Abstract
Lactation embodies a natural model of morphological, neurochemical, and functional brain plasticity. In this reproductive stage, the hippocampus of the female is less sensitive to excitotoxins in contrast to nulliparity. Growth hormone (GH) and insulin-like growth factor 1 (IGF1) are known to be neuroprotective in several experimental models of brain lesion. Here, activation of the GH–IGF1 pituitary–brain axis following kainic acid (7.5 mg/kg i.p. KA) lesion was studied in lactating and nulliparous rats. Serum concentrations of GH and IGF1 were uncoupled in lactation. Compared to virgin rats, the basal concentration of GH increased up to 40% but IGF1 decreased 58% in dams, and only GH increased further after KA treatment. In the hippocampus, basal expression of GH mRNA was higher (2.8-fold) in lactating rats than in virgin rats. GH mRNA expression in lactating rats increased further after KA administration in the hippocampus and in the hypothalamus, in parallel to GH protein concentration in the hippocampus of KA-treated lactating rats (43% vs lactating control), as detected by Western blot and immunofluorescence. Except for the significantly lower mRNA concentration in the liver of lactating rats, IGF1 expression was not altered by the reproductive condition or by KA treatment in the hippocampus and hypothalamus. Present results indicate upregulation of GH expression in the hippocampus after an excitotoxic lesion, suggesting paracrine/autocrine actions of GH as a factor underlying neuroprotection in the brain of the lactating dam. Since no induction of IGF1 was detected, present data suggest a direct action of GH.
Keywords: lactation, growth hormone, IGF1, hippocampus, neuroprotection, kainic acid
Introduction
In mammals, motherhood is accompanied by diverse adaptations as well as neural and behavioral plasticity, helping the female to cope with the demands of reproduction (1, 2, 3). These adaptations confer neuroprotection to the hippocampus of the dam against excitotoxic damage caused by kainic acid (KA) administration during lactation (4, 5, 6). KA acts as a neurotoxin inducing necrosis and apoptosis by generating reactive oxygen species and mitochondrial function disruption (7) in sensitive zones with high glutamate receptor concentration such as the hippocampus (8, 9).
Lactation is characterized by fluctuation of several hormones including growth hormone (GH) (10). GH is secreted mainly by the anterior pituitary gland, and it is known to regulate development and growth in vertebrates. During lactation, GH and other metabolic hormones increase to modulate the growth and metabolic features of the mammary gland (11), enhancing galactopoiesis in numerous mammalian species (12). GH is also synthesized in several, if not all, extra-pituitary tissues, including the nervous (13), immune (14) and reproductive systems (15). GH (protein or mRNA) is widely distributed in many brain regions, particularly in the hypothalamus (16) and hippocampus (17), both in neurons and in glial cells (18), indicating autocrine/paracrine actions involved in the regulation of neural growth, development and differentiation as well as in neurotransmission, behavior, neuroprotection and neuroplasticity (19, 20, 21).
Neuroprotective actions of GH have been studied in response to trauma, stroke and hypoxic–ischemic brain injury. GH enhances tissue repair and the recovery of some neuronal functions (22, 23). These actions can take place by binding of GH to its specific receptor (GHR), which is present in different regions of the CNS (24, 25). GH binding can have a direct effect on the target cell (26) or can be mediated by the induction of IGF1 synthesis (27, 28), which has been implied as neuroprotective.
To date, it remains unknown whether GH and IGF1 expression in the hippocampus and hypothalamus is modified by lactation, and whether these hormones could have a role as neurotrophic factors in neuroprotection after acute damage induced by KA lesion. Thus, in this study, we determined the expression of the GH–IGF1 system (mRNA and protein) in brain regions vulnerable to excitotoxic injury as well as their serum concentration.
Materials and methods
Animals
Adult virgin (200–250 g) or pregnant (250 g; 18 days) female Wistar rats were housed individually under a 12:12-h light/darkness cycle, with controlled temperature and lighting conditions, and food and water available ad libitum. One day after parturition, litter sizes were culled to ten. Mothers were used for experiments on postpartum (pp) days 14–19. Vaginal smears of virgin rats were followed for at least four estrous cycles. The Institutional Animal Care and Use Committee of the Institute of Neurobiology at UNAM approved all experimental protocols. Animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental design
One group of nulliparous or lactating rats (n = 11 per group) received 7.5 mg/kg of KA (Sigma-Aldrich) i.p. (nulliparous-KA and lactating-KA) or vehicle (nulliparous-vehicle, lactating-vehicle) and were killed 24 h later by an i.p. overdose of 20% urethane (2–3 mL) (4, 29). The pituitary gland, hypothalamus, hippocampus and liver were quickly dissected, frozen on dry ice and kept at −70°C until further processing. Whole blood was taken for serum measurements. Other groups of rats with similar experimental treatments were processed and perfused (see below) for histological analysis. We characterized hypothalamic damage by TUNEL and Fluoro-Jade C staining, and we observed high apoptosis and neurodegeneration in the arcuate nucleus of nulliparous rats compared to lactating rats (unpublished data).
Determination of GH and IGF1 concentration by ELISA
Trunk blood was immediately collected in tubes for serum isolation (BD Vacutainer, BD Diagnostics, NJ, USA). Blood was centrifuged (3000 g at 4°C for 15 min) to obtain serum. Aliquots were stored at −70°C. Serum GH was measured by competitive enzyme-linked immunosorbent assay (ELISA), as previously described (30). Briefly, 12 ng of rat recombinant GH (National Hormone and Peptide Program, NIDDKD, San Diego, CA, USA) were used to coat microtiter plates overnight at 4°C. After washing, we added the standards or samples (20 µL of serum). The primary antibody, a rabbit polyclonal anti-rat GH (Millipore), was used at a final dilution of 1:60,000. Later, the secondary antibody, a horseradish peroxidase-anti-rabbit immunoglobulin G conjugate (Invitrogen) was used at a dilution of 1:3000 in 1% (w/v) nonfat dry milk in TPBS (0.01 M sodium phosphate, 0.15 mM NaCl, 0.05% w/v Tween 20, pH 7). Bound secondary antibodies were then detected by reaction with HRP substrate (2,20-amino-di-[3-ethyl-benzothiazoline sulfate] (ABTS); Roche Diagnostics). All determinations were performed in triplicate. The inter- and intra-assay coefficients of variation were <4%.
