Abstract
IMPORTANCE
Leisure-time physical activity has been associated with lower risk of heart-disease and all-cause mortality, but its association with risk of cancer is not well-understood.
OBJECTIVE
To determine the association of leisure-time physical activity with incidence of common types of cancer and whether associations vary by body size and/or smoking.
DESIGN, SETTING, AND PARTICIPANTS
We pooled data from 12 prospective U.S. and European cohorts with self-reported physical activity (baseline 1987–2004). A total of 1.44 million participants (median age:59 years; range:19–98 years) and 186,932 cancers were included. We used multivariable Cox regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for associations of leisure-time physical activity with incidence of 26 types of cancer. Leisure-time physical activity levels were modeled as cohort-specific percentiles on a continuous basis and cohort-specific results were synthesized by random effects meta-analysis. Hazard ratios for high versus low levels of activity are based on a comparison of risk at the 90th versus 10th percentiles, respectively, of activity.
EXPOSURE
Leisure-time physical activity of a moderate to vigorous intensity.
MAIN OUTCOMES AND MEASURES
Incident cancer during follow-up.
RESULTS
High versus low levels of leisure-time physical activity were associated with lower risks of 13 cancers: esophageal adenocarcinoma (HR=0.58,CI:0.37–0.89), liver (HR=0.73,CI:0.55–0.98), lung (HR=0.74,CI:0.71–0.77), kidney (HR=0.77,CI:0.70–0.85), gastric cardia (HR=0.78,CI:0.64–0.95), endometrial (HR=0.79,CI:0.68–0.92), myeloid leukemia (HR=0.80,CI:0.70–0.92), myeloma (HR=0.83,CI:0.72–0.95), colon (HR=0.84,CI:0.77–0.91), head and neck (HR=0.85,CI:0.78–0.93), rectal (HR=0.87,CI:0.80–0.95), bladder (HR=0.87,CI:0.82–0.92), and breast (HR=0.90,CI:0.87–0.93). BMI adjustment modestly attenuated associations for several cancers, but 10 of 13 inverse associations remained statistically significant after BMI adjustment. Leisure-time physical activity was associated with higher risks of malignant melanoma (HR=1.27,CI:1.16–1.40) and prostate cancer (HR=1.05,CI:1.03–1.08). Associations were generally similar between the overweight/obese and the normal weight. Smoking status modified the association for lung cancer, but not other smoking-related cancers.
CONCLUSIONS AND RELEVANCE
In addition to associations with lower risk of heart-disease and mortality, leisure-time physical activity is also associated with lower risks of many cancer types. Health care professionals counseling inactive adults should emphasize that most of these associations were evident regardless of body size or smoking history, supporting broad generalizability of findings.
Keywords: Physical activity, cancer, pooled, meta-analysis, leisure-time, leisure time, epidemiology
INTRODUCTION
Physical activity is known to reduce risks of heart disease and all-cause mortality1, as well as risks of colon, breast, and endometrial cancers2. Less is known, however, about whether physical activity reduces risk of other cancers, which, together, comprise 75% of incident cancers in the United States3 and 61% of cancers worldwide4. Physical inactivity is highly prevalent, with an estimated 51% of people in the United States5 and 31% of people worldwide not attaining recommended physical activity levels6. Any decrease in risk of cancer associated with physical activity may therefore be public health relevant, and important for cancer prevention efforts.
To date, hundreds of prospective studies have examined associations between physical activity and cancer risk2 but, owing to small case numbers, results have been inconclusive for most cancer types. Meta-analyses, to a degree, mitigate the sample size issue by pooling the published studies2. However, pooled studies have typically been highly heterogeneous in study design (e.g. case-control vs. prospective cohort), physical activity types examined (e.g. leisure-time vs. occupational activity), and in the contrasts examined (tertiles vs. quintiles). Such heterogeneity can attenuate risk estimates, thereby masking true underlying associations.
In the current study, we examined leisure-time physical activity in relation to risk of 26 different cancer types in a pooled analysis of 12 prospective cohort studies and 1.44 million participants. We address several methodologic limitations in prior research by attaining case numbers comparable to or exceeding that of the literature for most cancer types (see eTable 1 in the supplement), by restricting analyses to a specific study design (prospective cohort) and type of physical activity (leisure-time), and by examining the same consistent and large contrast (90th vs. 10th percentile) across studies. Our objectives were to determine the cancers associated with leisure-time physical activity, and whether associations varied by excess bodyweight and smoking, among other factors of prior interest. Our hypothesis was that higher levels of leisure-time physical activity would be associated with lower risk of 26 cancers.
METHODS
Study population
The Physical Activity Collaboration of the National Cancer Institute’s Cohort Consortium was formed to estimate physical activity and disease associations using pooled prospective data and a standardized analytical approach. In a prior pooled analysis, we evaluated dose-response associations between leisure-time physical activity and mortality1.
Prospective studies in the National Cancer Institute Cohort Consortium were eligible for inclusion in the current study if they assessed leisure-time physical activity and had appropriate covariate data. For cohorts with key data missing at baseline but collected later (five cohorts), baseline was redefined as the later date. Twenty of 23 Cohorts (87%) met the inclusion criteria and 12 (52%) agreed to participate, including eight from the U.S. and four from Europe (Table 1)7–18.
Table 1.
