Abstract
Purpose: This study is to present a large cohort of extraskeletal osteosarcoma (ESOS) and evaluate prognostic factors and treatment options. Methods: Medical records were reviewed retrospectively for 41 patients with extraskeletal osteosarcoma that was diagnosed by pathology, and treated at our institution between 1960 and 2016. Kaplan-Meier analysis and Cox proportional hazards regression were used to identify variables that affect survival outcomes. Results: 41 patients were identified from 952 osteosarcomas. 32 patients had non-metastatic disease. Prognostic factors were identified by univariate analysis and multi-variate analysis. Surgery (p<0.001), and surgery type (p<0.001) both were shown to significantly affect overall survival (OS). Chemotherapy and radiation therapy (RT) did not show any significant effect on OS, local recurrence, or progression free survival as a whole. However for patients who had incomplete resection with residual tumor RT improved OS (p=0.03). The survival curve for ESOS follows more closely that of non-rhabdomyosarcoma soft tissue sarcomas (NRSTS). Conclusions: ESOS is a very rare tumor. Attempt to achieve wide resection is the treatment of choice. However for patients who are not able to achieve complete resection, RT may improve OS. The behavior of ESOS more closely follows that of NRSTS than osteosarcoma of the bone.
Keywords: Extraskeletal osteosarcoma, extraosseous osteosarcoma, adjuvant therapy, surgery
Introduction
Extraskeletal osteosarcoma (ESOS) is a very rare variant of osteosarcoma that is located in the soft tissue and is not attached to any bones. Although first described in 1941,1 there have been no more than 390 cases reported.2–6 The most recent study was from an international organization, Rare Cancer Network, which reported a cohort of 33 patients.5 The largest study including 60 patients in total with a time span from 1960 to 1999 was published in 2002. This study features the experience of a single institution over half a century and presents a relatively large series of ESOS.
Despite the increased awareness and knowledge of this rare tumor among clinicians, there is limited understanding of the clinical behavior and consensus in treatment plans of ESOS. Some studies suggest that ESOS behaves more like soft-tissue sarcomas and not bone osteosarcoma with poor response to chemotherapy.2 However, the role of adjuvant chemotherapy or radiation therapy (RT) has not been clear. This study was undertaken with the purpose of better understanding outcome predictors and treatment options especially adjuvant therapy.
Patients and methods
Patient selection
Our longitudinal Institutional Review Board (IRB)-approved institutional sarcoma data repository contains 12,961 sarcoma case records from 1960 to 2016. The data repository retrospectively and prospectively collects data on patients with sarcoma from our institution’s paper and electronic clinical records, including demographics, primary tumor characteristics, staging, pathology details, treatment, oncologic follow-up, vital status, and toxicities. Subject consent was waived for our retrospective, medical records-only, minimal risk clinical outcomes research and was approved by IRB of record, Partners Human Research Committee (Protocol# 2003P000854/PHS). A Health Insurance Portability and Accountability Act (HIPAA) compliant, IRB-approved web-based application (REDCap, Vanderbilt University) was employed to securely store and manage the database. We queried the database for patients who were diagnosed of ESOS that was confirmed by pathology and received some component of treatment at our institution.
Variables of interest
Patient demographic variables included age, gender, sex, medical history, family history, weight, smoking status, and race/ethnicity. Clinical features included method of diagnosis, radiation-associated status, primary tumor site, primary tumor size, histology subgroup, and extent of disease at diagnosis. Clinical staging was standardized using known tumor size, location, site, and grade based on American Joint Committee on Cancer (AJCC) 7th Edition staging system.7 Pathology features included French Federation of Comprehensive Cancer Centers (FNCLCC) tumor grade, as Grade I for well-differentiated tumors, Grade II for moderately differentiated tumors, and Grade III for poorly differentiated tumors. Further pathology details included lymphovascular invasion (positive, negative, and undetermined), percentage of necrosis (0, <90%, ≥90%, and undetermined), final surgical margins, and closest tumor margins (categorized as negative with >1 mm margin, negative with ≤1 mm margin, positive margin, and undetermined). Treatment options included local treatment combinations, as in surgery without RT, surgery plus RT, RT without surgery, none, and systemic treatment, including the type of chemotherapy given and whether it was more consistent with skeletal osteosarcoma regimen versus more soft tissue sarcoma type of regimen. Further RT information included total RT dose, modality used, and sequencing relative to surgery. Surgical extent was coded as amputation, radical resection, wide local resection, marginal, subtotal, or intralesional. Patient outcome was assessed for survival time (defined as time from the date of diagnosis until one of the following: date of event, date of death, last-contact date, or study cut-off date), vital status, and cause of death. For both overall survival (OS) and progression-free survival (PFS), patients who were lost to follow-up were coded as censored. For PFS, patients who had any of the following recurrences were censored: local recurrence, regional recurrence, distant metastasis, or disease progression.
