Abstract
Background
Given current drug-policy reforms to decriminalize or legalize cannabis in numerous countries worldwide, it is critically important to understand the potential impacts of cannabis use on the development of cancer. The current study aims to assess the relation between cannabis use and the development of testicular cancer.
Method
The current study relied on a population-based sample (n = 49 343) of young men aged 18–21 years who underwent conscription assessment for Swedish military service in 1969–1970. The conscription process included a non-anonymous questionnaire eliciting information about drug use. Individual-level conscription information was linked to Swedish health and social registry data. Testicular cancers diagnosed between 1970 and 2011 were identified by ICD-7/8/9/10 testicular cancer codes in the Swedish National Patient Register, the Cancer Register, or the Cause of Death Register. Cox regression modeling was used to estimate the hazards associated with cannabis use and time to diagnosis of testicular cancer.
Results
No evidence was found of a significant relation between lifetime “ever” cannabis use and the subsequent development of testicular cancer [n = 45 250; 119 testicular cancer cases; adjusted hazard ratio (AHR) 1.42, 95% CI, 0.83, 2.45]. “Heavy” cannabis use (defined as usage of more than 50 times in lifetime, as measured at conscription) was associated with the incidence of testicular cancer (n = 45 250; 119 testicular cancer cases; AHR 2.57, 95% CI, 1.02, 6.50).
Conclusion
The current study provides additional evidence to the limited prior literature suggesting cannabis use may contribute to the development of testicular cancer.
Keywords: cannabis, testicular cancer, incidence, youth, cohort, Sweden
Cannabis is the most widely used illicit drug worldwide (1). According to 2015 World Drug Report (1) approximately 180 million people (3.9% of the global population) consumed cannabis in the past year, and it is likely that these numbers are due, in part, to the common perception that its use confers minimal health risks (2).
Globally, a range of nations, including countries of the European Union, Australia, and the Americas have recently implemented or proposed reforms to the ways in which they control cannabis use (3–6), thereby departing from traditional approaches of criminal prohibition dominant throughout most of the 20th century (7). In light of the current international debates and policy changes regarding cannabis decriminalization/legalization in many regions (8–10), as well as its widespread global use, it is critically important to assess the impact of cannabis smoking on human health, especially on the development of cancers (11–13). Almost all research on the potential cannabis-cancer connection has focused on those cancers causally related to tobacco smoking (e.g., lung cancer, head and neck cancers) (11, 12), but a small group of recent studies (14–16) also has found a suggestive link between cannabis use and the development of testicular cancers, which are traditionally unassociated with tobacco use (17).
Testicular cancer is the most common type of cancer among young men, with a peak incidence occurring between the ages 15–40 years (18) – an age range in which cannabis use most frequently occurs (1). Rates of testicular cancer appear to be increasing rapidly over time in the United States and Europe (16, 19, 20), but the etiology remains unclear. Three US-based case-control studies (14–16) have found that cannabis use appears to confer an increased risk of developing testicular cancer. A meta-analytic summary of these three studies has shown that current, chronic (defined as cannabis use ≥ 10 years), and frequent (defined as at least once per week) cannabis use were all significantly associated with testicular cancer incidence (21). While a recent review on this topic (11) has called for longitudinal studies to address the potential weaknesses of recall and selection biases in the available case-control studies, it is important to note that the relatively low incidence of testicular cancer creates nearly insurmountable cohort-design challenges, such as enrollment of sufficiently large samples and follow-up over long time periods.
Drawing upon a uniquely large, population-based sample (n = 49 343) of Swedish young men aged 18–21 years undergoing extensive medical and psychiatric assessments required during the process of compulsory conscription into military service in Sweden in 1969–1970, the current retrospective cohort study aimed to assess the possible link between self-reported cannabis use, measured at conscription, and the subsequent development of testicular cancer over a 42-year follow-up period.
Method
Study cohort
The study cohort included 49 343 Swedish males born between 1949–1951 who underwent extensive medical and psychological assessment for the nationwide conscription for compulsory Swedish military service occurring in 1969–1970 (see Table 1 for initial sample details). Cohort members’ ages (at conscription assessment) ranged from 18–21 years. Only 2–3% of all young men were exempted from the conscription process due to severe mental or physical conditions.
Table 1.
