To the Editor:
With improving life expectancy in patients with cystic fibrosis (CF) and annual rates of decline in FEV1 as low as 1–2% predicted per year (1), the value of spirometry as a measurement of longitudinal change is limited. Spirometry is also insensitive to early changes in the CF lung, and the lung clearance index (LCI) derived from multiple breath washout (MBW) has emerged as a promising alternative for patients with well-preserved spirometry, including children (2).
Recent studies have highlighted the potential of LCI for the longitudinal assessment of CF lung disease (3), although there are still questions regarding the mechanisms behind the mixed LCI response seen in intervention studies (4–6). As a global metric, however, LCI offers little information as to the location of any abnormality and the associated pathophysiology.
Hyperpolarized gas ventilation magnetic resonance imaging (ventilation MRI) is a sensitive imaging technique that reveals the distribution of ventilation within the lung in exquisite detail, allowing regional ventilation heterogeneity to be assessed. Ventilation MRI has been shown to be highly sensitive to early lung function abnormalities in CF and other obstructive airways diseases before changes are manifested in spirometry (7–9).
In a previous study, we reported cross-sectional data from a cohort of 19 children with mild CF lung disease (8). In that study, ventilation MRI demonstrated lung defects when abnormality was often not detectable by either computed tomography or LCI. Our aim in the present study was to reassess these children at a second time point 1–2 years after the baseline visit and describe any observed longitudinal changes in lung function or ventilation MRI. Some of the results of these studies have been previously reported in the form of abstracts (10–12).
Methods
Fourteen children with CF from the previously described cohort (8) were reassessed at a second time point between 1.3–2.0 years after baseline using helium-3 (3He) ventilation MRI, MBW, spirometry, and body plethysmography as previously described (8). The patients were clinically stable (free from exacerbation and needing no new treatments) for at least 4 weeks before both visits and were on stable chronic medical regimens according to national guidelines. Two indices were calculated from the ventilation images: (1) the ventilation defect percentage (VDP), which quantifies the fraction of the lung volume that is not ventilated, and (2) the mean coefficient of variance of ventilated image signal intensity (CVmean), which is a metric of ventilation heterogeneity.
The percentage change (Δ) from baseline to visit 2 was calculated for all metrics. The Wilcoxon matched-pairs signed-rank test and Spearman correlations were performed owing to the small sample size.
Results
Patient demographics, lung function, and MRI metrics at baseline and visit 2 are presented in Table 1. At baseline all children with CF had visible ventilation defects, and VDP and CVmean were greater in CF patients compared with healthy controls, as reported previously (8). From baseline to visit 2, there were significant group increases in VDP, CVmean, and LCI, but not in spirometric indices (Table 1).
Table 1.
Demographics, Lung Function, and Ventilation Imaging Metrics for Patients with Cystic Fibrosis at Baseline and Visit 2
| Baseline | Visit 2 | P Value | |
|---|---|---|---|
| Age, yr | 10.30 (2.26) | 12.07 (2.28) | <0.001 |
| Height, cm | 139.2 (13.89) | 148.3 (12.88) | <0.001 |
| Weight, kg | 35.41 (13.26) | 40.85 (12.42) | <0.001 |
| FEV1, z-score | −0.12 (0.80) | −0.26 (0.66) | 0.349 |
| FEV1/FVC, z-score | −0.57 (0.65) | −0.47 (0.59) | 0.903 |
| RV/TLC, % | 26.80 (4.58) | 25.94 (4.38) | 0.636 |
| LCI | 7.29 (0.85) | 8.09 (1.44) | 0.029 |
| LCIsupine | 7.64 (1.03) | 8.77 (1.99) | 0.042 |
| Scond | 0.048 (0.025) | 0.055 (0.029) | 0.318 |
| Sacin | 0.132 (0.076) | 0.129 (0.076) | 0.985 |
| VDP, % | 4.37 (1.89) | 10.8 (4.62) | <0.001 |
| CVmean, % | 15.31 (2.30) | 17.94 (3.33) | 0.042 |
Definition of abbreviations: CVmean = mean coefficient of variance of ventilated image signal intensity; LCI = lung clearance index; RV = residual volume; Sacin = ventilation heterogeneity arising from intraacinar airways; Scond = ventilation heterogeneity arising from conducting airways; TLC = total lung capacity; VDP = ventilation defect percentage.