Statistical analysis
Descriptive statistics of patient characteristics were generated. Statistical significance between groups was analyzed using the chi-square tests for categorical variables and the student t-test or Wald tests for continuous variables. OS and disease-free survival were modeled using Kaplan–Meier analysis and compared by log-rank test. Statistical significance was based on p value of less than 0.05. Cox proportional hazards models were used to estimate hazard ratios (HRs) and to correlate between outcomes (OS and disease-specific survival (DSS)) and risk variables, including sex, age, stage, tumor size, site, and treatment options. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp, Armonk, NY, USA). Kaplan-Meier survival curves were generated in R (version 3.4.1; www.r-project.org), using the ‘survminer’ package and the ‘ggsurvplot’ function.
Results
Patient and tumor characteristics
A total number of 41 patients with ESOS were identified. The demographic and tumor characteristics are summarized in Table 1. Of the patients, 14 were female (34%) and 27 were male (66%). Median age was 60 (range: 18–92) years. All patients were greater than 18-year-old at the date of diagnosis. Five (12%) of the patients were between 18 and 30 years, 10 (24%) were between 31 and 50 years, and 26 (63%) patients were over 50 years. Five (12%) patients had prior RT treatment in the same anatomic region and thus were defined as radiation-associated sarcoma (RAS). Most of the primary tumors were located in lower extremities (23, 56%). The remaining tumor sites included chest and abdominal wall (12%), thorax (10%), abdomen (10%), upper extremity (7%), and retroperitoneum (5%). The primary tumor site was further categorized as extremities (63%) and trunk (37%) for analysis. In terms of tumor size, 9 (22%) cases were <5 cm, 9 (22%) between 5 and 8 cm, 21 (51%) >8 cm, and 2 (5%) undetermined. Histological distribution was 32 (78%) conventional osteosarcoma, 4 (10%) fibroblastic osteosarcoma, 2 (5%) small-cell osteosarcoma, 2 (5%) chondroblastic osteosarcoma, and 1 (2.4%) telangiectatic osteosarcoma. A total of 12 tumors (29%) were grade 2 and 29 (70%) were grade 3; none were grade 1. Of the patients, 15 (37%) presented with Stage 2 disease, 17 (42%) presented with Stage 3 disease, and 7 (17%) presented with Stage 4 disease. Among the 7 patients who presented with metastatic disease, 6 presented with metastasis in the lungs and 1 presented with metastasis in the liver. Lymphovascular invasion was found in 22 patients (54%). Final surgical margin was positive in 9 patients (22%), which was defined as positive microscopic margins. Six (15%) patients had over 90% necrosis rate on pathology; 11 (27%) patients had less than 90% necrosis and 3 (7%) patients had no necrosis at all.
Table 1.
Patient and tumor characteristics.
