Abstract
Antineuronal autoantibodies are associated with the involuntary movement disorder Sydenham chorea (SC) and paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) which are characterized by the acute onset of tics and/or obsessive compulsive disorder (OCD). In SC and PANDAS, autoantibodies signal human neuronal cells and activate calcium calmodulin-dependent protein kinase II (CaMKII). Animal models immunized with group A streptococcal antigens demonstrate autoantibodies against dopamine receptors and concomitantly altered behaviours. Human monoclonal antibodies (mAbs) derived from SC target and signal the dopamine D2L (long) receptor (D2R). Antibodies against D2R were elevated over normal levels in SC and acute-onset PANDAS with small choreiform movements, but were not elevated over normal levels in PANDAS-like chronic tics and OCD. The expression of human SC-derived anti-D2R autoantibody V gene in B cells and serum of transgenic mice demonstrated that the human autoantibody targets dopaminergic neurones in the basal ganglia and other types of neurones in the cortex. Here, we review current evidence supporting the hypothesis that antineuronal antibodies, specifically against dopamine receptors, follow streptococcal exposures and may target dopamine receptors and alter central dopamine pathways leading to movement and neuropsychiatric disorders.
Keywords: autoimmunity, chorea, dopamine, dopamine receptor, streptococci
Movement, behavioural and neuropsychiatric disorders affect millions worldwide, and there is a growing interest in the association between infections, autoimmunity and behavioural changes, and their impact on the genesis of neuropsychiatric disorders (Murphy et al. 2012). Studies suggesting that infection and antineuronal autoantibodies play a role in the pathogenesis of movement and behavioural disorders began with the studies of Sydenham chorea (SC) and autoantibodies against the brain in rheumatic fever (Zabriskie 1967, 1985, Zabriskie et al. 1970, Husby et al. 1976, Bronze & Dale 1993).
Sydenham chorea is well established as the major neurologic sequelae of Streptococcus pyogenes-induced rheumatic fever (RF) (Stollerman 2001). It is characterized by involuntary movements and neuropsychiatric disturbances, including obsessive–compulsive symptoms which may predate the chorea, hyperactivity, emotional lability, irritability and distractibility (Marques-Dias et al. 1997).
Sydenham chorea is associated with streptococcal pharyngitis (Taranta & Stollerman 1956, Taranta 1959), while tics and obsessive compulsive disorders may be associated with streptococcal and other types of infections (Kurlan & Kaplan 2004, Kurlan et al. 2008, Gause et al. 2009, Murphy et al. 2010), as well as with autoantibodies against neuronal antigens (Swedo et al. 1989, 1993, 1997, 1998, Swedo 1994, Kirvan et al. 2003, 2006a,b, 2007, Brilot et al. 2011). These disorders have been identified as paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) (Swedo et al. 1998) or paediatric acute-onset neuropsychiatric syndrome (PANS) (Swedo et al. 2012) in the presence of other types of infections (Swedo et al. 1997, Swedo & Grant 2005).
To more specifically describe the discovery of PANDAS, Swedo and colleagues identified children who appeared with a sudden acute onset of obsessive compulsive disorder which had a relapsing–remitting course. Five diagnostic criteria were reported from the first 50 cases: (i) presence of obsessive–compulsive disorder (by DSM criteria) or a tic disorder; (ii) symptom onset between the ages of 3 years and puberty; (iii) episodic symptoms, with abrupt and substantial symptom exacerbations; (iv) symptom onset and exacerbations associated temporally with group A streptococcal infections; and (v) presence of neurologic abnormalities during symptom exacerbations (Swedo et al. 1998). These cases also displayed piano playing choreiform movements of the fingers and toes.
Murphy also described acute-onset OCD/tics with severe hyperactivity, loss of fine motor skills (handwriting deterioration) or choreiform movements (Murphy et al. 2012). Psychiatric symptoms described by Murphy et al. included irritability, frequent mood changes, separation anxiety, hyperactivity, late-onset attention problems, personality change, oppositional behaviours, sleep disturbances and deterioration in mathematical skills. Historical accounts from the first 50 cases of PANDAS indicate that at least some cases with PANDAS were immediately following or during a group A streptococcal infection (Swedo et al. 1998), but it is still questioned whether group A streptococcal infections are coincidental or causal, or whether PANDAS could be a variant of acute rheumatic fever (Kurlan et al. 2008).
Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections have been proposed to develop due to post-infectious autoimmune processes (Swedo et al. 1998, Kirvan et al. 2006b). Thus, as in acute rheumatic fever, antibodies against the group A streptococcus would cross-react with brain antigens as their neuronal targets in susceptible hosts, by the process of molecular mimicry (Kirvan et al. 2003). The pathogenesis of PANDAS could be similar to Sydenham chorea where autoantibodies against neuronal cells in the basal ganglia may lead to neuropsychiatric and altered movement symptoms.
In the past, the basal ganglia has been implicated as a target of post-streptococcal immune responses (Swedo et al. 1993, Giedd et al. 1995). Sydenham chorea pathogenesis has been proposed to be an autoantibody-mediated disease with basal ganglia dysfunction where antibodies have an affinity for basal ganglia (Husby et al. 1976, Giedd et al. 1995), and anti-inflammatory treatments such as steroids, plasmaphoresis and intravenous immunoglobulin treatment are effective (Perlmutter et al. 1999). Plasmaphoresis has been found to reduce symptoms of Sydenham chorea and obsessive compulsive symptoms in PANDAS, which suggests that the removal of antineuronal autoantibodies abrogates symptoms (Swedo 1994, Perlmutter et al. 1999).
Antineuronal antibody titres have been associated with both severity and duration of choreic episodes in Sydenham chorea, and antibrain antibodies have been found in stained neurones in the basal ganglia (Husby et al. 1976, Bronze & Dale 1993). Husby et al. (Husby et al. 1976) found that chorea patient sera strongly reacted with cytoplasmic, but not nuclear, antigens in human caudate and subthalamic nuclei as well as cerebral cortex neurones. Autoantibodies, such as antilysoganglioside (LGN) GM1 (Kirvan et al. 2003, 2006b) and anti-β tubulin (Kirvan et al. 2006a), have been described in Sydenham chorea. Certainly, the removal of antibody by immunomodulatory therapies such as plasma exchange suggests that chorea may be caused by a pathogenic antibody response (Swedo 1994), and symptoms improved following plasmaphoresis (Allen et al. 1995, Perlmutter et al. 1999). PANDAS may also be associated with antineuronal antibody responses that may attack the basal ganglia (Singer et al. 2004, Kirvan et al. 2006b) and result in an abnormal brain MRI (Giedd et al. 2000).