GH immunoreactivity in tissue extracts from the pituitary, hypothalamus and hippocampus was detected as reported elsewhere (30) with minor modifications. Volumes of 400 µL for the hippocampus and 200 µL for the pituitary and hypothalamus of 0.1 M Tris-buffered saline (pH 10.3) containing Complete Protease Inhibitor Cocktail (Roche Diagnostics) and Teflon pistil were used to homogenate the tissue during 1 min on ice. Later, total proteins (0.3 µg for pituitary, 30 µg for hippocampus, and 50 µg for hypothalamus) were subjected to competitive ELISA as described earlier.
Serum-free IGF1 was quantified using a sandwich ELISA kit (ALPCO Immunoassays, Salem, NH, USA) according to the manufacturer’s instructions. Determinations were performed in duplicate, using 50 µL of serum (diluted 1:400 in sample buffer) per well, with inter- and intra-assay coefficients of variation <5%. For measurement of IGF1 content in the tissue, we followed the previously reported method (30) with minor modifications. In short, 400 μL of cold 1X RIPA buffer (Cell Signaling Technology) supplemented with Complete were added to liver fragments (~5 mm3) and the suspension was homogenized 5 times and centrifuged at 12,000 g for 20 min at 4°C. The supernatant was transferred to a new tube to remove significant lipid fraction, and it was subjected to a second centrifugation for 10 min. For whole hippocampus and hypothalamus, 150 μL of cold RIPA buffer supplemented with Complete Protease Inhibitor Cocktail were added and homogenized as described earlier, with one centrifugation at 12,000 g for 20 min at 4°C. The supernatant was aliquoted and stored at −70°C. Protein content of the three tissues was quantified by a microassay procedure based on the Bradford method following the supplier’s instructions (Bio-Rad). Total soluble protein amounts (15 μg for the hypothalamus, 25 μg for the hippocampus, and 5 μg for the liver) were subjected to ELISA as described earlier, and results were plotted as ng of IGF1 per μg of tissue protein.
GH and IGF1 gene expression: RNA extraction and cDNA synthesis
Total RNA was extracted from whole hypothalamus, hippocampus, pituitary and liver pieces (5 mm3) per experimental individual using PureLink RNA mini kit (Ambion, Life Technologies). Genomic DNA contamination was removed from the column with the PureLink DNase (Invitrogen, Life Technologies). First-strand cDNAs were synthesized from 2 µg total RNA using 100 U of Superscript II reverse transcriptase (Invitrogen, Life Technologies), 0.5 µg oligo d(T), 0.5 µg random hexamers and 1 mM dNTPs for 50 min at 42°C followed by 15 min at 70°C.
Quantification of GH and IGF1 mRNA gene expression
The relative abundance of GH and IGF1 mRNA expression was normalized with the endogenous reference genes hypoxanthine phosphoribosyl-transferase (HPRT) (31) and ribosomal protein S18 (RPS18) (32). Specific oligonucleotides were designed using Primer 7 software (Cascade, CO, USA) (Table 1). All real-time PCR reactions were carried out in a Roche LigthCycler 2.0 instrument (Roche Diagnostics) and using LigthCycler FastStart DNA Master SYBR Green I (Roche) in a final volume of 10 µL containing: 3 µL of cDNA (dilution 1:3 for hypothalamus and hippocampus, 1:5 for pituitary gland and 1:10 for liver), 2.5 mM MgCl2 and 0.5 µM of each primer. The reactions were performed under the following conditions: initial denaturation at 95°C for 10 min followed by 40 cycles of 95°C for 10 s, 65°C (for GH and IGF1) or 60°C (for HPRT and RPS18 oligonucleotides) for 10 s and 72°C for 15 s. The dissociation curve was run after each real-time PCR experiment to ensure that there was only one amplification product. Finally, the amplified product was sequenced to verify its identity. The relative expression of GH and IGF1 mRNAs was calculated using the comparative threshold cycle (CT) method and employing the formula 2−∆∆CT (33), where quantification is expressed relative to the geometric mean of HPRT and RPS18 (34).
Table 1.
Oligonucleotide primer sequences used in this study.
| Primer name | Synthesis direction | Sequence (5′–3′) |
|---|---|---|
| rGHqf | Forward | GGCCCAGCAGAGAACTGACAT |
| rGHqr | Reverse | ATCAGAGCCTGGATGCCCTC |
| rIGF1qf | Forward | ACCTTGCAAAAGTGGTCCTG |
| rIGF1qr | Reverse | AGGAATTTAGTGCAACCGAA |
| rHPRTqf | Forward | GACCGGTTCTGTCATGTCG |
| rHPRTqr | Reverse | ACCTGGTTCATCATCACTAATCAC |
| rRPS18qf | Forward | TTCAGCACATCCTGCGAGTA |
| rRPS18qr | Reverse | TTGGTGAGGTCAATGTCTGC |
Histology
Rats were deeply anesthetized with a urethane overdose and transcardially perfused with 4% paraformaldehyde (Sigma-Aldrich) in phosphate-buffered saline (PBS) (pH 9.5, 10°C). Brains were removed, postfixed overnight and cryoprotected in 30% sucrose-PBS solution at 4°C. Coronal sections (30 μm thick) were cut along the dorsal hippocampus on a freezing microtome (Leica 2000R), and five series were collected and stored in cryoprotectant solution (30% ethylene glycol and 20% glycerol in PBS) at −20°C. One series was analyzed using each of the staining methods.