| Variables | Number of patients (%) | ||
|---|---|---|---|
| Age (per year) | |||
| Age group (years) | 18–30 | 5 | 12.2% |
| 31–50 | 10 | 24.4% | |
| >50 | 26 | 63.4% | |
| Gender | Female | 14 | 34.1% |
| Male | 27 | 65.9% | |
| Radiation related | No | 36 | 87.8% |
| Yes | 5 | 12.2% | |
| Site | Lower extremity | 23 | 56.1% |
| Chest and abdominal wall | 5 | 12.2% | |
| Thorax | 4 | 9.8% | |
| Abdomen | 4 | 9.8% | |
| Upper extremity | 3 | 7.3% | |
| Retroperitoneum | 2 | 4.9% | |
| Size | Less than 5 cm | 9 | 22.0% |
| Between 5 and 8 cm | 9 | 22.0% | |
| More than 8 cm | 21 | 51.2% | |
| Undetermined | 2 | 4.9% | |
| Histology | Conventional | 32 | 78.0% |
| Fibroblastic | 4 | 9.8% | |
| Small cell | 2 | 4.9% | |
| Chondroblastic | 2 | 4.9% | |
| Telangiectatic | 1 | 2.4% | |
| T | T1 | 9 | 22.0% |
| T2 | 30 | 73.2% | |
| Undetermined | 2 | 4.9% | |
| M | M0 | 32 | 78.0% |
| M1 | 7 | 17.1% | |
| Undetermined | 2 | 4.9% | |
| AJCC | 2 | 15 | 36.6% |
| 3 | 17 | 41.5% | |
| 4 | 7 | 17.1% | |
| Undetermined | 2 | 4.9% | |
| Grade | G2 | 12 | 29.3% |
| G3 | 29 | 70.7% | |
| Lymphovascular invasion | Negative | 7 | 17.1% |
| Positive | 22 | 53.7% | |
| Undetermined | 12 | 29.3% | |
| Necrosis | 0 | 3 | 7.3% |
| <90% | 11 | 26.8% | |
| ≥90% | 6 | 14.6% | |
| Undetermined | 21 | 51.2% | |
| Margin | Negative >1 mm | 15 | 36.6% |
| Negative ≤1 mm | 12 | 29.3% | |
| Positive | 9 | 22.0% | |
| Undetermined | 5 | 12.2% | |
AJCC: American Joint Committee on Cancer.
Treatment details and complications
Almost all patients underwent surgical resection (39, 95%). In all, 16 (39%) patients received surgery only (Table 2) and 23 (56%) patients had both surgery and RT, among whom 11 (27%) received RT pre-operatively, 6 (14%) received RT postoperatively, and 2 (5%) both pre- and postoperatively. Most patients (30, 73%) underwent wide resection. Six (15%) patients had marginal resection and 2 (5%) had subtotal resection. Microscopic margin was negative in 27 (66%), positive in 9 (22%), and unknown in 3 (7%) of the cases, respectively. In all, 22 (54%) patients received chemotherapy: of the 22 patients, almost all patients received adriamycin (19, 86%). Of the 19 patients, 13 were treated with traditional skeletally based osteosarcoma cisplatin-containing regimens including methotrexate, adriamycin, and cisplatin (MAP) or vincristine, adriamycin, and cisplatin (VAC). Of the 19 patients, 5 received ifosfamide as part of soft-tissue sarcoma type of regimen: doxorubicin, ifosfamide, and mesna (MAI) or mesna, doxorubicin, ifosfamide, and dacarbazine (MAID). One patient received single-agent adriamycin.
Table 2.
Treatment details.
| Variables | Number of patients (%) | ||
|---|---|---|---|
| Surgery | No | 2 | 4.9% |
| Yes | 39 | 95.1% | |
| Surgery type | Subtotal resection | 2 | 4.9% |
| Marginal resection | 6 | 14.6% | |
| Wide resection | 30 | 73.2% | |
| Unknown type | 1 | 2.4% | |
| Chemotherapy | No | 19 | 46.3% |
| Yes | 22 | 53.7% | |
| Radiation therapy | No | 18 | 43.9% |
| Yes | 23 | 56.1% | |
| Radiation type | Preoperative | 11 | 26.8% |
| Postoperative | 6 | 14.6% | |
| Both preoperative and postoperative | 6 | 14.6% | |
Treatment complications were reviewed. For the 39 patients who underwent surgery, 11 (28%) patients had wound-healing complications, 2 (5%) patients had hardware failures, and 1 (2%) patient had fractures. Among 22 patients who received chemotherapy or RT, 2 (5%) patients had neutropenic fever, 1 (3%) patient had anemia, 1 (3%) had thromboembolic event, and 1 (3%) patient had leukemia secondary to chemotherapy which resulted in death.