The introduction has outlined the above evidence, suggesting that Sydenham chorea and potentially PANDAS are linked to autoantibodies against the brain. The antineuronal autoantibodies observed in acute episodes of Sydenham chorea and PANDAS are elevated during the acute stage of disease and decrease dramatically during the convalescent stages along with a reduction in symptoms as has been shown by Kirvan et al. in studies of human sera from Sydenham chorea (Kirvan et al. 2003) and PANDAS (Kirvan et al. 2006b). These studies also demonstrated IgG antineuronal antibody present in cerebrospinal fluid which reacted with human basal ganglia. The IgG antibodies reacted with lysoganglioside which is enriched in brain and present in the membranes and on the surface of human neuronal cells. Patients with the acute-onset type of PANDAS as well as Sydenham chorea respond positively to plasmaphoresis (Perlmutter et al. 1999), which also supports the hypothesis that antibodies may play a role in the disease. Intravenous immunoglobulin also led to a reduction in symptoms as well. Studies of human sera indicate that antineuronal antibodies including antilysoganglioside, antitubulin and antidopamine receptor antibodies are elevated during disease and are reduced in convalescence (Kirvan et al. 2003). A study by Ben-Pazi et al. (Ben-Pazi et al. 2013) has shown that the symptoms in Sydenham chorea directly correlate with the ratio of antibodies against the D1 and D2 receptors.
The antineuronal antibodies have also been found to signal human neuronal cells through the calcium calmodulin protein kinase II (CaMKII) activation which potentially would lead to increased dopamine release. To prove that these types of antibodies are present in Sydenham chorea which react with brain and the group A streptococcus, we produced human mAbs derived from Sydenham chorea and used them to study the mechanisms and the in vivo targets in the brain. The studies of chorea-derived human mAb 24.3.1 are described below as well as the transgenic mouse created to express this human antibody. The Tg mouse was used to empathize the brain regions targeted by the human antibody from chorea which signalled CaMKII and also reacted with lysoganglioside and the dopamine receptors D1 and D2.
Animal models will also be discussed in our review and further support the hypothesis that the antibodies are linked to symptoms (Hoffman et al. 2004, Yaddanapudi et al. 2010, Brimberg et al. 2012, Lotan et al. 2014a,b). These animal models include streptococcal exposure and immunization which led to the production of antibodies that deposited in the brain and also led to movement and neuropsychiatric symptoms in the mice or rats. The models were also recapitulated in passive antibody transfer experiments. These studies also further support the molecular mimicry hypothesis where the antibodies produced against the group A streptococcus target the brain.
Sydenham chorea-derived human mAbs
The hypothesis that Sydenham chorea may develop due to autoantibodies and inflammation which affect neuronal signalling mechanisms has been supported from studies of chorea-derived human monoclonal antibodies (mAbs) (Kirvan et al. 2003). Choreaderived human mAb 24.3.1 reacted with group A streptococcal carbohydrate epitope N-acetyl-β–D-glucosamine (GlcNAc) and neuronal surface antigen lysoganglioside GM1 and alpha helical intracellular brain protein tubulin (Kirvan et al. 2003, 2007) in what is called cross-reactivity or molecular mimicry, where antigenic structures are similar between host and microbe (Cunningham 2012, 2014).
The human chorea-derived mAb 24.3.1 induced antibody-mediated neuronal cell signalling by activating Ca++/calmodulin-dependent protein kinase (CaM kinase) II activity in a human neuronal cell line SK-N-SH (Kirvan et al. 2003), and intrathecal passive transfer of mAb 24.3.1 into Lewis rats induced elevated tyrosine hydroxylase in rat brain (Kirvan et al. 2006a). Sera and cerebrospinal fluid (CSF) IgG from Sydenham chorea demonstrated the same antigen cross-reactivities as well as similar neuronal cell signalling CaMKII activation in vitro (Kirvan et al. 2003). Our studies have shown Sydenham chorea and PANDAS IgG as well as chorea-derived mAb 24.3.1 all activated CaMKII in a human neuronal cell line, leading to tyrosine hydroxylase activation and subsequent dopamine release (Kirvan et al. 2003, 2006a,b). Tyrosine hydroxylase is the rate-limiting enzyme in dopamine synthesis, and was increased in the brain of Lewis rats after intrathecal infusion of mAb 24.3.1 (Kirvan et al. 2006a). Signalling properties of sera can be abrogated by the removal of the IgG (Brimberg et al. 2012), demonstrating that the CaMKII activation is associated with serum IgG.
Our evidence demonstrated that human choreaderived mAb 24.3.1 was similar to the IgG reactivity of Sydenham chorea sera not only from the mAb donor, but also from other cases of Sydenham chorea as well. Antibody-mediated cell signalling of the human neuronal cell line SK-N-SH appeared to cause excess dopamine release as shown in tritiated thymidine release assays from chorea-derived mAb and chorea serum-treated human SK-N-SH neuronal cells (Kirvan et al. 2006a). These data suggest that a possible alteration of central dopamine pathways may be involved in the immunopathogenesis of SC. Dopamine involvement in the disease is relevant and fulfils an important role in the pathophysiology of chorea, as treatment of Sydenham chorea has in the past relied on the use of antidopaminergic drugs (Shannon & Fenichel 1990, Sokol 2000, Dale 2005, Demiroren et al. 2007, Moretti et al. 2008).
Dopamine is the main catecholamine neurotransmitter in the central nervous system (CNS) and is synthesized from tyrosine via tyrosine hydroxylase and stored in vesicles in axon terminals. Dopaminergic signalling is a balance between dopamine release and re-uptake by the pre-synaptic terminal. The five dopamine receptor types are divided into two families: D1-like (stimulatory) and D2-like (inhibitory), both of which act through G-coupled protein receptors (Pollack 2004, Pivonello et al. 2007). Dopamine plays a pivotal role in modulating the functioning of the basal ganglia brain circuitry (Gerfen et al. 1990, Graybiel 2000, Pivonello et al. 2007), and abnormalities in the functioning of this neurotransmitter have been implicated in disorders like Sydenham chorea (Harris & Singer 2006). The dopamine receptors are associated with the regulation of the second messenger cAMP through G protein-mediated signalling (Beaulieu & Gainetdinov 2011). D1 receptors typically are associated with an increase in cAMP, but D2 receptors are inhibitory, with a decrease in cAMP, as was shown for mAb 24.3.1 as well as Sydenham chorea and PANDAS sera. D2 receptors are generally more complex than D1 because they are expressed pre- and post-synaptically (Beaulieu & Gainetdinov 2011).