Baseline characteristics of the study sample of 49 343 conscripts born in 1949–1951 and aged 18–21 at conscription by exposure status
| Study covariates | Total | Never drug use | “Ever” use of cannabis | p-valuea | Among “ever” cannabis usersb | Unclear/missing/use of other drugsc | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Use more than 50 times | p-valuea | Cannabis as “most frequently used drug” | p-valuea | ||||||
| Total, n (%)d | 49 343 (100) | 40 271 (81.6) | 5 326 (10.8) | 879 (1.8) | 4 323 (8.8) | 3 746 (7.6) | |||
| Birth year | <0.001 | <0.001 | <0.001 | ||||||
| 1949 | 2 833 (100) | 2 122 (74.9) | 336 (11.9) | 73 (2.6) | 265 (9.4) | 375 (13.2) | |||
| 1950 | 8 826 (100) | 6 741 (76.4) | 1 161 (13.1) | 194 (2.2) | 952 (10.7) | 924 (10.5) | |||
| 1951 | 37 684 (100) | 31 408 (83.3) | 3 829 (10.2) | 612 (1.6) | 3 106 (8.2) | 2 447 (6.5) | |||
| Cryptorchidism | 0.864 | 0.528 | 0.805 | ||||||
| No | 49 085 (100) | 40 059 (81.6) | 5 297 (10.8) | 873 (1.8) | 4 299 (8.8) | 3 729 (7.6) | |||
| Yes | 258 (100) | 212 (82.2) | 29 (11.2) | 6 (2.3) | 24 (9.3) | 17 (6.6) | |||
| Paternal testicular cancer | 0.139 | 0.001 | 0.476 | ||||||
| No | 49 267 (100) | 40 214 (81.6) | 5 314 (10.8) | 874 (1.8) | 4 315 (8.8) | 3 739 (7.6) | |||
| Yes | 76 (100) | 57 (75.0) | 12 (15.8) | 5 (6.6) | 8 (10.5) | 7 (9.2) | |||
| Tobacco smoking | <0.001e | <0.001e | <0.001e | ||||||
| Never | 20 906 (100) | 18 273 (87.4) | 790 (3.8) | 75 (0.4) | 628 (3.0) | 1 843 (8.8) | |||
| 1–5 cig/day | 5 428 (100) | 4 557 (83.9) | 477 (8.8) | 57 (1.1) | 396 (7.3) | 394 (7.3) | |||
| 6–10 cig/day | 10 058 (100) | 8 114 (80.7) | 1 294 (12.9) | 194 (1.9) | 1 059 (10.5) | 650 (6.4) | |||
| 11–20 cig/day | 11 198 (100) | 8 229 (73.5) | 2 268 (20.2) | 405 (3.6) | 1 861 (16.6) | 701 (6.3) | |||
| More than 20 cig/day | 1 747 (100) | 1 098 (62.8) | 497 (28.5) | 148 (8.5) | 379 (21.7) | 152 (8.7) | |||
| Missing | 6 (100) | 0 | 0 | 0 | 0 | 6 (100) | |||
| Alcohol consumption (g of 100% alc/week) | <0.001e | <0.001e | <0.001e | ||||||
| Abstainers (0 g) | 2 957 (100) | 2 738 (92.6) | 44 (1.5) | 20 (0.7) | 35 (1.2) | 175 (5.9) | |||
| Light (1–100 g) | 36 253 (100) | 31 134 (85.9) | 2 761 (7.6) | 395 (1.1) | 2 217 (6.1) | 2 358 (6.5) | |||
| Moderate (101–250 g) | 7 904 (100) | 5 475 (69.3) | 1 883 (23.8) | 277 (3.5) | 1 564 (19.8) | 546 (6.9) | |||
| Heavy (> 250 g) | 1 454 (100) | 730 (50.2) | 594 (40.8) | 176 (12.1) | 470 (32.32) | 130 (8.9) | |||
| Missing | 775 (100) | 194 (25.0) | 44 (5.7) | 11 (1.4) | 37 (4.8) | 537 (69.3) | |||
P-values for trend in comparison with “Never drug use”.
Percentages for “Use more than 50 times” and “Cannabis as most frequently used drug” are calculated out of total (100%).
Exposure to any drug use ever, but not to cannabis or missing/unclear information on drug use.
Categories “Never drug use”, “Ever use of cannabis” and “Unclear/missing/use of other drugs” sum up to 100%.
P-value calculated for categories with non-missing observations.
Data collection: process, sources, and linkage
Conscription assessment
The Swedish conscription process included a large battery of evaluations, including physical and psychological assessments, as well as non-anonymous self-report questionnaires on familial, social, and behavioral items (22). The 1969–1970 conscription also collected additional self-reported information about use of alcohol, drugs, tobacco, as well as questions on familial, social and behavioral items. All men underwent a medical examination, and diagnoses were recorded according to ICD-8. Persons who reported or showed signs of mental disorder were referred to a psychiatrist, who recorded a diagnosis, if a disorder was identified (23).
Linkage of conscription-assessment data with other Swedish health and social registries
All individuals living in Sweden are assigned a unique personal number in national Swedish registers, enabling linkage of data across registries (24). The Stockholm Regional Ethical Review Board provided permission for data linkage (Dnr 2016/3:7).
Our cohort study relied on the linkage of 6 data sources: 1) information from Swedish young men undergoing conscription assessment in 1969–1970; 2) the Swedish Patient Register, 1964–2011; 3) the National Cancer Register, 1958–2010; 4) the National Cause of Death Register, 1952–2011; 5) the Swedish Total Population Register, 1968–2011 (which captures date of emigration out of and immigration into Sweden); and 6) the Swedish Multigenerational Register, 1973–2011.
Study outcome: Testicular cancer
Diagnoses of testicular cancer were identified by use of the Swedish version of ICD-7/8/9/10 codes appearing in the National Patient Register, the Cancer Register, or the Cause of Death Register (see Supplemental Table 1). Incident cases of testicular cancer were identified in the main and supplemental diagnoses in the National Patient Register and the primary diagnoses in the Cancer Register, as well as in the underlying and contributory causes of death diagnoses in the Cause of Death Register. Additionally, in the initial sample, no cases of testicular cancer were identified during the period before conscription or at the conscription assessment. The current study was not able to access histological subtype information on testicular cancer and, as a result, assessment of the potential association between cannabis use and specific subtypes was not possible.