Survival analysis
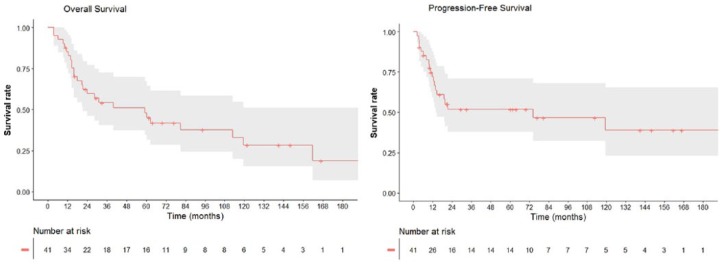
The median follow-up time for the cohort was 29.6 months (range: 3.7–213.2 months). The median survival time was 59.3 months (95% confidence interval (CI): 23.0–95.6 months). The 3-year and 5-year OS for all patients (Figure 1) were 51% (95% CI: 34%–67%) and 41% (95% CI: 25%–58%), respectively. The 3-year and 5-year OS for patients with non-metastatic disease at presentation were 60% (95% CI: 42%–78%) and 51% (95% CI: 32%–70%), respectively. The both 3-year and 5-year PFS were 51% (95% CI: 35%–68%). The both 3-year and 5-year local failure free survival were 76% (95% CI: 62%–91%).
Figure 1.
(a) Overall survival (OS) and (b) progression-free survival (PFS) curves for 41 patients with ESOS.
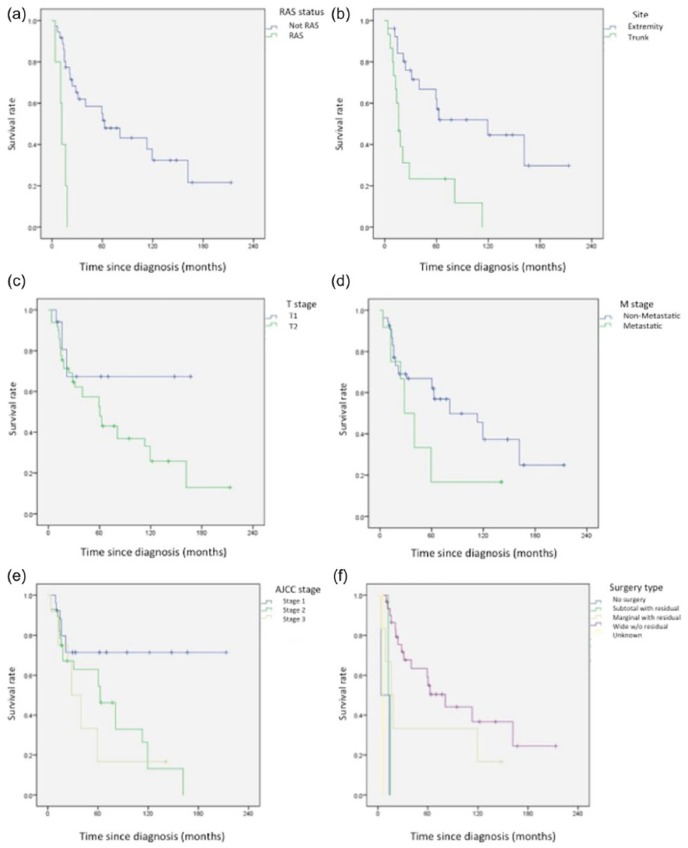
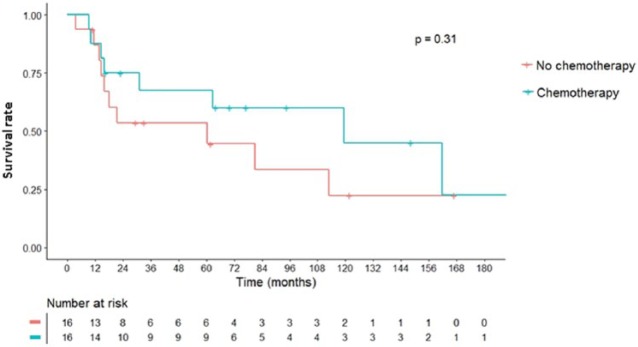
On univariate analysis (Figure 2), we identified negative prognostic factors for OS including RAS (p < 0.001), trunk as primary site of tumor (p < 0.001), higher T stage (p = 0.04), metastasis (p = 0.04), AJCC stage 4 (p = 0.02), microscopic positive margin (p < 0.001) and subtotal or marginal resection with residual tumor (R2; p < 0.001). Multivariate analysis (Table 3) identified older age (HR = 1.04 per year, p = 0.04), RAS (HR = 306, p < 0.001), undetermined margins (HR = 748, p = 0.001), metastasis (HR = 6.53, p = 0.01), and subtotal resection (HR = 232, p < 0.001) as independent predictors of worse OS. Chemotherapy was not shown to be a significant factor for OS among patients as a whole and among patients who presented with non-metastatic disease (Figure 3).