CAM kinase activation has been reported in association with heterodimers of dopamine receptors containing both D1 and D2 (Hasbi et al. 2011). Our recent report indicates that the ratio of antibodies against both D1R and D2R were correlated with the USCRS symptom scale of Sydenham chorea. We investigated further the possibility that dopamine receptors such as the D1 and D2 receptors may be involved in disease (Cox et al. 2013, 2015). Studies using the membrane antigen targets in an ELISA as well as dopamine receptor signalling assays in transfected cell lines and FLAG-tagged epitopes on the D2 dopamine receptor all confirmed the reaction of mAb 24.3.1, and IgG from chorea serum and CSF additionally confirmed reactivity with the D2 dopamine receptor (Cox et al. 2013). The D2R might be expected to be involved in Sydenham chorea as haloperidol, a D2R blocker, is used to treat symptoms of Sydenham chorea (Shannon & Fenichel 1990, Sokol 2000, Dale 2005, Demiroren et al. 2007, Moretti et al. 2008).
Sydenham chorea-derived mAb 24.3.1 and SC IgG reacted with FLAG-tagged D2R, while PANDAS IgG did not (Cox et al. 2013). However, human chorea mAb 24.3.1 and sera from the human SC mAb B cell donor as well as PANDAS sera all induced inhibitory signalling of D2R, which may be the consequence of antibody targeting of dopaminergic neurones (Cox et al. 2013). Our data support the hypothesis that in human disease, antibodies against D2R in SC or PANDAS with fine choreiform movements may target dopaminergic neurones in the basal ganglia and cortex and alter brain function to contribute to movement and behavioural disorders. Fine choreiform movements were reported in 95% of the first 50 cases of PANDAS (Swedo et al. 1998). Our novel findings suggest that autoantibodies which may signal neurones in chorea and other movement and behavioural disorders may signal through dopamine receptors.
Chorea-derived antibody targets dopaminergic neurones in basal ganglia in transgenic mice
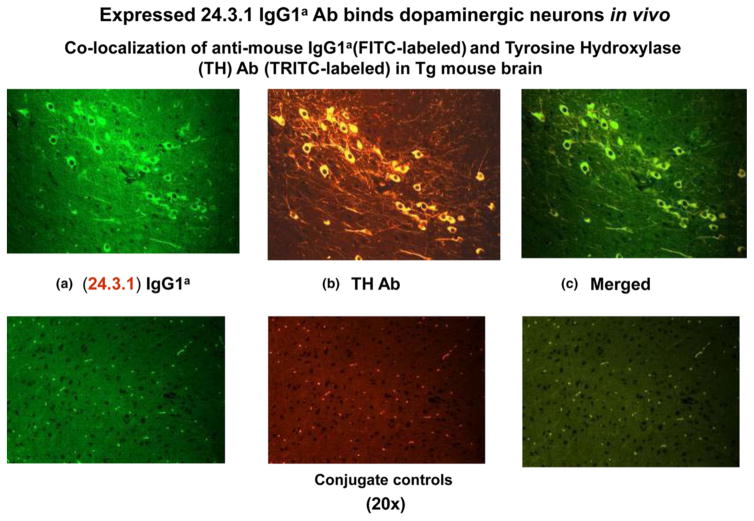
To study the in vivo deposition of chorea-derived human mAb 24.3.1, we created transgenic (Tg) mice expressing chorea-derived human mAb 24.3.1 heavy and light chain variable region (VH and VL) genes as part of a chimeric (human V gene/mouse constant region) IgG1a antibody (Cox et al. 2013). Chimeric Tg antibody 24.3.1 was expressed by B cells and was present in serum of the Tg-24.3.1 mice. Tg serum Ab and IgG1a hybridoma mAbs produced from the Tg-24.3.1 mice demonstrated the same strong reactivity with D2R as human SC-derived mAb 24.3.1. Our Tg-24.3.1-IgG1a transgenic mice were treated with lipopolysaccharide (LPS) and streptococcal cell walls to open the blood–brain barrier (BBB), thus allowing antibody penetration of the brain. To determine where chimeric Tg 24.3.1-IgG1a antibody (IgG1a) was localized in the brain in vivo, we investigated tissue sections of brains from Tg VH24.3.1 mice. Anti-IgG1a allotypic Ab detected Tg24.3.1-IgG1a within neuronal cell bodies in the basal ganglia, most likely the substantia nigra or ventral tegmental area (Fig. 1). Antibody to tyrosine hydroxylase (stained in Fig. 1b Yellow/TRITC) was used to identify dopaminergic neurones of Tg mouse brain because tyrosine hydroxylase converts tyrosine to dopamine and is a marker for dopaminergic neurones. As shown in Figure 1, the Tg-24.3.1 chimeric IgG1a antibody penetrated the brain and was located in dopaminergic neurones in the basal ganglia of Tg mice (green/FITC Fig. 1a), most likely in the substantia nigra or ventral tegmental area (Cox et al. 2013). Tg-24.3.1-IgG1a antibody was also associated with neurones in the cortex (Cox et al. 2013). It is worth noting that the Tg-24.3.1-IgG1a was found within the cytoplasm of dopaminergic neurones in vivo sparing the nucleus and was similar in appearance to what was observed in actual disease sections of human brain taken from Sydenham chorea (Husby et al. 1976). Colocalization studies gave the appearance that in vivo Tg-24.3.1-IgG1a antibody was internalized within TH+ neuronal cells in cross sections of the Tg mouse basal ganglia. Internalization might be expected as previous studies have shown that Abs to neuronal receptors can be internalized (Hughes et al. 2010), and it is well established that dopamine receptors undergo recycling (Parton & Richards 2003, Sudhof 2004, Wolstencroft et al. 2007). The substantia nigra and ventral tegmental area are part of the basal ganglia and contain dopaminergic neuronal cell bodies with axons which project to the striatum. D2 receptors are expressed on the dopaminergic neurones in the substantia nigra and ventral tegmental area as well as on neurones in the striatum (Gerfen et al. 1995, Kawaguchi et al. 1995, Surmeier et al. 2011, Anzalone et al. 2012). However, dopaminergic neuronal cell bodies are only seen in the substantia nigra and ventral tegmental area and the axons of these cells project into the striatum. Requirements for BBB penetration were established previously by Kowal (DeGiorgio et al. 2001, Kowal et al. 2004, 2006). Tg24.3.1-IgG1a antibody-producing mice not receiving the BBB stimulants did not have Tg24.3.1-IgG1a antibody in the brain. This evidence supports the hypothesis that infections would not only promote antibody production but also have the potential to break the BBB. Disease such as Sydenham chorea and PANDAS are associated with group A streptococcal infections and are considered sequelae of the suppurative infection.