Immunohistochemistry
Free-floating tissue sections were incubated with rabbit antisera raised against pure rat GH (1:5000; Millipore) at 4°C for 48 h in potassium phosphate-buffered saline (KPBS). The bound primary antibody was detected by using a Cy3-goat anti-rabbit IgG secondary antibody (Abcam; 1:5000), the labeled sections were placed with DAPI (Sigma, D-9542, at a final concentration of 0.1 µg/mL) stocks solution and washed. The sections were mounted, dehydrated and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA, USA). Microphotographs of the dorsal hippocampus (CA1 and CA3 (not shown) and dentate gyrus subfields) were obtained with a confocal microscope (Zeiss LSM 780).
Statistical analysis
Values were averaged by group and analyzed by GraphPad Prism, version 6.01 (GraphPad Software) to conduct a two-way ANOVA to determine whether factors, such as reproductive status and treatment, conferred statistical differences among groups. Then, a post hoc analysis was performed with the Tukey’s test. Pair comparison was estimated using Student’s t-test (statistical significance was established at P < 0.05). Data were plotted on bar graphs and data are expressed as mean ± s.e.m.
Results
Systemic GH–IGF1 axis is distinctly modulated by reproductive status and KA insult
Serum concentration of GH (Fig. 1A) and free IGF1 (Fig. 1B) were determined in vehicle- or KA-treated nulliparous and lactating rats, respectively. Basal serum GH increased significantly by lactation (40%) as compared to virgin rats (P < 0.05), and it increased further after KA treatment (72%, P < 0.001 vs virgin rats). The two-way ANOVA confirmed that the reproductive condition affected the response to the treatment (F(1,26) = 25.52; P < 0.0001), showing enhanced GH release in lactating rats. In contrast (Fig. 1B), the free IGF1 serum concentration in both control and KA-treated lactating rats significantly decreased by 58% (P < 0.01 vs nulliparous-vehicle) and 48% (P < 0.05 vs nulliparous-KA), respectively. The two-way ANOVA showed significant differences due to the reproductive status, F(1,12) = 31.71; P < 0.0001.
Figure 1.
Serum concentration of GH/IGF1 in virgin and lactating rats after kainic acid (KA) or vehicle administration. White bars represent virgin rats and black bars represent lactating rats. Data are plotted as mean ± s.e.m. (A) Seric concentration of GH; symbols denote *P < 0.05 and ***P < 0.001 vs nulliparous rats from each treatment, by unpaired Student’s t-test n = 7–8. (B) Seric concentration of IGF1; symbols denote *P < 0.05 and **P < 0.01 vs nulliparous rats from each treatment, by unpaired Student’s t-test, n = 4.
Relative GH gene expression in pituitary gland, hippocampus and hypothalamus
Assessment of GH relative gene expression showed a significant decrease of GH mRNA in the pituitary gland (3.12-fold, P < 0.001) and hypothalamus (11.1-fold, P < 0.001) of lactating rats (Fig. 2A and C) compared to virgin rats. The two-way ANOVA for pituitary GH mRNA expression showed significant differences depending on the reproductive status (F(1,32) = 56.06; P < 0.0001) and on the interaction between reproductive status and treatment (F(1,32) = 5.84; P < 0.05). In contrast, lactation triggered a 2.8-fold (P < 0.05) increase in GH mRNA levels in the hippocampus (Fig. 2B).
Figure 2.
Relative gene expression of GH mRNA in virgin and lactating rats after kainic acid (KA) or vehicle administration. White bars represent virgin nulliparous rats and black bars represent lactating rats. Data are plotted as mean ± s.e.m. Fold-change in GH mRNA relative gene expression in (A) pituitary gland, n = 4–5; (B) hippocampus, n = 4–5 and (C) hypothalamus, n = 3–5. Symbols denote *P < 0.05 and ***P < 0.001 vs nulliparous rats from each treatment; + P < 0.05 and ++ P < 0.01 inter-treatment differences by unpaired Student’s t-test.
KA treatment differentially affected GH expression in the analyzed tissues. KA in the pituitary gland of nulliparous rats significantly decreased GH mRNA (1.34-fold) vs nulliparous vehicle (P < 0.05). This response to the excitotoxin was absent in lactating rats. GH mRNA levels in the hippocampus of lactating rats increased 7.6-fold (P < 0.001) after KA vs nulliparous-KA rats and increased 2-fold (P < 0.05) vs lactating control (Fig. 2B). The two-way ANOVA showed significant differences based on reproductive status (F(1,32) = 28.66; P < 0.0001) and on the interaction between reproductive status and treatment (F(1,32) = 6.94; P < 0.05). No lesion effects were detected in the hippocampus of nulliparous rats.
KA injection induced a significant 3.3-fold (P < 0.01) decrease in GH mRNA relative gene expression in the hypothalamus of virgin rats (Fig. 2C) compared to virgin-vehicle group, while KA administration to lactating rats induced a 4.2-fold (P < 0.01) increase in GH mRNA relative gene expression vs the lactating-vehicle group. The two-way ANOVA showed the significant effects of reproductive status, (F(1,30) = 14.81; P < 0.001), treatment (F(1,30) = 4.40; P < 0.05) and the interaction between both factors (F(1,30) = 19.82; P < 0.0001).
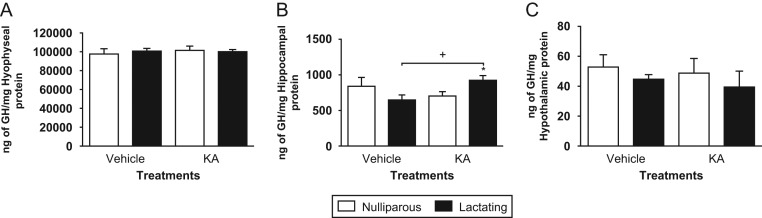
In order to correlate GH protein content to GH mRNA in the analyzed tissues, concentration of GH, as determined by ELISA, was normalized to total protein in the pituitary gland, hippocampus and hypothalamus of virgin and lactating rats (Fig. 3). Pituitary and hypothalamic GH remained unchanged regardless of the treatments (Fig. 3A and C), but KA treatment significantly increased GH content in the hippocampus of lactating rats (43%, P < 0.05) compared to lactating control (Fig. 3B). The two-way ANOVA showed an interaction between reproductive status and treatment factors (F(1,12) = 5.99; P < 0.05).