Measurement of cannabis use
Lifetime, ever cannabis use
The variable instantiating lifetime, “ever” use of cannabis was created from the following 3 questions in the self-reported, non-anonymous conscription-assessment questionnaire: 1) Have you ever tried the following substances? (Give an answer yes or no about every drug); 2) What substance did you take first?; and 3) What substance have you taken most frequently? If the participant indicated cannabis use in response to any of these three questions, the variable of lifetime, ever cannabis use was coded as “yes”.
Lifetime frequency of cannabis use
The variable capturing lifetime frequency of cannabis use was constructed from the following conscription survey question: “How many times have you used drugs?” (response options: 1, 2–4, 5–10, 11–50, more than 50 times). For those conscripts indicating “ever” cannabis use, it was assumed that the lifetime drug-use frequency question applied to their cannabis use, especially as cannabis use was, by far, the most frequently used drug in the sample. Of the 5326 persons who indicated ever using cannabis (with or without use of other drugs), 4323 (81.2%) indicated cannabis as the drug most frequently used. Among the 879 young men who reported use of cannabis more than 50 times (with or without use of other drugs), 743 (84.5% out of 879) individuals indicated cannabis as the drug most frequently used.
Other covariates included in statistical modeling
The selection of covariates for the statistical analyses in the current study included, where possible, the variables selected for the statistical modeling in the three prior case-control studies (14–16), as well as the modeling covariates recommended in a recent systematic review of the cannabis-testicular cancer area (11): age, cryptorchidism, family history of testicular cancer, tobacco use, and alcohol use.
Cryptorchidism
Cryptorchidism was identified by retrieving individual-level information from the National Patient Register [using Swedish ICD-7/8/9/10 codes (see Supplemental Table 1)] and from the results of medical examination performed at the conscription evaluation.
Paternal history of testicular cancer
Using the Swedish Multigenerational Register, the conscripts were linked to their biological fathers. Paternal diagnoses of testicular cancer were identified from the National Patient Register, the Cancer Register and the Cause of Death Register using the same ICD-7/8/9/10 codes defining the testicular cancer outcome (see Supplemental Table 1).
Tobacco smoking
Data on frequency of tobacco smoking reported at the conscription assessment were retrieved from individuals’ survey responses and categorized as: never smoking tobacco (reference group); 1–5 cig/day; 6–10 cig/day; 11–20 cig/day; more than 20 cig/day.
Alcohol consumption
Alcohol consumption was calculated by combining data on self-reported quantity and frequency of consumption of beer, wine, and spirits and expressed as grams of 100% alcohol (ethanol) per week: light drinkers [(0–100 g 100% alc/week); reference group]; abstainers (0 g 100% alc/week); moderate drinkers (101–250 g 100% alc/week); heavy drinkers (more than 250 g 100% alc/week). The Swedish alcohol retail monopoly provided information about the alcohol content (ethanol) for all alcohol beverages available in Sweden in 1969–1970 (25).
Statistical analyses
We evaluated whether lifetime cannabis use and lifetime frequency of cannabis use were related to incidence of testicular cancer. We applied Cox proportional hazards modelling to estimate the hazard ratio (HR) and 95% confidence intervals (CI) associated with cannabis use and time to diagnosis of testicular cancer. In multivariate analyses, we applied a stepwise adjustment strategy, based on the stepwise modeling recommendations of a recent systematic review of the cannabis-testicular cancer area (11). In Model 1 we adjusted for the conscripts’ birth year and for established risk factors for testicular cancer (i.e., conscripts’ cryptorchidism and paternal testicular cancer); in Model 2, we adjusted for the conscripts’ birth year and factors associated with cannabis use (i.e., frequency of tobacco smoking and alcohol consumption volume); and finally, Model 3 was a fully-adjusted model where all abovementioned risk factors were included. The presentation of model results in each stepwise strategy gives the reader an important description of the stability of the estimates across covariate combinations in each model.
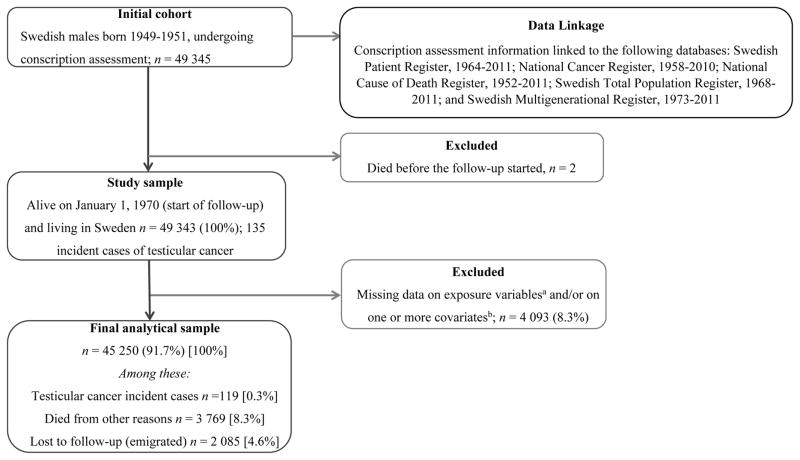
All analyses relied on list-wise deletion of individuals missing data on any variables included in the analyses. A flowchart (Figure 1) describes the initial sample and final cohort based on list-wise deletion, along with the linkage of the conscription-assessment information with other population-based health registries used in the study. Person-time was counted from January 1, 1970 until the date of diagnosis, date of death due to other reasons, date of emigration or until end of follow-up on December 31, 2011, whichever occurred first. Person-time comprised 1 803 490.9 person-years for the analyses associated with lifetime “ever” use of cannabis and 1 799 273.2 person-years for those analyses associated with lifetime frequency of cannabis use. The proportional hazard assumption was checked by the log-rank test for equality of survival function and the log-survival plots and was met in all models. All reported p-values were two-sided, with p < 0.05 considered as statistically significant. Statistical analyses were performed using STATA version 13.1.