Figure 1.
Human Sydenham chorea 24.3.1 V gene expressed as human V gene-mouse. IgG1a constant region in transgenic (Tg) mice targets dopaminergic neurons. Chimeric Tg24.3.1 VH IgG1a Ab expressed in Tg mouse sera penetrated dopaminergic neurons in Tg mouse brain in vivo. Colocalization of Tg 24.3.1 IgG1a (anti-IgG1a Ab, green, left panel) and tyrosine hydroxylase antibody (anti-TH Ab, yellow, middle panel). TH is a marker for dopaminergic neurons. Left panel shows IgG1a (FITClabelled), centre panel shows TH Ab (TRITC-labelled), and right panel is merged image (FITC-TRITC). Brain sections (basal ganglia) of VH24.3.1 Tg mouse (original magnification 320), showing FITC-labeled anti-mouse IgG1a (a), TRITC-labelled anti-TH Ab (b) and merged image (c). Colocalization by double immunostaining used Abs conjugated to FITC and TRITC. For localization of chimeric Tg24.3.1 VH IgG1a, primary Ab was biotin-conjugated mouse anti-mouse IgG1a (BD Pharmingen) which was used in combination with FITC-conjugated streptavidin (Invitrogen). For immunostaining of dopaminergic neurons, rabbit polyclonal Ab (Abcam) was used as primary Ab with TRITC-conjugated sheep anti-rabbit Ab as the secondary Ab (Sigma). Conjugate controls, secondary Ab controls (secondary Ab, no primary Ab) shown: FITC-conjugated streptavidin (1 : 20) (left panel) and TRITC-conjugated sheep anti-rabbit Ab (1 : 100) (middle panel); right panel is merged image (FITC-TRITC) (Cox et al. 2013).
Mere cross-reactivity by antineuronal autoantibodies alone may not lead to disease, as suggested by the results with normal sera and other mAbs which do not signal human neuronal cells even though they may react with a neuronal antigen. However, when antibody avidity increases and leads to signalling of a receptor, the antibody may lead to symptoms. The similarity in antigen specificity but differences in signalling of D2R by a variety of mAbs in our studies has illustrated this principle that cross-reactivity by itself may not necessarily lead to signalling of a receptor (Kirvan et al. 2003, Cox et al. 2013).
Animal models
Animal models have supported the hypothesis that streptococcal and potentially other infections may lead to the development of antineuronal autoantibodies which can enter the brain and alter behaviour and movement. Hoffman et al. immunized mice with group A streptococcal antigen and found serum antibodies that deposited in brain tissues and reacted with deep cerebellar nuclei (DCN) (Hoffman et al. 2004). The anti-DCN antibodies were present in group A streptococcal immunized mice that developed behaviour and movement disturbances (Hoffman et al. 2004). Yaddanapudi et al. further demonstrated that antibodies from streptococcal immunized mice passively transferred the motor and behavioural alterations to naïve mice after the blood–brain barrier (BBB) was broken with lipopolysaccharide (Yaddanapudi et al. 2010). Infections themselves may be effective in opening the BBB. Brimberg et al. pioneered the studies of behaviour changes in the male Lewis rat after immunization to group A streptococcal antigens (Brimberg et al. 2012). Exposure of the Lewis rat to group A streptococcal antigens led to impaired food handling and impaired narrow, but not wide, beam walking by the rats, and rats obsessively groomed significantly longer than control rats after treatment with water mist. Serum IgG from the Lewis rats activated CaMKII signalling in a human neuronal cell line SK-N-SH similar to CaMKII activation by PANDAS or Sydenham chorea serum (Brimberg et al. 2012). Absorption of IgG from the serum abrogated the CaMKII signalling and activation. Serum IgG from the immunized rats reacted with human D1 and D2 dopamine receptor antigen (50KD) in Western immunoblots compared with no reactivity in control rat sera (Brimberg et al. 2012). Impaired food manipulation and obsessive grooming in group A streptococcal immunized rats were alleviated by treatment with the D2 blocker haloperidol or the selective serotonin reuptake inhibitor paroxetine, which are used to treat motor and compulsive symptoms in SC and PANDAS respectively (Brimberg et al. 2012).
Further studies in the male Lewis rat model demonstrated that when serum IgG purified from group A streptococcal immunized rats was infused into the striatum of naïve rats over a 21-day period, the rats demonstrated difficulty in walking towards a narrow beam similar to the streptococcal immunized rats and demonstrated obsessive marble burying (Lotan et al. 2014a) when compared to adjuvant control rats and naïve control rats. Thus, infusion of purified IgG from streptococcal exposed rats intrathecally into naïve rats led to behavioural and motor alterations similar to those seen in streptococcal immunized rats.