Figure 3.
Protein content of GH in virgin and lactating rats after kainic acid (KA) treatment or vehicle administration. White bars represent nulliparous rats and black bars represent lactating rats. Data are plotted as mean ± s.e.m. (A) Pituitary gland concentration of GH, n = 4, (B) hippocampal concentration of GH, n = 4 and (C) hypothalamic concentration of GH, n = 3–4. Symbols denote *P < 0.05 vs nulliparous rats from each treatment; + P < 0.05 inter-treatment differences by unpaired Student’s t-test.
Relative gene expression of IGF1 mRNA and protein in liver, hippocampus and hypothalamus
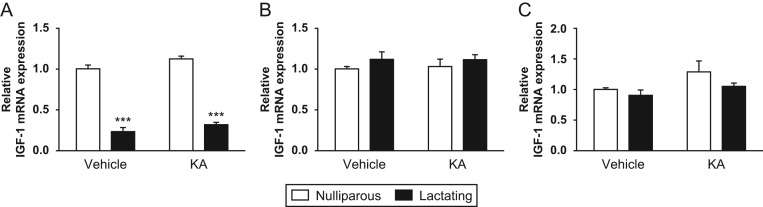
To determine whether IGF1 would increase in parallel with GH and thus mediate its effects, we quantified IGF1 mRNA levels in the main site of synthesis, the liver, as well as in the hippocampus and hypothalamus of rats in the different experimental groups. Hepatic levels of IGF1 were influenced by the reproductive status. The liver of lactating rats showed significantly reduced levels of IGF1, both in control (4.3-fold, P < 0.001) and in KA-treated (4.8-fold, P < 0.001) groups compared to corresponding nulliparous groups (Fig. 4A). The two-way ANOVA showed significant differences based on reproductive status (F(1,12) = 380.63; P < 0.0001) and treatment (F(1,12) = 6.62; P < 0.05). No differences were observed between relative gene expression of IGF1 in the hippocampus or hypothalamus based on the reproductive condition or KA treatment (Fig. 4B and C).
Figure 4.
Relative gene expression of IGF1 mRNA in female rats after kainic acid (KA) treatment or vehicle administration. White bars represent nulliparous rats and black bars represent lactating rats. Data are plotted as mean ± s.e.m. Fold change in IGF1 mRNA relative gene expression in (A) liver n = 4; (B) hippocampus, n = 5 and (C) hypothalamus, n = 4–5. Symbols denote ***P < 0.001 vs nulliparous rats from each treatment, by unpaired Student’s t-test.
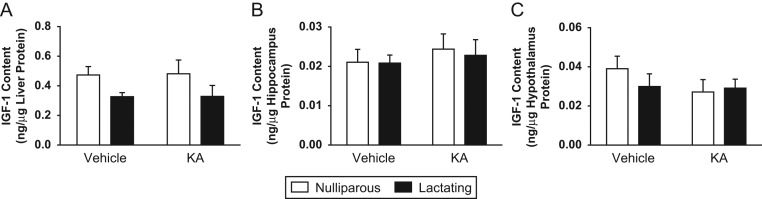
Quantification by ELISA showed no significant changes in IGF1 content normalized to hepatic protein in response to the reproductive condition or KA treatment (Fig. 5A). No changes in IGF1 concentration were detected in the hippocampus or in the hypothalamus; and as expected, IGF1 content was much lower than that in the liver (Fig. 5B and C).
Figure 5.
Protein content of IGF1 in female rats after kainic acid (KA) treatment or vehicle administration. White bars represent nulliparous rats and black bars represent lactating rats. Data are plotted as mean ± s.e.m. (A) Hepatic concentration of IGF1, n = 4. (B) Hippocampal concentration of IGF1, n = 3–4. (C) Hypothalamic concentration of IGF1, n = 4.
GH immunolocalization
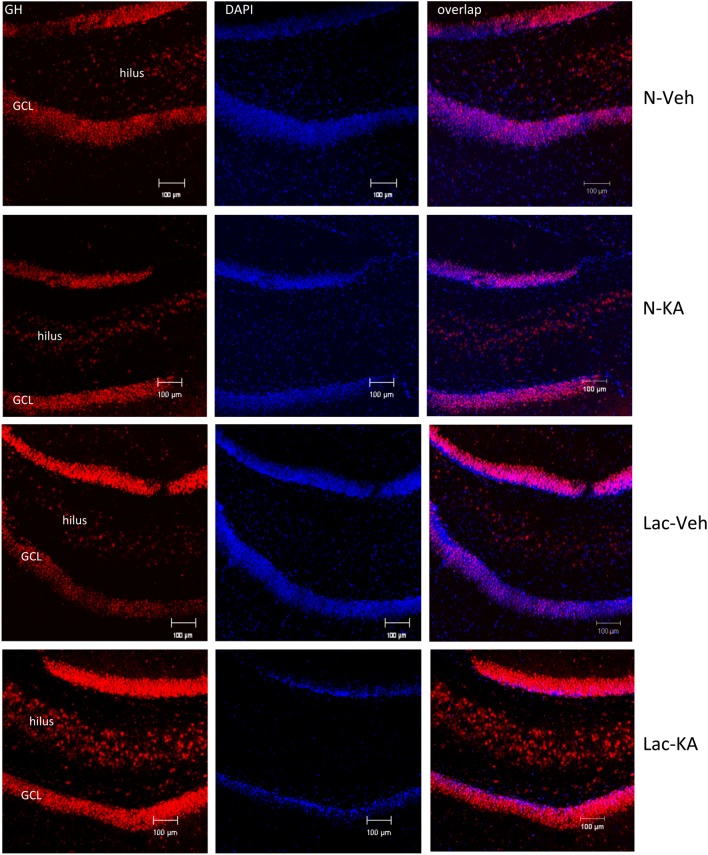
The immunohistochemical analysis revealed that GH immunoreactivity increased in the dentate gyrus of lactating rats treated with KA. Fig. 6 (upper panels) shows similar immunoreactivity and distribution that labeling for GH is in the granular cell layer (GCL) and hilus in dentate gyrus of nulliparous rats that received KA or vehicle, whereas KA strongly upregulated GH immunofluorescence in lactating dams (lower panels, Fig. 6), and similar results were observed in CA1 and CA3 (data no shown).