Figure 1.
Cohort profile and flow-chart for final analytic sample and 42-year follow-up
aExposure variables: “ever” use of cannabis before conscription and lifetime frequency of cannabis use; b Covariates: conscript’s birth year, history of cryptorchidism, parental testicular cancer, frequency of tobacco smoking and volume of alcohol drinking
Results
Testicular cancer cases
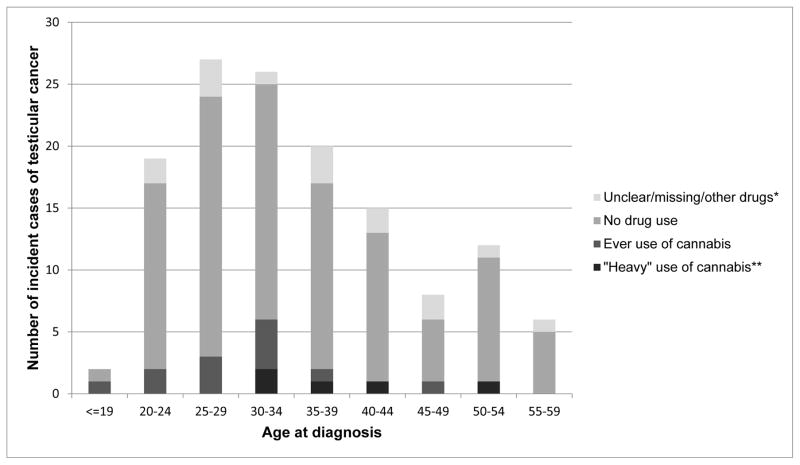
Figure 2 depicts the 135 testicular cancer cases identified in the initial sample (n = 49 343) during the follow-up between 1970 and 2011. Over 50% of the cases occurred among men aged 25–40 years.
Figure 2.
Number of incident cases (n = 135) of testicular cancer diagnosed during follow-up time (1970–2011) among original sample of conscripts born in 1949–1951 (n = 49 343) by age at diagnosis across levels of cannabis use.
* Information on drug use is unclear, missing or use of other drugs, but not cannabis indicated
** Defined as use of cannabis (with or without use of any other illicit drugs) ever before conscription more than 50+ times.
Cohort description
Table 1 provides a description of the baseline characteristics of cohort members in the initial study sample. More than half of the cohort members indicated that they were current smokers, and approximately 20% reported moderate-to-heavy alcohol consumption. Approximately 11% of the baseline sample reported lifetime “ever” cannabis, and among these “ever” cannabis users, approximately 16.5% (n = 879) indicated cannabis usage of more than 50 times in lifetime.
Cox modeling results
In Table 2, the fully adjusted model (Model 3) demonstrated no evidence of a significant relation between “ever” cannabis use and development of testicular cancer [Adjusted hazard ratio (AHR) 1.42, 95% CI, 0.83, 2.45]. In the fully adjusted model, cryptorchidism was the only variable significantly associated with development of testicular cancer (AHR 6.26, 95% CI, 2.30, 17.01).
Table 2.