Selected participant characteristics according to cohort study
| Cohort* | Participants | Men | Women | Study entry | Median years of follow-up (maximum) |
Median age in years (range) |
Median BMI |
% who ever smoked |
|---|---|---|---|---|---|---|---|---|
| AARP | 507,826 | 308,073 | 199,753 | 1995–1997 | 11 (11) | 62 (50–71) | 27 | 61 |
| BCDDP | 37,228 | 0 | 37,228 | 1987–1989 | 9 (11) | 60 (39–93) | 25 | 42 |
| COSM | 40,919 | 40,919 | 0 | 1998 | 10 (10) | 60 (45–80) | 26 | 63 |
| CPSII | 154,425 | 73,083 | 81,342 | 1992–1993 | 14 (17) | 63 (40–91) | 26 | 55 |
| EPIC | 410,165 | 126,664 | 283,501 | 1991–2001 | 12 (18) | 52 (19–98) | 26 | 49 |
| IWHS | 37,584 | 0 | 37,584 | 1986 | 20 (20) | 61 (52–70) | 26 | 34 |
| PHS | 27,890 | 27,890 | 0 | 1982–2001 | 21 (28) | 54 (40–87) | 25 | 47 |
| PLCO | 60,200 | 30,970 | 29,230 | 1993–2003 | 9 (13) | 62 (52–77) | 27 | 53 |
| SMC | 33,006 | 0 | 33,006 | 1998 | 10 (10) | 60 (47–83) | 25 | 46 |
| USRT | 57,967 | 12,357 | 45,610 | 1994–1998 | 9 (12) | 45 (31–88) | 26 | 44 |
| WHS | 39,414 | 0 | 39,414 | 1993–1996 | 17 (18) | 52 (39–90) | 26 | 49 |
| WLH | 30,000 | 0 | 30,000 | 2003–2004 | 7 (7) | 51 (40–62) | 25 | 51 |
| Total | 1,436,624 | 619,956 | 816,668 | 1982–2004 | 11 (28) | 59 (19–98) | 26 | 54 |
Abbreviations. AARP: NIH-AARP Diet and Health Study, BCDDP: Breast Cancer Detection and Demonstration Project, COSM: Cohort of Swedish Men, CPS II: Cancer Prevention Study II, EPIC: European Prospective Investigation into Cancer and Nutrition, IWHS: Iowa Women’s Health Study, PHS: Physician’s Health Study I and II, PLCO: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, SMC: Swedish Mammography Cohort, USRT: U.S. Radiologic Technologists Cohort, WHS: Women's Health Study, WLH: Women's Lifestyle and Health Study
Leisure-time physical activity assessment
Leisure-time physical activities are activities done at an individual’s discretion to improve or maintain fitness or health. Our analysis includes leisure-time activities of moderate intensity, defined as an intensity of three or more metabolic equivalents (MET), or vigorous intensity, defined as six or more METs; these are the intensity levels recommended by physical activity guidelines19.
Seven of the 12 cohorts9–11,14,15,18 (29% of the overall sample) assessed time per week in moderate and vigorous leisure-time physical activities, enabling calculation of MET-hours/week. These cohorts assessed physical activity by asking about discrete activities like walking, running, or swimming9, 11, or, alternately, by inquiring about overall weekly participation in moderate to vigorous intensity activities10, 14, 15, 18. The median activity level was 8 MET-hours/week overall, and in six of seven cohorts (eTable 2). This is equivalent to 150 minutes of moderate intensity activity (e.g. walking) per week, and comparable to the median activity level for the U.S. population5. Of the remaining cohorts, four evaluated only vigorous intensity leisure-time physical activity8,13,16,17, and one evaluated frequency of moderate to vigorous intensity activities, but not time spent12. Ten of twelve cohorts used questionnaires previously validated against objective criterion measures (eMethods).
Leisure-time physical activity levels were harmonized by converting them to cohort-specific percentiles, with values from 0 (low activity) to 100 (high activity). If physical activity was based on categorical responses, the percentile at the category midpoint was assigned, e.g. if 20% of participants indicated the lowest level of activity, they were assigned the 10th percentile.
Cancer ascertainment
Incident first primary cancers were identified by follow-up questionnaires and review of medical records7–9, cancer registry linkage10–12,18, or both13–17. Overall, 99% of cancer cases were confirmed by medical records or pathology reports. Cancer type was defined using the Surveillance Epidemiology and End Results site recode and the International Classification of Diseases for Oncology, Third Edition20 (eTable 3). Participants were followed from baseline to date of cancer diagnosis, death, or end of follow-up, whichever came first. Cancer types were selected for analysis if there were at least 300 cases across studies. For each cancer, only cohorts with at least 15 cases were included for analysis (eTable 4).
Statistical analysis
Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CIs) for the association between leisure-time physical activity and cancer. The linearity of physical activity and cancer associations was evaluated with cubic splines and likelihood ratio tests. Associations were predominantly linear (eFigure 1), therefore physical activity was modeled on a continuous linear basis for subsequent analyses. Hazard ratios (HR) for higher versus lower physical activity levels are estimated by comparing hazards at the 90th and 10th percentiles of cohort-specific distributions, respectively. Hazard ratios comparing higher versus lower activity levels were computed as e90β-10β, where β is the log HR from the model for the continuous physical activity percentile. DerSimonian and Laird random effects meta-analysis methods were used to summarize cohort- and cancer site-specific results21. P-values are considered statistically significant if less than 0.05. To account for an increased type I error rate due to testing of multiple outcomes, we also calculated the false discovery rate22 for primary findings. Statistical heterogeneity between studies was evaluated by Cochran’s Q23.
Models included age, gender, smoking, alcohol, race/ethnicity, education, and, for female-only cancers, hormone replacement therapy, oral contraceptive use, age at menarche, age at menopause, and parity. For ovarian and endometrial cancers, women who reported a history of oophorectomy and hysterectomy at baseline, respectively, were excluded from analysis. Covariates were selected based on known associations with cancer, and are similar to those used in a study by Park et al. of multiple cancer endpoints24. Multiple imputation procedures25 were used to accommodate missing data within each cohort, with the overall proportions of missing data as follows: smoking status (2.3%), alcohol intake (1.3%), race/ethnicity (1.7%), education (2.9%), hormone replacement therapy (2.2%), oral contraceptive use (0.8%), age at menarche (1.1%), age at menopause (1.2%), and parity (1.9%). We also evaluated the role of body mass index (BMI) in physical activity—cancer associations by running all models with and without adjustment for BMI.