Figure 6.
GH immunoreactivity (GH-IR) located in the dentate gyrus of the hippocampus of female rats after kainic acid (KA) or vehicle (Veh) administration. Representative confocal microphotographs stained for GH and secondary antibody goat anti-rabbit IgG Cy3 (red), counterstained with DAPI (blue) and merged for the different experimental groups (N, nulliparous; Lac, lactating rats). Increased GH-IR was detected in the hippocampus of lactating KA rats. Scale bar = 100 µm.
Discussion
The present research aimed to study responses of the GH/IGF1 axis during lactation and after a brain lesion induced by excitotoxins, which would suggest involvement in neuroprotective actions (2, 5, 35, 36). Findings showed that GH/IGF1 circulating levels were uncoupled. Serum GH increased, while IGF1 concentration decreased in lactating rats. Suckling stimulation provided by the litter produces a rapid and transient increase of plasmatic GH in lactating rats on 9–10 pp (37). This increase was observed throughout lactation, since we employed dams on 14–19 pp. Another study has shown increased plasma GH in rats during pregnancy but returned to basal levels in early lactation (day 8 pp). Moreover, plasma IGF1 decreased in pregnancy and remained unchanged in early lactation (38). These results are in contrast with ours, perhaps due to the stage of lactation: early vs late lactation (days 14–19), but the present study include diestrus virgin rats to compare GH or IGF1 levels to those of lactating rats (against non-lactating postpartum day 8 rats used by Escalada and coworkers (38). However, another study (39) showed that serum IGF1 concentrations and hepatic mRNA both decreased in mid-pregnancy and early lactation in mice, in contrast to GH levels in both reproductive stages; this reinforces our results regarding solely to divergence observed between increased serum GH during late lactation and decreased serum IGF-1 in this period. The contribution of peripheral GH to brain tissue levels is important because transport of this hormone through the blood–brain barrier (BBB) has been shown (19, 40). Moreover, KA excitotoxic damage increases the permeability of BBB in the hippocampus (41), which would allow circulating GH into the brain.
The GH/IGF1 ratio observed during middle and late lactation has been suggested as a GH-resisting state by Escalada and coworkers (38), who also found reduction in liver GHR mRNA in pregnant and early lactating rats. In agreement with our results, serum IGF1 on lactation days 12–14 has been reported as low as those of virgin rats (42). This GH-resisting state could explain the high GH/low IGF1 ratio found in the present study, and we hypothesize this could be due to the increased metabolic demands in lactating rats. Differences in the GH/IGF1 ratio due to changes in the metabolism have been demonstrated in fasting models where serum GH increased while levels of IGF1 are diminished (43).
Nevertheless, present results showed low relative GH gene expression and no effect of acute excitotoxic treatment in the pituitary gland of lactating rats (Fig. 2A). The lack of relationship between plasma and pituitary GH could result from a negative feedback of high levels of circulating GH upon its own transcription via negative short-loop regulation through hypothalamic somatostatin (SST, 44). In nulliparous rats, GH mRNA decreased after KA treatment, suggesting more sensitivity of the pituitary gland to excitotoxic damage (29). In contrast, GH content in the pituitary was not altered by lactation or KA administration. Escalada and coworkers (38) showed that pituitary GH content in early lactation was lower than in non-suckling rats, and they used a semi-quantitative method to show that GH mRNA did not undergo significant modifications. In the present study, a quantitative method was utilized to determine GH mRNA levels. GH expression in lactating rats was lower than that in virgin rats.
Interestingly, significant changes in GH expression were observed in the hippocampus. GH mRNA in the whole hippocampus of lactating rats was higher than that of nulliparous rats. Relative GH mRNA amount particularly increased after acute KA treatment in the hippocampus of lactating rats. In addition, we found that GH immunoreactivity augmented in the dentate gyrus of lactating rats treated with KA (Fig. 6), reflecting a high relative GH mRNA expression in the hippocampus. In previous studies, we found that the main areas of the dorsal hippocampus affected by KA in nulliparous rats were the CA1 and the hilus of the dentate gyrus, with high levels of apoptosis and neurodegeneration, in contrast to hippocampus of the lactating dams (2, 4, 19). Thus, these data suggest that GH may play an autocrine/paracrine role as a survival factor (19, 45), possibly interacting with the positive regulation of hippocampal GH expression by estradiol (46). Concerning GHR, its expression in the hippocampus of male rats (18) has been reported, as well as changes in GHR expression in peripheral tissues during pregnancy and lactation (38, 47). However, further analysis of GHR through lactation in murine hippocampus is needed.
The potential role or synergism of GH with other mitogens in the recovery of neuronal injury is not fully elucidated (48). Studies in male rats have shown that acute KA and exogenous GH stimulate the proliferation of hippocampal precursors and upregulate local GH mRNA and protein expression (48). Our results provide evidence of the possible role of local hippocampal GH expression as one of the factors underlying neuroprotection in lactation, but further research on the molecular mechanisms that regulate GH gene transcription in this tissue is required.