Incidence rates (IR), hazard ratios (HR) and 95% confidence intervals (CI) for testicular cancer (n = 119 cases) by exposure to “ever” cannabis use in 45 250 conscripts with data available on all study covariates
| Study covariates | Cases/Non-cases | Crude incidence rate per 10 000 PY | Crude model | Model 1a | Model 2b | Model 3c |
|---|---|---|---|---|---|---|
| N | IR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Ever use of cannabis | ||||||
| Never use of any drugs | 102/39975 | 0.64 (0.53, 0.77) | 1.00 | 1.00 | 1.00 | 1.00 |
| Ever use of cannabisd | 17/5156 | 0.85 (0.53, 1.37) | 1.32 (0.79, 2.21) | 1.32 (0.79, 2.21) | 1.44 (0.83, 2.47) | 1.42 (0.83, 2.45) |
| Birth year | ||||||
| 1949 | 10/2427 | 1.06 (0.57, 1.97) | 1.00 | 1.00 | 1.00 | 1.00 |
| 1950 | 16/7836 | 0.52 (0.32, 0.85) | 0.49 (0.22, 1.08) | 0.49 (0.22, 1.09) | 0.49 (0.22, 1.08) | 0.50 (0.22, 1.09) |
| 1951 | 93/34868 | 0.67 (0.54, 0.82) | 0.63 (0.33, 1.22) | 0.65 (0.34, 1.24) | 0.64 (0.33, 1.23) | 0.64 (0.34, 1.24) |
| Cryptorchidism | ||||||
| No | 115/44897 | 0.64 (0.54, 0.77) | 1.00 | 1.00 | 1.00 | |
| Yes | 4/234 | 4.23 (1.59, 11.27) | 6.59 (2.43, 17.86) | 6.42 (2.37, 17.42) | 6.26 (2.30, 17.01) | |
| Parental testicular cancer | ||||||
| No | 118/45063 | 0.66 (0.55, 0.79) | 1.00 | 1.00 | 1.00 | |
| Yes | 1/68 | 3.58 (0.50, 25.40) | 5.46 (0.76, 39.05) | 4.95 (0.69, 35.57) | 4.82 (0.67, 34.72) | |
| Tobacco smoking | ||||||
| Never | 46/18776 | 0.61 (0.46, 0.82) | 1.00 | 1.00 | 1.00 | |
| 1–5 cig/day | 16/5004 | 0.80 (0.49, 1.30) | 1.31 (0.74, 2.31) | 1.37 (0.77, 2.44) | 1.38 (0.77, 2.46) | |
| 6–10 cig/day | 18/9349 | 0.48 (0.30, 0.77) | 0.79 (0.46, 1.36) | 0.83 (0.47, 1.45) | 0.83 (0.47, 1.45) | |
| 11–20 cig/day | 33/10420 | 0.80 (0.57, 1.13) | 1.30 (0.83, 2.04) | 1.41 (0.88, 2.28) | 1.41 (0.88, 2.28) | |
| More than 20 cig/day | 6/1582 | 0.98 (0.44, 2.18) | 1.58 (0.68, 3.71) | 1.95 (0.80, 4.71) | 1.90 (0.79, 4.60) | |
| Alcohol consumption (g of 100% alc/week) | ||||||
| Abstainers (0 g) | 10/2770 | 0.90 (0.48, 1.67) | 1.00 | 1.00 | 1.00 | |
| Light (1–100 g) | 91/33731 | 0.67 (0.55, 0.83) | 0.75 (0.39, 1.44) | 0.67 (0.34, 1.32) | 0.67 (0.34, 1.32) | |
| Moderate (101–250 g) | 17/7311 | 0.59 (0.37, 0.95) | 0.65 (0.30, 1.42) | 0.49 (0.21, 1.14) | 0.49 (0.21, 1.14) | |
| Heavy (more than 250 g) | 1/1319 | 0.20 (0.03, 1.41) | 0.22 (0.03, 1.70) | 0.13 (0.02, 1.09) | 0.14 (0.02, 1.11) |
Model 1 adjusted for conscript’s birth year, conscript’s cryptorchidism, and paternal history of testicular cancer.
Model 2 adjusted for conscript’s birth year, conscript’s frequency of tobacco smoking and conscript’s alcohol consumption.
Model 3 adjusted for all covariates in the table.
Exposure to ever use of cannabis before conscription (with or without use of any other illicit drugs).
In Table 3, “heavy” cannabis use (AHR 2.57, 95% CI, 1.02, 6.50) and cryptorchidism (AHR 6.24, 95% CI, 2.30, 16.97) were significantly related to testicular cancer, whereas tobacco use and alcohol consumption showed no evidence of significant associations with the outcome.
Table 3.
Incidence rates (IR), hazard ratios (HR) and 95% confidence intervals (CI) for testicular cancer (n = 119 cases) by exposure to frequency of cannabis use in 45 250 conscripts with data available on all study covariates
| Study covariates | Cases/Non-cases | Crude incidence rate per 10 000 PY | Crude model | Model 1a | Model 2b | Model 3c |
|---|---|---|---|---|---|---|
| N | IR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Frequency of ever cannabis used | ||||||
| Never use of any drugs | 102/39975 | 0.64 (0.53, 0.77) | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–4 times | 6/2704 | 0.57 (0.25, 1.26) | 0.88 (0.39, 2.01) | 0.90 (0.40, 2.05) | 0.94 (0.41, 2.17) | 0.95 (0.41, 2.19) |
| 5–10 times | 4/861 | 1.20 (0.45, 3.19) | 1.86 (0.68, 5.05) | 1.90 (0.70, 5.15) | 2.13 (0.77, 5.90) | 2.15 (0.77, 5.95) |
| 11–50 times | 2/728 | 0.70 (0.18, 2.81) | 1.09 (0.27, 4.43) | 1.04 (0.26, 4.25) | 1.25 (0.30, 5.15) | 1.17 (0.28, 4.85) |
| More than 50 times | 5/863 | 1.56 (0.65, 3.74) | 2.40 (0.98, 5.88) | 2.32 (0.94, 5.70) | 2.68 (1.06, 6.77) | 2.57 (1.02, 6.50) |
| Birth year | ||||||
| 1949 | 10/2427 | 1.06 (0.57, 1.97) | 1.00 | 1.00 | 1.00 | 1.00 |
| 1950 | 16/7836 | 0.52 (0.32, 0.85) | 0.49 (0.22, 1.08) | 0.50 (0.23, 1.10) | 0.49 (0.22, 1.09) | 0.50 (0.23, 1.10) |