Figure 2.
Kaplan–Meier analysis of overall survival for different variables: (a) OS for RAS and non-RAS ESOS; (b) OS for ESOS in extremity and trunk; (c) OS for T1 and T2 ESOS; (d) OS for non-metastatic and metastatic ESOS; (e) OS for AJCC Stages 1, 2, and 3 ESOS; and (f) OS for ESOS with different types of surgery.
Table 3.
Multivariate analysis identified significant variables for overall survival.
| Variables | OS |
95% CI for HR |
|||
|---|---|---|---|---|---|
| p value | HR | Lower | Upper | ||
| Age (per year) | 0.035 | 1.039 | 1.002 | 1.077 | |
| Radiation associated | No | <0.001 | |||
| Yes | 305.9 | 15.1 | 6205.2 | ||
| M stage | M0 | 0.007 | |||
| M1 | 0.010 | 6.521 | 1.572 | 27.0 | |
| Undetermined | 0.009 | 56.5 | 2.683 | 1188.0 | |
| Microscopic margin | Negative >1 mm | 0.001 | |||
| Negative ≤1 mm | 0.435 | 1.569 | 0.507 | 4.856 | |
| Positive | 0.590 | 0.566 | 0.071 | 4.496 | |
| Undetermined | 0.001 | 747.7 | 14.503 | 38555.1 | |
| Surgery type | Wide resection | 0.019 | |||
| Subtotal resection | <0.001 | 231.6 | 9.962 | 5386.4 | |
| Marginal resection | 0.964 | 1.046 | 0.146 | 7.525 | |
| No surgery | 0.908 | 0.826 | 0.033 | 20.7 | |
| Unknown type | 0.643 | 0.338 | 0.003 | 33.1 | |
OS: overall survival; CI: confidence interval; HR: hazard ratio.
Figure 3.
Overall survival (OS) for extraskeletal osteosarcoma (ESOS) with non-metastatic disease who received chemotherapy versus those who did not.
Failure during follow-up
Patterns of failure including disease progression, local recurrence, regional recurrence, and distant metastasis were analyzed in patients who presented with non-metastatic disease. Median follow-up time at recurrence was 52 months from diagnosis. In all, 12 patients had at least one type of failure during follow-up: 7 patients developed distant metastasis (5 lungs, 1 spine, and 1 other soft tissue) and 5 had local recurrence. Association with prior RT (p = 0.009), metastasis status at presentation (p = 0.04), lymphovascular invasion (p = 0.04), and surgery type (p = 0.03) are significant factors identified by univariate analysis to affect PFS.
Discussion
ESOS is a very rare tumor. The incidence in our sarcoma database is 1.6% among all soft-tissue sarcomas and 4.3% of all osteosarcomas. Lower extremity is the most commonly presenting tumor site in our series, as is also shown in previous studies.5 On univariate analysis, truncal ESOS appears to do worse than extremity ESOS. However, in our multivariate analysis, trunk was not a significant negative factor affecting OS. ESOS presenting as RAS (five cases) was identified as an independent significant factor for OS and PFS. All five RAS cases all had prior malignancies treated with RT in the area of ESOS development. RT causes double-strand breaks in normal tissue, which increases the risk of gene mutation and secondary cancer, particularly sarcomas. Prognosis was poor for radiation-associated ESOS. Four out of the five RAS patients died of ESOS.
Margin is an important factor that affected OS and local recurrence in our series. We found that tumors with positive margins have high risk of local recurrence after 5 years and also have significantly worse OS after 5 years. The local control (LC) rate for patients presenting with non-metastatic disease who were found to have positive margins was 86% at 5 years and 29% at 10 years. In comparison, LC rate for patients with negative margins remained at 89% at 5 years and 10 years. Thus, patients with positive margins should continue oncologic surveillance even after 5 years.