We evaluated multiplicative effect modification by BMI (<25 kg/m2; ≥ 25 kg/m2), smoking status (current; former; never smokers), geographic region (U.S.; Europe), postmenopausal hormone therapy (women only: ever-user; never-user), gender, race (white; black), and follow-up time (<5 years of follow up; > 5 years of follow up) using the Wald test for homogeneity. Interactions were declared if p-values were less than 0.01.
We conducted selected cancer subgroup analyses using additional detailed data from the NIH-AARP Diet and Health Study (eMethods). Specifically, we examined associations for estrogen receptor positive (ER+) and negative (ER−) breast cancers and for non-advanced and advanced prostate cancers. These specific cancers and subtypes were selected based on prior data suggesting subtype-specificity of associations26, 27. We also examined malignant melanoma associations stratified by ground-level solar ultraviolet radiation of participant residence, as determined by linkage to the solar ultraviolet radiation dataset from the National Aeronautics and Space Administration (eMethods). We also evaluated diet as a potential confounder in this study by adding covariates for intake of kilocalories, multivitamins, use of individual vitamin supplements and intake of fruit, vegetables, and red meat. These diet covariates are the same as in Park et al24.
Analyses were done in SAS 9.4.
RESULTS
In our pooled dataset, 1.44 million of 1.65 million participants had complete leisure-time physical activity data and no history of cancer at baseline. Fifty five percent of participants were women, the median age at baseline was 59 years, and the median BMI was 26 (Table 1). Higher activity levels were associated with younger age, more education, lower BMI, and lower likelihood of being a current smoker (eTable 5). During a median 11 years of follow-up, 186,932 incident cancers were identified.
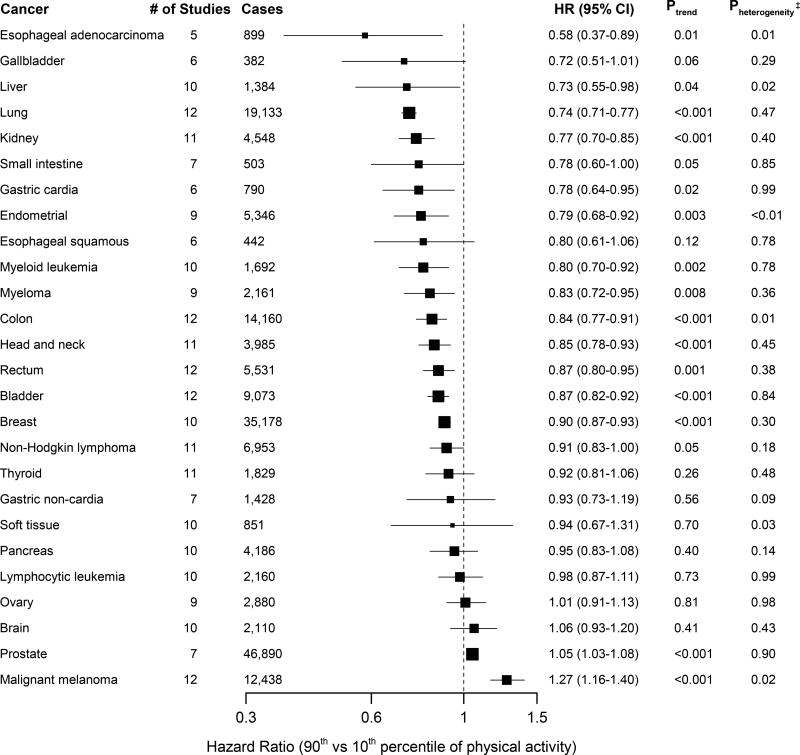
A higher level of leisure-time physical activity was associated with lower risk for 13 of the 26 types of cancer (Figure 1, eFigures 2a–2z). Compared with a lower level of leisure-time physical activity (10th percentile), higher level of activity (at the 90th percentile) had strong inverse associations (greater than 20% reduction in risk) for seven cancers: esophageal adenocarcinoma (HR=0.58,CI:0.37–0.89), and cancers of the liver (HR=0.73,CI:0.55–0.98), lung (HR=0.74,CI:0.71–0.77), kidney (HR=0.77,CI:0.70–0.85), gastric cardia (HR=0.78,CI:0.64–0.95), endometrium (HR=0.79,CI:0.68–0.92), and myeloid leukemia (HR=0.80,CI:0.70–0.92). Moderate inverse associations (10–20% reduction in risk) were observed for myeloma (HR=0.83,CI:0.72–0.95), colon cancer (HR=0.84,CI:0.77–0.91), head and neck cancer (HR=0.85,CI:0.78–0.93), rectal cancer (HR=0.87,CI:0.80–0.95), bladder cancer (HR=0.87,CI:0.82–0.92), and breast cancer (HR=0.90,CI:0.87–0.93). Suggestive inverse associations were also noted for gallbladder cancer (HR=0.72,CI:0.51–1.01), small intestine cancer (HR=0.78,CI:0.60–1.00), and Non-Hodgkin Lymphoma (HR=0.91,CI:0.83–1.00). Higher levels of PA were associated with an increased risk of prostate cancer (HR=1.05,CI:1.03–1.08) and malignant melanoma (HR=1.27,CI:1.16–1.40). Over the 26 cancers, the estimated false discovery rate is 7%. This low false discovery rate suggests that chance is unlikely to explain any more than one to two study findings. In aggregate, higher levels of physical activity were associated with a seven percent lower risk of total cancer (HR=0.93,CI:0.90–0.95).
