surface interactions at the vascular wall |
| SCGF-β | Q9Y240 | Positive regulation of cell proliferation | |
| GP114 | Q8IZF4 | Cell surface receptor signaling pathway | |
| TIMP-3 | P35625 | Sensory transduction | Platelet degranulation |
| MSPR | Q04912 | Immunity, innate immunity | Signaling by MST1 |
| MSP | P26927 | Hepatocyte growth factor receptor signaling pathway, negative regulation of gluconeogenesis, regulation of cAMP-dependent protein kinase activity | Signaling by MST1 |
| Bone proteoglycan ll | P07585 | Cytokine-mediated signaling pathway, negative regulation of angiogenesis, negative regulation of vascular endothelial growth factor signaling pathway | Degradation of the ECM, ECM proteoglycans |
Definition of abbreviations: APP = amyloid precursor protein; cGMP = cyclic guanosine monophosphate; ECM = extracellular matrix; GP114 = G-protein–coupled receptor 114; MMP7 = matrix metalloproteinase-7; MSP = macrophage-stimulating protein; MSPR = macrophage-stimulating protein receptor; MST1 = macrophage stimulating 1; NCK1 = noncatalytic region of tyrosine kinase adaptor protein 1; ON = osteonectin; PDGF = platelet-derived growth factor; PDE5A = phosphodiesterase 5A; PF4 = platelet factor 4; SCGF = stem cell growth factor; SPARC = secreted protein acidic and rich in cysteine; STK16 = serine/threonine-protein kinase 16; TIMP3 = tissue inhibitor of metalloproteinases 3; VEGFA = vascular endothelial growth factor A; VEGFR2 = vascular endothelial growth factor receptor 2.
Figure 1.
Partial plots from a random forest, illustrating the association between pulmonary vascular disease (PVD) and selected proteins (in relative fluorescent units) after averaging out the influence of the remaining proteins in the random forest. Large values for logit Pr (PVD) (y-axis) indicate higher probabilities for PVD, y-axis values below 0 indicate lower probabilities for PVD, and a 0 y-axis value indicates probabilities of 0.5 or no discrimination between PVD and no PVD. GP114 = G-protein–coupled receptor 114; MSP = macrophage-stimulating protein; NCK1 = noncatalytic region of tyrosine kinase adaptor protein 1.
Discussion
PVD has been identified as a risk factor for later respiratory disease, and there is interest in identifying potential biomarkers associated with early PVD. In this preliminary investigation, we identified 18 biomarkers associated with PVD at Day 7 in a cohort of 100 preterm infants.
Several of the identified proteins have known or probable associations with pulmonary outcomes, and several selected proteins were related to extracellular matrix, growth factors, and angiogenesis (ON [osteonectin], PDGF [platelet-derived growth factor]-AA, PDGF-BB, MMP7 [matrix metalloproteinase-7], APP [amyloid precursor protein], bone proteoglycan II, STK16 [serine/threonine-protein kinase 16], endocan, TIMP3 [tissue inhibitor of metalloproteinases 3], and survivin). In addition, some of the proteins identified suggest novel pathways (SCGF [stem cell growth factor]-α, SCGF-β, NCK1 [noncatalytic region of tyrosine kinase adaptor protein 1], PDE5A [phosphodiesterase 5A], PF4 [platelet factor 4], GP114 [G-protein–coupled receptor 114], MSPR [macrophage-stimulating protein receptor], and MSP [macrophage-stimulating protein]).
Limitations of this study include the use of subjects from a single center and the fact that circulating proteins may not represent what is occurring at the target organ.
Using an unbiased, aptamer-based approach, we identified several known angiogenesis-related proteins as well as novel proteins that may be involved in the early pathogenesis of PVD in preterm infants. This study highlights the usefulness of the aptamer-based approach, especially in neonates, given the small amount of sample that is required. Future studies are needed to validate these findings and to analyze the relationship between early proteomics and echo changes with late outcomes, including BPD.
Acknowledgments
Summer Institute for Biostatistics Participants: Ana E. Babinec, Charlie Carpenter, Samantha Gonzalez, Grace O’Brien, and Kara Rollock. SIBS Instructors: Brandie D. Wagner, Steven H. Abman, and John Kittelson.
Footnotes
This work was supported by the NIH/NHLBI Summer Institute in Biostatistics grant R25 HL131486-01 (John Kittelson, Principal Investigator [PI]). The study population consisted of subjects enrolled in a prospective study of premature infants at risk for bronchopulmonary dysplasia (NHLBI R01 HL085703; S.H.A., PI) and was also supported by Colorado Clinical and Translational Sciences Institute UL1 TR000154 and NIH/National Center for Research Resources K23RR021921.
Originally Published in Press as DOI: 10.1164/rccm.201703-0654LE on June 26, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, et al. Prematurity and Respiratory Outcomes Program. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35:313–321. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreiner-Møller E, Chawes BL, Vissing NH, Koppelman GH, Postma DS, Madsen JS, et al. VEGFA variants are associated with pre-school lung function, but not neonatal lung function. Clin Exp Allergy. 2013;43:1236–1245. doi: 10.1111/cea.12188. [DOI] [PubMed] [Google Scholar]
- 4.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 5.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2015;112:7153–7158. doi: 10.1073/pnas.1507719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. 2016;315:2532–2541. doi: 10.1001/jama.2016.5951. [DOI] [PubMed] [Google Scholar]
- 8.Ngo D, Sinha S, Shen D, Kuhn EW, Keyes MJ, Shi X, et al. Aptamer-based proteomic profiling reveals novel candidate biomarkers and pathways in cardiovascular disease. Circulation. 2016;134:270–285. doi: 10.1161/CIRCULATIONAHA.116.021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatine MS. Using aptamer-based technology to probe the plasma proteome for cardiovascular disease prediction. JAMA. 2016;315:2525–2526. doi: 10.1001/jama.2016.6110. [DOI] [PubMed] [Google Scholar]
- 10.Rohloff JC, Gelinas AD, Jarvis TC, Ochsner UA, Schneider DJ, Gold L, et al. Nucleic acid ligands with protein-like side chains: modified aptamers and their use as diagnostic and therapeutic agents. Mol Ther Nucleic Acids. 2014;3:e201. doi: 10.1038/mtna.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]