Regarding GH mRNA in the hypothalamus, both basal and KA-induced levels of expression were different depending on the reproductive status. Basal level was high in nulliparous rats and low in lactating rats. Meanwhile, KA lesions provoked a reduction in nulliparous rats and a 4.2-fold increase in lactating rats. In parallel, we quantified GH protein content in the hypothalamus and did not find changes. This can be explained in part by the low expression level of GH protein per total protein tissue, which was <1% of that found in the pituitary gland.
A primary GH action is the promotion of IGF1 synthesis and release, which mediates some effects of GH (49). Circulating concentration and hepatic expression of IGF1 mRNA were decreased in lactating-vehicle and lactating-KA groups compared to virgin rats. Moreover, the acute KA injection had no effect on reproductive condition. Unlike GH in the hippocampus of lactating rats, we observed no changes in hippocampal IGF1 synthesis in the experimental groups. This result indicates that potential GH action during hippocampal neuroprotection would be direct and non-mediated by IGF1 (50). Similarly, neuroprotective effects of GH in the hypoxic-ischemic brain injury model are not mediated by IGF1 (26). Nevertheless, chronic treatment with GH caused an augmented synthesis of IGF1 mRNA in the hypothalamus and hippocampus of male rats, along with cell survival and neuroprotective pathway activation (51). Moreover, intrahippocampal administration of IGF1 with KA showed neuroprotective effects (52). It has been shown that GH has protective effects on the cerebellum of chicken embryos subjected to hypoxia-ischemia conditions by increasing the concentration of survival factors such as Bcl-2 and p-Akt (53). Furthermore, administration of GH, or its local overexpression, promotes cell survival and IGF1 expression in quail retinal ganglion cell lines (QNR/D) exposed to excitotoxic conditions (54). Also, both endogenous GH and IGF1 expression were significantly increased in the iguana neuroretina in response to KA injury, and it was shown that GH was able to exert, either directly or mediated by IGF1, a neuroprotective action against excitotoxic damage (55).
Conclusion
Present studies demonstrate that the GH/IGF1 axis responds to lactation and acute KA lesions. The later upregulates GH mRNA and protein expression in the hippocampus, particularly in the dentate gyrus, without modifying IGF1 expression. This suggests that local GH might play a role in hippocampal neuroprotection against excitotoxic damage. Further analysis is needed to quantify the presence and anatomical distribution of GHR in the hippocampus of the lactating rat, and the intracellular pathways that mediate its actions. Consideration should be given to the crosstalk with prolactin signaling, since GH seems to signal via the prolactin receptor to promote the migration of neurogenic radial glia from fetal human forebrains (56).
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by UNAM-DGAPA-PAPIIT: IN202315, IN204718; IN206115, and IN201817.
Acknowledgements
The authors thank Martha Carranza, Dr Anaid Antaramian, Adriana González-Gallardo and Nydia Hernández for their technical support; Dr Mauricio Díaz-Muñoz for his laboratory facilities; Dr Martín García and Dr Alejandra Castilla for animal care and Jessica González Norris for editing the manuscript.
References
- 1.Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Archives of Sexual Behavior 2008. 37 43–56. ( 10.1007/s10508-007-9277-x) [DOI] [PubMed] [Google Scholar]
- 2.Morales T. Recent findings on neuroprotection against excitotoxicity in the hippocampus of female rats. Journal of Neuroendocrinology 2011. 23 994–1001. ( 10.1111/j.1365-2826.2011.02141.x) [DOI] [PubMed] [Google Scholar]
- 3.Hillerer KM, Jacobs VR, Fischer T, Aigner L. The maternal brain: an organ with peripartal plasticity. Neural Plasticity 2014. 2014 574159 ( 10.1155/2014/574159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanoye-Carlo A, Morales T, Ramos E, Mendoza-Rodríguez A, Cerbón M. Neuroprotective effects of lactation against kainic acid treatment in the dorsal hippocampus of the rat. Hormones and Behavior 2008. 53 112–123. ( 10.1016/j.yhbeh.2007.09.004) [DOI] [PubMed] [Google Scholar]
- 5.Cabrera V, Cantú D, Ramos E, Vanoye-Carlo A, Cerbón M, Morales T. Lactation is a natural model of hippocampus neuroprotection against excitotoxicity. Neuroscience Letters 2009. 461 136–139. ( 10.1016/j.neulet.2009.06.017) [DOI] [PubMed] [Google Scholar]
- 6.Cabrera V, Ramos E, González-Arenas A, Cerbón M, Camacho-Arroyo I, Morales T. Lactation reduces glial activation induced by excitotoxicity in the rat hippocampus. Journal of Neuroendocrinology 2013. 25 519–527. ( 10.1111/jne.12028) [DOI] [PubMed] [Google Scholar]
- 7.Djebaili M, Lerner-Natoli M, Pascale M, Baille V, Bockaert J, Rondouin G. Molecular events involved in neuronal death induced in the mouse hippocampus by in-vivo injection of kainic acid. Molecular Brain Research 2001. 93 190–198. ( 10.1016/S0169-328X(01)00197-8) [DOI] [PubMed] [Google Scholar]
- 8.Wisden W, Seeburg P. A complex mosaic of high-affinity kainate receptors in rat brain. Journal of Neuroscience 1993. 13 3582–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollard H, Charriaut-Marlangue C, Cantagrel S, Represa A, Robain O, Moreau J, Ben-Ari Y. Kainate-induced apoptotic cell death in hippocampal neurons. Neuroscience 1994. 63 7–18. ( 10.1016/0306-4522(94)90003-5) [DOI] [PubMed] [Google Scholar]