Figure 1. Summary multivariable* hazard ratios (HR) and 95% confidence intervals (CI) for a higher (90th percentile) versus lower (10th percentile) level of leisure-time physical activity by cancer type†.
* Multivariable models were adjusted for age, gender, smoking status (never, former, current), alcohol consumption (0, 0.1–14.9, 15.0–29.9 and 30.0+ g/day), education (did not complete high school, completed high school, post high-school training, some college, completed college), and race/ethnicity (white, black, other). Models for endometrial, breast, and ovarian cancers are additionally adjusted for hormone replacement therapy use (ever, never), oral contraceptive use (ever, never), age at menarche (<10 years, 10–11 years, 12–13 years, 14+ years), age at menopause (premenopausal, 40–44 years, 45–49 years, 50–54 years, 55+ years), and parity (0 children, 1 child, 2 children, 3+ children).
† The Surveillance Epidemiology and End Results site recode and the International Classification of Diseases for Oncology, Third Edition code corresponding to each cancer type are shown in Supplementary Table 1.
‡ Pheterogeneity indicates the P-value for heterogeneity of hazard ratios across participating studies.
Heterogeneity between studies was modest, with nominal heterogeneity (p<0.05) for esophageal adenocarcinoma, liver cancer, soft tissue cancer, colon cancer, and melanoma, and endometrial cancer. Exact causes of heterogeneity could not be determined with certainty, but for esophageal adenocarcinoma, liver cancer, and soft tissue cancer, variability in hazard ratios may reflect small case numbers. For colon cancer, associations were weaker in female cohorts, and, for melanoma, associations were stronger in European studies (possibly reflecting skin tone). For endometrial cancer, one outlying result appears to drive heterogeneity, but the reason for the outlier is not understood. Despite quantitative heterogeneity, point estimates for each study were generally consistent in direction. In an influence analysis, excluding each study in turn only modestly impacted hazard ratios (eTable 6).
Adjusting for BMI attenuated associations for esophageal adenocarcinoma and cancers of the liver, kidney and gastric cardia (i.e. increase of 5–11% in HRs; see Table 2 and eFigure 3) and nullified the association for endometrial cancer, i.e. the hazard ratio increased from 0.79 (statistically significant) to 0.98 (non-significant). Associations for liver and gastric cardia were no longer statistically significant in BMI-adjusted models, though hazard ratios were still consistent with 15 to 20 percent lower risk. Otherwise, the effects of adjustment for BMI were modest, and 10 of 13 inverse associations remained statistically significant after adjustment.
Table 2.
Comparison of multivariable hazard ratios (HRs)* and 95% confidence intervals (CIs) for a higher (90th percentile) versus lower (10th percentile) level of leisure-time physical activity by cancer type, without and with adjustment for BMI†
| Cancer‡ | HR (95% CI) Not BMI-adjusted |
HR (95% CI) BMI-adjusted |
Percent difference in HR§ |
|---|---|---|---|
| Esophageal adenocarcinoma | 0.58 (0.37–0.89) | 0.62 (0.40–0.97) | 6.9 |
| Gallbladder | 0.72 (0.51–1.01) | 0.78 (0.57–1.06) | 8.3 |
| Liver | 0.73 (0.55–0.98) | 0.81 (0.61–1.09) | 11.0 |
| Lung | 0.74 (0.71–0.77) | 0.73 (0.70–0.76) | −1.4 |
| Kidney | 0.77 (0.70–0.85) | 0.84 (0.77–0.91) | 9.1 |
| Small intestine | 0.78 (0.60–1.00) | 0.81 (0.62–1.05) | 3.8 |
| Gastric cardia | 0.78 (0.64–0.95) | 0.85 (0.69–1.04) | 9.0 |