In terms of treatment, almost all patients received surgery, which was previously shown to significantly improve OS in ESOS.5 There were only two patients who did not receive surgery. One presented with advanced age of 92 years and did not pursue surgery due to life expectancy and potential risks and complications. The other one presented with a large retroperitoneal mass involving the mesentery of the descending colon that could not be resected. In our study, we have about the same number of patients who received chemotherapy as those who did not. Multi-agent chemotherapy has been known to significantly improve survival in primary skeletal osteosarcoma.8 One previous study showed that ESOS has a favorable prognosis when treated like conventional osteosarcoma with MAP-based therapy.9 In our study, the majority cases received MAP-based chemotherapy and a minority received soft-tissue sarcoma type of regimen (adriamycin- and ifosfamide-based therapy); however, we did not identify any survival benefit between different chemotherapy regimens or between the patients who received chemotherapy and the ones who did not receive chemotherapy. A previous study of ESOS focusing more on the imaging response rate has also observed similar results.2 Adjuvant RT, whether given preoperatively or postoperatively,10 was thought to be beneficial in decreasing local failure in intermediate- to high-risk soft-tissue sarcomas.11 In our study, although RT was not shown to improve OS, LC, or PFS as a whole, RT improved OS (p = 0.03) for the patients who were not able to achieve negative margins (R1 and R2). However, given the small size of this cohort due to rarity of the disease, the limitations of this study cannot be ignored. The very low incidence of ESOS as a very rare tumor makes prospective disease-specific clinical trials very hard to perform. The future of research for better understanding ESOS most likely lies in potentially multi-center studies.
In an attempt to elaborate whether ESOS resembles its counterpart, osteosarcoma, or soft-tissue sarcoma, we found that the survival curves of ESOS resembled non-rhabdomyosarcoma soft-tissue sarcoma (NRSTS) the most. We were also able to compare survival of ESOS to bone oseosarcoma and NRSTS in our sarcoma data repository. We found that ESOS resembles NRSTS most of all (p = 0.16). On the contrary, the OS curves of ESOS and primary skeletal osteosarcoma were significantly different (p = 0.009). Another notable feature is that ESOS, unlike osteosarcoma, does not respond to chemotherapy, while on the contrary, RT was shown to improve OS in our study with similarity to soft-tissue sarcomas. Therefore, we conclude that our results, consistent with existing literature, suggest that ESOS behaves more like NRSTS versus primary skeletal osteosarcomas. Thus, our current institutional recommendation is to treat ESOS like soft-tissue sarcomas.
Conclusion
ESOS is a very rare tumor that makes prospective study difficult to perform. Our retrospective analysis of a relatively large single-institutional series show that these behave more like other soft-tissue sarcomas in survival and that wide resection is the treatment of choice. Unlike primary osteosarcoma of the bone, chemotherapy does not appear to impact survival. For patients who are not able to achieve R0 resection, RT may improve OS. These findings suggest that ESOS has its own features distinct from primary osteosarcoma of the bone and might benefit more from treatment similar to primary STS. Further multi-center studies might be useful given the rarity of ESOS.
Acknowledgments
We thank our colleagues who provided insight and expertise that greatly assisted the research.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Wilson H. Extraskeletal ossifying tumors. Ann Surg 1941; 113(1): 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol 2002; 20(2): 521–527. [DOI] [PubMed] [Google Scholar]
- 3. Lee JS, Fetsch JF, Wasdhal DA, et al. A review of 40 patients with extraskeletal osteosarcoma. Cancer 1995; 76(11): 2253–2259. [DOI] [PubMed] [Google Scholar]
- 4. McCarter MD, Lewis JJ, Antonescu CR, et al. Extraskeletal osteosarcoma: analysis of outcome of a rare neoplasm. Sarcoma 2000; 4(3): 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an International Rare Cancer Network Study. Am J Clin Oncol 2016; 39(1): 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torigoe T, Yazawa Y, Takagi T, et al. Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci 2007; 12(5): 424–429. [DOI] [PubMed] [Google Scholar]
- 7. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17(6): 1471–1474. [DOI] [PubMed] [Google Scholar]
- 8. Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol 2007; 19(4): 341–346. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein-Jackson SY, Gosheger G, Delling G, et al. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol 2005; 131(8): 520–526. [DOI] [PubMed] [Google Scholar]
- 10. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002; 359(9325): 2235–2241. [DOI] [PubMed] [Google Scholar]
- 11. Kachare SD, Brinkley J, Vohra NA, et al. Radiotherapy associated with improved survival for high-grade sarcoma of the extremity. J Surg Oncol 2015; 112(4): 338–343. [DOI] [PubMed] [Google Scholar]