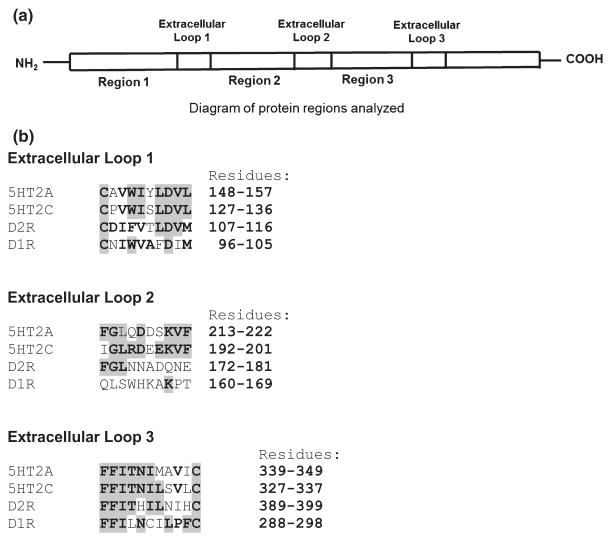
In the study by Lotan et al., serum antibody (IgG) confirmed reactivity with the D1 and D2 dopamine receptors and demonstrated for the first time the reactivity of antibodies (IgG) from streptococcal immunized mice with serotonin receptors 5HT-2A and 5HT-2C in Western immunoblots (Lotan et al. 2014a). Protein sequence similarities were found between dopamine receptors and serotonin receptors (Fig. 2). Sequence identity was found between the dopamine D1 and D2 receptors and the serotonin receptors, 5HT-2A and 2C, including three extracellular regions (loops) of D2R (Fig. 2a,b). Of the three extracellular loop regions, extracellular loop 1 (10 residues) (Fig. 2b) had the highest overall per cent sequence identity (76%) among the above receptors. Serotonin receptors 5HT-2A and 2C had the highest per cent identity (80%). Extracellular loop 3 (11 residues) (Fig. 2b) had 71% sequence identity among the receptors, with 5HT-2A and 2C again sharing the greatest per cent identity (73%) among the receptors. Overall per cent sequence identity of extracellular loop 2 (10 residues) (Fig. 2b) among all receptors analysed was 44% (60% between 5HT-2A and 2C). The finding of high sequence identity between the dopamine receptors and serotonin receptors in three extracellular regions of the receptors suggests that the same antibodies could bind to the four receptors. It is therefore possible that the antibody reactivity against D2 dopamine receptors found in GAS-related neuropsychiatric disorders might also react with other receptors sharing similar structures, thus altering not only the functioning of the dopaminergic system but also the functioning of the serotonergic system.
Figure 2.
Protein sequence similarities between dopamine D1 and D2 receptors and serotonin receptors. Protein sequence alignments were performed between 5HT-2A and 2C serotonin receptors and dopamine receptors D1 and D2. (a) is a diagram of protein regions analyzed, including three extracellular regions (extracellular loop 1, extracellular loop 2, extracellular loop 3) of D2R. (b) shows residues analysed in each extracellular loop (1, 2 and 3). Identical residues are indicated in bold and highlighted (grey). Protein sequence alignments were performed using the basic local alignment search tool analysis program (BLAST) (National Center for Biotechnology Information) and WUR MUSCLE multiple alignment analysis program (www.bioinformatics.nl/tools/muscle.html).
Most importantly in the infused rat animal model, IgG deposited in vivo in striatum of infused rats and colocalized with dopamine receptors, the serotonin transporter and other neuronal proteins. The rat animal model suggested that the antibodies in streptococcal immunized rats target neuronal cells in the brain, colocalize with dopamine receptors and potentially cause behavioural or movement alterations (Lotan et al. 2014a).
Evidence is accumulating which reinforces the link between exposure to group A streptococcal antigen and alteration of central dopamine pathways and associated movement and behavioural disorders.
Anti-dopamine receptor autoantibodies in Sydenham chorea, PANDAS and related disorders
Several studies provide supportive evidence for antineuronal autoantibodies in PANDAS, Sydenham chorea and related disorders such as Tourette syndrome (Husby et al. 1976, Kiessling et al. 1993, 1994, Singer et al. 1998). Successful immunosuppressive treatments including plasmaphoresis, intravenous immunoglobulin (IVIG) and prednisone, which have been reported to result in suppression of acute childhood OCD and Tourette syndrome, support the autoantibody hypothesis (Kondo & Kabasawa 1978, Matarazzo 1992, Swedo 1994, Allen et al. 1995, Perlmutter et al. 1999). The search for neuronal antibody targets has been ongoing for some time and is reported in the literature (Husby et al. 1976, Church et al. 2002, 2003, Kirvan et al. 2003, 2006b, Singer et al. 2005, Dale et al. 2006, 2012, Pavone et al. 2006, Brilot et al. 2011, Mohammad et al. 2013, Pathmanandavel et al. 2013, Ramanathan et al. 2013). Most recently, the dopamine D1 and D2 receptors were reported in Sydenham chorea and PANDAS (Brimberg et al. 2012, Cox et al. 2013) and by Ben Pazi et al. where the ratio of anti-D1/anti-D2 receptor antibodies correlated with USCRS symptoms (Ben-Pazi et al. 2013). These antibody-targeted antigens namely, dopamine receptors D1R and D2R, are key components in the regulation of the dopaminergic pathways which are considered to be the source of the chorea and behavioural symptoms. Consequently, both are successfully targeted with antidopaminergic drugs (Shannon & Fenichel 1990, Sokol 2000, Dale 2005, Demiroren et al. 2007, Moretti et al. 2008).
Children with PANDAS-like conditions can be categorized into at least 2 groups: those with abnormally elevated antibodies against the D2L receptor (Brimberg et al. 2012, Cox et al. 2013), which include Sydenham chorea and the first 50 cases of reported PANDAS (Swedo et al. 1998), and a second group without abnormally elevated autoantibodies against the D2L receptor. In the second group with chronic tics and/or OCD, antibodies may be abnormally elevated against D1R and/or lysoganglioside antigens (Cox et al. 2015, Singer et al. 2015). Both groups have markedly elevated activity in serum which signals the CaMKII in human neuronal cells. In our study of over 260 children, sera IgG from chronic OCD, tics or both reacted significantly with human D1 receptor or lysoganglioside antigens and had significantly elevated CaMKII activation compared with normal controls (Cox et al. 2015). Chronic tics and OCD did not react above normal levels with D2L receptor (Cox et al. 2015, Singer et al. 2015). This is in contrast to cases of PANDAS with fine piano playing choreiform movements (Swedo et al. 1998) which demonstrate abnormally elevated anti-D2R antibodies. In our group of over 260 youth and young adults, chronic OCD and/or tics was positively associated with streptococcal infection status (Cox et al. 2015) and with autoantibodies against neuronal antigens, D1R and lysoganglioside. The antibody-mediated neuronal cell signalling of CaMKII activation in human neuronal cells in these diseases suggests that the antibodies may target the receptors and alter dopamine neurotransmission leading to neuropsychiatric symptoms.