| Endometrial | 0.79 (0.68–0.92) | 0.98 (0.89–1.09) | 24.1 |
| Esophageal squamous | 0.80 (0.61–1.06) | 0.76 (0.58–1.01) | −5.0 |
| Myeloid leukemia | 0.80 (0.70–0.92) | 0.85 (0.73–0.97) | 6.2 |
| Myeloma | 0.83 (0.72–0.95) | 0.87 (0.77–0.98) | 4.8 |
| Colon | 0.84 (0.77–0.91) | 0.87 (0.80–0.94) | 3.6 |
| Head and neck | 0.85 (0.78–0.93) | 0.85 (0.77–0.94) | 0.0 |
| Rectum | 0.87 (0.80–0.95) | 0.88 (0.81–0.96) | 1.1 |
| Bladder | 0.87 (0.82–0.92) | 0.88 (0.83–0.94) | 1.1 |
| Breast | 0.90 (0.87–0.93) | 0.93 (0.90–0.96) | 3.3 |
| Non–Hodgkin lymphoma | 0.91 (0.83–1.00) | 0.94 (0.85–1.04) | 3.3 |
| Thyroid | 0.92 (0.81–1.06) | 0.95 (0.81–1.11) | 3.3 |
| Gastric non–cardia | 0.93 (0.73–1.19) | 0.92 (0.73–1.15) | −1.1 |
| Soft tissue | 0.94 (0.67–1.31) | 0.97 (0.70–1.34) | 3.2 |
| Pancreas | 0.95 (0.83–1.08) | 0.98 (0.86–1.12) | 3.2 |
| Lymphocytic leukemia | 0.98 (0.87–1.11) | 0.99 (0.88–1.12) | 1.0 |
| Ovary | 1.01 (0.91–1.13) | 1.03 (0.92–1.15) | 2.0 |
| Brain | 1.06 (0.93–1.20) | 1.06 (0.92–1.22) | 0.0 |
| Prostate | 1.05 (1.03–1.08) | 1.04 (1.01–1.07) | −1.0 |
| Malignant melanoma | 1.27 (1.16–1.40) | 1.28 (1.17–1.41) | 0.8 |
All models were adjusted for age, gender, smoking status (never, former, current), alcohol consumption (0, 0.1–14.9, 15.0–29.9 and 30.0+ g/day), education (did not complete high school, completed high school, post high-school training, some college, completed college), and race/ethnicity (white, black, other). Models for endometrial, breast, and ovarian cancers are additionally adjusted for postmenopausal hormone therapy use (ever, never), oral contraceptive use (ever, never), age at menarche (<10 years, 10–11 years, 12–13 years, 14+ years), age at menopause (premenopausal, 40–44 years, 45–49 years, 50–54 years, 55+ years), and parity (0 children, 1 child, 2 children, 3+ children)
Body mass index categories used for adjustment were as follows: <18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, 40+ kg/m2
The Surveillance Epidemiology and End Results site recode and the International Classification of Diseases for Oncology, Third Edition code20 corresponding to each cancer type are shown in eTable 3
Bolded numbers indicate an increase or decrease of five or more percent in hazard ratio after adjusting for BMI
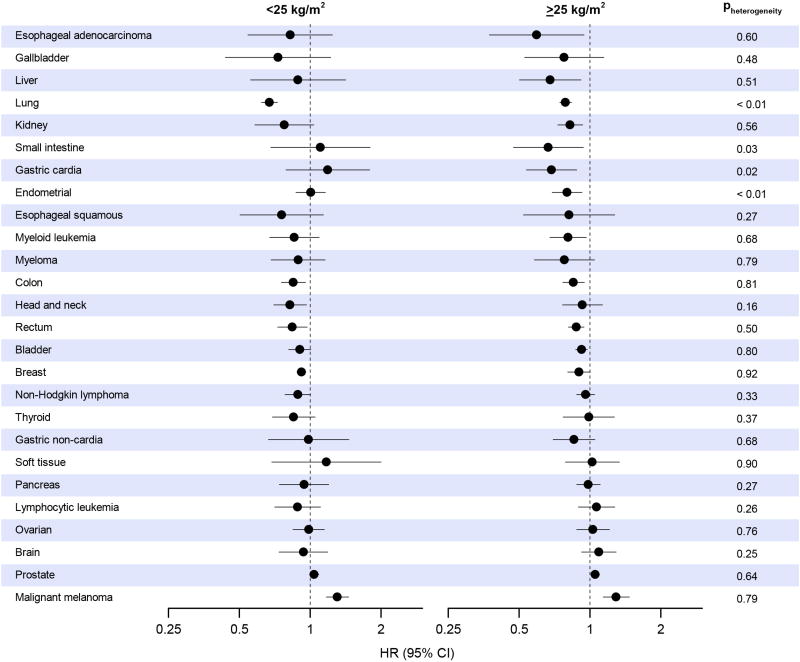
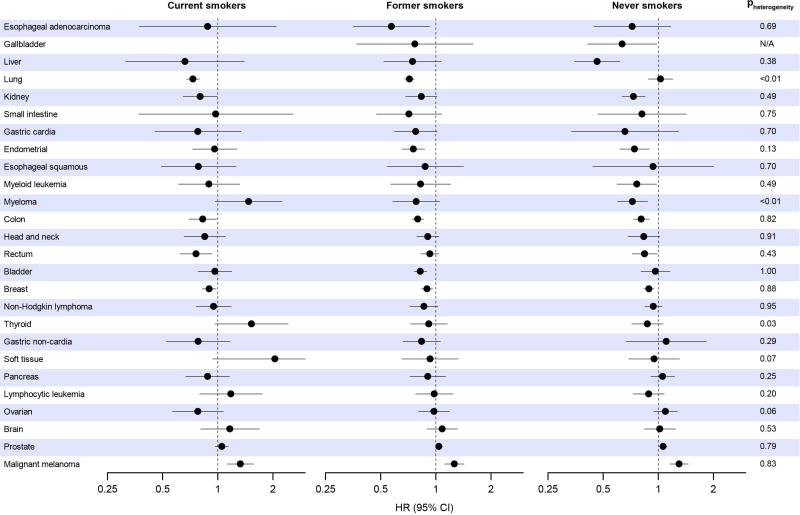
Effect modification by BMI was modest (Figure 2) except for a slightly stronger lung cancer association (Pheterogeneity=0.002) and a null endometrial cancer association (Pheterogeneity<0.001) in those with a BMI lower than 25. Effect modification by smoking history (Figure 3) was also modest, except for a null lung cancer association in never smokers (Pheterogeneity<0.001), and an inverse myeloma association in never smokers that became positive in current smokers (Pheterogeneity=0.002). There was no effect modification of associations by geographic region (eFigure 4), hormone replacement therapy use (eFigure 5), gender (eFigure 6), race (limited subset of cancers, eFigure 7), or follow-up time (eFigure 8), except for myeloma, for which case numbers were small. Restriction to studies with validated questionnaires resulted in no changes in hazard ratios greater than six percent, and associations did not become uniformly stronger or weaker (eTable 7).
Figure 2. Summary multivariable* hazard ratios (HR) and 95% confidence intervals (CI) for a higher (90th percentile) versus lower (10th percentile) level of leisure-time physical activity by cancer type, stratified by body mass index of less than 25 kg/m2 or 25 kg/m2 or higher.