| 1951 | 93/34868 | 0.67 (0.54, 0.82) | 0.63 (0.33, 1.22) | 0.65 (0.34, 1.25) | 0.64 (0.33, 1.24) | 0.65 (0.34, 1.25) |
| Cryptorchidism | ||||||
| No | 115/44897 | 0.64 (0.54, 0.77) | 1.00 | 1.00 | 1.00 | |
| Yes | 4/234 | 4.23 (1.59, 11.27) | 6.59 (2.43, 17.86) | 6.37 (2.35, 17.28) | 6.24 (2.30, 16.97) | |
| Parental testicular cancer | ||||||
| No | 118/45063 | 0.66 (0.55, 0.79) | 1.00 | 1.00 | 1.00 | |
| Yes | 1/68 | 3.58 (0.50, 25.40) | 5.46 (0.76, 39.05) | 4.84 (0.67, 34.91) | 4.62 (0.64, 33.60) | |
| Tobacco smoking | ||||||
| Never | 46/18776 | 0.61 (0.46, 0.82) | 1.00 | 1.00 | 1.00 | |
| 1–5 cig/day | 16/5004 | 0.80 (0.49, 1.30) | 1.31 (0.74, 2.31) | 1.37 (0.77, 2.44) | 1.38 (0.77, 2.46) | |
| 6–10 cig/day | 18/9349 | 0.48 (0.30, 0.77) | 0.79 (0.46, 1.36) | 0.82 (0.47, 1.44) | 0.82 (0.47, 1.44) | |
| 11–20 cig/day | 33/10420 | 0.80 (0.57, 1.13) | 1.30 (0.83, 2.04) | 1.40 (0.87, 2.26) | 1.40 (0.87, 2.26) | |
| More than 20 cig/day | 6/1582 | 0.98 (0.44, 2.18) | 1.58 (0.68, 3.71) | 1.87 (0.77, 4.54) | 1.84 (0.76, 4.46) | |
| Alcohol consumption (g of 100% alc/week) | ||||||
| Abstainers (0 g) | 10/2770 | 0.90 (0.48, 1.67) | 1.00 | 1.00 | 1.00 | |
| Light (1–100 g) | 91/33731 | 0.67 (0.55, 0.83) | 0.75 (0.39, 1.44) | 0.68 (0.35, 1.34) | 0.68 (0.35, 1.34) | |
| Moderate (101–250 g) | 17/7311 | 0.59 (0.37, 0.95) | 0.65 (0.30, 1.42) | 0.50 (0.22, 1.14) | 0.50 (0.22, 1.15) | |
| Heavy (more than 250 g) | 1/1319 | 0.20 (0.03, 1.41) | 0.22 (0.03, 1.70) | 0.12 (0.01, 1.01) | 0.13 (0.02, 1.04) |
Model 1 adjusted for conscript’s birth year, conscript’s cryptorchidism, and paternal history of testicular cancer.
Model 2 adjusted for conscript’s birth year, conscript’s frequency of tobacco smoking and conscript’s alcohol consumption.
Model 3 adjusted for all covariates in the table.
Exposure to ever use of cannabis before conscription (with or without use of any other illicit drugs).
Discussion
Our large Swedish data-linkage project found that self-reported “heavy” cannabis use – defined as self-reported use of more than 50 times in lifetime at the conscription assessment period – was significantly associated with a 2.5-fold increased hazard of subsequent testicular cancer. The study found no evidence of a significant relation between “ever” cannabis use and the development of testicular cancer. This null finding may be due to heterogeneity of cannabis use in the “ever” group, as this category contained only a minority who reported “heavy” cannabis use and a majority of individuals indicating minimal lifetime cannabis exposure (e.g., 1–4 times in lifetime). In addition to the current study finding a significant relation between cannabis use and testicular cancer, our prior work drawing upon the same Swedish conscript cohort found that cannabis use was significantly associated with the development of lung cancer (12).
The available three prior case-control studies in the area have shown a significant pooled association between testicular cancer and current, frequent (i.e., ≥ weekly use) and lengthy cannabis use (i.e., ≥ 10 years), as well as a much stronger relation between cannabis use and the development of nonseminomatous germ cell tumors rather than seminomatous germ cell tumors (21). The Daling et al. (14) and Lacson et al. (15) case-control studies found a modest but statistically significant association between “ever” cannabis use and testicular cancer, whereas Trabert et al. (16) found no evidence of a relation between “ever” cannabis use and testicular cancer. Meta-analytic estimates have shown no evidence of a statistically significant pooled relation between “ever” use and testicular cancer, when these three case-control studies were aggregated (11, 21). While the current study and the three previous studies have shown a relation between frequency and duration of cannabis use and the development of testicular cancer, the field still lacks evidence of a clear dose-response curve – a prerequisite for establishing a persuasive argument for the causal link. The current study showed no evidence of a gradient of risk across cannabis-use frequency variables, and the three prior case-controls studies relied on dichotomized cannabis-use frequency variables, such as “≥ weekly use: yes/no”– a design structure which does not allow for the assessment of a dose-response relation.