Finally, as Sydenham chorea is the established neurologic manifestation of group A streptococcal induced rheumatic fever, it may be a prototype for other group A streptococcal or infection-related movement and neuropsychiatric conditions. Evidence suggests that human anti-D2R titres in Sydenham chorea correlated with antistreptolysin O titres, a common serologic test for diagnosis of prior group A streptococcal infection, which strengthens the hypothesis that the streptococcal infection may lead to anti-D2R antibody production ultimately causing clinical symptoms (Ben-Pazi et al. 2013). Furthermore, evidence from animal models supports the hypothesis that streptococcal infections are associated with the induction of antibodies against the dopamine receptors in human disease.
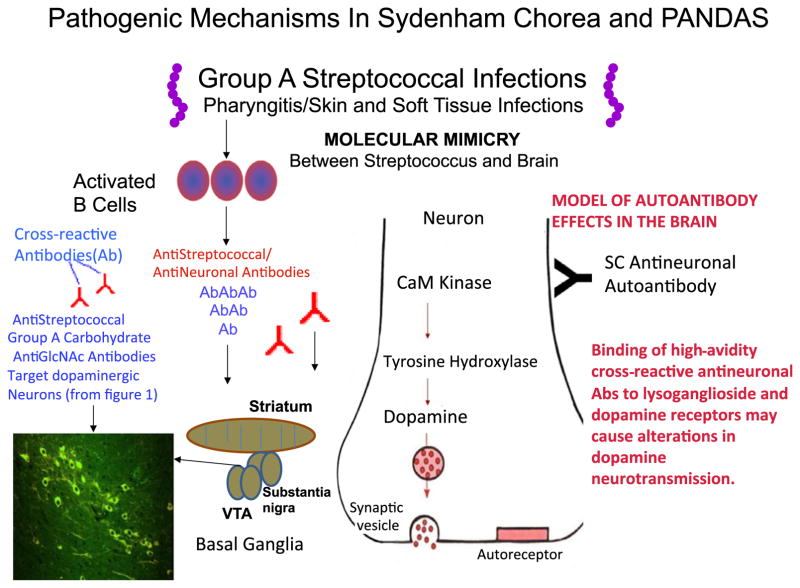
Figure 3 summarizes the evidence for the role of streptococcal infections in the induction of antibodies that may lead to movement or neuropsychiatric disorders. The pathogenesis of Sydenham chorea and PANDAS begins with an infection with group A streptococci in the throat or soft tissues or skin. The susceptible host may produce antibodies which react with brain tissues and in particular the dopamine receptors or neural antigens such as tubulin or lysoganglioside (Kirvan et al. 2003, 2006a,b, 2007)(Ben-Pazi et al. 2013, Cox et al. 2013). Antibodies target primarily the basal ganglia, in particular the ventral tegumental area or the substantia nigra which project to the striatum. The mechanism of the antibodies as shown would signal the neuronal cell through activation of CaMKII and lead to excess dopamine release. Binding of high-avidity cross-reactive antistreptococcal/antineuronal antibodies to lysoganglioside or dopamine receptors on the surface of neurones may lead to alterations in dopamine transmission. Previous studies suggest that intrathecal passive transfer of antibodies leads to increased tyrosine hydroxylase activity in neurones which is in the CamKII pathway as shown in Figure 3 and also leads to neuropsychiatric symptoms in the animals receiving the passive transfer of antibody (Kirvan et al. 2006a).
Figure 3.
Summary of evidence for the role of streptococcal infections in the induction of antibodies that may lead to movement or neuropsychiatric disorders. The pathogenesis of Sydenham chorea and PANDAS begins with an infection with group A streptococci in the throat or soft tissues or skin. The susceptible host may produce antibodies which react with brain tissues and in particular the dopamine receptors or neural antigens such as tubulin or lysoganglioside (Kirvan et al. 2003, 2006a,b, 2007, Ben-Pazi et al. 2013, Cox et al. 2013). Antibodies target primarily the basal ganglia, in particular the ventral tegumental area or the substantia nigra which project to the striatum. The mechanism of the antibodies as shown would signal the neuronal cell through activation of CaMKII and lead to excess dopamine release. Binding of high-avidity cross-reactive antistreptococcal/antineuronal antibodies to lysoganglioside or dopamine receptors on the surface of neurons may lead to alterations in dopamine transmission.
Future and ongoing studies will investigate the possible receptors and mechanisms which may directly affect antibody-mediated CaMKII activation in human neuronal cells and the role of other types of receptors such as the serotonin receptor which may play a role in disease as well as the dopamine receptors and the autoantibodies that signal them. Studies already in progress suggest that antibody binding to the dopamine D1 receptor in transfected cells leads to sensitization of the receptor to the neurotransmitter dopamine (J. Zuccolo, E.V. Edwards, A.J. Zuccolo, A.I. Mascaro-Blanco, H. Ben-Pazi, S.E. Swedo & M.W. Cunningham, Manuscript in preparation). In addition, further studies of the role and effects of cytokines and T cells and their responses on the brain will all be important areas for future studies. Affects of the immune system on the brain may not be limited to the neurones directly, but may also affect microcirculation and blood vessels in the brain and lead to damage to the brain due to vasculitis and fibrosis with blockage of the blood supply to certain regions of the brain.
Footnotes
Conflict of interest
Madeleine Cunningham is the chief scientific officer and co-founder with financial interest in Moleculera Labs, a commercial laboratory for diagnostic testing of antineuronal antibodies. Carol J. Cox declares financial interest in Moleculera Labs.