Data are mean (SD). P values from the Wilcoxon signed-rank test are given for baseline and visit 2 group comparison.
Longitudinal changes in ventilation MRI
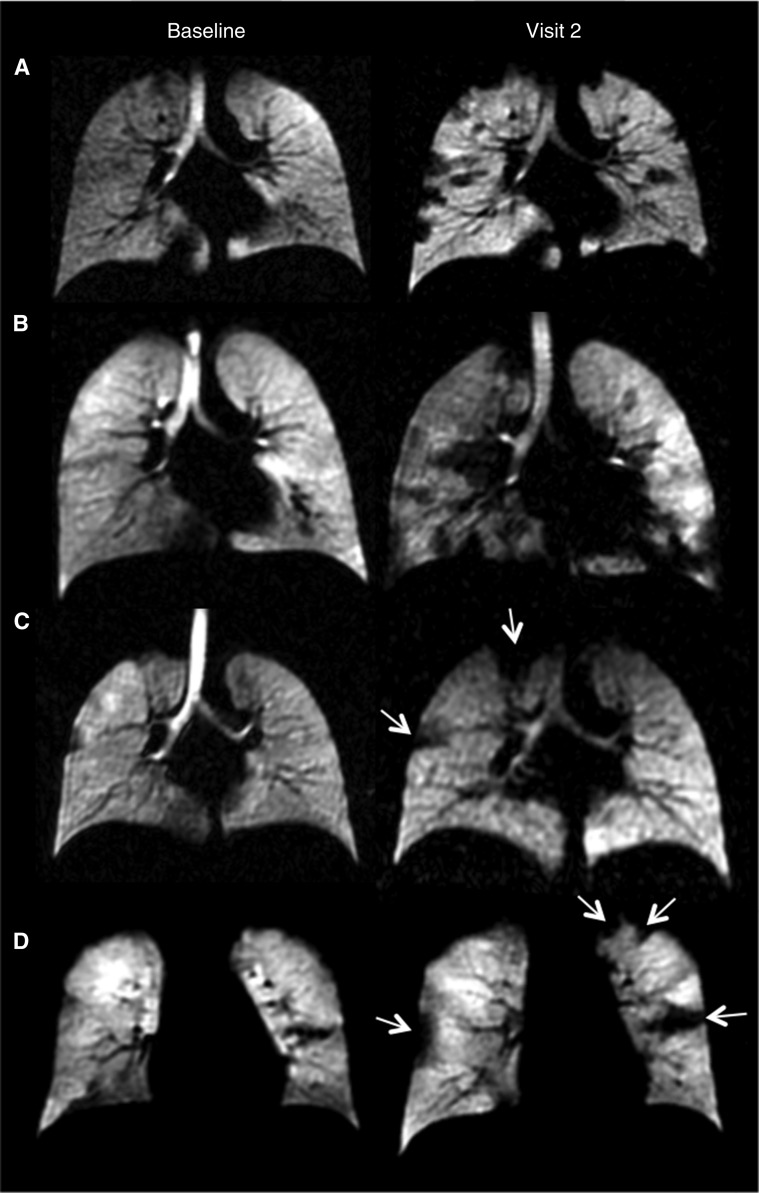
VDP increased over time in 13 of 14 children, and 10 of 14 children showed increased CVmean at visit 2 compared with baseline. There was no single regional pattern of disease progression: in some patients, multiple small ventilation defects became apparent that were not visible at baseline (Figure 1, subjects A and B). Other patients had a visible progression in regions of ventilation abnormalities that were already present at baseline, but had minimal new defects (Figure 1, subjects C and D). One child showed improvement in VDP (and LCI) between baseline and visit 2, and the ventilation defects that were evident at baseline were either completely resolved or had reduced in size.
Figure 1.