The mechanism by which cannabis affects the development of testicular cancer is not well elucidated. It is known, however, that active compounds in phytocannabinoids, such as tetrahydrocannabinol (THC) and cannabidiol (CBD), bind to cannabinoid receptors (CB1 and CB2) in many human organs, including the testes (26, 27). Experimental animal studies have shown that by binding to CB1 and CB2 in Leydig cells and Sertoli cells of the testes, levels of testosterone, follicle-stimulating hormone and luteinizing hormone are affected, as is the survival of Sertoli cells (28–31). Aberration in both steroid hormone levels and gonadotropin levels suggests that cannabinoids may cause a general perturbation to the hypothalamic-pituitary-gonadal axis which could result in tumorigenesis (32). Much more research in this area, including timing of exposure, is required, however.
Our results should be interpreted with caution, given a number of important study limitations. The key variable instantiating conscripts’ lifetime frequency of cannabis use relied on an indirect assessment of cannabis use. It was assumed that for those conscripts indicating “ever” cannabis use, the conscription survey question eliciting information about lifetime drug-use frequency (i.e., “How many times have you used drugs?”) applied to individuals’ cannabis use. This appears to be a reasonable assumption, given that cannabis users in the sample typically indicated cannabis as their most frequently used drug. For example, approximately 81% of “ever” cannabis users and 84% of “heavy” cannabis users indicated cannabis as their most frequently used drug. In addition, the current study did not have information about cannabis use after the conscription-assessment period, but European studies have demonstrated that adolescent cannabis use, especially those patterns associated with regular use, abuse, or dependence, tend to persist and remain relatively stable throughout the developmental years of the 20s and early 30s (33, 34). Even though unmeasured post-conscription changes in cannabis use may have affected our results, such misclassification biases would tend to attenuate our hazard ratio estimates and push our findings toward the null. For example, if “heavy” cannabis users (at baseline) later became nonusers of cannabis, this pattern would lead to a diminished hazard of testicular cancer associated with the cannabis-use variable; if nonusers of cannabis (at baseline) became cannabis users during the follow-up, this pattern would lead to an underestimate of the testicular-cancer risk in the initial cannabis-exposed groups, as this pattern would create an inflated risk in the non-using reference group (defined at baseline). An additional limitation was that the study had no information on the histology of the testicular cancers. While meta-analytic pooled estimates of the three existing case control studies have shown a relation between cannabis-use frequency and testicular cancer, especially the non-seminoma subtype, the current study was not able to estimate the hazards of cannabis use and the subsequent development of seminoma or non-seminoma subtypes.
While the current study does have limitations, it is important also to acknowledge the project’s strengths. The study included a very large population-based sample of young men, who underwent an extensive conscription assessment. This individual-level conscription information was also linked to population-based health registries, including the Swedish cancer registry. This data-linking procedure allowed the study to incorporate a very lengthy 42-year follow-up for members of the initial cohort. As a result, the project is the first cohort study assessing the potential relation between cannabis use and the development of testicular cancer, and it makes an important contribution to the small literature finding a potential association between cannabis use and the incidence of testicular cancer. Given current international developments to decriminalize or legalize cannabis use in a number of countries worldwide, it is critically important for legislation initiatives to consider the possible health consequences of cannabis use in the cost-benefit analysis underpinning rational drug-policy development.
Supplementary Material
Acknowledgments
Claire Benny, BHSc (Honours) provided invaluable assistance during the manuscript-preparation and manuscript-revision phases of the project.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report 2015 (United Nations publication, Sales No. E.15.XI.6) Geneva, Switzerland: United Nations Office on Drugs and Crime; 2015. [Google Scholar]
- 2.Berg CJ, Stratton E, Schauer GL, Lewis M, Wang Y, Windle M, et al. Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: Marijuana, hookah, and electronic cigarettes win. Subst Use Misuse. 2014;50(1):79–89. doi: 10.3109/10826084.2014.958857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall WD. The contribution of research to the development of a national cannabis policy in Australia. Addiction. 2008;103(5):712–20. doi: 10.1111/j.1360-0443.2008.02169.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall W, Lynskey M. The challenges in developing a rational cannabis policy. Curr Opin Psychiatry. 2009;22(3):258–62. doi: 10.1097/YCO.0b013e3283298f36. [DOI] [PubMed] [Google Scholar]
- 5.Davenport S, Caulkins JP, Kleiman MAR. Controlling underage access to cannabis. Case W Reserve L Rev. 2015;65(3):541–66. [Google Scholar]