References
- Allen AJ, Leonard HL, Swedo SE. Case study: a new infection-triggered, autoimmune subtype of pediatric OCD and Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1995;34:307–311. doi: 10.1097/00004583-199503000-00015. [DOI] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, Caron MG, Bonci A, Sulzer D, Borrelli E. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32:9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Ben-Pazi H, Stoner JA, Cunningham MW. Dopamine receptor autoantibodies correlate with symptoms in Sydenham’s Chorea. PLoS ONE. 2013;8:e73516. doi: 10.1371/journal.pone.0073516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilot F, Merheb V, Ding A, Murphy T, Dale RC. Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology. 2011;76:1508–1513. doi: 10.1212/WNL.0b013e3182181090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, Klein J, Moses AE, Somnier FE, Leckman JF, Swedo SE, Cunningham MW, Joel D. Behavioral, Pharmacological, and Immunological Abnormalities after Streptococcal Exposure: A Novel Rat Model of Sydenham Chorea and Related Neuropsychiatric Disorders. Neuropsychopharmacology. 2012;37:2076–2087. doi: 10.1038/npp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronze MS, Dale JB. Epitopes of streptococcal M proteins that evoke antibodies that cross-react with human brain. J Immunol. 1993;151:2820–2828. [PubMed] [Google Scholar]
- Church AJ, Cardoso F, Dale RC, Lees AJ, Thompson EJ, Giovannoni G. Anti-basal ganglia antibodies in acute and persistent Sydenham’s chorea. Neurology. 2002;59:227–231. doi: 10.1212/wnl.59.2.227. [DOI] [PubMed] [Google Scholar]
- Church AJ, Dale RC, Lees AJ, Giovannoni G, Robertson MM. Tourette’s syndrome: a cross sectional study to examine the PANDAS hypothesis. J Neurol Neurosurg Psychiatry. 2003;74:602–607. doi: 10.1136/jnnp.74.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CJ, Sharma M, Leckman JF, Zuccolo J, Zuccolo A, Kovoor A, Swedo SE, Cunningham MW. Brain human monoclonal autoantibody from Sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J Immunol. 2013;191:5524–5541. doi: 10.4049/jimmunol.1102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CJ, Zuccolo AJ, Edwards EV, Mascaro-Blanco A, Alvarez K, Stoner J, Chang K, Cunningham MW. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2015;25:76–85. doi: 10.1089/cap.2014.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012;24:408–416. doi: 10.1097/BOR.0b013e32835461d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW. Rheumatic fever, autoimmunity, and molecular mimicry: the streptococcal connection. Int Rev Immunol. 2014;33:314–329. doi: 10.3109/08830185.2014.917411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC. Post-streptococcal autoimmune disorders of the central nervous system. Dev Med Child Neurol. 2005;47:785–791. doi: 10.1017/S0012162205001647. [DOI] [PubMed] [Google Scholar]
- Dale RC, Candler PM, Church AJ, Wait R, Pocock JM, Giovannoni G. Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J Neuroimmunol. 2006;172:187–197. doi: 10.1016/j.jneuroim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, Ben-Pazi H, Varadkar S, Aumann TD, Horne MK, Church AJ, Fath T, Brilot F. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- Demiroren K, Yavuz H, Cam L, Oran B, Karaaslan S, Demiroren S. Sydenham’s chorea: a clinical follow-up of 65 patients. J Child Neurol. 2007;22:550–554. doi: 10.1177/0883073807302614. [DOI] [PubMed] [Google Scholar]
- Gause C, Morris C, Vernekar S, Pardo-Villamizar C, Grados MA, Singer HS. Antineuronal antibodies in OCD: comparisons in children with OCD-only, OCD+chronic tics and OCD+PANDAS. J Neuroimmunol. 2009;214:118–124. doi: 10.1016/j.jneuroim.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Kruesi MJ, Parker C, Schapiro MB, Allen AJ, Leonard HL, Kaysen D, Dickstein DP, Marsh WL. Sydenham’s chorea: magnetic resonance imaging of the basal ganglia. Neurology. 1995;45:2199–2202. doi: 10.1212/wnl.45.12.2199. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157:281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Harris K, Singer HS. Tic disorders: neural circuits, neurochemistry, and neuroimmunology. J Child Neurol. 2006;21:678–689. doi: 10.1177/08830738060210080901. [DOI] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain. 2011;4:26. doi: 10.1186/1756-6606-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci. 2004;24:1780–1791. doi: 10.1523/JNEUROSCI.0887-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balice-Gordon RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G, van de Rijn I, Zabriskie JB, Abdin ZH, Williams RC. Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med. 1976;144:1094–1110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies in movement disorders. Pediatrics. 1993;92:1788–1791. [PubMed] [Google Scholar]
- Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies – tics and obsessive-compulsive symptoms. J Dev Behav Pediatr. 1994;15:421–425. [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity. 2006a;39:21–29. doi: 10.1080/08916930500484757. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006b;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Cox CJ, Swedo SE, Cunningham MW. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J Immunol. 2007;178:7412–7421. doi: 10.4049/jimmunol.178.11.7412. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kabasawa T. Improvement in Gilles de la Tourette syndrome after Corticosteroid-therapy. Ann Neurol. 1978;4:387. doi: 10.1002/ana.410040423. [DOI] [PubMed] [Google Scholar]
- Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, Diamond B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics. 2004;113:883–886. doi: 10.1542/peds.113.4.883. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Johnson D, Kaplan EL, Group TSS. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: a prospective blinded cohort study. Pediatrics. 2008;121:1188–1197. doi: 10.1542/peds.2007-2657. [DOI] [PubMed] [Google Scholar]
- Lotan D, Benhar I, Alvarez K, Mascaro-Blanco A, Brimberg L, Frenkel D, Cunningham MW, Joel D. Behavioral and neural effects of intra-striatal infusion of anti-streptococcal antibodies in rats. Brain Behav Immun. 2014a;38:249–262. doi: 10.1016/j.bbi.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan D, Cunningham M, Joel D. Antibiotic treatment attenuates behavioral and neurochemical changes induced by exposure of rats to group a streptococcal antigen. PLoS ONE. 2014b;9:e101257. doi: 10.1371/journal.pone.0101257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Dias MJ, Mercadante MT, Tucker D, Lombroso P. Sydenham’s chorea. Psychiatr Clin North Am. 1997;20:809–820. doi: 10.1016/s0193-953x(05)70346-4. [DOI] [PubMed] [Google Scholar]