* Multivariable models were adjusted for age, gender, smoking status (never, former, current), alcohol consumption (0, 0.1–14.9, 15.0–29.9 and 30.0+ g/day), education (did not complete high school, completed high school, post high-school training, some college, completed college), and race/ethnicity (white, black, other). Models for endometrial, breast, and ovarian cancers are additionally adjusted for hormone replacement therapy use (ever, never), oral contraceptive use (ever, never), age at menarche (<10 years, 10–11 years, 12–13 years, 14+ years), age at menopause (premenopausal, 40–44 years, 45–49 years, 50–54 years, 55+ years), and parity (0 children, 1 child, 2 children, 3+ children).
Figure 3. Summary multivariable* hazard ratios and 95% confidence intervals for a higher (90th percentile) versus lower (10th percentile) level of leisure-time physical activity by cancer type, stratified by current, former, and never smokers.
* Multivariable models were adjusted for age, gender, alcohol consumption (0, 0.1–14.9, 15.0–29.9 and 30.0+ g/day), education (did not complete high school, completed high school, post high-school training, some college, completed college), and race/ethnicity (white, black, other). Models for endometrial, breast, and ovarian cancers are additionally adjusted for hormone replacement therapy use (ever, never), oral contraceptive use (ever, never), age at menarche (<10 years, 10–11 years, 12–13 years, 14+ years), age at menopause (premenopausal, 40–44 years, 45–49 years, 50–54 years, 55+ years), and parity (0 children, 1 child, 2 children, 3+ children). For gallbladder cancer among current smokers, case numbers were inadequate to provdie an estimate.
In additional analyses in the NIH-AARP study (eFigure 9), leisure-time physical activity was inversely associated with risk of ER+ breast cancers (HR=0.89,CI:0.82–0.97), and especially ER- cancers (HR=0.72,CI:0.59–0.88; Pheterogeneity=0.05). Leisure-time physical activity was associated with higher risk of non-advanced prostate cancer (HR=1.08,CI:1.03,1.12), but not advanced prostate cancer (HR=0.99,CI:0.88–1.10; Pheterogeneity=0.14). The leisure-time physical activity—melanoma association was statistically significant in U.S. regions with higher levels of solar ultraviolet radiation (HR=1.26,CI:1.14–1.38), but not in regions with lower levels (HR=1.12,CI:0.97–1.30; Pheterogeneity=0.21). Lastly, adjustment for dietary factors resulted in modest increases in HRs for esophageal adenocarcinoma (seven percent), liver cancer (five percent) and rectal cancer (five percent), but for other physical activity associated cancers, the attenuation was minimal, i.e. less than five percent (eTable 8).
DISCUSSION
In this pooled analysis of 1.44 million participants, higher levels of leisure-time physical activity (at the 90th percentile), as compared with lower levels (at the 10th percentile) were associated with lower risk of 13 of 26 types of cancer examined, with risk reductions of 20 percent or more for seven of the cancers. Leisure-time physical activity was also associated with higher risk of malignant melanoma, and higher risk of non-advanced prostate cancer. A higher level of leisure-time physical activity was associated with a seven percent lower risk of total cancer.
Our results suggest that leisure-time physical activity may be associated with lower risk of a wider breadth of types of cancer than previously described, and they further bolster the evidence base for associations that were previously only weakly supported. For example, associations for esophageal adenocarcinoma and gastric cardia cancer were among our strongest findings, but previous prospective studies found small effects compared to our own28. For kidney and bladder cancers, we observed clear inverse associations, while recent meta-analyses reported suggestive, but not significant, associations in prospective studies29,30. For myeloid leukemia and myeloma, we found robust inverse associations, whereas a 2015 meta-analysis found null associations31. For liver cancer, inverse associations had been observed, but few studies had been conducted32 and additional confirmatory data were needed. We also observed suggestive inverse associations for cancers of the gallbladder and small intestine, while existing studies found no associations32,33. Our findings further confirm the previously reported inverse associations between physical activity and risk of colon, endometrial, and breast cancers, and further extend the observation of inverse associations to the ER- subtype of breast cancers (in the AARP study).
An additional contribution of our study is its systematic exploration of the role of BMI in associations between physical activity and the full breadth of cancer types. Longitudinal studies34,35 and randomized exercise trials36 show that physical activity helps prevent weight gain and that exercise reduces levels of cancer-relevant biomarkers such as estradiol, mostly as a consequence of weight loss37,38. These combined observations have given rise to a hypothesis that physical activity may reduce cancer risk primarily through lowering body weight. Our finding that most physical activity and cancer associations were BMI-independent argues against this hypothesis for many cancers. For esophageal adenocarcinoma and cancers of the liver, gastric cardia, kidney, and endometrium—cancers known to be obesity-related39—the associations were not completely BMI-independent, however. BMI could be viewed as a mediator underlying associations between physical activity and lower risk of these cancers. Unfortunately, we did not have information about the trajectories of physical activity and body weight and therefore could not distinguish between BMI’s mediating and confounding roles.
We additionally observed that leisure-time physical activity was strongly inversely associated with lung cancer and unassociated with endometrial cancer in those with a BMI lower than 25. For lung cancer, this may reflect higher smoking prevalence among the lean, and thus higher potential for residual confounding. For endometrial cancer, this may reflect the effect of removing body weight (because all participants in this group are lean) from the causal path connecting physical activity to lower risk. For all other cancers, there was little evidence for effect modification, suggesting that among the overweight and obese, a higher physical activity level is still associated with lower cancer risk. This is important because not all persons who engage in high levels of physical activity have low body weights. This finding may help encourage those who are overweight or obese to be physically active.