- 6.Evans-Whipp T, Plenty SM, Catalano RF, Herrenkohl TI, Toumbourou JW. Research and practice: Longitudinal effects of school drug policies on student marijuana use in Washington state and Victoria, Australia. Am J Public Health. 2015;105(5):994–1000. doi: 10.2105/AJPH.2014.302421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Room R, Fischer B, Hall W, Lenton S, Reuter P. Cannabis policy: Moving beyond stalemate. Oxford Univ Press; 2010. [Google Scholar]
- 8.Ghosh T, Van Dyke M, Maffey A, Whitley E, Gillim-Ross L, Wolk L. The public health framework of legalized marijuana in Colorado. Am J Public Health. 2016;106(1):21–7. doi: 10.2105/AJPH.2015.302875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wall MM, Mauro C, Hasin DS, Keyes KM, Cerda M, Martins SS, et al. Prevalence of marijuana use does not differentially increase among youth after states pass medical marijuana laws: Commentary on and reanalysis of US National Survey on Drug Use in Households data 2002–2011. Int J Drug Policy. 2016;29:9–13. doi: 10.1016/j.drugpo.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickenlooper GJW. Experimenting with pot: The state of Colorado’s legalization of marijuana. Milbank Q. 2014;92(2):243–9. doi: 10.1111/1468-0009.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YJ, Zhang Z, Tashkin DP, Feng B, Straif K, Hashibe M. An epidemiologic review of marijuana and cancer: An update. Cancer Epidemiol Biomarkers Prevent. 2015;24(1):15–31. doi: 10.1158/1055-9965.EPI-14-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: A 40-year cohort study. Cancer Causes & Control. 2013;24(10):1811–20. doi: 10.1007/s10552-013-0259-0. [DOI] [PubMed] [Google Scholar]
- 13.Hall Wayne, Renström Maria, Poznyak Vladimir., editors. The health and social effects of nonmedical cannabis use. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 14.Daling JR, Doody DR, Sun X, Trabert BL, Weiss NS, Chen C, et al. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115(6):1215–23. doi: 10.1002/cncr.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacson JC, Carroll JD, Tuazon E, Castelao EJ, Bernstein L, Cortessis VK. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer. 2012;118(21):5374–83. doi: 10.1002/cncr.27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer. 2011;117(4):848–53. doi: 10.1002/cncr.25499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewett MAS, Bokemeyer C. Testicular germ cell tumours. The Lancet. 2016;387(10029):1762–74. doi: 10.1016/S0140-6736(15)00991-5. [DOI] [PubMed] [Google Scholar]
- 18.Winter C, Albers P. Testicular germ cell tumors: Pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2011;7(1):43–53. doi: 10.1038/nrendo.2010.196. [DOI] [PubMed] [Google Scholar]
- 19.Nigam M, Aschebrook-Kilfoy B, Shikanov S, Eggener S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World J Urol. 2015;33(5):623–631. doi: 10.1007/s00345-014-1361-y. [DOI] [PubMed] [Google Scholar]
- 20.Ghazarian AA, Trabert B, Devesa SS, McGlynn KA. Recent trends in the incidence of testicular germ cell tumors in the United States. Andrology. 2015;3(1):13–18. doi: 10.1111/andr.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: A systematic review and meta-analysis. BMC Cancer. 2015;15(1):1. doi: 10.1186/s12885-015-1905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto U. A sociopsychiatric study of a total annual population of Swedish adolescent boys. Acta Psychiatr Scand Suppl. 1976;264(1):5–312. [PubMed] [Google Scholar]
- 23.Andréasson S, Engström A, Allebeck P, Rydberg U. Cannabis and schizophrenia: A longitudinal study of Swedish conscripts. The Lancet. 1987;330(8574):1483–6. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: Possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreasson S, Allebeck P, Romelsjö A. Alcohol and mortality among young men: Longitudinal study of Swedish conscripts. Br Med J (Clin Res Ed) 1988;296(6628):1021–1025. doi: 10.1136/bmj.296.6628.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda LA, Bonner TI, Lolait SJ. Cannabinoid receptors: Which cells, where, how, and why? NIDA Res Monogr. 1993;126:48. [PubMed] [Google Scholar]
- 27.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 28.Kolodny RC, Masters WH, Kolodner RM, Toro G. Depression of plasma testosterone levels after chronic intensive marihuana use. N Engl J Med. 1974;1974(290):872–874. doi: 10.1056/NEJM197404182901602. [DOI] [PubMed] [Google Scholar]
- 29.Mandal TK, Das NS. Testicular toxicity in cannabis extract treated mice: Association with oxidative stress and role of antioxidant enzyme systems. Toxicol Ind Health. 2010;26(1):11–23. doi: 10.1177/0748233709354553. [DOI] [PubMed] [Google Scholar]
- 30.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–613. [PubMed] [Google Scholar]
- 31.Wenger T, Ledent C, Csernus V, Gerendai I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem Biophys Res Commun. 2001;284(2):363–368. doi: 10.1006/bbrc.2001.4977. [DOI] [PubMed] [Google Scholar]
- 32.Skeldon SC, Goldenberg SL. Urological complications of illicit drug use. Nat Rev Urol. 2014;11(3):169–77. doi: 10.1038/nrurol.2014.22. [DOI] [PubMed] [Google Scholar]
- 33.Perkonigg A, Goodwin RD, Fiedler A, Behrendt S, Beesdo K, Lieb R, et al. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103(3):439–449. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- 34.Perkonigg A, Lieb R, Hofler M, Schuster P, Sonntag H, Wittchen HU. Patterns of cannabis use, abuse and dependence over time: Incidence, progression and stability in a sample of 1228 adolescents. Addiction. 1999;94(11):1663–78. doi: 10.1046/j.1360-0443.1999.941116635.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.