Representative ventilation magnetic resonance imaging slices from four separate subjects with cystic fibrosis. All four subjects had normal FEV1 at both visits. Subjects A and B demonstrated widespread ventilatory defects visible at visit 2. The ventilation defect percentage (VDP) increased from 3.1% to 19.7%, the mean coefficient of variance of ventilated image signal intensity (CVmean) increased from 15.9% to 20.1%, and the lung clearance index (LCI) increased from 6.6 to 8.7 for subject A. VDP increased from 3.4% to 19.4%, CVmean increased from 11.9% to 24.5%, and LCI increased from 6.6 to 9.1 for subject B. Subjects C and D demonstrated localized ventilatory defects that largely increased in size from baseline to visit 2 (as shown by white arrows). VDP increased from 4.4% to 9.4%, CVmean demonstrated a small decline from 15.2% to 14.9%, and LCI increased from 6.2 to 6.6 but remained well within the normal range for subject C. There was an increase in VDP from 7.5% to 10.3%, CVmean from 13.3% to 16.6%, and LCI from 7.6 to 7.8 for subject D.
Longitudinal change in lung physiology
Six of 14 children had an abnormally elevated LCI (>7.4) at baseline. By visit 2, the LCI values had increased in 11 of 14, with eight children demonstrating abnormal LCI values. At baseline, all children had normal spirometry (FEV1 and FEV1/FVC z-score > −1.96) and only one child had an FEV1 < −1.64. At visit 2, all children had an FEV1 z-score > −1.64 and 13 of 14 children had an FEV1/FVC z-score > −1.96.
Correlation between imaging and physiology
At both baseline and visit 2, there were statistically significant correlations between VDP and LCI (r = 0.66, P = 0.013, r = 0.82, P = 0.001, respectively), and a significant correlation between LCI and CVmean was observed at visit 2 (r = 0.62, P = 0.02). There was no significant correlation between FEV1 and FEV1/FVC with either LCI or MRI metrics. ΔLCI showed significant strong correlations with ΔCVmean (r = 0.75, P = 0.003) and ΔVDP (r = 0.6, P = 0.025). ΔFEV1 and ΔFEV1/FVC demonstrated no significant correlation with other metrics.
Discussion
With the current slow rate of decline in FEV1 in stable CF lung disease, sensitive outcome measures of longitudinal changes in lung function are needed to guide therapy to maintain lung health. Ventilation MRI is capable of detecting significant lung function changes in the follow-up of children with CF and normal spirometry, which are not always evident using MBW. Although there was a significant group mean increase in LCI values between visits, highlighting the potential of LCI as a longitudinal assessment method, LCI was abnormal in only two more children at visit 2 (8/14) compared with baseline (6/14). However, ventilation MRI was abnormal in all children at both time points and showed increased ventilation impairment over time, as quantified by a highly significant increase in VDP. The nature of the changes in ventilation imaging varied between subjects, with new unventilated regions present at visit 2 in some patients and others showing an increase in the volume of unventilated regions, which were already present at baseline. The sensitivity of ventilation MRI to longitudinal therapy response in patients with CF has previously been demonstrated (13), but this is the first study to compare ventilation MRI and LCI for longitudinal assessment of lung disease progression in children with CF.
The observed improved sensitivity of ventilation MRI to longitudinal disease progression may be due to its ability to identify small changes within specific lung regions, in early disease, whereas LCI is a global assessment of lung function measured at a single point. In addition, unventilated regions of the lung will not contribute signal to outcomes that rely on gas mixing, causing an underestimate of measures such as the LCI.
In conclusion, although further data are still required, in children with CF and clinically stable lung function and normal spirometric values, hyperpolarized gas ventilation MRI appears to identify longitudinal changes in early lung disease earlier than other physiological methods.
Footnotes
Supported by grants from the National Institute for Health Research (NIHR-RP-R3-12-027), Medical Research Council (MR/M008894/1), and Cystic Fibrosis Trust. L.S., A.H., and J.W. were supported by the NIHR, L.S. was supported by a Health Education England/NIHR clinical doctoral research fellowship, A.H. was supported by an NIHR Clinician Scientist award, and J.W. was supported by an NIHR Professorship award. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Author Contributions: Concept and design: J.W., C.J.T., and A.H. Analysis and interpretation: L.S., H.M., D.H., A.H., G.C., and J.W. Manuscript writing: L.S., H.M., A.H., and J.W. Manuscript review: all authors. Pulmonary function testing: L.S. MRI technical work: H.M., F.H., G.C., and J.W. Patient recruitment/consent/support: I.A., N.W., and C.J.T. Underwriting of the work: J.W.