We also separately examined risk associations among current, former, and never smokers, and aside from lung cancer and myeloma, found little evidence for effect modification. For lung cancer, variability association by smoking status could reflect an inability to completely adjust for smoking habits among current or former smokers, i.e. residual confounding. It is also conceivable, however, that the different findings in current or former smokers—who comprise the overwhelming majority of cases—are indicative of distinct etiologic and biologic features of their lung cancers compared with never smokers40. Effect modification by smoking status was not observed for other smoking-related cancers, e.g. head and neck cancer. This provides some evidence against a generic bias due to residual confounding by smoking, although case numbers were too small to rule this out definitively. For myeloma, smoking is not a risk factor and effect modification may therefore be due to small case numbers and/or chance.
Leisure-time physical activity was positively associated with prostate cancer risk but there is no known biologic rationale to explain this association. Physically active men are more likely than inactive men to receive digital rectal exams and/or prostate-specific antigen screening26, which increases the likelihood of diagnosing indolent prostate cancers. The positive association we observed could therefore be due to screening bias. To circumvent this potential bias, we analyzed advanced prostate cancers in the AARP study, as advanced cases are less likely to remain indolent, and found no physical activity and advanced prostate cancer association. This difference in associations for overall prostate cancers and advanced prostate cancers implies that results for overall prostate cancers were influenced by screening bias, though we cannot fully rule out etiologic heterogeneity.
The higher risk of melanoma with increased leisure-time physical activity was notable, particularly as this association has only been examined in one prior study. This case-control study found that higher activity levels were associated with a 30% lower melanoma risk41, a finding that our analysis strongly refutes. Of the 12 cohorts we examined, eight found higher activity levels to be associated with at least a 20% higher melanoma risk. Greater incidental sun exposure seems to be the likely reason for this increase in melanoma risk, as physical activity is frequently done outdoors in light clothing and has been associated with substantially increased risk of sun burn42. Moreover, we found that the physical activity—melanoma association was stronger in high UV areas, implying that sun exposure is an important factor underlying this association. Physically active people thus appear to be a vulnerable population for melanoma and cancer prevention efforts focused on physical activity should emphasize sun safety (e.g.,http://www.cancer.org/healthy/besafeinthesun/).
Physical activity’s biological link with cancer has been hypothesized to be mediated through three hormonal systems: sex steroids, insulin and insulin-like growth factors, and adipokines43. Among other evidence for a link between physical activity and these hormonal systems, randomized exercise trials show that randomization to a one year physical activity intervention reduces levels of estrone and estradiol37, 38, and insulin44 in postmenopausal women, with effects mediated, at least in part, through reduced adiposity. Several non-hormonal mechanisms have been hypothesized to link physical activity to cancer risk, including inflammation, immune function, oxidative stress, and for colon cancer, reduced gastrointestinal transit time43. Some of these non-hormonal mechanisms could potentially explain why physical activity was more robustly inversely associated with ER- than ER+ breast cancer. For some physical activity-associated cancers in our study, e.g. esophageal adenocarcinoma or bladder cancer, less is known about the potential mechanisms underlying the physical activity association and our results suggest that further mechanistic research is warranted.
The primary strength of our study is that it is the largest ever conducted on physical activity and cancer risk. This afforded us the statistical precision to examine uncommon and rare cancers that together constitute most incident cancers. Another strength is our consistent methodological approach, including restriction to prospective cohort studies, and leisure-time physical activity, as well as analyzing the same large contrast in physical activity level across studies. This approach minimizes heterogeneity, improves consistency of results, and maximizes power. Finally, our results are not susceptible to publication bias because our analysis is not restricted to published data.
The main limitation of our study is that, in the context of an observational study of lifestyle factors, we cannot fully exclude the possibility that residual confounding by diet, smoking, or other factors may affect our results. However, we did control for many of the known cancer risk factors, and adjusted for diet in sensitivity analyses, with little overall effect on results. We also carefully evaluated effects of adjusting for BMI, and evaluated potential residual confounding by smoking by estimating associations separately in never smokers. We also conducted sensitivity analyses to explore potential screening bias for prostate cancer and the role of sun exposure for melanoma.
An additional limitation is that we used self-reported physical activity, which entails some error in recall. Mitigating this concern is that many physical activity assessments were previously validated and that the discrete, structured nature of leisure-time physical activities makes them comparatively easy to recall45. A further concern is that assessments of physical activity differed somewhat by study; however, for most cancers, results were still highly consistent between studies. Finally, not all cohorts assessed moderate and vigorous intensity activities separately, and several cohorts lacked key details needed to calculate MET-hours/week of physical activity, a measure that would have enabled benchmarking our findings against national guidelines. Our collaborative group will conduct future studies targeting in greater detail the type, intensity, and amount of physical activity needed to reduce overall cancer risk in subsets with the relevant data.
In conclusion, increasing levels of leisure-time physical activity were associated with lower risks of 13 of the 26 cancers we investigated, extending our current evidence-base beyond colon, breast, and endometrial cancers. Furthermore, our results support that these associations are broadly generalizable to different populations, including the overweight or obese, or those with a history of smoking. These findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health (NIH). Details regarding funding for the individual studies are listed in the eAcknowledgments of the online supplement.
Footnotes
Disclosure of potential conflicts of interest: The authors have no conflicts of interest to report.