- 10.Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. Journal of Mammary Gland Biology and Neoplasia 2002. 7 49–66. ( 10.1023/A:1015770423167) [DOI] [PubMed] [Google Scholar]
- 11.Harvey S. Extrapituitary growth hormone. Endocrine 2010. 38 335–359. ( 10.1007/s12020-010-9403-8) [DOI] [PubMed] [Google Scholar]
- 12.Trott JF, Vonderhaar BK, Hovey RC. Historical perspectives of prolactin and growth hormone as mammogens, lactogens and galactagogues-Agog for the future! Journal of Mammary Gland Biology and Neoplasia 2008. 13 3–11. ( 10.1007/s10911-008-9064-x) [DOI] [PubMed] [Google Scholar]
- 13.Arámburo C, Alba-Betancourt C, Luna M, Harvey S. Expression and function of growth hormone in the nervous system: a brief review. General and Comparative Endocrinology 2014. 203 35–42. ( 10.1016/j.ygcen.2014.04.035) [DOI] [PubMed] [Google Scholar]
- 14.Weigent DA. High molecular weight isoforms of growth hormone in cells of the immune system. Cellular Immunology 2011. 271 44–52. ( 10.1016/j.cellimm.2011.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luna M, Martínez-Moreno CG, Ahumada-Solórzano MS, Harvey S, Carranza M, Arámburo C. Extrapituitary growth hormone in the chicken reproductive system. General and Comparative Endocrinology 2014. 203 60–68. ( 10.1016/j.ygcen.2014.02.021) [DOI] [PubMed] [Google Scholar]
- 16.Addison ML, Rissman EF. Sexual dimorphism of growth hormone in the hypothalamus: regulation by estradiol. Endocrinology 2012. 153 1898–1907. ( 10.1210/en.2011-1982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang H, Zhang Y, Yu X, Song J, Xu C, Wan Y. Changes in growth hormone (GH), GH receptor, and GH signal transduction in hippocampus of congenital hypothyroid rats. Journal of Neuroscience Research 2011. 89 248–255. ( 10.1002/jnr.22540) [DOI] [PubMed] [Google Scholar]
- 18.Lobie PE, García-Aragón J, Lincoln DT, Barnard R, Wilcox JN, Waters MJ. Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Brain Research: Developmental Brain Research 1993. 74 225–233. ( 10.1016/0165-3806(93)90008-X) [DOI] [PubMed] [Google Scholar]
- 19.Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Scientific World Journal 2006. 6 53–80. ( 10.1100/tsw.2006.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aberg D. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocrine Development 2010. 17 63–76. ( 10.1159/000262529) [DOI] [PubMed] [Google Scholar]
- 21.Isgaard J, Aberg D, Nilsson M. Protective and regenerative effects of the GH/IGF-I axis on the brain. Minerva Endocrinologica 2007. 32 103–113. [PubMed] [Google Scholar]
- 22.Arce VM, Devesa P, Devesa J. Role of growth hormone (GH) in the treatment on neural diseases: from neuroprotection to neural repair. Neuroscience Research 2013. 76 179–186. ( 10.1016/j.neures.2013.03.014) [DOI] [PubMed] [Google Scholar]
- 23.Devesa J, Reimunde P, Devesa P, Barberá M, Arce V. Growth hormone (GH) and brain trauma. Hormones and Behavior 2013. 63 331–344. ( 10.1016/j.yhbeh.2012.02.022) [DOI] [PubMed] [Google Scholar]
- 24.Harvey S, Lavelin I, Pines M. Growth hormone (GH) action in the brain: neural expression of a GH-response gene. Journal of Molecular Neuroscience 2002. 18 89–95. ( 10.1385/JMN:18:1-2:89) [DOI] [PubMed] [Google Scholar]
- 25.Schneider HJ, Pagotto U, Stalla GK. Central effects of the somatotropic system. European Journal of Endocrinology 2003. 149 377–392. ( 10.1530/eje.0.1490377) [DOI] [PubMed] [Google Scholar]
- 26.Scheepens A, Sirimanne ES, Breier BH, Clark RG, Gluckman PD, Williams CE. Growth hormone as a neuronal rescue factor during recovery from CNS injury. Neuroscience 2001. 104 677–687. ( 10.1016/S0306-4522(01)00109-9) [DOI] [PubMed] [Google Scholar]
- 27.Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Developmental Neurobiology 2010. 70 384–396. ( 10.1002/dneu.20778) [DOI] [PubMed] [Google Scholar]
- 28.Puche JE, Castilla-Cortázar I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. Journal of Translational Medicine 2012. 10 224 ( 10.1186/1479-5876-10-224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Molecular Neurobiology 2005. 31 3–16. ( 10.1385/MN:31:1-3:003) [DOI] [PubMed] [Google Scholar]
- 30.Arellanes-Licea EC, Báez-Ruiz A, Carranza ME, Arámburo C, Luna M, Díaz-Muñoz M. Daily patterns and adaptation of the ghrelin, growth hormone and insulin-like growth factor-1 system under daytime food synchronisation in rats. Journal of Neuroendocrinology 2014. 26 282–295 ( 10.1111/jne.12145) [DOI] [PubMed] [Google Scholar]
- 31.Xing W, Deng M, Zhang J, Huang H, Dirsch O, Dahmen U. Quantitative evaluation and selection of reference genes in a rat model of extended liver resection. Journal of Biomolecular Techniques 2009. 20 109–115. [PMC free article] [PubMed] [Google Scholar]
- 32.Hvid H, Ekstrøm CT, Vienberg S, Oleksiewicz MB, Klopfleisch R. Identification of stable and oestrus cycle-independent housekeeping genes in the rat mammary gland and other tissues. Veterinary Journal 2011. 190 103–108. ( 10.1016/j.tvjl.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001. 25 402–428. ( 10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 34.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 2002. 3 research0034.1–research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales T, Lorenson M, Walker AM, Ramos E. Both prolactin (PRL) and a molecular mimic of phosphorylated PRL, S179D-PRL, protect the hippocampus of female rats against excitotoxicity. Neuroscience 2014. 258 211–217. ( 10.1016/j.neuroscience.2013.11.015) [DOI] [PubMed] [Google Scholar]