Originally Published in Press as DOI: 10.1164/rccm.201705-0894LE on June 29, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Que C, Cullinan P, Geddes D. Improving rate of decline of FEV1 in young adults with cystic fibrosis. Thorax. 2006;61:155–157. doi: 10.1136/thx.2005.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horsley A, Wild JM. Ventilation heterogeneity and the benefits and challenges of multiple breath washout testing in patients with cystic fibrosis. Paediatr Respir Rev. 2015;16:15–18. doi: 10.1016/j.prrv.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Stanojevic S, Ratjen F. Physiologic endpoints for clinical studies for cystic fibrosis. J Cyst Fibros. 2016;15:416–423. doi: 10.1016/j.jcf.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax. 2010;65:379–383. doi: 10.1136/thx.2009.125831. [DOI] [PubMed] [Google Scholar]
- 5.Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J. 2011;37:806–812. doi: 10.1183/09031936.00072510. [DOI] [PubMed] [Google Scholar]
- 6.Horsley AR, Davies JC, Gray RD, Macleod KA, Donovan J, Aziz ZA, Bell NJ, Rainer M, Mt-Isa S, Voase N, et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax. 2013;68:532–539. doi: 10.1136/thoraxjnl-2012-202538. [DOI] [PubMed] [Google Scholar]
- 7.Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros. 2017;16:275–282. doi: 10.1016/j.jcf.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall H, Horsley A, Taylor CJ, Smith L, Hughes D, Horn FC, Swift AJ, Parra-Robles J, Hughes PJ, Norquay G, et al. Detection of early subclinical lung disease in children with cystic fibrosis by lung ventilation imaging with hyperpolarised gas MRI. Thorax. 2017;72:760–762. doi: 10.1136/thoraxjnl-2016-208948. [DOI] [PubMed] [Google Scholar]
- 9.Kanhere N, Couch MJ, Kowalik K, Zanette B, Rayment JH, Manson D, Subbarao P, Ratjen F, Santyr G. Correlation of LCI with hyperpolarized (129)Xe magnetic resonance imaging in pediatric CF subjects. Am J Respir Crit Care Med. doi: 10.1164/rccm.201611-2228LE. [online ahead of print] 28 Feb 2017; DOI: 10.1164/rccm.201611-2228LE. [DOI] [PubMed] [Google Scholar]
- 10.Smith L, Aldag I, Hughes P, Horn F, Marshall H, Norquay G, Collier G, Hughes D, Taylor C, Horsley A, et al. Longitudinal monitoring of disease progression in children with mild CF using hyperpolarised gas MRI and LCI. Eur Respir J. 2016;48(Suppl 60) OA284. [Google Scholar]
- 11.Smith L, Aldag I, Hughes P, Horn F, Marshall H, Norquay G, Collier G, Hughes D, Taylor C, West N, et al. Longitudinal monitoring of disease progression in children with mild CF using 3-helium MRI and LCI. Presented at the North American Cystic Fibrosis Conference; October 27–29, 2016, Orlando, FL. [Google Scholar]
- 12.Smith L, Aldag I, Hughes P, Horn F, Marshall H, Norquay G, Collier G, Hughes D, Taylor C, Horsley A, et al. Longitudinal monitoring of disease progression in children with mild cystic fibrosis using hyperpolarised gas MRI and lung clearance index. Presented at the 25th Annual Meeting of the International Society for Magnetic Resonance in Medicine; April 22–27, 2017, Honolulu, HI. [Google Scholar]
- 13.Altes TA, Johnson M, Fidler M, Botfield M, Tustison NJ, Leiva-Salinas C, de Lange EE, Froh D, Mugler JP., 3rd Use of hyperpolarized helium-3 MRI to assess response to ivacaftor treatment in patients with cystic fibrosis. J Cyst Fibros. 2017;16:267–274. doi: 10.1016/j.jcf.2016.12.004. [DOI] [PubMed] [Google Scholar]