Reference List
- 1.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2014. 2014 http://wwwcancerorg/research/cancerfactsstatistics/cancerfactsfigures2014/
- 4.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase No 11 Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 5.CDC Exercise or Physical Activity Fastats. [Last accessed 2-22-2016]; [Google Scholar]
- 6.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 7.Lee IM, Manson JE, Ajani U, Paffenbarger RS, Jr, Hennekens CH, Buring JE. Physical activity and risk of colon cancer: the Physicians' Health Study (United States) Cancer Causes Control. 1997;8(4):568–574. doi: 10.1023/a:1018438228410. [DOI] [PubMed] [Google Scholar]
- 8.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–1880. doi: 10.1001/jama.2012.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang SM, Moore SC, Lin J, et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol. 2006;163(2):108–115. doi: 10.1093/aje/kwj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Hakansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst. 2006;98(6):407–413. doi: 10.1093/jnci/djj094. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand JS, Gapstur SM, Campbell PT, Gaudet MM, Patel AV. Recreational physical activity and leisure-time sitting in relation to postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1906–1912. doi: 10.1158/1055-9965.EPI-13-0407. [DOI] [PubMed] [Google Scholar]
- 12.Sinner P, Folsom AR, Harnack L, Eberly LE, Schmitz KH. The association of physical activity with lung cancer incidence in a cohort of older women: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2359–2363. doi: 10.1158/1055-9965.EPI-06-0251. [DOI] [PubMed] [Google Scholar]
- 13.Moore SC, Rajaraman P, Dubrow R, et al. Height, body mass index, and physical activity in relation to glioma risk. Cancer Res. 2009;69(21):8349–8355. doi: 10.1158/0008-5472.CAN-09-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashibe M, Hunt J, Wei M, Buys S, Gren L, Lee YC. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head Neck. 2013;35(7):914–922. doi: 10.1002/hed.23052. [DOI] [PubMed] [Google Scholar]
- 15.Howard RA, Leitzmann MF, Linet MS, Freedman DM. Physical activity and breast cancer risk among pre- and postmenopausal women in the U.S. Radiologic Technologists cohort. Cancer Causes Control. 2009;20(3):323–333. doi: 10.1007/s10552-008-9246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steindorf K, Ritte R, Eomois PP, et al. Physical activity and risk of breast cancer overall and by hormone receptor status: the European prospective investigation into cancer and nutrition. Int J Cancer. 2013;132(7):1667–1678. doi: 10.1002/ijc.27778. [DOI] [PubMed] [Google Scholar]
- 17.Leitzmann MF, Moore SC, Peters TM, et al. Prospective study of physical activity and risk of postmenopausal breast cancer. Breast Cancer Res. 2008;10(5):R92. doi: 10.1186/bcr2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roswall N, Sandin S, Adami HO, Weiderpass E. Cohort Profile: The Swedish Women's Lifestyle and Health cohort. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv089. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. 2008 physical activity guidelines for Americans. Washington, DC: 2008. [Google Scholar]
- 20.World Health Organization. International Classification of Diseases for Oncology. Third. Geneva, Switzerland: 2000. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57(1):289–300. [Google Scholar]
- 23.Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150(2):206–215. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 24.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169(4):391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman and Hall; 1997. [Google Scholar]
- 26.Moore SC, Peters TM, Ahn J, et al. Physical activity in relation to total, advanced, and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2458–2466. doi: 10.1158/1055-9965.EPI-08-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters TM, Schatzkin A, Gierach GL, et al. Physical activity and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):289–296. doi: 10.1158/1055-9965.EPI-08-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behrens G, Jochem C, Keimling M, Ricci C, Schmid D, Leitzmann MF. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol. 2014;29(3):151–170. doi: 10.1007/s10654-014-9895-2. [DOI] [PubMed] [Google Scholar]
- 29.Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer. 2013;108(4):798–811. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. The association between physical activity and bladder cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(7):1862–1870. doi: 10.1038/bjc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jochem C, Leitzmann MF, Keimling M, Schmid D, Behrens G. Physical activity in relation to risk of hematologic cancers: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(5):833–846. doi: 10.1158/1055-9965.EPI-13-0699. [DOI] [PubMed] [Google Scholar]
- 32.Behrens G, Matthews CE, Moore SC, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIH-AARP Diet and Health Study. Eur J Epidemiol. 2013;28(1):55–66. doi: 10.1007/s10654-013-9767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross AJ, Hollenbeck AR, Park Y. A large prospective study of risk factors for adenocarcinomas and malignant carcinoid tumors of the small intestine. Cancer Causes Control. 2013;24(9):1737–1746. doi: 10.1007/s10552-013-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee IM, Djousse L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA. 2010;303(12):1173–1179. doi: 10.1001/jama.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hankinson AL, Daviglus ML, Bouchard C, et al. Maintaining a high physical activity level over 20 years and weight gain. JAMA. 2010;304(23):2603–2610. doi: 10.1001/jama.2010.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ennour-Idrissi K, Maunsell E, Diorio C. Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015;17(1):139. doi: 10.1186/s13058-015-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedenreich CM, Woolcott CG, McTiernan A, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28(9):1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell KL, Foster-Schubert KE, Alfano CM, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30(19):2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 40.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 41.Shors AR, Solomon C, McTiernan A, White E. Melanoma risk in relation to height, weight, and exercise (United States) Cancer Causes Control. 2001;12(7):599–606. doi: 10.1023/a:1011211615524. [DOI] [PubMed] [Google Scholar]
- 42.Holman DM, Berkowitz Z, Guy GP, Jr, Hartman AM, Perna FM. The association between demographic and behavioral characteristics and sunburn among U.S. adults - National Health Interview Survey, 2010. Prev Med. 2014;63:6–12. doi: 10.1016/j.ypmed.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46(14):2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 44.Friedenreich CM, Neilson HK, Woolcott CG, et al. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr Relat Cancer. 2011;18(3):357–369. doi: 10.1530/ERC-10-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews CE, Moore SC, George SM, Sampson J, Bowles HR. Improving self-reports of active and sedentary behaviors in large epidemiologic studies. Exerc Sport Sci Rev. 2012;40(3):118–126. doi: 10.1097/JES.0b013e31825b34a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.