- Matarazzo E. Tourette’s syndrome treated with ACTH and prednisone: report of two cases. J Child Adolesc Psychopharmacol. 1992;2:215–226. doi: 10.1089/cap.1992.2.215. [DOI] [PubMed] [Google Scholar]
- Mohammad SS, Ramanathan S, Brilot F, Dale RC. Autoantibody-associated movement disorders. Neuropediatrics. 2013;44:336–345. doi: 10.1055/s-0033-1358603. [DOI] [PubMed] [Google Scholar]
- Moretti G, Pasquini M, Mandarelli G, Tarsitani L, Biondi M. What every psychiatrist should know about PANDAS: a review. Clin Pract Epidemiol Ment Health. 2008;4:13. doi: 10.1186/1745-0179-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Kurlan R, Leckman J. The immunobiology of Tourette’s disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: a way forward. J Child Adolesc Psychopharmacol. 2010;20:317–331. doi: 10.1089/cap.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman W. Clinical factors associated with Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. J Pediatr. 2012;160:314–319. doi: 10.1016/j.jpeds.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Pathmanandavel K, Starling J, Dale R, Brilot F. Autoantibodies and the immune hypothesis in psychotic brain diseases: challenges and perspectives. Clin Dev Immunol. 2013;2013:1–10. doi: 10.1155/2013/257184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone P, Parano E, Rizzo R, Trifiletti RR. Autoimmune neuropsychiatric disorders associated with streptococcal infection: sydenham chorea, PANDAS, and PANDAS variants. J Child Neurol. 2006;21:727–736. doi: 10.1177/08830738060210091401. [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354:1153–1158. doi: 10.1016/S0140-6736(98)12297-3. [DOI] [PubMed] [Google Scholar]
- Pivonello R, Ferone D, Lombardi G, Colao A, Lamberts SW, Hofland LJ. Novel insights in dopamine receptor physiology. Eur J Endocrinol. 2007;156(Suppl 1):S13–S21. doi: 10.1530/eje.1.02353. [DOI] [PubMed] [Google Scholar]
- Pollack A. Coactivation of D1 and D2 dopamine receptors: in marriage, a case of his, hers, and theirs. Sci STKE. 2004;2004:pe50. doi: 10.1126/stke.2552004pe50. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Mohammad SS, Brilot F, Dale RC. Autoimmune encephalitis: recent updates and emerging challenges. J Clin Neurosci. 2013;21:722–730. doi: 10.1016/j.jocn.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Shannon KM, Fenichel GM. Pimozide treatment of Sydenham’s chorea. Neurology. 1990;40:186. doi: 10.1212/wnl.40.1.186. [DOI] [PubMed] [Google Scholar]
- Singer HS, Giuliano JD, Hansen BH, Hallett JL, Laurino JP, Benson M, Kiessling LS. Antibodies against human putamen in children with Tourette syndrome. Neurology. 1998;50:1618–1624. doi: 10.1212/wnl.50.6.1618. [DOI] [PubMed] [Google Scholar]
- Singer HS, Loiselle CR, Lee O, Minzer K, Swedo S, Grus FH. Anti-basal ganglia antibodies in PANDAS. Mov Disord. 2004;19:406–415. doi: 10.1002/mds.20052. [DOI] [PubMed] [Google Scholar]
- Singer HS, Hong JJ, Yoon DY, Williams PN. Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology. 2005;65:1701–1707. doi: 10.1212/01.wnl.0000183223.69946.f1. [DOI] [PubMed] [Google Scholar]
- Singer HS, Mascaro-Blanco A, Alvarez K, Morris-Berry C, Kawikova I, Ben-Pazi H, Thompson CB, Ali SF, Kaplan EL, Cunningham MW. Neuronal antibody biomarkers for Sydenham’s chorea identify a new group of children with chronic recurrent episodic acute exacerbations of tic and obsessive compulsive symptoms following a streptococcal infection. PLoS ONE. 2015;10:e0120499. doi: 10.1371/journal.pone.0120499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol MS. Infection-triggered anorexia nervosa in children: clinical description of four cases. J Child Adolesc Psychopharmacol. 2000;10:133–145. doi: 10.1089/cap.2000.10.133. [DOI] [PubMed] [Google Scholar]
- Stollerman GH. Rheumatic fever in the 21st century. Clin Infect Dis. 2001;33:806–814. doi: 10.1086/322665. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Carrillo-Reid L, Bargas J. Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience. 2011;198:3–18. doi: 10.1016/j.neuroscience.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE. Sydenham’s chorea: a model for childhood autoimmune neuropsychiatric disorders. J Am Med Assoc. 1994;272:1788–1791. doi: 10.1001/jama.272.22.1788. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Grant PJ. Annotation: PANDAS: a model for human autoimmune disease. J Child Psychol Psychiatry. 2005;46:227–234. doi: 10.1111/j.1469-7610.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Rapoport JL, Cheslow DL, Leonard HL, Ayoub EM, Hosier DM, Wald ER. High prevalence of obsessive-compulsive symptoms in patients with Sydenham’s chorea. Am J Psychiatry. 1989;146:246–249. doi: 10.1176/ajp.146.2.246. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Schapiro MB, Casey BJ, Mannheim GB, Lenane MC, Rettew DC. Sydenham’s chorea: physical and psychological symptoms of St. Vitus dance. Pediatrics. 1993;91:706–713. [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Mittleman BB, Allen AJ, Rapoport JL, Dow SP, Kanter ME, Chapman F, Zabriskie J. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psych. 1997;154:110–112. doi: 10.1176/ajp.154.1.110. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (Pediatric acute-onset neuropsychiatric syndrome) Pediatr Therapeut. 2012;2:2. [Google Scholar]
- Taranta A. Relation of isolated recurrences of Sydenham’s chorea to preceding streptococcal infections. N Engl J Med. 1959;260:1204–1210. doi: 10.1056/NEJM195906112602402. [DOI] [PubMed] [Google Scholar]
- Taranta A, Stollerman GH. The relationship of Sydenham’s chorea to infection with group A streptococci. Am J Med. 1956;20:170–175. doi: 10.1016/0002-9343(56)90186-3. [DOI] [PubMed] [Google Scholar]
- Wolstencroft EC, Simic G, thi Man N, Holt I, Lam le T, Buckland PR, Morris GE. Endosomal location of dopamine receptors in neuronal cell cytoplasm. J Mol Histol. 2007;38:333–340. doi: 10.1007/s10735-007-9106-5. [DOI] [PubMed] [Google Scholar]
- Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban A, Villar G, Lipkin WI. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry. 2010;15:712–726. doi: 10.1038/mp.2009.77. [DOI] [PubMed] [Google Scholar]
- Zabriskie JB. Mimetic relationships between group A streptococci and mammalian tissues. Adv Immunol. 1967;7:147–188. doi: 10.1016/s0065-2776(08)60128-5. [DOI] [PubMed] [Google Scholar]
- Zabriskie JB. Rheumatic fever: the interplay between host, genetics, and microbe. Lewis A. Conner memorial lecture. Circulation. 1985;71:1077–1086. doi: 10.1161/01.cir.71.6.1077. [DOI] [PubMed] [Google Scholar]
- Zabriskie JB, Hsu KC, Seegal BC. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970;7:147–159. [PMC free article] [PubMed] [Google Scholar]