- 36.Tejadilla D, Cerbón M, Morales T. Prolactin reduces the damaging effects of excitotoxicity in the dorsal hippocampus of the female rat independently of ovarian hormones. Neuroscience 2010. 169 1178–1185. ( 10.1016/j.neuroscience.2010.05.074) [DOI] [PubMed] [Google Scholar]
- 37.Wehrenberg WB, Gaillard RC. Neuroendocrine mechanisms regulating growth hormone and prolactin secretion during lactation. Endocrinology 1989. 124 464–467. ( 10.1210/endo-124-1-464) [DOI] [PubMed] [Google Scholar]
- 38.Escalada J, Sánchez-Franco F, Velasco B, Cacicedo L. Regulation of growth hormone (GH) gene expression and secretion during pregnancy and lactation in the rat: role of insulin-like growth factor-I, somatostatin, and GH-releasing hormone. Endocrinology 1997. 138 3435–3443. ( 10.1210/endo.138.8.5342) [DOI] [PubMed] [Google Scholar]
- 39.Travers MT, Madon RJ, Vallance AJ, Barber MC. Circulating concentrations and hepatic expression of IGF-1 during pregnancy and lactation in the mouse. Biochemical Society Transactions 1990. 18 1268. [DOI] [PubMed] [Google Scholar]
- 40.Pan W, Yu Y, Cain CM, Nyberg F, Couraud PO, Kastin AJ. Permeation of growth hormone across the blood-brain barrier. Endocrinology 2005. 146 4898–4904. ( 10.1210/en.2005-0587) [DOI] [PubMed] [Google Scholar]
- 41.Chen ZL, Indyk JA, Bugge TH, Kombrinck KW, Degen JL, Strickland S. Neuronal death and blood-brain barrier breakdown after excitotoxic injury are independent processes. Journal of Neuroscience 1999. 19 9813–9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barber MC, Travers MT, Finley E, Flint DJ, Vernon RG. Growth-hormone-prolactin interactions in the regulation of mammary and adipose-tissue acetyl-CoA carboxylase activity and gene expression in lactating rats. Biochemical Journal 1992. 285 469–475. ( 10.1042/bj2850469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beauloye V, Willems B, de Coninck V, Frank SJ,, Edery M, Thissen JP. Impairment of liver GH receptor signaling by fasting. Endocrinology 2002. 143 792–800. ( 10.1210/endo.143.3.8692) [DOI] [PubMed] [Google Scholar]
- 44.Aguila MC, McCann SM. Growth hormone increases somatostatin release and messenger ribonucleic acid levels in the rat hypothalamus. Brain Research 1993. 623 89–94. ( 10.1016/0006-8993(93)90014-E) [DOI] [PubMed] [Google Scholar]
- 45.Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends in Endocrinology and Metabolism 2001. 12 252–257. ( 10.1016/S1043-2760(01)00423-4) [DOI] [PubMed] [Google Scholar]
- 46.Quinnies KM, Bonthuis PJ, Harris EP, Shetty SR, Rissman EF. Neural growth hormone: regional regulation by estradiol and/or sex chromosome complement in male and female mice. Biology of Sex Differences 2015. 6 8 ( 10.1186/s13293-015-0026-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi K, Tanaka M, Ohkubo T, Yoshizato H, Hanai Y, Fujikawa T, Kaneko H, Nakashima K. Tissue-specific regulation of growth hormone receptor and growth hormone binding protein gene expression during pregnancy and lactation in the rat. Endocrine Journal 1998. 45 S105–S107. ( 10.1507/endocrj.45.Suppl_S105) [DOI] [PubMed] [Google Scholar]
- 48.Devesa P, Reimunde P, Gallego R, Devesa J, Arce VM. Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury. Brain Injury 2011. 25 503–510. ( 10.3109/02699052.2011.559611) [DOI] [PubMed] [Google Scholar]
- 49.Chia DJ. Minireview: mechanisms of growth hormone-mediated gene regulation. Molecular Endocrinology 2014. 28 1012–1025. ( 10.1210/me.2014-1099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathews LS, Norstedt G, Palmiter RD. Regulation of insulin-like growth factor I gene expression by growth hormone. PNAS 1986. 83 9343–9347. ( 10.1073/pnas.83.24.9343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frago LM, Pañeda C, Dickson SL, Hewson AK, Argente J, Chowen JA. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology 2002. 143 4113–4122. ( 10.1210/en.2002-220261) [DOI] [PubMed] [Google Scholar]
- 52.Miltiadous P, Stamatakis A, Stylianopoulou F. Neuroprotective effects of IGF-I following kainic acid-induced hippocampal degeneration in the rat. Cell Molecular Neurobiology 2010. 30 347–360. ( 10.1007/s10571-009-9457-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alba-Betancourt C, Luna-Acosta JL, Ramírez-Martínez CE, Avila-González D, Granados-Ávalos E, Carranza M, Martínez-Coria H, Arámburo C, Luna M. Neuro-protective effects of growth hormone (GH) after hypoxia-ischemia injury in embryonic chicken cerebellum. General and Comparative Endocrinology 2013. 183 17–31. ( 10.1016/j.ygcen.2012.12.004) [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Moreno CG, Ávila-Mendoza J, Wu Y, Arellanes-Licea ED, Louie M, Luna M, Arámburo C, Harvey S. Neuroprotection by GH against excitotoxic-induced cell death in retinal ganglion cells. General and Comparative Endocrinology 2016. 234 68–80. (10.1016/j.ygcen.2016.03.023) [DOI] [PubMed] [Google Scholar]
- 55.vila-Mendoza J, Mora J, Carranza M, Luna M, Arámburo C. Growth hormone reverses excitotoxic damage induced by kainic acid in the green iguana neuroretina. General and Comparative Endocrinology 2016. 234 57–67. ( 10.1016/j.ygcen.2016.04.004) [DOI] [PubMed] [Google Scholar]
- 56.Pathipati P, Gorba T, Scheepens A, Goffin V, Sun Y, Fraser M. Growth hormone and prolactin regulate human neural stem cell regenerative activity. Neuroscience 2011. 190 409–427. ( 10.1016/j.neuroscience.2011.05.029) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a